Abstract
Methuselah (mth) is a chromosome 3 Drosophila mutant with an increased lifespan. A large number of studies have investigated the genetic, molecular, and biochemical mechanisms of the mth gene. Much less is known about the effects of mth on preservation of sensorimotor abilities throughout Drosophila’s lifespan, particularly in late life. The current study investigated functional senescence in mth and its parental-control line (w1118) in two experiments that measured age-dependent changes in flight functions and locomotor activity. In experiment 1, a total of 158 flies (81 mth and 77 controls) with an age range from 10 to 70 days were individually tethered under an infrared laser-sensor system that allowed monitoring of flight duration during phototaxic flight. We found that mth has a statistically significant advantage in maintaining continuous flight over control flies at age 10 days, but not during middle and late life. At age 70 days, the trend reversed and parental control flies had a small but significant advantage, suggesting an interaction between age and genotype in the ability to sustain flight. In experiment 2, a total of 173 different flies (97 mth and 76 controls) with an age range from 50 to 76 days were individually placed in a large well-lit arena (60 × 45 cm) and their locomotor activity quantified as the distance walked in a 1-min period. Results showed that mth flies had lower levels of locomotor activity relative to controls at ages 50 and 60 days. These levels converged for the two genotypes at the oldest ages tested. Findings show markedly different patterns of functional decline for the mth line relative to those previously reported for other life-extended genotypes, suggesting that different life-extending genes have dissimilar effects on preservation of sensory and motor abilities throughout an organism’s lifespan.
Keywords: Drosophila, Longevity, mth, Methuselah, Aging, Sensorimotor, Locomotor, Behavior
Introduction
Drosophila is an important model system for the study of aging and functional senescence (Curtsinger et al. 1995; Osiewacz 1997). The wild-type Drosophila has a maximum lifespan of approximately 1.5 to 2 months, a large repertoire of interesting motor, sensory, and perceptual functions (e.g., visual, locomotor, olfactory, auditory, and memory), and a high homology of genes to other species including mammals (Rose et al. 1992; Curtsinger et al. 1995; Osiewacz 1997; Parkes et al. 1998; Butler 1999; Phillips et al. 2000). Fortini et al. (2000), for example, have reported that approximately 65 % of human disease genes are conserved in Drosophila (187 of 287 examined). Drosophila’s short lifespan, high reproductive rate, and long history of use in experimental genetics (Loeb and Bancroft 1911), has made it a practical species for population-level studies of aging and functional decline.
Several studies have investigated life extension in Drosophila using genetic strategies. These studies have identified genes that extend lifespan between 35 % to 60 % by a variety of mechanisms including antioxidant intervention in aerobic cells, e.g., motorneurons (superoxide dismutase gene; Parkes et al. 1998; Phillips et al. 2000), modulating metabolic state (Rogina et al. 2002; the INDY gene: I’m not dead yet), and controlling synaptic efficacy (Song et al. 2002; methuselah) among others (Clancy et al. 2001; Kapahi et al. 2004; Wang et al. 2003; Giannakou et al. 2004). While a large number of studies have investigated the genetic, biochemical, and neural mechanisms of life-extending genes, much less is known about their influence on sensory and behavioral abilities of Drosophila throughout its lifespan.
The current study investigates lifespan changes in behavioral abilities of methuselah (mth), a chromosome 3 Drosophila mutant that outlives its parental control line by 35 % (Lin et al. 1998; Wang et al. 2004). Life extension by mth expression is speculated to involve the regulation of Drosophila’s antioxidant defense system (Lin et al. 1998) with intermediate expression levels associated with maximum extension of life. Two prior studies have examined lifespan variation in sensorimotor and behavioral abilities of mth relative to their parental-control lines with seemingly contradictory findings. Cook-Wiens and Grotewiel (2002), for example, reported no significant difference between functional aging in mth and its parental line in odor avoidance, electric-shock avoidance, phototaxis, geotaxis, and exploratory behavior, concluding that life extension in mth is dissociated form delayed functional senescence. Petrosyan et al. (2007), however, reported distinct differences in sensorimotor abilities of mth and controls in optomotor efficiency during in-flight tracking of a visual target, wingbeat frequency in tethered flight, and onset of first flight after eclosion.
The experiments reported here were motivated by two specific considerations. First, different life-extending genes, such as mth, SOD1, and INDY, will likely yield different patterns of gerontological decline in sensorimotor and other behavioral abilities. Our earlier work on mth and SOD1 has shown markedly distinct patterns of age-dependent functional decline across genotypes (Petrosyan et al. 2007, 2012a, b). Such differences point to the importance of line-specific investigation of functional senescence because different life-extending genes likely preserve different abilities to various degrees at different stages of the aging process. Second, the apparent discrepancy between findings that chronological aging is dissociated from functional aging in mth, and that it is influenced by mth expression, necessitates further investigation. To this end, we examined two behavioral measures, age-dependent changes in flight duration and locomotor (exploratory) activity. We found significantly longer periods of sustained flight by younger mth flies relative to age-matched controls, significantly longer flight duration by controls relative to mth at old age, suggesting an interaction between age and genotype, and significantly lower levels of locomotor activity by mth relative to controls in late life.
