Abstract
While a negative correlation between reproduction and life span is commonly observed, specialized reproductive individuals outlive their non-reproductive nestmates in all eusocial species, including the honeybee, Apis mellifera (L). The consequences of reproduction for individual life expectancy can be studied directly by comparing reproductive and non-reproductive workers. We quantified the life span consequences of reproduction in honeybee workers by removal of the queen to trigger worker reproduction. Furthermore, we observed the social behavior of large cohorts of workers under experimental and control conditions to test for associations with individual life expectancy. Worker life expectancy was moderately increased by queen removal. Queenless colonies contained a few long-lived workers, and oviposition behavior was associated with a strong reduction in mortality risk, indicating that a reproductive role confers a significant survival advantage. This finding is further substantiated by an association between brood care behavior and worker longevity that depends on the social environment. In contrast, other in-hive activities, such as fanning, trophallaxis, and allogrooming did not consistently affect worker life expectancy. The influence of foraging varied among replicates. An earlier age of transitioning from in-hive tasks to outside foraging was always associated with shorter life spans, in accordance with previous studies. In sum, our studies quantify how individual mortality is affected by particular social roles and colony environments and demonstrate interactions between the two. The exceptional, positive association between reproduction and longevity in honeybees extends to within-caste plasticity, which may be exploited for mechanistic studies.
Keywords: Biodemography, Reproduction, Behavioral profiles, Social evolution, Division of Labor, Mortality dynamics
Introduction
The universal process of aging is increasingly studied in a variety of organisms but our understanding of its causes and consequences remain incomplete. Various studies of the classic gerontological model species, Saccharomyces cerevisiae, Canorhabditis elegans, Drosophila melanogaster, and Mus musculus have revealed a complex determination of life span with numerous interacting genetic and environmental influences (Mackay 2002; Kenyon 2005; de Magalhaes et al. 2009). However, additional studies of long-lived model organisms under natural conditions need to complement the previous progress in classic models to gain a more comprehensive understanding of aging (Finch and Tanzi 1997). Social insects, in particular the honeybee (Apis mellifera), present an attractive alternate model for aging studies (Rueppell et al. 2004; Keller and Genoud 1997).
Honeybees are also an attractive aging model for other reasons, such as the potential to experimentally manipulate social environment and a pronounced aging plasticity across and within different castes (Page and Peng 2001; Omholt and Amdam 2004; Rueppell et al. 2004). The queen is typically the sole reproductive bee of the colony, laying up to 2,000 eggs per day to replace dying workers, expand the colony, and produce sexual individuals of the next generation. Significantly, the queen outlives her daughter and sister workers by an order of magnitude under normal conditions without a systematic genetic difference (Page and Peng 2001). In addition to the queen, honeybee colonies typically contain thousands of female but functionally sterile workers that perform all nonreproductive tasks in and outside the hive (Winston 1987). The life expectancy of these workers is influenced by their social environment, such as the age structure (Rueppell et al. 2008) and colony size (Rueppell et al. 2009), the external environment influencing foraging risks (Rueppell et al. 2007), and individual physiology and behavior (Amdam et al. 2009). Most significantly, worker life span is affected by the timing of their transition from inside tasks to foraging outside of the hive (Rueppell et al. 2007).
Comparatively, little is known about influences on the life expectancy of honeybee queens but several studies have compared the long-lived queens with short-lived workers to identify the mechanisms that underlie this pronounced aging plasticity (Remolina and Hughes 2008). Molecular differences have been found in patterns of gene expression related to oxidative stress (Corona et al. 2005), mitochondrial maintenance (Aamodt 2009), the yolk protein and antioxidant vitellogenin (Corona et al. 2007), and lipid composition (Haddad et al. 2007). Other possible mechanisms may depend on systemic reprogramming of the genome, for example, by differential methylation of DNA during the development of the two female castes (Omholt and Amdam 2004; Kucharski et al. 2008; De Loof 2011; Ford 2012; but see Herb et al. 2012). However, comparisons between the queen and worker castes to identify the underlying causes of their differences in life expectancy are problematic because the two castes differ not only in their mortality risk but also in many other aspects of morphology, physiology, development, and behavior (Rueppell et al. 2004; Page and Peng 2001).
In the absence of a queen, honeybee workers can activate their ovaries and become reproductive (Winston 1987; Hoover et al. 2003). However, the presence of young brood can also repress the reproductive activity of the workers (Backx et al. 2012). Each worker can only produce a small number of the eggs because their ovaries are usually less than 5 % the size of a queen ovary (Rueppell et al. 2011). Furthermore, workers generally lack the ability to produce fertilized eggs and consequently cannot sustain the colony but produce only sons by arrhenotokous parthenogenesis (Winston 1987). Only a portion of workers in a queenless colony become reproductively active while the social homeostasis and cooperation of the colony seem compromised (Page and Robinson 1994). Nevertheless, queenless colonies can persist for prolonged periods (Page and Metcalf 1984).
Compared to queen–worker comparisons, the study of reproductive and nonreproductive workers can result in fewer but functionally more meaningful differences (Grozinger et al. 2007; Hartmann and Heinze 2003) without confounding developmental and morphological influences. Surprisingly, the mortality patterns of reproductive and nonreproductive worker honeybees have not yet been compared. Here we studied the mortality consequences of removing the queen and brood from honeybee colonies to induce worker reproduction. We measured individual behavior and mortality of large experimental cohorts of worker honeybees across the life span in observation hives and corroborated our results with a mortality study in cohorts of workers in multiple small experimental hives. Our results quantify the mortality consequences of brood and queen presence and observations of individual behavior, including reproduction, in worker honeybees.
Methods and material
Small hive experiment
Mature honeybee workers of random age from two full hives of European honeybees, Apis mellifera, were used to set up 21 experimental hives in Greensboro, NC at the beginning of June 2009. The experimental hives consisted of three-frame mating nucs with one frame of honey, one empty frame, and one frame with small amounts of pollen. Directly after introducing approximately 1,000 workers, six hives received a mature, caged queen (Laidlaw and Page 1997), while the other 15 nuclear hives were kept queenless. Nine of the 15 queenless colonies absconded, and the queen in one of the queenright colonies disappeared, leaving five queenright and six queenless experimental colonies. Queen excluder gates were placed in the entrance of each hive to prevent queens from leaving or entering the colonies.
One week after colony establishment, a focal cohort of approximately 125 workers was introduced into each experimental colony. A second focal cohort consisting of 55 workers was introduced 3 weeks later. Both focal cohorts consisted of a random mixture of newly emerged workers from six mature, unrelated hives. After overnight emergence in a temperature- (33 °C) and humidity- (60 % R.H.) controlled incubator, these workers were paint-marked (Testor’s®) and introduced into the experimental colonies. Workers that carried signs of disease or Varroa mites were excluded. The entrance to each hive was blocked for several hours after introduction in order to prevent forcible removal of the newly introduced workers from the hive.
Throughout the ensuing experiment, a mixture of sugar, pollen, and honey was regularly fed to the colonies to keep all experimental hives well-provisioned. Estimates of total colony size were performed weekly and newly emerged workers were added as necessary to keep colony sizes constant. The queens were occasionally released from their cages for short periods of time to maintain comparable brood levels of queenless and queenright colonies. The number of focal workers in each hive was counted daily until less than 10 % remained from the introduced cohorts.
