Abstract
Magnetic resonance spectroscopy (MRS) can explore aging at a molecular level. In this study, we investigated the relationships between regional concentrations of metabolites (such as choline, creatine, myo-inositol, and N-acetyl-aspartate) and normal aging in 30 cognitively normal subjects (15 women and 15 men, age range 22–82, mean = 49.9 ± 18.3 years) using quantitative proton magnetic resonance spectroscopy. All MR scans were performed using a 3 T scanner. Point resolved spectroscopy was used as the volume selection method for the region-of-interest and the excitation method for water suppression. Single voxel spectroscopy with short echo time of 39 ms and repetition time of 2,000 ms was employed. Single voxels were placed in the limbic regions, i.e., anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), and left and right hippocampi. Cerebrospinal fluid normalization and T1 and T2 correction factors were implemented in the calculation of absolute metabolite concentrations. A standardized T1W 3D volumetric fast field echo and axial T2-weighted fast spin-echo images were also acquired. Our results showed significant positive correlation of choline (r = 0.545, p = 0.002), creatine (r = 0.571, p = 0.001), and N-acetyl-aspartate (r = 0.674, p < 0.001) in the ACC; choline (r = 0.614, p < 0.001), creatine (r = 0.670, p < 0.001), and N-acetyl-aspartate (r = 0.528, p = 0.003) in the PCC; and NAA (r = 0.409, p = 0.025) in the left hippocampus, with age. No significant gender effect on metabolite concentrations was found. In aging, increases in choline and creatine might suggest glial proliferation, and an increase in N-acetyl-aspartate might indicate neuronal hypertrophy. Such findings highlight the metabolic changes of ACC and PCC with age, which could be compensatory to an increased energy demand coupled with a lower cerebral blood flow.
Keywords: Aging, Anterior cingulate cortex, Posterior cingulate cortex, Magnetic resonance spectroscopy, Neuronal hypertrophy, Absolute quantification
Introduction
Aging is accompanied by cognitive decline in a major segment of the population and is the primary risk factor for Alzheimer’s disease and other prevalent neurodegenerative disorders. Despite this central role in disease pathogenesis and morbidity, the aging of the brain has not been well understood at a molecular level (Yankner et al. 2008).
Proton magnetic resonance spectroscopy (1H-MRS) can measure metabolite levels by using proton signals from the metabolites (Martin 2007). It is a noninvasive clinical tool and has been applied to study neurodegenerative diseases and aging (Kantarci et al. 2000). A previous 1H-MRS study of purified brain cell cultures found that each neural cell type had a characteristic metabolic pattern, and N-acetyl aspartate (NAA) was specific for mature neurons (Urenjak et al. 1993).
Many studies of 1H-MRS in normal aging have been published and a literature review of these studies in the normal aging brain can be found in a recent publication (Reyngoudt et al. 2012). Some studies employed absolute quantification (Brooks et al. 2001; Chang et al. 1996; Charlton et al. 2007; Gruber et al. 2008; Harada et al. 2001; Leary et al. 2000; Reyngoudt et al. 2012; Saunders et al. 1999; Schuff et al. 2001), while others employed relative quantification (Angelie et al. 2001; Grachev et al. 2001). Many researchers found that metabolite concentrations expressed as relative metabolite ratios could be misleading as the referenced metabolite (such as creatine) could change with aging (Chang et al. 1996; Leary et al. 2000; Reyngoudt et al. 2012; Saunders et al. 1999). Moreover, there was clear evidence that relative quantification introduced larger errors than absolute quantification (Jansen et al. 2006).
Absolute quantification in 1H-MRS can be achieved by several methods, mainly classified as using either internal or external references. For absolute quantification using internal reference, internal water is the standard reference (Jansen et al. 2006). External methods include external concentration reference and phantom replacement external concentration reference. There is another relatively new external technique called “electric reference to access in vivo concentrations” (Heinzer-Schweizer et al. 2010). Both internal and external methods were used in previous aging studies (Brooks et al. 2001; Chang et al. 1996; Charlton et al. 2007; Gruber et al. 2008; Harada et al. 2001; Leary et al. 2000; Reyngoudt et al. 2012; Saunders et al. 1999; Schuff et al. 2001).
There appears to be a general consensus that there are age-related increases in total creatine (Cr) and choline (Cho) in either gray matter (Chang et al. 1996) or white matter (Gruber et al. 2008; Leary et al. 2000) or both (Angelie et al. 2001; Pfefferbaum et al. 1999), although no significant change in either Cho (Charlton et al. 2007; Harada et al. 2001; Reyngoudt et al. 2012; Saunders et al. 1999; Schuff et al. 2001) or Cr or both (Brooks et al. 2001) with age has been also reported. Such age-related increases might be explained by glial proliferation in normal aging (Finch 2003; Mrak and Griffin 2005), as higher concentrations of these metabolites were found in glial cells (Urenjak et al. 1993).
Another metabolite which is also related to glial cells is myo-inositol (mI). Most of the mI is present in the glial cells (Glanville et al. 1989). mI levels were reported to be increased with age (Chang et al. 1996; Gruber et al. 2008; Raininko and Mattsson 2010; Reyngoudt et al. 2012) or not change with age (Leary et al. 2000; Saunders et al. 1999). Increased mI levels might indicate glial proliferation and gliosis (Ross et al. 1998).
The most controversial metabolite in the normal aging brain is NAA. In most of the prior studies, NAA was reported to decrease (Brooks et al. 2001; Gruber et al. 2008; Harada et al. 2001) or have no change with age (Chang et al. 1996; Harada et al. 2001; Leary et al. 2000; Pfefferbaum et al. 1999; Reyngoudt et al. 2012; Saunders et al. 1999). However, an increase in NAA in the frontal and parietal cortices was found in two 1H-MRS imaging studies (Charlton et al. 2007; Schuff et al. 2001). In one recent study, absolute concentration of NAA showed a nonsignificant trend to increase with age (Reyngoudt et al. 2012.)