Materials and methods
Fly stocks
The mth and w1118 parental-control stock (from which mth was derived) were a generous gift from the laboratory of the late Dr. Seymour Benzer (California Institute of Technology, Pasadena, CA, USA). The mth stock had been backcrossed to w1118 for several generations, and then extensively out-crossed to avoid nonspecific effects of genetic background. We verified increased longevity in mth relative to w1118 (Fig. 1, top panel).1 Data collection on behavioral traits began within days after confirmation of increased longevity using flies collected from the same colony as that used for measurement of survival functions. All flies were maintained in small groups of no more than 50 per vial (Genesee Scientific Corp., San Diego, CA, USA; polystyrene, O.D. × H 25 × 95 mm) containing fresh food media (standard cornmeal-agar medium, IU Bloomington formula). Vials were kept in a low-temperature incubator at 25 °C and constant humidity on a 12/12-h light/dark cycle (VWR Scientific, Model 2015).2 Flies were transferred to fresh food vials every 3 to 4 days. Only male flies were used in the current study, consistent with use of males in the original longevity study of mth mutants, as well as several other prior studies of Drosophila behavior (Lin et al. 1998; Petrosyan et al. 2007; Bland et al. 2009; Slawson et al. 2011). A total of 158 flies (81 mth and 77 controls) were individually tethered and tested in the flight-duration experiment, and 173 different flies (97 mth and 76 controls) in the locomotion (exploratory walking) experiment.
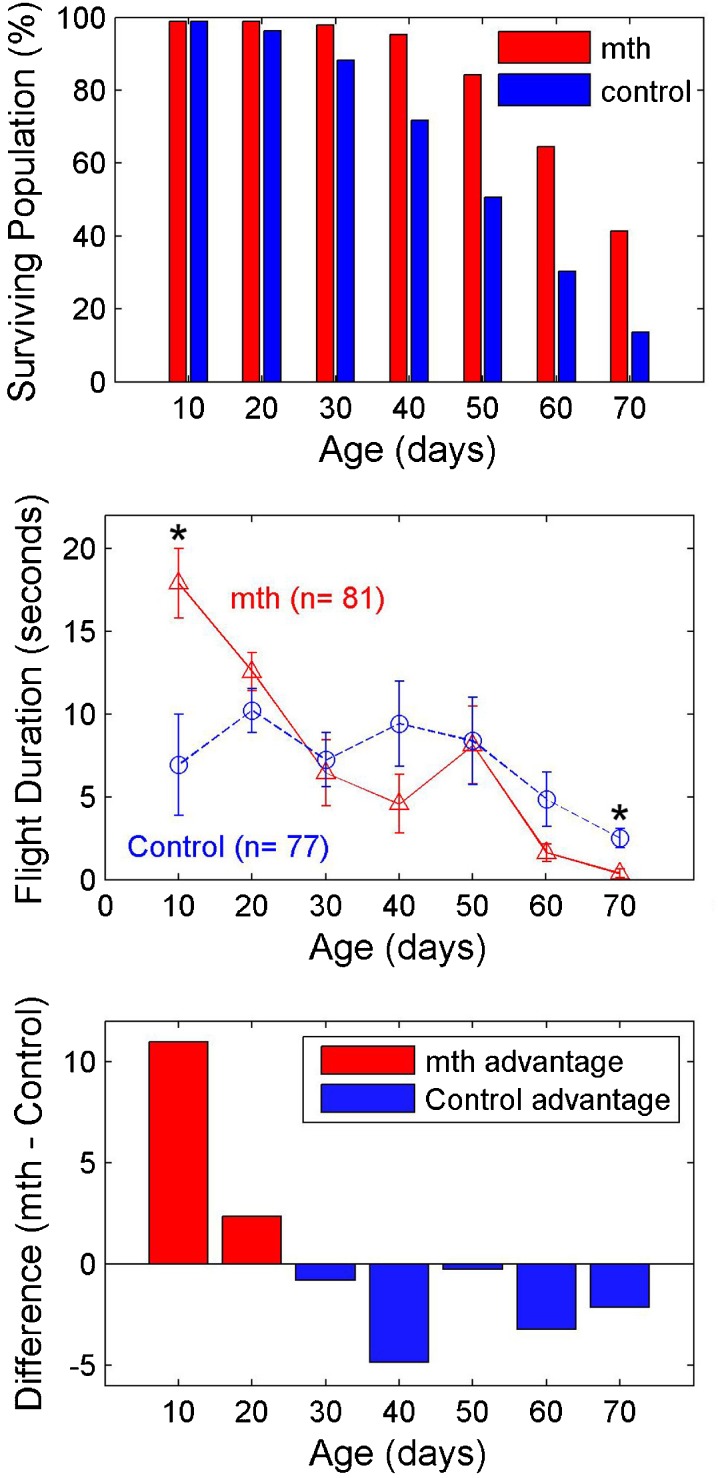
Fig. 1.