To assess the treatment effect, 37 focal workers from queenright hives and 38 workers from queenless hives were randomly selected for assessing reproductive status by ovary dissections between the third and eighth week of the experiment. Workers were dissected and the ovary activation assessed as previously described (Graham et al. 2011). Based on the size and maturity of the oocytes in the ovaries, workers were labeled as either reproductively “inactive” (individual oocytes not exceeding the width of the ovariole) or “active” (discernible oocytes deforming their ovariole).
Observation hive experiment 1
Prior to the start of the experiment in June 2009, three glass-walled, four-frame observation hives were set up, each containing approximately 3,000 workers of mixed ages. A queen from an existing colony was introduced into one of these hives (Q+), while the other two were kept queenless. One of the queenless hives was provided with a frame of young brood (Q−B), while the other queenless hive (Q−) and the queenright hive were provided with an empty frame instead. Additionally, each hive was provided with honey (three fourths of a frame) and pollen (one eighth of a frame) and one empty frame to provide all three treatment groups with equal food resources and empty cells. Newly constructed queen cells in the Q−B treatment were destroyed as soon as they were perceived.
Two weeks after the setup, newly emerged workers from unrelated colonies in the UNCG bee yard were collected as described above to serve as focal cohorts. In this experiment, 665 workers per treatment were marked within 24 h of emergence by gluing a plastic tag (Graze, Germany) with a unique color/number combination onto the thorax of each bee. Workers that were visibly diseased or observed carrying Varroa mites were excluded. Bees were introduced into the respective colony environment immediately after marking. The entrance to each hive was blocked for 18 h after the introduction in order to prevent forcible removal of the newly introduced workers from the hive. Initial aggression against the introduced bees, such as biting and pulling, was observed but subsided within 2 h.
Queen excluder gates were placed in the entrance of each hive to prevent queen exit or entry. Any accumulated dead drones in front of these gates were manually removed daily to allow workers to pass. Food levels and overall colony sizes were assessed weekly and kept comparable between the three treatments by adding food and young workers to the colonies as needed.
A census of all focal cohort workers present in the hive was performed daily to measure survival. In addition, the following were determined for each worker by repeated scan-sampling of the observation hives: (1) foraging (e.g., performing waggle dance, tremble dances, or entering the ramp of the observation hive), (2) pollen foraging (e.g., carrying visible pollen loads), (3) queen tending, (4) brood care, and (5) ovipositing (e.g., inserting abdomen into an empty cell, sometimes combined with retinue of workers surrounding the reproductive bee). Only stationary workers were considered to be engaged in queen tending (standing on the queen cage) or brood care (standing on capped brood cells or with head inserted into uncapped brood cell) (Stout et al. 2011). When fewer than 5 % of the workers survived, the remaining bees were dissected to determine their ovary activation as described before.
Observation hive experiment 2
At the end of May 2011, six experimental colonies were set up in glass-walled, four-frame observation hives, each containing approximately 5,000 workers of mixed ages. These six hives were organized in two replicates that were housed apart. As before, each replicate included one colony that received a queen from an existing colony (Q+), one colony that was provided with one frame of young brood (Q−B), and a third colony was set up without queen or brood (Q−). All colonies were provided with comparable amounts of honey (three fourths of a frame) and pollen (one eighth of a frame).
As described before, focal cohorts were obtained by transferring ready-to-emerge brood combs from multiple UNCG colonies to a humidity- and temperature-controlled incubator and tagging newly emerged workers within 24 h of emergence for individual identification. Approximately 600 newly emerged workers of healthy appearance were tagged and added to each of the six observation hives. The entrance to each hive was blocked for 18 h after introduction in order to prevent forcible removal of the newly introduced workers from the hive.
Queen excluder gates were placed in the entrance of each hive to prevent queen exits or intrusions. Estimates of colony size and food stores were performed weekly and newly emerged workers and food were added as necessary. Queens were occasionally released from their cages for short periods of time to produce brood in the queenright colonies and keep brood levels comparable between treatment groups.
A census was taken daily of the focal cohort of workers present in each hive. Each day worker behavior was also recorded by repeated scan sampling. In addition to the behaviors recorded in the 2009 observation experiment (foraging, pollen foraging, queen tending, brood care, and ovipositing), (6) fanning, (7) grooming others (allogrooming), (8) trophallaxis (Tezze and Farina 1999), and (9) prolonged inactivity (motionless for >10 min) were also recorded. Ten tagged workers from queenright hives and 23 tagged workers from queenless hives were randomly selected for ovary dissections. Workers were labeled as either reproductively “active” or “inactive” as described above.
Analyses
The survivorship of the focal workers in the small hive experiments was compared between queenless and queenright conditions with a General Linear Model, using treatment as a fixed factor and time as a covariate. In addition, we calculated the age-specific mortality risk of workers in both experimental groups and plotted its smoothed average (5-day window). Fisher’s exact test was used to assess whether there was a significant difference in the ovary development of focal workers in queenless and queenright conditions.
A preliminary log-rank test was conducted to determine whether the data sets from the observation hives could be combined for analysis. Significant differences in survival were found for each treatment (Q+: χ2 = 41.6, p < 0.001; Q−B: χ2 = 37.8, p < 0.001; Q−: χ2 = 43.9, p < 0.001). Therefore, experiment one and each replicate of the second experiment were analyzed separately. We analyzed the influence of the focal workers’ hive environment and specific behavioral activity (for each worker the proportion of observations in a specific behavioral state) on their life expectancy separately using a Cox proportional hazard model. The hazard rate for the age of first foraging (AFF) was calculated in separate analyses for each of the nine cohorts. Fisher’s exact test was used to assess differences in the ovary development between workers from the queenless and queenright hives.
In order to avoid confounding treatment effects with potential injuries caused by the tagging and hive introduction of the focal bees, data collected during the initial 5 days (and in a subset of the 2011A replicate 8 days) were not included in any analyses. This resulted in the following number of individuals included in the data analyses: 2009: Q+ = 51, Q−B = 73, Q− = 94; 2011A: Q+ = 47, Q−B = 71, Q− = 331; 2011B: Q+ = 344, Q−B = 289, Q− = 355. Data for the focal workers randomly selected for ovary dissections or individuals that had not died at the end of the experimental observations were handled as censored data. Analyses were performed in “R” (The R Development Core Team 2005) and SPSS 20.0 (IBM, NY).