Early neuropathological studies suggested some neuronal loss with age in all cortical layers (Brody 1955) and a 10–60 % decline in cortical neuron density with aging (Coleman and Flood 1987). The accuracy of these studies was questioned with the development of stereological method in the early 1990s (Morrison and Hof 1997; West et al. 1994). The resulting conclusion was that significant cell death in the hippocampus and neocortex was not characteristic of normal aging (Bishop et al. 2010; Burke and Barnes 2006; Yankner et al. 2008).
Recently, no brain atrophy and, in fact, neuronal hypertrophy were found in asymptomatic Alzheimer’s disease (Iacono et al. 2008; O’Brien et al. 2009; Riudavets et al. 2007). In the Baltimore Longitudinal Study of Aging autopsy series, asymptomatic Alzheimer’s disease represented approximately 50 % of individuals with preserved cognition beyond 75 years of age, who tended to overlap with normal aging clinically. This group of individuals seemed to be resistant to the toxic effects of Alzheimer’s disease (AD) pathology (O’Brien et al. 2009).
Functional neuroimaging techniques such as 18 F-fluorodeoxyglucose positron emission tomography (18 F-FDG PET) and functional magnetic resonance imaging (fMRI) have provided insights into the aging brain (Hedden and Gabrieli 2004). A recent PET study showed that the anterior cingulate cortex (ACC) was involved in normal aging (Pardo et al. 2007). The ACC has recently become a focus for aging research because of its implicated role in attention and mood regulation. A fMRI study in older adults also demonstrated an increase in blood oxygen level-dependent (BOLD) signal in the ACC during the Stroop task (Milham et al. 2002).
A molecular imaging modality such as 1H-MRS could be useful to look for any metabolic evidence to address the above neuropathogical and neuroimaging findings. Of all brain regions which are believed to be closely associated with AD (i.e., posterior cingulate cortex (PCC), ACC, and hippocampus), only the ACC has not been subject to thorough investigation (Hirono et al. 1998; Liang et al. 2008; Minoshima et al. 1997; Reiman et al. 1996; Wang et al. 2009).
In the current study, we employed 3 T 1H-MRS to study metabolic changes in the limbic system with aging. Internal water was used as a reference for absolute quantification and several correction factors, such as cerebrospinal fluid (CSF) normalization and T1 and T2 relaxation times, were taken into account to enhance the accuracy (Charlton et al. 2007; Harada et al. 2001; Reyngoudt et al. 2012). We attempted to evaluate (a) any changes in neural tissue composition with aging, as measured by absolute quantification of metabolites such as NAA, Cho, Cr, and mI using 1H-MRS at 3 T, and (b) any metabolic changes in the limbic system (ACC, PCC, left and right hippocampi) with aging.
Methods
Subjects
Thirty healthy cognitively normal, right-handed Chinese controls (15 women, 15 men) were recruited in the study. Subjects were within the age range of 22 to 82 (mean = 49.9 ± 18.3) years. They were stratified into three age groups, 20–39, 40–59, and 60–89, with ten subjects for each. All of their Mini-Mental State Examination (MMSE) scores were 28 or above. Subjects were screened and excluded for high systolic blood pressure (>140 mmHg), previous cerebrovascular events, and claustrophobia. For all subjects, the exclusion criteria also included history of stroke; head injury; seizures; migraine; cancer within 5 years; active infection; end-stage renal or other organ failure; nonambulatory, psychiatric diseases; regular alcohol drinkers; and drug abusers.
We interviewed subjects aged 60–89 to gather sociodemographic data, self-reported smoking and alcohol history, drug or substance abuse, history of memory impairment and cognitive complaints, past medical history, and related medications. These subjects underwent full physical and neurological examination, standardized cognitive assessments, and a 1-hour battery of neuropsychological tests, as reported in our previous studies (Chu et al. 2009). Standardized cognitive assessment tools including the Chinese versions of the MMSE (Chiu et al. 1994), Alzheimer’s Disease Assessment Scale—cognitive subscale (Chu et al. 2000), and Delayed 10-Word Recall Test were used. For verbal memory, the Logical Memory subtest from the Wechsler Memory Scale—third edition and the Free and Cued Selective Reminding Test were administered (Petersen et al. 1992; Weschler 1997).
Subjects aged 20–59 were recruited from the university staff and students. They did not have any history of neurological disease and were not taking any psychiatric drug. They attended the university health clinic for routine check-up, which included blood pressure checking as well as clinical and physical examination by a registered medical practitioner. No memory deficit was detected on assessment. All subjects gave their informed consent to participate. The study was approved by the Institutional Review Board of The University of Hong Kong/Hong Kong West Cluster.
Data acquisition
All MR scans were performed using a 3 T scanner (Achieva 2.6.3, Philips Healthcare, Best, The Netherlands). A sensitivity encoding (SENSE)-head-8-coil was used. A standardized T1W 3D volumetric fast field echo (FFE) sequence was employed with the following imaging parameters: repetition time TR/TE = 7.0/3.2 ms, voxel size = 1 × 1 × 1 mm3, field of view = 256 × 256 × 167 mm3, reconstruction matrix = 256, and turbo field echo factor = 240. Images acquired from T1W 3D FFE were employed for the positioning of single voxel spectroscopy (SVS) for MRS. Axial T2-weighted fast spin-echo images (TR/TE = 3,000/80 ms, flip angle 90°, slice thickness 5 mm, ETL 16) were also acquired to exclude structural abnormalities.
T1 and T2 images were interpreted by an experienced neuroradiologist (HKFM). All T1 images were normal. Four subjects within the 60–89 age group had scattered nonspecific punctuate T2 abnormalities in subcortical and deep white matter. Therefore, they were not excluded from the study (Reyngoudt et al. 2012).