Top panel shows proportion of surviving populations of male mth and control flies at the same age categories as those tested in the flight-duration experiment. The starting populations at age 1 day were 290 mth flies and 346 control flies. Middle panel shows duration of the longest segment of sustained flight as a function of age and genotype. Asterisks mark ages at which a statistically significant difference in flight duration was observed between genotypes. Bottom panel plots the difference between flight duration of mth and control flies as a function of age, showing a significant mth advantage in maintaining sustained flight over control flies in early life and a disadvantage after age 30 days. A statistically significant age by genotype interaction effect was observed as expected from the crossover of the two functions (see text)
Flight duration
Flight duration was measured in a tethered-flight paradigm at ∼5 h after onset of subjective day. The tethering process involved several steps. First, an individual fly was lightly CO2 anesthetized and transferred to a custom-made aluminum block in a Peltier cooler (Boekel Scientific, Model 260014) on which a small opening (2 x 1 × 1 mm3) had been drilled to allow accurate positioning of an anesthetized fly. The fly remained under cold anesthesia at 4 °C. Individual flies were gently handled either with a small brush or a jeweler’s vacuum tweezers. The tip of a tungsten wire (130 μ in diameter) was dipped in glass glue (Loctite, New York, NY, USA), and under a stereo microscope (Olympus SZ40) lowered using a micropositioner (Stoelting Co./Prior, England) onto the anesthetized fly’s thorax. The glue was cured with a UV gun (Electro-Lite Corp., Model ELC-403) for 20 s, and the fly was removed from the Peltier cooler using the micropositioner. Flies usually recovered from cold anesthesia and began flight within 3 to 4 min. Tethered flies were moved to the experimental chamber, fed with a small piece of filter paper dipped in sucrose-water, and allowed to rest and become acclimated to the experimental environment for an additional 30–60 min prior to data collection.
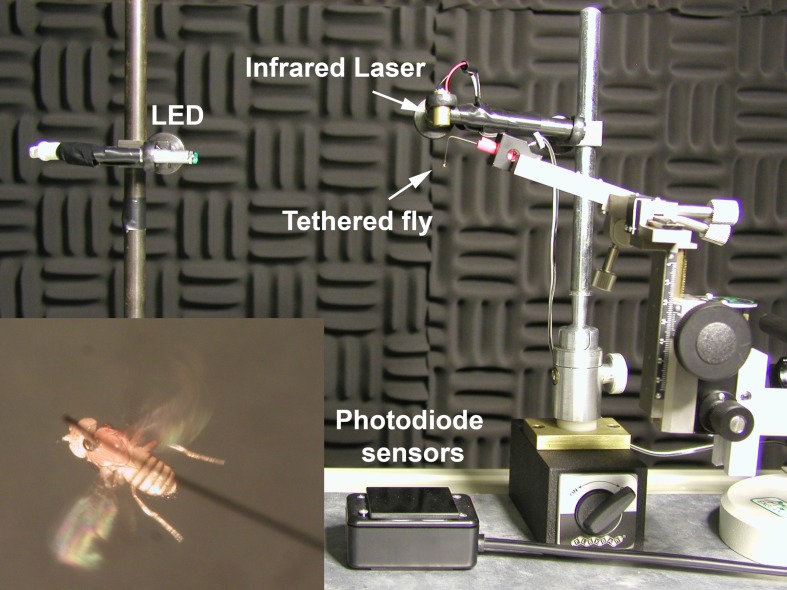
The tethered fly (Fig. 2) was positioned under a solid-state infrared (IR) laser (Lasermate Group Inc., Pomona, Ca, USA, Model PLC8082AE) with an adjustable focus that cast shadows of the wing beats onto fast-response IR photodiode sensors Photonic Detectors Inc., Simi Valley, CA, USA, Part no. PDB-C615-2). The IR wavelength (808 nm) was outside the fly’s range of visible spectrum (Hernfindez de Salomon and Spatz 1983). The sensors were placed in a small plastic box covered with an IR bandpass filter (Edmund Industrial Optics, Barrington, NJ, USA, Part. no. NT32769). The experiment was run in complete darkness in a steel chamber (2 × 2 × 2 m3; IAC) with only a single green LED (555 nm) positioned directly in the fly’s line of sight at a distance of 15 cm to provide a visual target for phototaxis (Hadler 1964; Miller et al. 1981). The output of the photodiode sensors was sent to an amplifier and fed into an analog-to-digital converter (Sound Blaster Live, −120 dB noise floor) positioned outside the chamber, and recorded at a sampling rate of 10 kHz. Since the main interest here was to examine stability of sensorimotor abilities as a function of age, maximum continuous flight duration was measured at older age categories of at least 10 days. Flies that are 3 days or younger may fly uninterrupted for several minutes (up to 30 min or longer). As they age, their ability to maintain continuous flight rapidly declines. For these older flies, we measured the longest continuous stretch of flight in a 20-s period after initiation of flight. If a fly stopped flying during this period, it was stimulated with a puff of air to reinitiate flight. After each experimental run, flies were CO2 anesthetized, removed from tether, and discarded in citrus oil.
Fig. 2.
Apparatus used for measurement of flight duration. A tethered fly was positioned under a solid-state infrared (IR) laser with an adjustable focus. Wingbeat shadows were cast onto IR photodiode sensors. The experiment was run in complete darkness in a steel chamber with only a single green LED (555 nm) positioned directly in the fly’s line of sight to stimulate phototaxic flight. Inset shows a parental-line fly (w1118) in tethered flight
Flight duration measured at 20 days of age conditioned on lifespan
Previous research has shown that the wingbeat frequencies of mth are conditioned on lifespan (Petrosyan et al. 2007). Measured at age 5 days, highest wingbest frequencies are associated with those flies that live an average lifespan, whereas the slowest wingbeat frequencies are associated with flies that either have the shortest or longest lifespan in the population. In the current study, we examined flight duration of mth and control flies, conditioned on lifespan. We measured flight duration for groups of mth and parental flies at age 20 days, and maintained the tested flies in individual vials to track their longevity with the goal of determining if individual differences in flight duration at a given age are correlated with lifespan. The testing age of 20 days was selected because from the first experiment we determined that it is a reasonable age for testing flight duration as nearly all flies can maintain sustained flight, but not for extended periods, hence avoiding ceiling or floor effects during the 20-s measurement period. All procedures were identical to those described earlier, with the following exceptions. After measuring flight duration at age 20 days, flies were CO2 anesthetized and removed from tether under a microscope by gently pressing on their thorax. Each fly was then placed in an individually numbered fresh-food vial and maintained in the incubator. Their behavior, after recovery from anesthesia was informally monitored and appeared to be normal. Fourteen male mth flies and 12 male parental-line flies were tested in this experiment.