Results
Small hive experiment
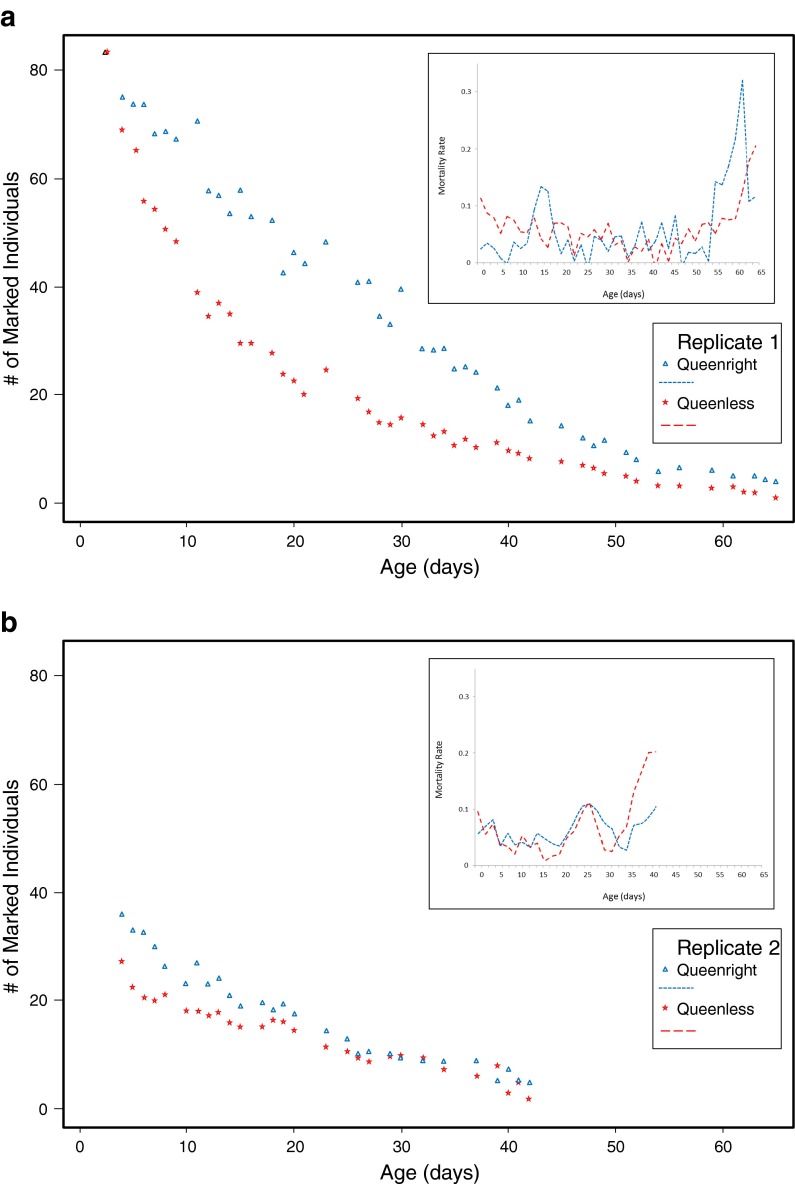
In the first replicate, survivorship differed significantly among queenless and queenright nuclear hive workers (F(1,449) = 25.6, p < 0.001). Visual inspection suggested an initial sharp decline in the number of marked bees in the queenless hives, followed by a period of lower mortality compared to the queenright hives (Fig. 1a). Overall, the worker decline in the second replicate was also significantly affected by treatment (F(1,272) = 3.2, p < 0.001), but worker numbers under queenless conditions declined more slowly (Fig. 1b). Ovary dissections of workers from both cohorts revealed that queenless workers displayed significantly greater ovary activation than workers in the queenright hives (Table 1).
Fig. 1.
Average counts of color-marked workers in small nuclear hives when a queen is present (blue triangles) and in the absence of the queen (red stars) are shown with corresponding average mortality rates (inserts). The first replicate (a) showed an initially rapid decline of workers in the queenless hives, indicated by a high initial mortality rate (insert: red, long-dashed line). This initial effect is also apparent to a lesser degree in the second replicate (b). However, the initial declines do not present an age-related mortality but rather mis-orientation and failure to remain in the experimental hives due to the lack of attractive queen pheromones. At the relevant later ages, mortality in the queenright hives (insert: blue, short-dashed line) generally exceeds the mortality of workers under queenless conditions
Table 1.
Comparison of ovary development in queenright and queenless experimental hives
| Replicate | Hive status | Dissected workers with “inactive” ovaries | Dissected workers with “active” ovaries | Fisher’s exact probability of randomness |
|---|---|---|---|---|
| Nuclear hives | Queenright | 31 | 6 | p = 0.039 |
| Queenless | 23 | 15 | ||
| 2009 Observation hives | Queenright | 14 | 2 | p = 1.000 |
| Queenless | 10 | 1 | ||
| 2011A observation hives | Queenright | 8 | 0 | p = 0.029 |
| Queenless | 4 | 5 | ||
| 2011B observation hives | Queenright | 2 | 0 | p = 0.500 |
| Queenless | 8 | 6 | ||
| Observation hives combined | Queenright | 24 | 2 | p = 0.015 |
| Queenless | 22 | 12 |
Observation hive experiments
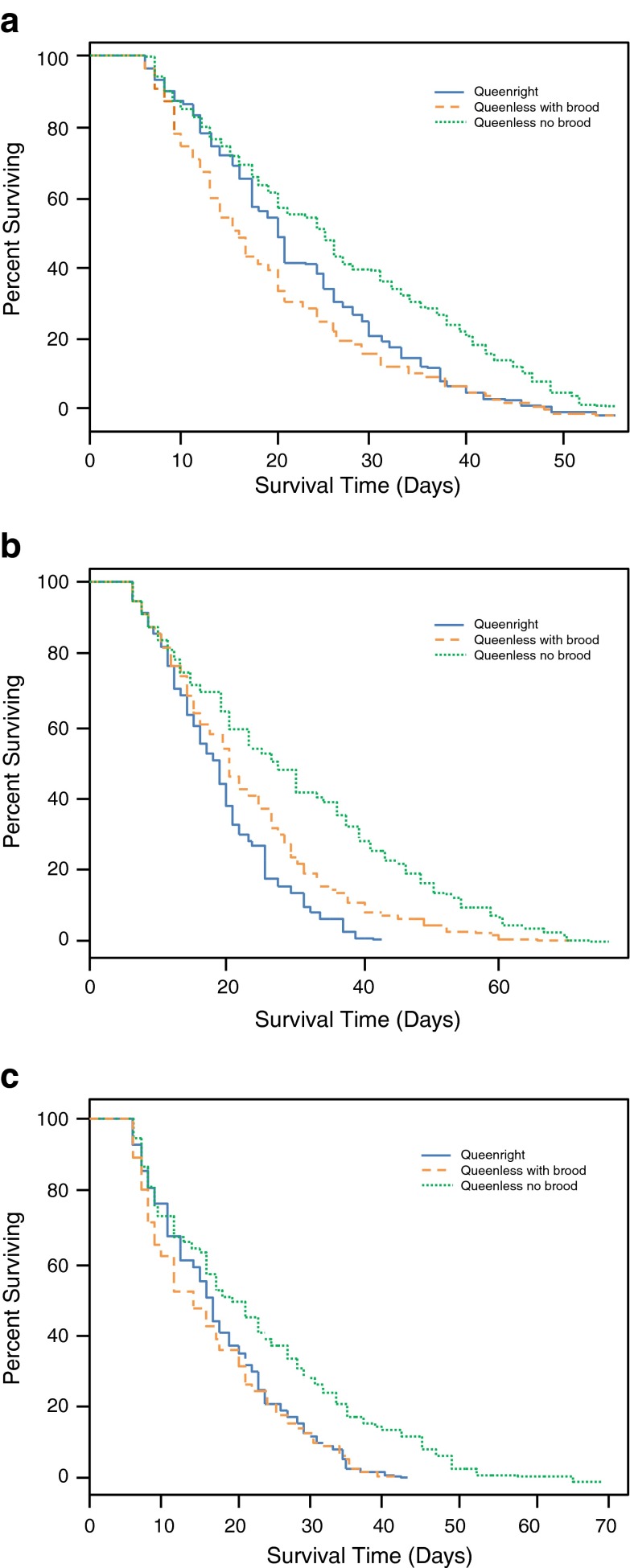
In 2009, workers in the queenless colony without initial brood demonstrated lower mortality rates than workers in the queenright colony (hazard ratio: HR = 0.67, p = .009), while workers in the queenless colony with initial brood exhibited higher mortality rates (HR = 1.2, p = .009) compared to the workers in the control environment (Fig. 2a). These differences translated into life expectancy for Q− workers of 26.8 days (95 % CI, 25.2–28.4), for Q+ workers of 22.3 days (21.2–23.5), and for Q−B workers of 19.3 days (18.0–20.7). Workers in the queenless colony without initial brood demonstrated lower mortality than their queenright counterparts in both the second (HR = 0.41, p < 0.001; Fig. 2b) and third (HR = 0.61, p < 0.001; Fig. 2c) observation hive replicates. Workers in the queenless colony with brood also showed lower mortality than the control workers in the second replicate (HR = 0.68, p < 0.001; Fig. 2b) but no significant difference was observed in the third replicate (HR = 1.1, p = 0.292; Fig. 2c). These mortality differences resulted in the following life expectancy in the 2011A and 2011B replicates respectively: Q−, 29.7 days (27.8–31.6) and 22.4 days (20.9–23.8); Q−B, 22.7 days (21.2–24.3) and 16.5 days (15.4–17.6), and Q+, 18.9 days (18.0–19.8) and 17.7 days (16.7–18.7).