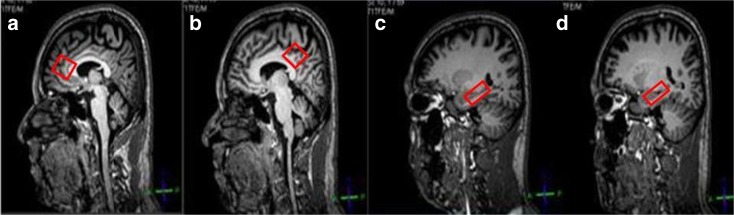
Point resolved spectroscopy (PRESS) was used as the volume selection method for the region-of-interest and the excitation method for water suppression. Scanning parameters are TR/TE = 2,000/39 ms, number of signals averaged = 128, phase cycles = 16, spectral width = 2,000 Hz with spectral resolution of 1.95 Hz per point, and free induction decay = 1,024. For shimming, pencil-beam-auto was employed. Voxels of size 2 × 2 × 2 cm3 were placed in the ACC (Figs. 1 and 2) and PCC (Fig. 1). For the left and right hippocampi, voxels were of size 2.5 × 1.5 × 1 cm3 (Fig. 1). The whole scan time was approximately 50 min.
Fig. 1.
a–d Positions of voxels placed on a 41-year-old male subject in the a anterior cingulate cortex, b posterior cingulate cortex, c left hippocampus, and d right hippocampus. Voxel size of a and b is 2 × 2 × 2 cm3. Voxel size of c and d is 2.5 × 1.5 × 1 cm3
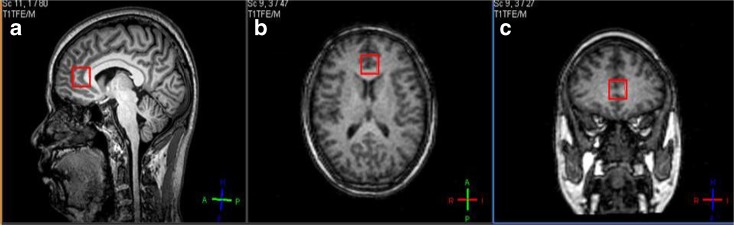
Fig. 2.
a–c Positions of voxels placed on a 63-year-old male subject in the anterior cingulate cortex: a sagittal view, b axial view, and c coronal view
In addition, using PRESS as a method for volume selection and the excitation method for water suppression, T1 and T2 relaxation times of various metabolites and water were measured in five subjects in the young age group of 20–39. T1 relaxation times were measured by a series of SVS MRS scans, with TR = 900, 1,200, 2,000, 3,000 and 5,000 ms and TE = 144 ms. For the determination of T2 relaxation times, similar techniques were employed, with a series of SVS MRS scans at TE = 35, 60, 90, 120, 150, 180, 288, and 408 ms and TR = 2,250 ms. Voxel sizes in both T1 and T2 metabolite determinations were 2 × 2 × 2 cm3 for ACC and PCC and 2.5 × 1.5 × 1 cm3 for the left and right hippocampi.
Data analysis
MRS
In this study, two MRS files were generated simultaneously by our scanner. One file was the actual (suppressed) MRS data, and the other file was the unsuppressed water signal intensity MRS file. For our absolute concentration calculation, the unsuppressed water signal intensity was used as internal reference.
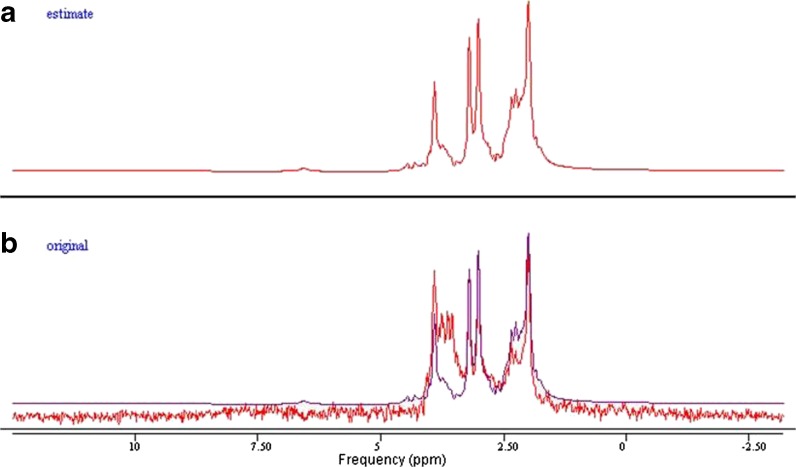
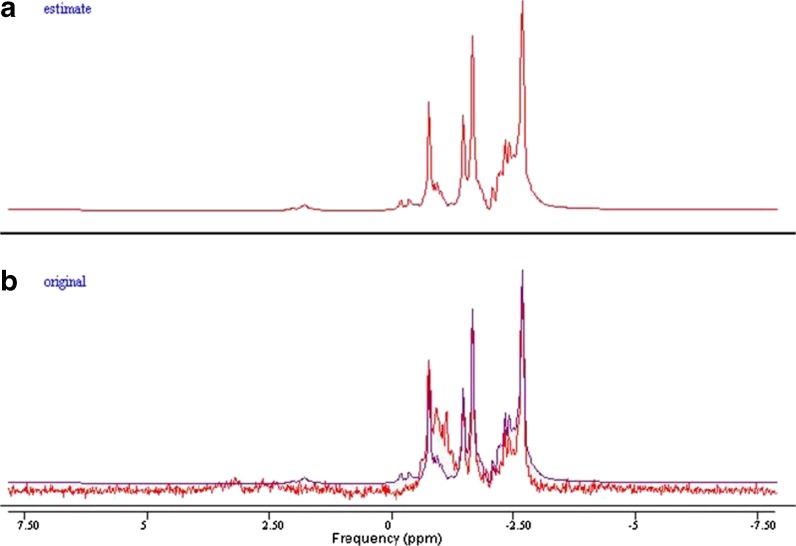
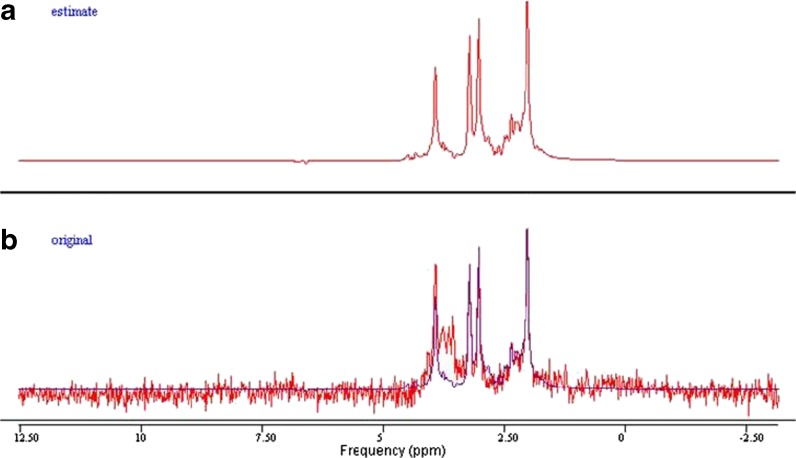
The MRS spectra were processed with an offline Java-based version of jMRUI (4.0) software. Spectrum simulation of various metabolites was completed using the built-in NMR-SCOPE. Signal amplitudes were determined using QUEST (quantification based on quantum estimation). The unsuppressed water signal was also measured using jMRUI. Cho, Cr, mI, and NAA were measured and quantified. T1 and T2 values were calculated as described in a previous study (Träber et al. 2004) (Figs. 3, 4, 5, and 6).