Locomotor ability
The distance walked in a fixed period of time has previously been used as a measure of exploratory behavior and locomotor ability in Drosophila (Strauss et al. 1992; Hayward et al. 1993; Roberts 1998; Petrosyan et al. 2007). The current experiment measured average walking distance for both genotypes in a large well-lit arena (60 × 45 cm). Since the main goal of the experiment was to examine sensorimotor senescence at old age, the minimum age tested was 50 days. At this age, flies cannot sustain their weight in free flight and therefore nearly always walk to explore their environment.
A total of 173 flies (97 mth and 76 control) were individually tested in this experiment, with an average of approximately 12 flies per age group and genotype. The arena was visually divided into a grid of 2.54 cm (1-in.) squares. On the day of the experiment, none of the flies were anesthetized. Each fly was gently positioned, using a small brush, in the center grid square. A timer was started immediately after the fly crossed the first grid line. This protocol was adopted because when placed in a new setting some flies tended to remain stationary for a brief period of time. The experimenter thus waited until the fly decided to initiate exploratory movements before starting the timer. The number of line crossings during a 1-min period was counted and recorded. If a fly did not leave the center square within 3 min after being placed in the arena, a score of zero was recorded for that fly.
Results
Flight duration as a function of age
Middle panel of Fig. 1 shows flight-duration data from mth and control flies. The mth has a statistically significant advantage in maintaining sustained flight over control flies at age 10 days (t(9) = 2.84, p < 0.05), but not during middle and late life. At age 70 days, the trend reverses and parental control flies have a small advantage (t(10) = −4.03, p < 0.01). Bottom panel of Fig. 1 shows the difference in flight duration between the mth and control lines. The reversal point occurs at age 30 days and is maintained at every age category up to the oldest age tested. A two-way independent-groups analysis of variance (ANOVA) on the entire data set showed a significant effect of age (F(6,144) = 7.74, p < 0.001), no significant effect of genotype (F(1,144) = 0.07, n.s.), and as expected, a significant interaction between age and genotype (F(6,144) = 2.56, p < 0.05). This interaction effect reflects the generally better performance (longer sustained flight) of mth at younger stages of life, and poorer performance at older ages.
Flight duration conditioned on lifespan
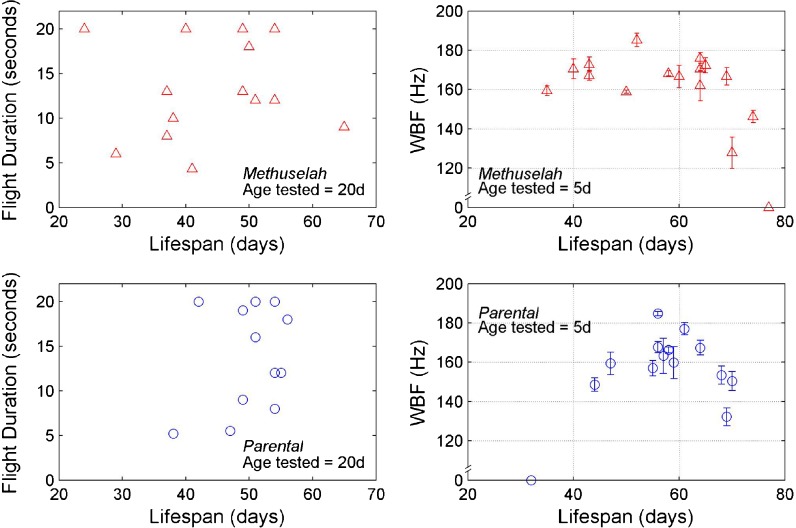
Left panels of Fig. 3 show results of this experiment for 14 mth and 12 control flies. These flies were tested at age 20 days for their ability to maintain continuous flight, but were kept alive in individual fresh food vials until they died. We found no pattern between the ability to maintain flight at 20 days and longevity. This finding is contrary to that reported for mth wingbeat frequency, shown in the right panels of Fig. 3, which has been reported to be highest for flies with an average lifespan, and lower for long-lived and short-lived flies (Petrosyan et al. 2007).
Fig. 3.