Fig. 2.
Survival of large experimental worker cohorts in observation hives under three different social conditions. Honeybee workers lived longest when observation hives were kept without queen and set up without initial brood, conditions which are most conducive to reproductive activation in workers. Queen removal with initially leaving brood in the hive resulted in shortened worker life span in the first replicate (a), longer worker life span in the second replicate (b), and no effect on worker life span in the third replicate (c) relative to the queenright control treatment. Cohorts that exhibited increased worker life expectancy due to queen removal also contained some exceptionally long-lived workers
The sample of old workers in the 2009 replicate exhibited a low degree of ovary activation without significant differences between queenless and queenright conditions (Table 1). The degree of ovary activation differed significantly among queenless and queenright workers in the second but not in the third replicate. The combined data for dissections of workers from the three observation hive replicates showed a significantly higher degree of ovary activation in queenless colonies than in queenright colonies (Table 1).
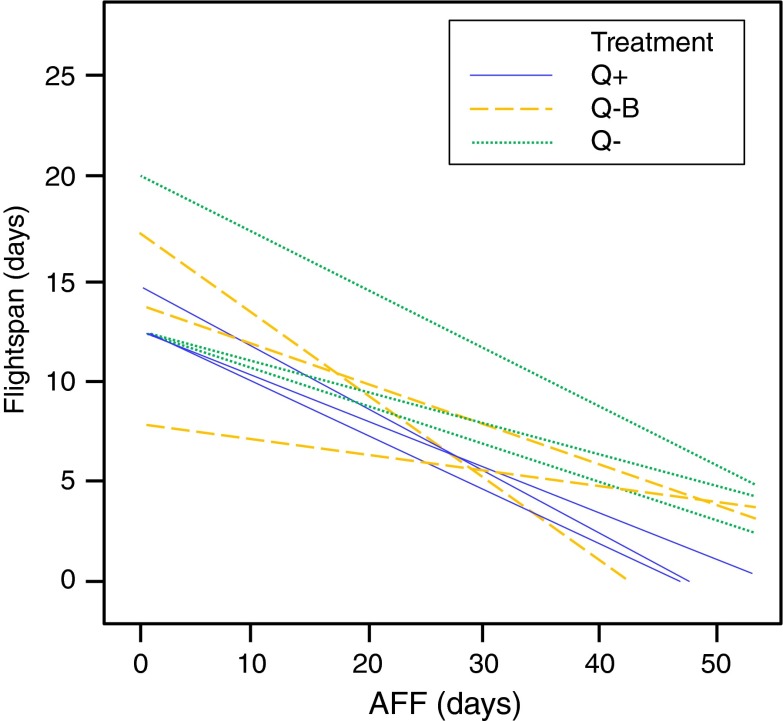
A later AFF was associated with significantly lower mortality rates in all three experimental conditions and replicates (Table 2). A negative relation existed between the AFF and the time spent foraging (flightspan) in all colonies, which was most consistent among replicates of the Q+ treatments and least consistent for the Q−B cohorts (Fig. 3). In 2009, general foraging activity increased mortality risk, but the effect was only significant under Q+ conditions. In contrast, general foraging decreased mortality risk consistently in all experimental cohorts in 2011, although the effect was weaker in the Q− cohorts, where it was nonsignificant in the first 2011 replicate. Workers that were observed foraging did not differ from workers that were not observed foraging in the relative performance of most in-hive tasks. However, they displayed less brood care under Q+ and Q−B conditions across replicates (respectively: F(1,1040) = 1.3, p < 0.001 and F(1,832) = 2.3, p < 0.001) and exhibited a more convex mortality dynamic than workers that were not observed foraging (Fig. 4). The more specialized pollen foraging was associated with a reduced mortality risk in seven cohorts, although only three of these associations were significant. Within each replicate, pollen foraging showed the strongest negative association with mortality risk in the Q+ cohorts (Table 2).
Table 2.