Fig. 3.
a, b Example of spectra in jMRUI. The simulated spectrum of a 41-year-old subject’s anterior cingulate cortex, using the parameter QUEST in jMRUI (a). In b, the spectrum obtained from the subject (red) was superimposed on the estimated spectrum (blue) from QUEST
Fig. 4.
a, b Example of spectra in jMRUI. The simulated spectrum of a 41-year-old subject’s posterior cingulate cortex, using the parameter QUEST in jMRUI (a). In b, the spectrum obtained from the subject (red) was superimposed on the estimated spectrum (blue) from QUEST
Fig. 5.
a, b Example of spectra in jMRUI. The simulated spectrum of a 41-year-old subject’s left hippocampus, using the parameter QUEST in jMRUI (a). In b, the spectrum obtained from the subject (red) was superimposed on the estimated spectrum (blue) from QUEST
Fig. 6.
a, b Example of spectra in jMRUI. The simulated spectrum of a 41-year-old subject’s right hippocampus, using the parameter QUEST in jMRUI (a). In b, spectrum obtained from the subject (red) was superimposed on the estimated spectrum (blue) from QUEST
Image processing
Variations in water content in gray matter (GM), white matter (WM), and CSF account for metabolite concentration differences, which affect the accuracy in absolute quantification. To ensure metabolite concentration differences were not due to tissue composition, voxel-based morphometry (VBM) was used to determine the GM, WM, and CSF composition within the voxel of each of the four regions investigated (Mak et al. 2011).
VBM segmentation
High-resolution 3D FFE T1 images were processed using the VBM8 toolbox (C. Gaser, structural Brain Imaging Group, Department of Psychiatry, University of Jena; http://dbm.neuro.uni-jena.de/vbm/) within an SPM8 environment (Wellcome Department of Imaging Neuroscience Group, London, UK; http://www.fil.ion.ucl.ac.uk/spm) on the MATLAB R2009a (MathWorks, Natrick, MA, USA) platform. Segmenation was performed using the default parameters in VBM8 with Hidden Markov Random Field Model. Segmented WM, GM, and CSF images were normalized to the ICBM-152 template (Montreal Neurological Institute) and saved in native space for later analysis.
Coregistration of the four SVS with the 3D FFE T1 images was performed and each individual single voxel was then overlapped with the segmented masks generated from SPM. The percentages of GM, WM, and CSF were calculated using MATLAB.
Absolute quantification
The T1 and T2 values for different metabolites were employed in the absolute quantification as mentioned above. The equation for absolute quantification is (de Graff 2007)
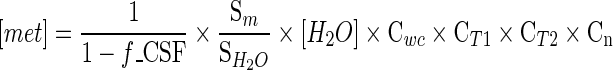 |
[met] is the concentration of the metabolite; f_CSF is the fraction of CSF; Sm is the signal intensity of the metabolite; 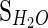 is the signal intensity of the unsuppressed water signal intensity; [H2O] is the concentration of water which is 55,509 mmol kg−1; Cwc is the correction factor of the relative water content (GM constitutes 82 % water, WM constitutes 73 % water, CSF constitutes 98 % water) (de Graff 2007); CT1 is the correction factor of the metabolite T1 value calculated as
is the signal intensity of the unsuppressed water signal intensity; [H2O] is the concentration of water which is 55,509 mmol kg−1; Cwc is the correction factor of the relative water content (GM constitutes 82 % water, WM constitutes 73 % water, CSF constitutes 98 % water) (de Graff 2007); CT1 is the correction factor of the metabolite T1 value calculated as 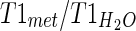 (T1mI was obtained from the literature) (Reyngoudt et al. 2010); CT2 is the correction factor of the metabolite T2 value calculated as
(T1mI was obtained from the literature) (Reyngoudt et al. 2010); CT2 is the correction factor of the metabolite T2 value calculated as 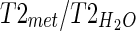 ; and Cn is the correction factor for the number of magnetically equivalent nuclei, found by the number of
; and Cn is the correction factor for the number of magnetically equivalent nuclei, found by the number of 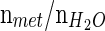 (n = 2 for H2O and mI, n = 9 for Cho, n = 3 for Cr, and n = 3 for NAA).
(n = 2 for H2O and mI, n = 9 for Cho, n = 3 for Cr, and n = 3 for NAA).
GM, WM, and CSF percentages of each SVS (as discussed in “Image processing”) were used in CSF normalization factor (1 / 1 − f_CSF) and Cwc. As for Cwc, the percentages of GM, WM, and CSF (as discussed in “Absolute quantification”) were used to calculate the relative water content (de Graff 2007).