Left panels show maximum flight duration measured at 20 days of age, conditioned on lifespan. Data are from 14 mth and 12 parental-line flies. Right panels show data re-plotted from Petrosyan et al. (2007) on wingbeat frequencies measured at 5 days of age, conditioned on lifespan
Locomotor activity
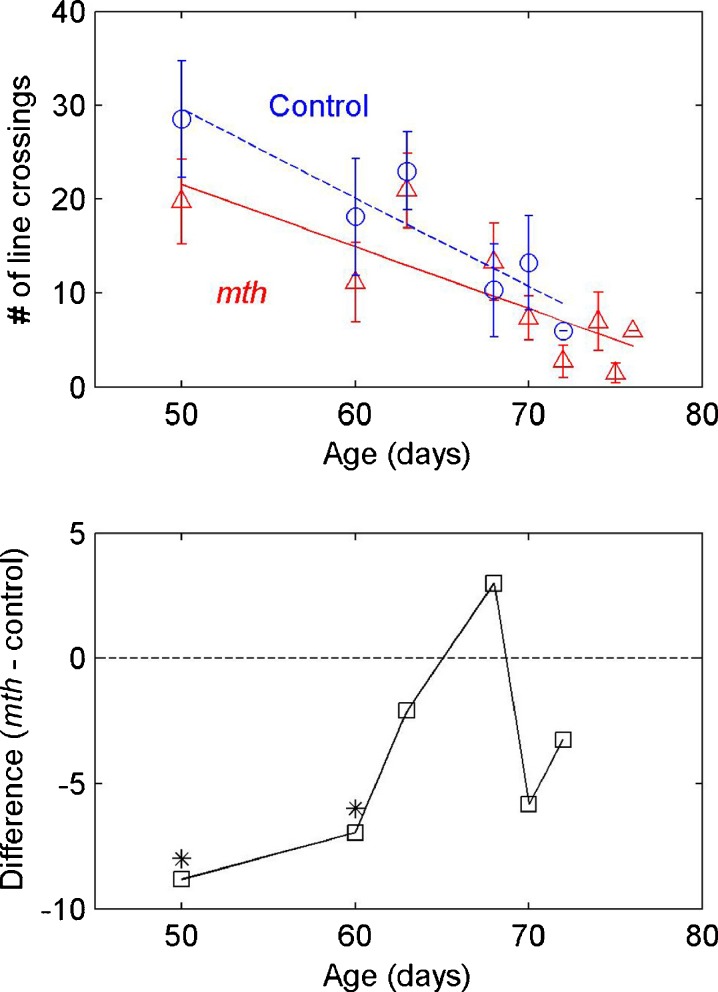
Figure 4 shows results of the locomotor (exploratory walking) experiment. Top panel shows the number of line crossings as a measure of distance traveled in a 1-min period as a function of age and genotype. The lines are least-squares regression fits. The bottom panel shows the difference at the six age categories for which we had matched data across genotypes. The level of exploratory behavior is clearly reduced with age for both genotypes. In addition, mth shows a lower level of exploratory activity relative to controls, particularly at ages 50 and 60 days. These activity levels converge for the two genotypes at the oldest ages tested. A two-factor independent-groups analysis of variance showed a significant effect of age (F(1,148) = 10.45, p < 0.001), a significant effect of genotype (F(4,148) = 5.93, p < 0.05), but no significant interaction between age and genotype (F(4,148) = 1.63, n.s.). The observed genotype effect results from significant differences in activity levels at ages 50 and 60 days. Post hoc independent-group t tests showed a significant genotype effect for both the 50-day age groups (t(33) = 2.57, p < 0.05) and the 60-day groups (t(42) = 2.17, p < 0.05).
Fig. 4.
Number of line crossings as a measure of locomotor (walking) activity for mth (triangles) and control groups (circles) as a function of age. Error bars are one standard deviation, and lines are regression fits. Bottom panel shows differences in activity levels between mth and control flies, with significant differences observed at ages 50 and 60 days
Discussion
The experiments described here provide new insights into functional aging of a Drosophila line whose chronological aging has been delayed by a chromosome 3 mutation. We found that mth maintains continuous flight significantly longer than controls in early stages of life. No differences were observed in middle stages of life, and a small disadvantage (i.e., shorter flight durations) was observed in late life suggesting an interaction between genotype and age for the ability to sustain flight.
In earlier work, we found that mth mutants have a higher wingbeat frequency in early and middle, but not late life (Petrosyan et al. 2007). Flight abilities of mth, therefore, appear to be more robust during the first few days of life, but the decline and failure of these functions in late life is not delayed to parallel their increased lifespan. Although mth mutants outlive parental control flies by of 35 % to 45 %, their flight abilities fail on the same schedule or earlier than that of controls. This is surprising because the mth gene is speculated to be involved in regulation of Drosophila’s antioxidant defense system (Lin et al. 1998) which mitigates the cumulative oxidative damage to DNA and cell structure that leads to eventual systemic failure during the normal aging process (Harman 1995, 2003; Barja 2004; Bokov et al. 2004; Landis and Tower 2005). Oxidative stress results from the presence of oxygen radicals or reactive oxygen species (ROS; e.g., hydrogen peroxide H2O2) which are byproducts of oxygen consumption by all aerobic organisms. Drosophila that are null for the mth gene die before adulthood, but mth mutation which reduces the expression of this gene significantly increases lifespan (Lin et al. 1998). This suggests that the gene is essential for survival but its full expression exceeds optimum levels. It is unclear why antioxidant intervention by mth mutation leads to improved sensorimotor performance during early stages of life when cumulative oxidative damage has likely not yet had time to cause significant systemic dysfunction, but does not delay the failure of these functions in late-life in spite of leading to a significantly increased lifespan.