Hazard ratios (with 95 % CI and p value) of behavioral variables for honeybee workers in queenright (Q+), queenless with initial brood (Q−B), and queenless without initial brood (Q−) observation hives
| Replicate 1 (2009) | Replicate 2 (2011A) | Replicate 3 (2011B) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Q− | Q−B | Q+ | Q− | Q−B | Q+ | Q− | Q−B | Q+ | |
| Oviposition | 2 × 10−5 [9 × 10−11–2.9] | 10−5 [10−16–106] | N/Oa | 4,932 [0.0073–3 × 109] | 9 × 10−40 [3 × 10−79−2.6] | N/Oa | 2 × 10−17 [10−39–4 × 105] | 4 × 10−4 [4 × 10−17–4 × 109] | N/Oa |
| p = 0.738 | p = 0.373 | p = 0.215 | p = 0.052 | p = 0.143 | p = 0.610 | ||||
| Queen tending | N/Oa | N/Oa | 0.082 [0.0074–0.907] | N/Oa | N/Oa | 0.24 [0.025–2.4] | N/Oa | N/Oa | 2.3 [0.20–25.9] |
| p = 0.041 | p = 0.224 | p = 0.502 | |||||||
| Brood tending | 4.7 [0.96–22.6] | 6 × 10−5 [3 × 10−6–10−3] | 1.6 [0.22–12.1] | 7 × 10−6 [4 × 10−7–10−4] | 7.1 [3.0–16.6] | 3.8 [1.8–7.9] | 3 × 10−16 [6 × 10−27–10−5] | 3.5 [2.0–6.0] | 8.4 [4.7–14.9] |
| p = 0.057 | p < 0.001 | p = 0.642 | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.004 | p < 0.001 | p < 0.001 | |
| Foraging | 16.7 [0.29–951] | 1.0 [0.20–5.0] | 4.6 [1.4–15.0] | 0.0076 [7 × 10−7–80.0] | 2 × 10−4 [10−6–0.033] | 3 × 10−4 [5 × 10−6–0.018] | 0.014 [5 × 10−4–0.37] | 10−5 [2 × 10−7–6 × 10−4] | 4 × 10−4 [10−5–0.011] |
| p = 0.173 | p = 0.996 | p = 0.011 | p = 0.302 | p = 0.001 | p < 0.001 | p = 0.011 | p < 0.001 | p < 0.001 | |
| Pollen foraging | 3.5 [0.92–13.2] | 0.72 [0.085–6.2] | 0.58 [5 × 10−4–673] | 0.019 [3 × 10−6–123] | 2.5 [0.009–668] | 4 × 10−4 [4 × 10−6–0.48] | 0.11 [10−4–128.1] | 2 × 10−6 [10−11–0.22] | 9 × 10−4 [9 × 10−6–0.083] |
| p = 0.065 | p = 0.768 | p = 0.882 | p = 0.377 | p = 0.754 | p = 0.001 | p = 0.545 | p = 0.027 | p = 0.002 | |
| Grooming | N/Aa | N/Aa | N/Aa | 0.29 [0.0062–13.3] | 0.17 [0.012–2.4] | 0.013 [5 × 10−5–3.3] | 0.93 [0.062–13.9] | 0.011 [6 × 10−5–1.9] | 0.014 [4 × 10−5–5.5] |
| p = 0.524 | p = 0.195 | p = 0.123 | p = 0.957 | p = 0.084 | p = 0.163 | ||||
| Fanning | N/Aa | N/Aa | N/Aa | 0.14 [4 × 10−4–52.0] | 5.0 [0.51–48.7] | 0.024 [0.0018–0.31] | 3.0 [0.51–17.5] | 0.097 [0.010–0.92] | 0.75 [0.11–5.3] |
| p = 0.517 | p = 0.168 | p = 0.004 | p = 0.227 | p = 0.042 | p = 0.774 | ||||
| Trophallaxi ng | N/Aa | N/A | N/A | 3.0 [0.14–63.6] | 0.31 [0.018–5.4] | 0.26 [0.014–4.9] | 1.9 [0.24–14.8] | 1.5 [0.31–7.3] | 1.0 [0.12–7.9] |
| p = 0.484 | p = 0.424 | p = 0.373 | p = 0.554 | p = 0.613 | p = 0.993 | ||||
| Inactivity | N/Aa | N/Aa | N/Aa | 0.28 [0.012–6.5] | 1.4 [0.012–165] | 16.2 [0.28–937] | 3 × 10−7 [10−12–0.081] | 17.9 [4 × 10−17–4 × 109] | 58.6 [0.0031–106] |
| p = 0.427 | p = 0.883 | p = 0.178 | p = 0.019 | p = 0.610 | p = 0.418 | ||||
| AFF | 0.93 [0.89–0.97] | 0.90 [0.87–0.93] | 0.92 [0.90–0.96] | 0.93 [0.88–0.97] | 0.94 [0.91–0.97] | 0.89 [0.86–0.93] | 0.94 [0.91–0.98] | 0.86 [0.79–0.94] | 0.90 [0.86 - 0.95] |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.003 | p = 0.001 | p < 0.001 | |
N/A was not measured
aN/O denotes a behavioral status that was not observed
Fig. 3.
The age of transitioning from in-hive tasks to foraging (= age of first foraging: AFF) is negatively related to the remaining life span after this life history transition (= flight span). The association reflects the coupling between the two life history stages and the relative mortality costs of in-hive tasks and foraging. The differences in consistency among the treatments indicate the importance of social organization for worker aging and suggest a central role of queen presence for honeybee worker life history
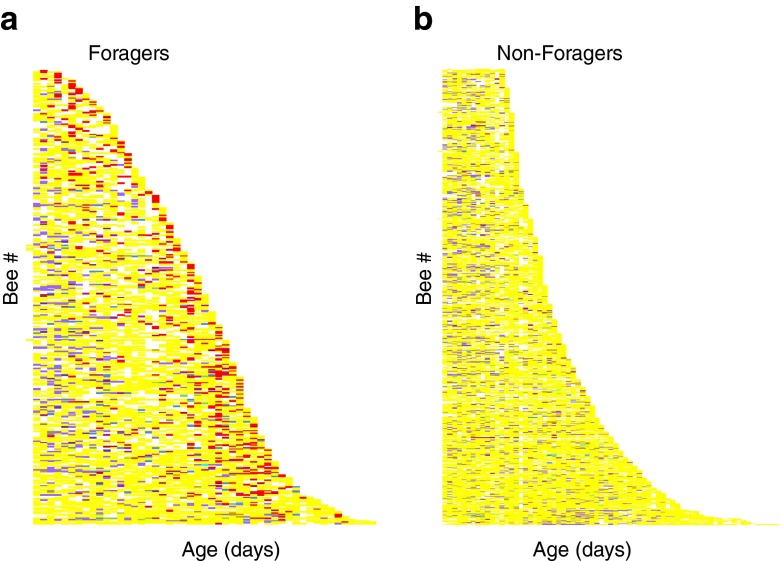
Fig. 4.
Event history charts across all treatments and replicates for the minority of individuals that were observed foraging (a) and workers that were never observed foraging (b). Each row represents an individual bee, sorted according to life span, and each column a daily observation period. The color of each cell indicates whether the bee was just recorded alive (yellow) or observed performing one of the following behaviors: “foraging” (red), “pollen foraging” (dark brown), “brood care” (light blue), “fanning” (dark blue) “trophallaxis” (purple), “queen tending” (violet), “grooming” (light green), “ovipositing” (turquoise), and “inactivity” (light brown)
In-hive behavioral variables also exhibited mortality effects that were dependent on experimental treatments and replicates. “Oviposition” was only observed in Q−B and Q− worker bees. In five of these six cohorts, it was associated with a strongly reduced mortality rate, although few observations of oviposition resulted in wide confidence intervals precluding statistical significance. Thus, a combined analysis of all six queenless cohorts was performed, which confirmed a strong negative association of oviposition with mortality: the hazard ratio was 6 × 10−7 (95 % CI: 10−11–0.00026, p < 0.001). Brood care decreased life expectancy under Q+ conditions consistently (significantly in two of three replicates) and under Q−B in both 2011 replicates. However, it was significantly associated with increased survival in the 2009 Q−B cohort and in both 2011 Q− cohorts (Table 2). Queen tending was only observed in the Q+ colonies and exhibited a significant hazard ratio only in 2009, reducing mortality risk.
The remaining behavior was only evaluated in 2011. Observations of grooming behavior were nonsignificantly but consistently associated with lower mortality risk in all six cohorts. Fanning was significantly associated with lower mortality in the 2011A Q+ and the 2011B Q−B cohorts, but trophallaxis did not show any significant association with mortality. Inactivity was associated with lower mortality in the Q− treatments (one of two replicates significant) and exhibited nonsignificant, opposite effects in the remaining four cohorts (Table 2).