Statistical analysis
SPSS 20.0 was employed for statistical analysis. A Pearson correlation coefficient was calculated to assess the correlation between metabolite concentrations and age. A one-way ANOVA was used to further explore the differences in metabolite concentrations among the three age groups. To investigate the gender effect on the metabolite concentrations, a two-samples t-test was used. A linear regression analysis was conducted to assess the effect of age and gender on the metabolite concentrations. The level of significance was set at 0.05.
Results
Mean concentrations
Mean absolute concentrations of Cho, Cr, mI, and NAA in the ACC, PCC, left hippocampus, and right hippocampus are shown in Table 1.
Table 1.
Mean concentrations of different metabolites in various limbic regions
| ACC | PCC | Left hippocampus | Right hippocampus | |
|---|---|---|---|---|
| Cho (mmol kg−1) | 1.00 ± 0.25 | 0.51 ± 0.07 | 0.65 ± 0.11 | 1.19 ± 0.28 |
| Cr (mmol kg−1) | 5.93 ± 1.29 | 6.52 ± 0.66 | 6.14 ± 0.81 | 8.18 ± 1.53 |
| NAA (mmol kg−1) | 12.06 ± 2.91 | 12.88 ± 1.30 | 15.98 ± 2.49 | 11.57 ± 2.86 |
| mI (mmol kg−1) | 0.19 ± 0.20 | 0.23 ± 0.21 | 0.10 ± 0.17 | 0.14 ± 0.24 |
ACC anterior cingulate cortex, Cho choline, Cr creatine, mI myo-inositol, mmol kg −1 millimole per kilogram per brain tissue, NAA N-acetyl-aspartate, PCC posterior cingulate cortex
Age effect on metabolite concentrations
Clearly, the ACC and PCC revealed significant positive correlation of Cho, Cr, and NAA concentrations with age (Table 2). The left and right hippocampi showed no significant result, except a positive correlation was detected for NAA with age in the left hippocampus.
Table 2.
Summary of statistical results of different metabolites in various limbic regions with age using Pearson correlation
| Region | Cho | Cr | NAA | mI | ||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| ACC | 0.545** | 0.002 ↑ | 0.571** | 0.001 ↑ | 0.674** | <0.001 ↑ | 0.254 | 0.192 − |
| PCC | 0.614** | <0.001 ↑ | 0.670** | <0.001 ↑ | 0.528** | 0.003 ↑ | 0.179 | 0.344 − |
| Left hippocampus | 0.283 | 0.129 − | 0.130 | 0.492 − | 0.409* | 0.025 ↑ | 0.289 | 0.122 − |
| Right hippocampus | 0.004 | 0.983 − | 0.258 | 0.169 − | 0.076 | 0.692 − | −0.024 | 0.902 – |
ACC anterior cingulate cortex, Cho choline, Cr creatine, mI myo-inositol, NAA N-acetyl-aspartate, PCC posterior cingulate cortex, ↑ significant increase, − no significant change
*p < 0.05, **p < 0.01
Further analysis by age groups also revealed that the significant differences in Cho, Cr, and NAA concentrations were clearly found in the ACC and PCC (Table 3). In the ACC, an increasing trend was observed for Cho, Cr, and NAA. In the PCC, the young age group (20–39 years) showed significantly lower levels of Cho and NAA than the other two age groups (40–89 years), while an increasing trend was observed for Cr. No significant correlation of mI with age is seen in all regions.
Table 3.
Summary of statistical results of different metabolites in various limbic regions by age group using one-way ANOVA
| Region/age group | Cho | Cr | NAA | mI | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20–39 | 40–59 | 60–89 | p | 20–39 | 40–59 | 60–89 | p | 20–39 | 40–59 | 60–89 | p | 20–39 | 40–59 | 60–89 | p | |
| ACC | 0.875 | 0.952 | 1.178 | 0.014* | 5.209 | 5.841 | 6.738 | 0.022* | 9.952 | 11.807 | 14.418 | 0.001* | 0.119 | 0.168 | 0.294 | 0.171 |
| PCC | 0.444 | 0.535 | 0.550 | <0.001** | 6.038 | 6.517 | 7.011 | 0.002** | 11.850 | 13.442 | 13.354 | 0.005** | 0.249 | 0.153 | 0.287 | 0.249 |
| Left hippocampus | 0.600 | 0.686 | 0.659 | 0.181 | 5.952 | 6.334 | 6.147 | 0.589 | 14.894 | 15.887 | 16.875 | 0.210 | 0.034 | 0.122 | 0.133 | 0.374 |
| Right hippocampus | 1.206 | 1.146 | 1.226 | 0.803 | 7.972 | 7.595 | 8.985 | 0.099 | 11.164 | 11.864 | 11.682 | 0.855 | 0.212 | 0.037 | 0.195 | 0.210 |
ACC anterior cingulate cortex, Cho choline, Cr creatine, mI myo-inositol, NAA N-acetyl-aspartate, PCC posterior cingulate cortex
*p < 0.05, **p < 0.01
Gender effect on metabolite concentrations
No significant gender effect on metabolite concentrations was found, except that a significantly lower level of Cr in males in the PCC was observed (Table 4).
Table 4.
Summary of statistical results of different metabolites in various limbic regions by gender group using two-samples t test
| Region/gender group | Cho | Cr | NAA | mI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | p | Female | Male | p | Female | Male | p | Female | Male | p | |
| ACC | 0.994 | 1.001 | 0.861 | 6.199 | 5.660 | 0.259 | 12.389 | 11.729 | 0.544 | 0.221 | 0.147 | 0.333 |
| PCC | 0.525 | 0.494 | 0.245 | 6.772 | 6.271 | 0.034* | 13.243 | 12.520 | 0.130 | 0.232 | 0.228 | 0.960 |
| Left hippocampus | 0.668 | 0.629 | 0.324 | 6.099 | 6.190 | 0.763 | 16.521 | 15.249 | 0.165 | 0.099 | 0.093 | 0.919 |
| Right hippocampus | 1.282 | 1.103 | 0.071 | 8.317 | 8.051 | 0.637 | 12.152 | 10.988 | 0.264 | 0.140 | 0.149 | 0.923 |
ACC anterior cingulate cortex, Cho choline, Cr creatine, mI myo-inositol, NAA N-acetyl-aspartate, PCC posterior cingulate cortex
*p < 0.05
Age and gender effect on metabolite concentrations
Using linear regression analysis, results of age and gender effects on metabolite concentrations are presented in Table 5. Broadly consistent with the findings in Tables 2 to 4, positive age effect was only found in the ACC and PCC, and no significant gender effect on metabolite concentrations was detected.