These results are also informative when compared to patterns of functional decline observed for another life-extended Drosophila line that carries the human gene superoxide dismutase (SOD1), an enzyme whose function is to remove oxidants from the system (Mccord and Fridovic 1969; Parkes et al. 1998; Phillips et al. 2000). While broad expression of the human SOD1 gene has no effect on Drosophila longevity (Kirby et al. 2008) selective expression of this gene in motorneurons extends lifespan by 40 % (Parkes et al. 1998). This increased lifespan is coupled with significant improvements to complex functions that include optomotor efficiency in tracking a visual target during flight (Petrosyan et al. 2012a), and longer sustained flight during early and middle stages of life (Petrosyan et al. 2012b). These patterns are partly similar to those of the mth mutant. However, the largest advantage in flight duration for the SOD1 line was during middle stages of life (30 days), an age at which no advantage was observed for the mth line. Another difference between these two life-extended lines is the pattern of change in wingbeat frequency as a function of age. The mth mutant has higher wingbeat frequencies relative to its parental control during early and middle, but not late stages of life. The SOD1 line has the same wingbeat frequency as its control line in early life, but progressively lower wingbeat frequency as it ages, with the largest difference observed in late life. These patterns appear to be opposite of each other. None of these differences are likely due to differences in metabolic rate, since measurements of respiration rate for both lines (mth and SOD1) appear equivalent to their respective control lines (Mockett and Sohal 2006; Parkes et al. 1998).
Our results also show a significant difference between mth and control flies in their locomotor activity in late life. We found that the mth line displays significantly lower levels of locomotion than controls at ages 50 and 60 days. This is opposite to that previously reported for the SOD1 life-extended line which shows a significantly higher level of locomotor activity in late life relative to its control line (Petrosyan et al. 2012b), and different than that reported in another study of mth locomotor activity (Cook-Wiens and Grotewiel 2002). In the latter study, no genotype difference in exploratory (walking) behavior was observed, suggesting a dissociation between functional decline and extension of lifepspan for the mth line. Our results, however, do not support such dissociation, at least for flight and locomotion. Mockett and Sohal (2006) have, in addition, reported a higher walking speed for relatively young (10 days) mth flies in a negative geotaxis experiment, whereas Cook-Weins and Grotewiel (2002) observed no difference between mth and control flies in negative geotaxis (2002). Possible differences between our findings on walking behavior and those of Cook-Weins and Grotewiel (2002) may be related either to the age tested or to the experimental environments within which exploratory behavior was measured. The flies we tested were 50 days of age or older, whereas in their study all flies were younger than 45 days. In addition, they used a small 8 cm diameter Petri dish under dim red light; whereas, we used a significantly larger open arena (60 × 45 cm) under broadband white light.
To be clear, our experiment on locomotion shows only that mth flies are less active in walking behavior late in life. One interpretation of this finding, given similar levels of metabolic rates at old age (Lin et al. 1998), is that mth flies have less robust motor functions in late life relative to age-matched controls. A second interpretation is that their locomotor system is intact, but their tendency to explore their environment has diminished. We should caution that while we observed lower levels of locomotor activity for mth flies relative to controls in late life, we have not measured exploratory walking behavior in early life. Unless a fly’s wings are clipped, potentially causing damage, young flies tend to walk only short distances, preferring instead to jump or fly when placed in a large open arena. We favored a large well-lit arena as a more “natural” environment when contrasted to a small-enclosed Petri dish in red background lighting (Cook-Weins and Grotewiel 2002). We also preferred to avoid clipping a fly’s wings, as the damage caused to these flies would be unclear and possibly different across individual flies, and may thus have affected measures of locomotor activity. Although we did not measure locomotor activity in young flies, at least one prior study does provide evidence that young (<10 days) mth flies display more robust locomotor functions relative to age-matched controls, as indicated by their faster walking speeds and hence longer distances per unit time (Mockett and Sohal 2006). This finding is opposite to what we have found for old flies, and suggestive of an age by genotype interaction in walking behavior.
Several life-extension strategies have been used to increase longevity in Drosophila. These include screening genetic mutants for longevity phenotypes (Lin et al. 1998), overexpression of genes known to enhance antioxidant defense mechanisms (Parkes et al. 1998), generational selection studies (Rose et al. 1992), and environmental strategies such as drug consumption (e.g., 4-phenylbutyrate; PBA; Kang et al. 2002) or caloric restriction (Clancy et al. 2002; Mair et al. 2003). It is becoming increasingly clear that different genetic and environmental strategies for life extension lead to different patterns of gerontological decline in functional abilities of Drosophila (e.g., flight, locomotion, visuo-motor coordination, memory, and learning). Decline of some functions are delayed in late life for some life-extended lines, and are unaffected for others, whereas some life-extended lines show more robust abilities relative to control lines in early but not late life. These diverse findings suggest significant interaction between different life-extension genes, age, and sex on functional senescence, necessitating line-specific explorations of functional decline and raising the question of whether different strategies to life extension may be used in concert to optimally preserve a broad range of functions in late life.
Acknowledgments
The mth and control flies were graciously provided by the laboratory of the late Prof. Seymour Benzer. We thank Rosana Magalhães, Eugénia Fernandes, and Jorge Alves for helpful discussions. This work was supported by funding from the University of California, Irvine, and from the University of Minho, Portugal.
Footnotes
These survival functions are in line with several previous studies of mth (Cook-Wiens and Grotewiel 2002; Mockett and Sohal 2006), shorter than those reported by Lin et al. (1998), and longer than those reported by others (Cevjic et al. 2004; Petrosyan et al. 2007; Paaby and Schmidt 2008). These differences may be due to a variety of factors as partly described by Cook-Wiens and Grotewiel (2002), including differences in genetic backgrounds (Finch 1990), frequency of changing food media (Baldal et al. 2006; Cook-Wiens and Grotewiel 2002), and number and length of times exposed to anesthesia during transfer. Significant increase in longevity by mth mutation, however, is reported in all these studies.