Discussion
Our experiments demonstrate that the social environment and individual behavior can have profound influences on the individual life expectancy of honeybee workers. Workers assuming reproductive roles in the absence of queen inhibition generally have a higher life expectancy than nonreproductive workers, as would be predicted based on the caste differences in life expectancy between queens and workers under normal circumstances. Our manipulations of the colony composition establish a causal relation between social environment and individual life expectancy. However, the documented relation between behavior and life expectancy is correlative. Behavior may influence life expectancy (Rueppell et al. 2007; Amdam et al. 2009), but remaining life expectancy may also be a determinant of social behavior (Woyciechowski and Kozlowski 1998; Woyciechowski and Moron 2009; Kuszewska and Woyciechowski 2013). Regardless of causality, we also found evidence for an interaction between the social environment and the relation between social behavior and life expectancy.
Under normal hive conditions, a combination of queen pheromones inhibits worker reproduction (Le Conte and Hefetz 2008), but under queenless conditions, some workers activate their ovaries and assume a reproductive role (Makert et al. 2006). Our dissection results confirm that only a portion of workers becomes reproductively activated and we were able to observe very few actual oviposition events in workers in the Q− and Q−B treatment groups. Thus, the overall worker mortality in these treatments does not specifically quantify the mortality risk of reproductive workers compared to nonreproductive workers. On average, workers lived longer under queenless conditions, and the majority of Q− and Q−B cohorts contained some very long-lived individuals. However, even the longest-lived “reproductive” workers were much shorter lived than typical queens (Page and Peng 2001) or diutinus workers (Omholt and Amdam 2004). These results suggest that removal of the reproductive inhibition results in a reproductive worker phenotype that shows an intermediate mode of aging. Reproductive workers confirm the positive association between reproduction and longevity in honeybees and may present an important model for understanding the aging plasticity of honeybees. Furthermore, more sophisticated experimental manipulations or the inclusion of distinct worker genotypes (Lattorff et al. 2007) could be used to mechanistically investigate the apparent conundrum of life extension through increased reproduction in social insects (Hartmann and Heinze 2003; Rueppell et al. 2004).
The initial mortality of workers in small nuclear hives was apparently higher under queenless than under queenright conditions, but observations of workers drifting from queenless to queenright nuclear hives suggest that failure to return to the queenless and broodless small hives may be a better explanation of these results. After the establishment of the colonies with brood in the second half of the first replicate and during the second replicate, no further drifting was observed, and the worker mortality in queenright colonies was slightly higher than under queenless conditions. Thus, the nuclear hive experiment corroborates the finding of the observation hive experiments that the removal of reproductive inhibition leads to a moderate increase of life expectancy in worker honeybees. These results conflict with findings of a previous study (Delaplane and Harbo 1987). However, survival and drifting in this earlier study were only quantified for older workers and in larger colonies, which may be responsible for the different findings because drifting and survival are affected by colony size and worker age (Rueppell et al. 2009; Free 1958).
The life-shortening effect of brood care (Amdam et al. 2009) and brood pheromone (Smedal et al. 2009) are well-established and therefore we predicted the Q−B cohorts to live shorter than the Q− cohorts. However, the effect of the Q−B treatment was less consistent relative to the Q+ control: in the first replicate the Q−B workers lived shorter than the Q+ workers, in 2011A they lived longer, and in 2011B the mortality of Q−B and Q+ workers was statistically indistinguishable. The Q−B treatment was also most variable among the replicates with respect to individual behavioral associations with life expectancy. The relation between the age of the transition from in-hive tasks to outside foraging (AFF) and flight span varied most among the three cohorts of this treatment group. The variability may be due to more variable social dynamics that led to a fast onset of worker reproduction in the first but not the two remaining replicates. In the first replicate, brood patterns suggested that workers developed their ovaries before the focal cohorts were introduced, resulting in a subsequent reproductive inhibition of the focal cohort workers (Page and Erickson 1988) and relatively strong brood care demands.
At the individual level, a higher AFF was consistently associated with lower mortality, in accordance with all previous studies (Rueppell et al. 2007). The negative relation between AFF and remaining life span demonstrates that senescence occurs before the onset of foraging, although at a lower rate. The relationship between AFF and flight span was most consistent in the Q+ cohorts, which suggests that colony internal factors in the Q− and Q−B treatments were more important for causing variability among replicates than variable outside foraging conditions (Rueppell et al. 2007). The hazard ratios associated with AFF are consistent with previous studies (Rueppell et al. 2007, 2008, 2009) and indicate a similar influence of AFF on worker life expectancy in all treatment and replicate cohorts. In contrast to the AFF effect that indicated a higher mortality in workers that started foraging early in life, observations of foraging or pollen foraging activity were mostly associated with an increased life expectancy in the respective workers. This counterintuitive result can be explained by the different mortality dynamics exhibited by workers that were observed as foragers (Fig. 4a) compared to workers that were never observed foraging (Fig. 4b). Very few workers were observed dying in the hive, suggesting that most of the “non-foragers” died during their initial foraging attempts, shortening their life. This interpretation is corroborated by the type III survivorship (Fig. 4) and an initial mortality peak in foraging honeybee workers (Rueppell et al. 2007, but see Dukas 2008). Our event history charts (Carey et al. 2006) also show a concentration of nursing behavior at younger ages in both groups, with foraging activity increasing at older ages in the “forager” group. Furthermore, they indicate that only a fraction of foraging activity was recorded, assuming that workers commit entirely to foraging after reaching their AFF (Winston 1987). We may have only recorded the more successful or conspicuous foragers that may represent particularly healthy and long-lived individuals. These arguments, the consistent AFF effect, and previous results (Rueppell et al. 2007; Neukirch 1982) suggest that the positive associations of foraging and life expectancy are probably an analytical artifact instead of a causal relationship.
Oviposition behavior was associated with a drastic reduction in mortality in five out of six cohorts, with more than a thousand-fold reduction of mortality risk. However, the association was significant only in a combined analysis due to the small number of observations of ovipositing workers. The combined analysis confirmed the predicted drastic reduction in mortality, but the mortality reduction did not extend the life span of any worker beyond 75 days. Therefore, the survival benefit of reproductive workers is most likely occurring before reaching advanced ages but declining colony sizes during experiments may have also contributed to reproductive workers not reaching their full life span potential. Thus, the results from our experiments are a conservative measure of the effect of reproductive activation on honeybee worker life expectancy.
Among the remaining in-hive behaviors, brood care exhibited distinct differences between the Q− and the other two treatments. While it was associated with a prolonged life span in the Q− treatments, it exhibited the opposite effect in the Q−B and Q+ cohorts, in accordance with previous observations (Amdam et al. 2009). This contingency of the behavioral effect on the social environment may be explained by different individuals engaging in brood care in the different social circumstances. The only brood present in the Q− colonies is the drone brood produced by the reproductive workers, and it might be these reproductive workers that are also caring for their brood or cannibalizing the brood (Robinson et al. 1990, but see Page and Robinson 1994), which was not distinguished in our study. The social role of reproductive workers may outweigh the physiological costs of brood care, resulting in an overall reduction of mortality. In contrast, the perception of worker brood in the Q−B and Q+ colonies may result in brood care by random workers without reproductive activation. Consequently, these workers only suffer the physiological costs of brood care, shortening their life (Amdam et al. 2009). This interpretation is supported by a significant correlation between oviposition and brood care that existed only in the Q− colonies and a significantly smaller proportion of Q− workers engaging in brood care (post hoc analyses: results not shown).