Table 5.
Summary of statistical results of different metabolites in various limbic regions to assess the age and gender effects using linear regression analysis
| Region/effect | Coefficient (p value in bracket) | |||||||
|---|---|---|---|---|---|---|---|---|
| Cho | Cr | NAA | mI | |||||
| Age | Gender | Age | Gender | Age | Gender | Age | Gender | |
| ACC | 0.009 (0.001)** | 0.131 (0.119) | 0.040 (0.002)** | −0.017 (0.969) | 0.116 (<0.001)** | 0.851 (0.330) | 0.002 (0.348) | −0.034 (0.701) |
| PCC | 0.002 (0.001)** | 0.001 (0.981) | 0.022 (<0.001)** | −0.216 (0.273) | 0.035 (0.009)** | −0.269 (0.552) | 0.002 (0.328) | 0.026 (0.756) |
| Left hippocampus | 0.001 (0.219) | −0.020 (0.629) | 0.008 (0.399) | 0.191 (0.558) | 0.049 (0.063) | −0.630 (0.496) | 0.003 (0.111) | 0.033 (0.622) |
| Right hippocampus | −0.002 (0.490) | −0.205 (0.057) | 0.021 (0.205) | 0.013 (0.983) | 0.000 (0.997) | −1.166 (0.305) | <0.001 (0.923) | 0.006 (0.952) |
ACC anterior cingulate cortex, Cho choline, Cr creatine, mI myo-inositol, NAA N-acetyl-aspartate, PCC posterior cingulate cortex
**p < 0.01
Discussion
Our study highlighted the metabolic changes in the ACC and PCC of the normal aging brain (Table 2). In our study, we found that Cho significantly increased with age, with a Pearson correlation coefficient of r = 0.545 (p = 0.002) and r = 0.614 (p < 0.001) in the ACC and PCC, respectively. These results were in concordance with previous studies (Chang et al. 1996; Gruber et al. 2008; Leary et al. 2000). Chang et al. suggested that since Cho was a marker of cell membrane turnover, an increase of Cho might imply rapid membrane phospholipid synthesis and related degradation. Leary et al. reported that the biological significance of increasing Cr and Cho with age was unclear, although higher concentrations of these metabolites were found in glial cells (Urenjak et al. 1993).
Increasing glial cell population was previously found to correlate with normal aging in the midfrontal cortex in a neuropathological study based on cell size (Terry et al. 1987). Another study employed an immunohistochemical technique in the mesial temporal lobe, which showed that normal aging was accompanied by dramatic activation of, and cytokine overexpression in, microglia and astrocytes (Mrak and Griffin 2005). Activation of astrocytes and microglia early in aging was also found in other studies (Finch 2003). A later neocortical immunocytochemical study found that the microglia was significantly increased in asymptomatic AD cases compared to controls (O’Brien et al. 2009; Vehmas et al. 2003). The absolute Cho concentration increase in our study supported glial proliferation during aging. We suspect that, in addition to the aging effect, potential lesions of asymptomatic AD patients might have exacerbated the Cho increase.
Our finding of an age-related Cr increase with age in the ACC and PCC, with a Pearson correlation coefficient of r = 0.571 (p = 0.001) and r = 0.670 (p < 0.001), was consistent with recent studies (Chang et al. 1996; Charlton et al. 2007; Gruber et al. 2008; Leary et al. 2000; Reyngoudt et al. 2012; Saunders et al. 1999; Schuff et al. 2001). However, another study claimed that there was no significant change of Cr with age (Brooks et al. 2001). Cr is considered as a cerebral metabolism marker and an energy reservoir (Imamura 2003). Charlton et al. postulated that an increase in total Cr might indicate reduction of cellular capacity to generate energy with age, evidenced by a prior rat study (Smith et al. 1997). Our finding might otherwise signify increasing number of glial cells in the related region as suggested by other researchers (Charlton et al. 2007; Leary et al. 2000; Reyngoudt et al. 2012; Saunders et al. 1999). Reyngoudt et al. found an age-related increase of Cr in the posterior cingulate gyrus (Pearson correlation coefficient = 0.57), but not in the left hippocampus. There was also an age-related increase in mI in both the posterior cingulate gyrus and the left hippocampus in their study, which seemed to support gliosis as an underlying cause.
In our study, we found no significant correlation between mI and aging, likely the result of a high degree of error in the measurement of mI (Table 1). Since mI is strongly coupled, some of the peaks will overlap. Elevated mI levels had been observed in mild cognitively impaired and AD patients. The mI levels in cognitively normal subjects are low and easily affected by a distorted baseline, overlapping of peaks, and noise from the background, as observed in our results.
It was interesting to discover a significant NAA increase with age in the ACC (r = 0.674, p < 0.001), PCC (r = 0.528, p = 0.003), and left hippocampus (r = 0.409, p = 0.025). Although most studies reported a significant age-related decrease (Brooks et al. 2001; Gruber et al. 2008; Harada et al. 2001), an increase in NAA was also found in two previous studies, involving the centrum semiovale white matter (Charlton et al. 2007) and the frontal white matter (Schuff et al. 2001). However, both authors attributed this observation to be technically related, i.e., variations in T1 and T2 metabolite relaxation times in aging. Since NAA is thought to reflect density and integrity of neurons, this result appears counterintuitive (Charlton et al. 2007). However, we postulated that it might serve as an important link or explanation for the recent findings reported in the literature by various fMRI, 18 F-FDG PET, and neuropathological studies.