Temperature has a significant effect on mth longevity with most studies maintaining mth stocks at an optimum temperature of 25 °C (Lin et al. 1998; Cvejic et al. 2004; Wang et al. 2004; Martinez et al. 2007; Petrosyan et al. 2007). Humidity, however, has been reported to have little or no effect on mth life extension for relative humidity ranging from 15 % to 90 % (Mockett and Sohal 2006).
References
- Baldal EA, Baktawar W, Brakefield PM, Zwaan BJ. Methuselah life history in a variety of conditions, implications for the use of mutants in longevity research. Exp Gerontol. 2006;41:1126–1135. doi: 10.1016/j.exger.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Barja G. Free radicals and aging. Trends Neurosci. 2004;27:595–600. doi: 10.1016/j.tins.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Bland ND, Robinson P, Thomas JE, Shirras AD, Turner AJ, Isaac RE. Locomotor and geotactic behavior of Drosophila melanogaster over-expressing neprilysin. Peptides. 2009;30:571–574. doi: 10.1016/j.peptides.2008.10.020. [DOI] [PubMed] [Google Scholar]
- Bokov A, Chaudhuri A, Richardson A. The role of oxidative damage and stress in aging. Mech Aging Dev. 2004;125:811–826. doi: 10.1016/j.mad.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Butler D. Venter's Drosophila 'success' set to boost human genome efforts. Nature. 1999;401:729–730. doi: 10.1038/44410. [DOI] [PubMed] [Google Scholar]
- Cvejic S, Zhu Z, Felice SJ, Berman Y, Huang XY. The endogenous ligand Stunted of the GPCR Methuselah extends lifespan in Drosophila. Nat Cell Biol. 2004;6:540–546. doi: 10.1038/ncb1133. [DOI] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Hafen E, Leevers SJ, Partridge L. Dietary restriction in long-lived dwarf flies. Science. 2002;296:319. doi: 10.1126/science.1069366. [DOI] [PubMed] [Google Scholar]
- Cook-Wiens E, Grotewiel MS. Dissociation between functional senescence and oxidative stress resistance in Drosophila. Exp Gerontol. 2002;37:1345–1355. doi: 10.1016/S0531-5565(02)00096-7. [DOI] [PubMed] [Google Scholar]
- Curtsinger JW, Fukui HH, Khazaeli AA, Kirscher A, Pletcher SD, Promislow SEL, Tatar M. Genetic variation and aging. Annu Rev Genet. 1995;29:553–575. doi: 10.1146/annurev.ge.29.120195.003005. [DOI] [PubMed] [Google Scholar]
- Finch CE. Longevity, Senescence, and the Genome. Chicago: University of Chicago Press; 1990. [Google Scholar]
- Fortini ME, Skupski MP, Boguski MS, Hariharan IK. A survey of human disease gene counterparts in the Drosophila genome. J Cell Biol. 2000;150:F23–F29. doi: 10.1083/jcb.150.2.F23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakou ME, Goss M, Junger MA, Hafen E, Leevers SJ, et al. Longlived Drosophila with overexpressed dFOXO in adult fat body. Science. 2004;305:361. doi: 10.1126/science.1098219. [DOI] [PubMed] [Google Scholar]
- Hadler NM. Genetic influence on phototaxis in Drosophila Melanogaster. Biol Bull. 1964;126:264–273. doi: 10.2307/1539524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. The free radical theory of aging. Antioxid Redox Signal. 2003;5:557–561. doi: 10.1089/152308603770310202. [DOI] [PubMed] [Google Scholar]
- Harman D. Free radical theory of aging: Alzheimer's disease pathogenesis. Age. 1995;18:97–119. doi: 10.1007/BF02436085. [DOI] [Google Scholar]
- Hayward DC, Delaney SJ, Campbell HD, Ghysen A, Benzer S, et al. The Sluggish-A Gene of Drosophila melanogaster is Expressed in the Nervous System and Encodes Proline Oxidase, a Mitochondrial Enzyme Involved in Glutamate Biosynthesis. Proc Nat Acad Sci USA. 1993;90:2979–2983. doi: 10.1073/pnas.90.7.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernfindez de Salomon C, Spatz HC. Colour vision in Drosophila melanogaster: Wavelength discrimination. J Comp Physiol. 1983;150:31–37. doi: 10.1007/BF00605285. [DOI] [Google Scholar]
- Kang HL, Benzer S, Min KT. Life extension in Drosophila by feeding a drug. Proc Nat Acad Sci USA. 2002;99:838–843. doi: 10.1073/pnas.022631999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, et al. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby K, Jensen LT, Binnington J, Hilliker AJ, Ulloa J, Culotta VC, Phillips JP. Instability of superoxide dismutase 1 of Drosophila in mutants deficient for its cognate copper chaperone. J Biol Chem. 2008;283:35393–35401. doi: 10.1074/jbc.M807131200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis GN, Tower J. Superoxide dismutase evolution and life span regulation. Mech Aging Dev. 2005;126:365–379. doi: 10.1016/j.mad.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Lin YJ, Seroude L, Benzer S. Extended life-span and stress resistance in the Drosophila mutant Methuselah. Science. 1998;282:943–946. doi: 10.1126/science.282.5390.943. [DOI] [PubMed] [Google Scholar]