The effect of prolonged inactivity was similarly modulated by social environment. It was associated with lower mortality rate in the Q− but not in the Q−B and Q+ treatments. Thus, it might also be an indicator of different functions, dependent on colony status. For example, it could be a sign of frailty but it could also represent sleep (Eban-Rothschild and Bloch 2008). However, inactivity was only measured in two of the three replicates and associations were mostly nonsignificant. It also was not significantly associated with oviposition behavior, and its relative frequency did not differ among the three treatment groups.
The observation of grooming, fanning, and trophallaxis showed few significant effects on mortality rate. However, the lack of significance may reflect a true lack of relation between these behaviors and life expectancy because these results were not caused by too few observations, in contrast to oviposition behavior. While oviposition was only recorded in 24 workers of the 2,855 total observed workers, grooming, fanning, and trophallaxis were all observed in over 30 % of the workers. Grooming is an important contributor to social immunity in bees (Wilson-Rich et al. 2009), but it may not be very costly in physiological terms. Therefore, it might not have a significant association with mortality. Fanning is a homeostatic activity that is exhibited to regulate nest temperature (Winston 1987). It is conceptually related to flight, which may bear physiological costs (Dukas 2008; Neukirch 1982), but our results suggest the contrary. We also studied trophallaxis based on an expectation that it would affect worker longevity because it is a main route for resource transfer (Amdam and Page 2005). However, hazard ratios did not even indicate a consistent trend for an association with mortality risk.
In sum, our study demonstrates a moderately lowered mortality by removing reproductive inhibition in worker honeybees. The longevity gain is not comparable to the queen’s longevity (Page and Peng 2001) or longevity gains in other social insects for workers assuming a sole reproductive role (Hartmann and Heinze 2003). In addition, the correlations between specific in-hive behavior of honeybee workers and their mortality risk suggest a more complex relationship between social behavior and mortality risk than what has been realized to date. Despite large sample sizes, our estimates of hazard ratios for individual behaviors exhibited a high degree of uncertainty and more detailed behavioral profiling of individual workers may be necessary to resolve the intricate relation between social role, behavior, and mortality. The complexity of the “social dimension of aging” is further increased by paramount interaction effects of individual behavior and social environment on mortality risk.
Acknowledgments
This study would not have been possible without the practical help of Tara McCray. We would also like to acknowledge the encouragement and help by the other members of the Social Insect Lab at UNCG. Financial support for this project was provided by the National Science Foundation (grants: #0850465 and # 0926288) and the Agriculture and Food Research Initiative of the USDA National Institute of Food and Agriculture (#2010-65104-20533) to OR.
Footnotes
Authors Luke Dixon and Ryan Kuster contributed equally to this work.
References
- Aamodt RM. Age-and caste-dependent decrease in expression of genes maintaining DNA and RNA quality and mitochondrial integrity in the honeybee wing muscle. Exp Gerontol. 2009;44(9):586–593. doi: 10.1016/j.exger.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Amdam GV, Page RE. Intergenerational transfers may have decoupled physiological and chronological age in a eusocial insect. Aging Res Rev. 2005;4(3):398–408. doi: 10.1016/j.arr.2005.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Rueppell O, Fondrk MK, Page RE, Nelson CM. The nurse's load: early-life exposure to brood-rearing affects behavior and life span in honeybees (Apis mellifera) Exp Gerontol. 2009;44(6–7):467–471. doi: 10.1016/j.exger.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backx AG, Guzman-Novoa E, Thompson GJ. Factors affecting ovary activation in honeybee workers: a meta-analysis. Insect Soc. 2012;59(3):381–388. doi: 10.1007/s00040-012-0230-1. [DOI] [Google Scholar]
- Carey JR, Papadopoulos N, Kouloussis N, Katsoyannos B, Muller HG, Wang JL, Tseng YK. Age-specific and lifetime behavior patterns in Drosophila melanogaster and the Mediterranean fruit fly, Ceratitis capitata. Exp Gerontol. 2006;41(1):93–97. doi: 10.1016/j.exger.2005.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona M, Hughes KA, Weaver DB, Robinson GE. Gene expression patterns associated with queen honeybee longevity. Mech Ageing Dev. 2005;126(11):1230–1238. doi: 10.1016/j.mad.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Corona M, Velarde RA, Remolina S, Moran-Lauter A, Wang Y, Hughes KA, Robinson GE. Vitellogenin, juvenile hormone, insulin signaling, and queen honeybee longevity. Proc Natl Acad Sci U S A. 2007;104(17):7128–7133. doi: 10.1073/pnas.0701909104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Loof A. Longevity and aging in insects: is reproduction costly; cheap; beneficial or irrelevant? A critical evaluation of the “trade-off” concept. J Ins Physiol. 2011;57(1):1–11. doi: 10.1016/j.jinsphys.2010.08.018. [DOI] [PubMed] [Google Scholar]
- de Magalhaes JP, Curado J, Church GM. Meta-analysis of age-related gene expression profiles identifies common signatures of aging. Bioinformatics. 2009;25(7):875–881. doi: 10.1093/bioinformatics/btp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaplane KS, Harbo JR. Effect of queenlessness on worker survival, honey gain and defense behavior in honeybees. J Apic Res. 1987;26(1):37–42. [Google Scholar]
- Dukas R. Mortality rates of honeybees in the wild. Insect Soc. 2008;55(3):252–255. doi: 10.1007/s00040-008-0995-4. [DOI] [Google Scholar]
- Eban-Rothschild AD, Bloch G. Differences in the sleep architecture of forager and young honeybees (Apis mellifera) J Exp Biol. 2008;211(Pt 15):2408–2416. doi: 10.1242/jeb.016915. [DOI] [PubMed] [Google Scholar]
- Finch CE, Tanzi RE. Genetics of aging. Science. 1997;278(5337):407–411. doi: 10.1126/science.278.5337.407. [DOI] [PubMed] [Google Scholar]
- Ford D. Honeybees and cell lines as models of DNA methylation and aging in response to diet. Exp Gerontol. 2012 doi: 10.1016/j.exger.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Free JB. The drifting of honeybees. J Agric Sci. 1958;51:294–306. doi: 10.1017/S0021859600035103. [DOI] [Google Scholar]
- Graham AM, Munday MD, Kaftanoglu O, Page RE, Jr, Amdam GV, Rueppell O. Support for the reproductive ground plan hypothesis of social evolution and major QTL for ovary traits of Africanized worker honeybees (Apis mellifera L.) BMC Evol Biol. 2011;11:95. doi: 10.1186/1471-2148-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozinger CM, Fan YL, Hoover SER, Winston ML. Genome-wide analysis reveals differences in brain gene expression patterns associated with caste and reproductive status in honeybees (Apis mellifera) Mol Ecol. 2007;16(22):4837–4848. doi: 10.1111/j.1365-294X.2007.03545.x. [DOI] [PubMed] [Google Scholar]