A prior fMRI study involving the Stroop task showed an increased activation of the ACC in older participants (Milham et al. 2002). Elderly adults attained higher sensitivity to competing color information in order to accomplish the same task as young adults. This finding reflected an age-related decrease in effectiveness of attentional control in the anterior cingulate. Other fMRI studies have also found that elderly individuals showed greater functional activation of brain regions (usually in the prefrontal cortex) than younger adults and that such additional activations were often seen in high-performing older adults (Cabeza et al. 2002; Reuter-Lorenz and Cappell 2008). The authors proposed the “compensation hypothesis” to account for this phenomenon. Decline in frontal glucose metabolism involving the ACC/medial frontal cortex was correlated with a decline in cognitive function in a PET study of normal aging (Pardo et al. 2007). The authors suggested that decreased glucose uptake in the ACC or other medial regions might reflect structural atrophy.
Besides ACC, PCC and hippocampi activations were also reported in the BOLD fMRI studies of aging. One fMRI study reported that older adults had a significantly larger magnitude of activation than younger adults when performing famous name recognition (Nielson et al. 2006). The regions which showed such activation included the right hippocampus and the bilateral PCC. Another fMRI study based on an associative encoding task showed that there was no significant difference in hippocampal activation between young and old subjects (Sperling et al. 2003), while another study on autobiography memory retrieval only showed that the right hippocampus of older subjects was more activated than the younger group when retrieving autobiographical events (Maguire and Frith 2003).
Our current findings thereby provide a neural basis for their observations. Although the neural mechanism underlying the compensation activity was far from being understood (Hedden and Gabrieli 2004), there is a rapidly growing amount of literature linking NAA to cognitive functions such as intelligence, working memory, attention, and logical and verbal memory (Ross and Sachdev 2004). A prior neurodevelopmental study using 1H-MRS suggested that better working memory performance was associated with greater levels of NAA due to increased dendritic branching (Yeo et al. 2000). A recent neuropathological study (O’Brien et al. 2009) proposed that the group of “successful aging” (i.e., without clinical or neurological impairment) elderly individuals was not homogeneous. In their study, some individuals were practically free of neuropathology but others bore abundant AD lesions. The authors concluded that this latter group (termed as asymptomatic AD) with marked neuronal hypertrophy (in the hippocampus, ACC, and PCC) might account for the functional compensation reflected in the different clinical outcome compared to mild cognitive impairment and AD subgroups.
Interestingly, a recent 1H-MRS study (Shinno et al. 2007) found that an increase in NAA in the ACC might protect Alzheimer’s disease patients from developing behavioral and psychological symptoms. The study demonstrated that the neuropsychological scores (on a four-point scale of increasing severity) obtained in two categories of BEHAVE-AD (delusional thought and activity disturbance) were negatively related to NAA/Cr in the ACC, but not in the posterior cingulate gyrus.
Arterial spin labeling fMRI was employed (Restom et al. 2007) to compare measures of CBF and BOLD responses in the medial temporal lobe to a memory encoding task between healthy young and elderly adults. The older adults showed higher BOLD response, which was related to lower baseline CBF, via the so-called “M” factor in the calibrated fMRI literature (Ances et al. 2008). However, it is important to emphasize that lower baseline CBF does not mean lower brain metabolism (oxygen or glucose) in the context of aging. In fact, recent literature suggested that baseline brain oxygen metabolic rate is greater in the older individuals (Lu et al. 2011), presumably related to lower efficiency in mitochondria and neuronal functions. This concomitant increase in metabolic demand and decrease in blood supply of course will result in a higher oxygen extraction fraction (OEF) with age, which has been confirmed as well (Lu et al. 2011).
In summary, our interpretation of the present findings and the literature is that, with age, brain glucose and oxygen utilization increases to compensate for lower efficiency, which results in an increase in NAA. On the other hand, vascular function deteriorates with age (due to possibly multiple factors such as hypertension, atherosclerosis, arteriosclerosis, and amyloid angiopathy), thus CBF is decreased. This combination of higher demand and lower supply results in a greater OEF and lower baseline venous oxygenation, which according to BOLD signal model, would correspond to a greater fMRI response.
In our study, no significant gender effect on metabolite concentrations was found, except that a significantly lower level of Cr in males in the PCC was observed. Gender-related differences had been reported in prior studies but gender effect on metabolite concentrations is still controversial (Reyngoudt et al. 2012).
The current study had several methodological improvements compared to prior studies. Firstly, CSF normalization, T1 and T2 correction factors were implemented in the calculation of the absolute metabolite concentrations. In absolute quantification, correction factors for T1 and T2 are essential. Therefore, T1 and T2 correction factors were decided to be measured within the cohort, rather than relying on literature values to acquire more accurate results. To be ideal, metabolite concentrations should be corrected for individual T1 and T2, given interparticipant variability. However, the whole scan took around 150 min and the elderly subjects might not be able to withstand such a relatively long scan time. Therefore, a reasonable sample of five participants (17 % of the cohort) was chosen for the T1 and T2 relaxation time measurement.
To our knowledge, only three prior 1H-MRS studies corrected for T1 and T2 values (Brooks et al. 2001; Gruber et al. 2008; Reyngoudt et al. 2012), and these studies found no significant age dependence. Nevertheless, one study showed that there was a significant relation between T1 and T2 of various metabolites and age. For example, T2 of NAA showed a significant increase with age (Kreis et al. 2005).
In addition, T1 and T2 values of various metabolites vary in different regions, performance of scanners, and sequence designs. Compared to a study (Mlynarik et al. 2001) which used stimulated echo acquisition method as a method for volume selection and inversion times (TI) for calculation of T1, our measured T1 values of Cho, Cr, and NAA in ACC are 22.6, 28.6, and 35.4 %, respectively, larger using our PRESS method. Also, in contradistinction to Mlynariky’s study, we used eight time points instead of five for the measurement of T2 values. In short, the accuracy of this study was higher since we measured individual T1 and T2 values for each metabolite within each region of interest.