- Loeb J, Bancroft FW. Some experiments on the production of mutants in Drosophila. Science. 1911;33:781–783. doi: 10.1126/science.33.855.781. [DOI] [PubMed] [Google Scholar]
- Mair W, Goymer P, Pletcher SD, Partridge L (2003) Demography of dietary restriction and death in Drosophila. Science 301:1731–1733 [DOI] [PubMed]
- Martinez VG, Javadi CS, Ngo E, Lagow RD, Zhang B. Age-related changes in climbing behavior and neural circuit physiology in Drosophila. Dev Neurobiol. 2007;67:778–791. doi: 10.1002/dneu.20388. [DOI] [PubMed] [Google Scholar]
- Mccord JM, Fridovic I. Superoxide Dismutase an enzymic function for erythrocuprein (Hemocuprein) J Biol Chem. 1969;244:6049. [PubMed] [Google Scholar]
- Miller GV, Hansen KN, Stark WS. Phototaxis in Drosophila – R1-6 input and interaction among ocellar and compound eye receptors. J Insect Physiol. 1981;27:813–819. doi: 10.1016/0022-1910(81)90073-1. [DOI] [Google Scholar]
- Mockett RJ, Sohal RS. Temperature-dependent trade-offs between longevity and fertility in the Drosophila mutant, methuselah. Exp Gerontol. 2006;41:566–573. doi: 10.1016/j.exger.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Osiewacz HD. Genetic regulation of aging. J Mol Med. 1997;75:715–727. doi: 10.1007/s001090050158. [DOI] [PubMed] [Google Scholar]
- Paaby AB, Schmidt PS. Functional significance of allelic variation at Methuselah, an aging gene in Drosophila. PLoS One. 2008;3:1–8. doi: 10.1371/journal.pone.0001987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes TL, Elia AJ, Dickinson D, Hilliker AJ, Phillips JP, Boulianne GL. Extension of drosophila lifespan by overexpression of human SOD1 in motorneurons. Nat Genet. 1998;19:171–174. doi: 10.1038/534. [DOI] [PubMed] [Google Scholar]
- Petrosyan A, Hsieh I, Saberi K. Age-dependent stability of sensorimotor functions in the life-extended Drosophila mutant Methuselah. Behav Genet. 2007;37:585–594. doi: 10.1007/s10519-007-9159-y. [DOI] [PubMed] [Google Scholar]
- Petrosyan A, Goncalves OF, Hsieh I, Phillips JP, Saberi K (2012a) Enhanced optomotor efficiency by selective expression of the human gene superoxide dismutase primarily in Drosophila motorneurons. J Neurogenetics 27:59–67 [DOI] [PubMed]
- Petrosyan A, Goncalves OF, Hsieh I, Phillips JP, Saberi K (2012b) Enhanced flight and locomotion by selective expression of the human gene SOD1 in Drosophila motorneurons. (submitted)
- Phillips JP, Parkes TL, Hilliker AL. Targeted neuronal gene expression and longevity in Drosophila. Exp Gerontol. 2000;35:1157–1164. doi: 10.1016/S0531-5565(00)00117-0. [DOI] [PubMed] [Google Scholar]
- Roberts DB. Drosophila: A practical approach. 2. New York: Oxford University Press; 1998. [Google Scholar]
- Rogina B, Helfand SL, Frankel S. Longevity regulation by Drosophila Rpd3 deacetylase and caloric restriction. Science. 2002;298:1745–1745. doi: 10.1126/science.1078986. [DOI] [PubMed] [Google Scholar]
- Rose MR, Vu LN, Park SU, Graves JL. Selection on stress resistance increases longevity in Drosophila-Melanogaster. Exp Gerontol. 1992;27:241–250. doi: 10.1016/0531-5565(92)90048-5. [DOI] [PubMed] [Google Scholar]
- Slawson JB, Kuklin EA, Ejima A, Mukherjee K, Ostrovsky L, Griffith LC. Central regulation of locomotor behavior of drosophila melanogaster depends on a CASK isoform containing CaMK-Like and L27 domains. Genetics. 2011;187:171–184. doi: 10.1534/genetics.110.123406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Ranjan R, Dawson-Scully K, Bronk P, Marin L, et al. Presynaptic regulation of neurotransmission in Drosophila by the G protein-coupled receptor methuselah. Neuron. 2002;36:105–119. doi: 10.1016/S0896-6273(02)00932-7. [DOI] [PubMed] [Google Scholar]
- Strauss R, Hanesch U, Kinkelin M, Wolf R, Heisenberg M. No-bridge of Drosophila melanogaster- Portrait of a structural brain mutant of the central complex. J Neurogenet. 1992;8:125–155. doi: 10.3109/01677069209083444. [DOI] [PubMed] [Google Scholar]
- Wang HD, Kazemi-Esfarjani P, Benzer S. Multiple-stress analysis for isolation of Drosophila longevity genes. Proc Nat Acad Sci USA. 2004;101:12610–12615. doi: 10.1073/pnas.0404648101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MC, Bohmann D, Jasper H. JNK signaling confers tolerance to oxidative stress and extends lifespan in Drosophila. Dev Cell. 2003;5:811–816. doi: 10.1016/S1534-5807(03)00323-X. [DOI] [PubMed] [Google Scholar]