- Haddad LS, Kelbert L, Hulbert AJ. Extended longevity of queen honeybees compared to workers is associated with peroxidation-resistant membranes. Exp Gerontol. 2007;42(7):601–609. doi: 10.1016/j.exger.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Hartmann A, Heinze J. Lay eggs, live longer: division of labor and life span in a clonal ant species. Evolution. 2003;57(10):2424–2429. doi: 10.1111/j.0014-3820.2003.tb00254.x. [DOI] [PubMed] [Google Scholar]
- Herb BR, Wolschin F, Hansen KD, Aryee MJ, Langmead B, Irizarry R, Amdam GV, Feinberg AP. Reversible switching between epigenetic states in honeybee behavioral subcastes. Nat Neurosci. 2012;15(10):1371–1373. doi: 10.1038/nn.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover SER, Keeling CI, Winston ML, Slessor KN. The effect of queen pheromones on worker honeybee ovary development. Naturwissenschaften. 2003;90(10):477–480. doi: 10.1007/s00114-003-0462-z. [DOI] [PubMed] [Google Scholar]
- Keller L, Genoud M. Extraordinary life spans in ants: a test of evolutionary theories of ageing. Nature. 1997;389:958–960. doi: 10.1038/40130. [DOI] [Google Scholar]
- Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120(4):449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Kucharski R, Maleszka J, Foret S, Maleszka R. Nutritional control of reproductive status in honeybees via DNA methylation. Science. 2008;319(5871):1827–1830. doi: 10.1126/science.1153069. [DOI] [PubMed] [Google Scholar]
- Kuszewska K, Woyciechowski M. Reversion in honeybee, Apis mellifera, workers with different life expectancies. Anim Behav. 2013;85(1):247–253. doi: 10.1016/j.anbehav.2012.10.033. [DOI] [Google Scholar]
- Laidlaw HH, Page RE. Queen rearing and bee breeding. Cheshire: Wicwas Press; 1997. [Google Scholar]
- Lattorff HMG, Moritz RFA, Crewe RM, Solignac M. Control of reproductive dominance by the thelytoky gene in honeybees. Biol Lett. 2007;3(3):292–295. doi: 10.1098/rsbl.2007.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Conte Y, Hefetz A. Primer pheromones in social hymenoptera. Annu Rev Entomol. 2008;53:523–542. doi: 10.1146/annurev.ento.52.110405.091434. [DOI] [PubMed] [Google Scholar]
- Mackay TF. The nature of quantitative genetic variation for Drosophila longevity. Mech Ageing Dev. 2002;123(2–3):95–104. doi: 10.1016/S0047-6374(01)00330-X. [DOI] [PubMed] [Google Scholar]
- Makert GR, Paxton RJ, Hartfelder K. Ovariole number—a predictor of differential reproductive success among worker subfamilies in queenless honeybee (Apis mellifera L.) colonies. Behav Ecol Sociobiol. 2006;60(6):815–825. doi: 10.1007/s00265-006-0225-x. [DOI] [Google Scholar]
- Neukirch A. Dependence of the life span of the honeybee (Apis mellifica) upon flight performance and energy consumption. J Comp Physiol. 1982;146:35–40. [Google Scholar]
- Omholt SW, Amdam GV. Epigenic regulation of aging in honeybee workers. Sci Aging Knowl Environ. 2004;26:pe28. doi: 10.1126/sageke.2004.26.pe28. [DOI] [PubMed] [Google Scholar]
- Page RE, Erickson EH. Reproduction by worker honeybees (Apis mellifera L) Behav Ecol Sociobiol. 1988;23(2):117–126. doi: 10.1007/BF00299895. [DOI] [Google Scholar]
- Page RE, Metcalf RA. A population investment sex ratio for the honeybee (Apis mellifera L.) Am Nat. 1984;124:680–702. doi: 10.1086/284306. [DOI] [Google Scholar]
- Page RE, Peng Y-SC. Aging and development in social insects with emphasis on the honeybee, Apis mellifera L. Exp Gerontol. 2001;36(4–6):695–711. doi: 10.1016/S0531-5565(00)00236-9. [DOI] [PubMed] [Google Scholar]
- Page RE, Robinson GE. Reproductive competition in queenless honeybee colonies (Apis mellifera L) Behav Ecol Sociobiol. 1994;35(2):99–107. doi: 10.1007/BF00171499. [DOI] [Google Scholar]
- Remolina SC, Hughes KA. Evolution and mechanisms of long life and high fertility in queen honeybees. AGE. 2008;30(2–3):177–185. doi: 10.1007/s11357-008-9061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GE, Page RE, Fondrk MK. Intracolonial behavioral variation in worker oviposition, oophagy, and larval care in queenless honeybee colonies. Behav Ecol Sociobiol. 1990;26:315–323. doi: 10.1007/BF00171096. [DOI] [Google Scholar]
- Rueppell O, Amdam GV, Page RE, Jr, Carey JR. From genes to society: Social insects as models for research on aging. Sci Aging Knowl Environ. 2004;5:pe5. doi: 10.1126/sageke.2004.5.pe5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueppell O, Bachelier C, Fondrk MK, Page RE., Jr Regulation of life history determines life span of worker honeybees (Apis mellifera L.) Exp Gerontol. 2007;42:1020–1032. doi: 10.1016/j.exger.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueppell O, Linford R, Gardner P, Coleman J, Fine K. Aging and demographic plasticity in response to experimental age structures in honeybees (Apis mellifera L) Behav Ecol Sociobiol. 2008;62:1621–1631. doi: 10.1007/s00265-008-0591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueppell O, Kaftanouglu O, Page RE. Honeybee (Apis mellifera) workers live longer in small than in large colonies. Exp Gerontol. 2009;44(6–7):447–452. doi: 10.1016/j.exger.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueppell O, Phaincharoen M, Kuster R, Tingek S. Cross-species correlation between queen mating numbers and worker ovary sizes suggests kin conflict may influence ovary size evolution in honeybees. Naturwissenschaften. 2011;98(9):795–799. doi: 10.1007/s00114-011-0822-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedal B, Brynem M, Kreibich CD, Amdam GV. Brood pheromone suppresses physiology of extreme longevity in honeybees (Apis mellifera) J Exp Biol. 2009;212(23):3795–3801. doi: 10.1242/jeb.035063. [DOI] [PubMed] [Google Scholar]
- Stout TL, Slone JD, Schneider SS. Age and behavior of honeybee workers, Apis mellifera, that interact with drones. Ethology. 2011;117(5):459–468. doi: 10.1111/j.1439-0310.2011.01895.x. [DOI] [Google Scholar]
- Tezze AA, Farina WM. Trophallaxis in the honeybee, Apis mellifera: the interaction between viscosity and sucrose concentration of the transferred solution. Anim Behav. 1999;57:1319–1326. doi: 10.1006/anbe.1999.1110. [DOI] [PubMed] [Google Scholar]
- The “R” Development Core Team (2005) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
- Wilson-Rich N, Spivak M, Fefferman NH, Starks PT. Genetic, individual, and group facilitation of disease resistance in insect societies. Annu Rev Entomol. 2009;54:405–423. doi: 10.1146/annurev.ento.53.103106.093301. [DOI] [PubMed] [Google Scholar]
- Winston ML. The biology of the honeybee. Cambridge: Harvard University Press; 1987. [Google Scholar]
- Woyciechowski M, Kozlowski J. Division of labor by division of risk according to worker life expectancy in the honeybee (Apis mellifera L.) Apidologie. 1998;29:191–205. doi: 10.1051/apido:19980111. [DOI] [Google Scholar]
- Woyciechowski M, Moron D. Life expectancy and onset of foraging in the honeybee (Apis mellifera) Insect Soc. 2009;56(2):193–201. doi: 10.1007/s00040-009-0012-6. [DOI] [Google Scholar]