Secondly, there are few high-field 1H-MRS studies on aging (Gruber et al. 2008; Reyngoudt et al. 2012). In high field in vivo 1H-MRS, signal-to-noise intensity is tremendously improved, thus giving a higher quality 1H-MRS (Ugurbil et al. 2000). High field 1H-MRS can give spectral lines of higher resolution due to improved chemical shift dispersion and reduced higher order coupling effect. Therefore, accuracy in measuring metabolite concentrations using absolute quantification could be enhanced (Gruetter et al. 1998).
Thirdly, as for the data analysis tool, the LCModel was mostly employed in other studies (Gruber et al. 2008; Leary et al. 2000), while QUEST was chosen to be the quantification parameter in jMRUI due to its robustness in this study. LCModel spectra are based on individual metabolite solutions. For QUEST, the spectrum is simulated using the built-in NMR-SCOPE algorithm. Since signals obtained at short echo time contain overlapping spectral components from many metabolites, QUEST can provide the optimal fitting of metabolite basis-set signals to contaminated data by a parametric nonlinear least squares fitting of the untangled metabolite signals (Stefan et al. 2009).
The major limitation of the current study would be the relatively small sample size of 30 subjects. Hence, our study is a pilot investigation and a further large-scale study is warranted. Secondly, no significant correlation of the metabolite concentrations of bilateral hippocampi with aging was found (except NAA in the left hippocampus), which might be due to technical factors such as smaller voxel size (hippocampal voxel of 3.75 cm3 versus cingulate voxel of 8 cm3). Future study with appropriate voxel size is suggested to clarify the issue. Thirdly, comparisons between different studies were difficult due to inherent variable study designs, i.e., different brain regions under investigation, MRS sequences (SVS versus chemical shift imaging), and CSF adjustment methods.
In conclusion, our 1H-MRS findings highlighted the metabolic changes of the ACC and PCC in normal aging. Significant increases in Cho and Cr might suggest glial proliferation, and an increase in NAA might indicate neuronal hypertrophy. These findings seemingly provide the neuronal basis of a compensatory response to increased oxygen utilization despite a lower resting cerebral blood flow with age, evidenced by recent BOLD fMRI studies. Our study revealed an unrecognized role of the ACC in the aging brain, which might help to distinguish normal from pathological age-related cognitive changes. This study also showed that implementation of various correction factors is essential for absolute quantification of metabolites.
Acknowledgments
This work was supported by The University of Hong Kong seeding funding for research [grant number 20460015].
References
- Ances BM, Leontiev O, Perthen JE, Liang C, Lansing AE, Buxton RB. Regional differences in the coupling of cerebral blood flow and oxygen metabolic changes in response to activation: implications for BOLD-fMRI. NeuroImage. 2008;29:1510–1521. doi: 10.1016/j.neuroimage.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelie E, Bonmartin A, Boudraa A, Gonnaud PM, Mallet JJ, Sappey-Marinier D. Regional differences and metabolic changes in normal aging of the human brain: proton MR spectroscopic imaging study. AJNR Am J Neuroradiol. 2001;22:119–127. [PMC free article] [PubMed] [Google Scholar]
- Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature. 2010;464:529–35. doi: 10.1038/nature08983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody H. Organization of the cerebral cortex. III. A study of aging in the human cerebral cortex. J Comp Neurol. 1955;102:511–16. doi: 10.1002/cne.901020206. [DOI] [PubMed] [Google Scholar]
- Brooks JCW, Roberts N, Kemp GJ, Gosney MA, Lye M, Whitehouse GH. A proton magnetic resonance spectroscopy study of age-related changes in frontal lobe metabolite concentrations. Cereb Cortex. 2001;11:598–605. doi: 10.1093/cercor/11.7.598. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. NeuroImage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Poland RE, Jenden DJ. In vivo proton magnetic resonance spectroscopy of the normal aging human brain. Life Sci. 1996;58:2049–2056. doi: 10.1016/0024-3205(96)00197-X. [DOI] [PubMed] [Google Scholar]
- Charlton RA, McIntyre DJO, Howe FA, Morris RG, Markus HS. The relationship between white matter brain metabolites and cognition in normal aging: the GENIE study. Brain Research. 2007;1164:108–16. doi: 10.1016/j.brainres.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Chiu HFK, Lee HC, Chung WS, Kwong PK. Reliability and validity of the Cantonese version of Mini-Mental State Examination—a preliminary study. J Hong Kong Coll Psychiatr. 1994;4:25–28. [Google Scholar]
- Chu LW, Chiu KC, Hui SL, Yu GKK, Tsui WJC, Lee PWH. The reliability and validity of the Alzheimer’s Disease Assessment Scale cognitive Subscale (ADAS-cog) among the elderly Chinese in Hong Kong. Annals Academy of Singapore. 2000;29:478–85. [PubMed] [Google Scholar]
- Chu LW, Tam S, Lee PWH, Yik PY, Song YQ, Cheung BMY, Lam KSL. Late-life body mass index and waist circumference in amnestic mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2009;17:223–32. doi: 10.3233/JAD-2009-1043. [DOI] [PubMed] [Google Scholar]
- Coleman PD, Flood DG. Neuron numbers and dendritic extent in normal aging and Alzheimer’s disease. Neurobiol Aging. 1987;8:521–545. doi: 10.1016/0197-4580(87)90127-8. [DOI] [PubMed] [Google Scholar]
- de Graff RA. In vivo NMR spectroscopy: principles and techniques. 2. Chichester: Wiley; 2007. [Google Scholar]
- Finch CE. Neurons, glia and plasticity in normal brain aging. Neurobiol Aging. 2003;24:S123–S127. doi: 10.1016/S0197-4580(03)00051-4. [DOI] [PubMed] [Google Scholar]
- Glanville NT, Byers DM, Cook HW, Spence MW, Palmer FB. Differences in the metabolism of inositol and phosphoinositides by cultured cells of neuronal and glial origin. Biochem Biophys Acta. 1989;1004:169–79. doi: 10.1016/0005-2760(89)90265-8. [DOI] [PubMed] [Google Scholar]
- Grachev ID, Swarnkar A, Szeverenyi NM, Ramachandran TS, Apkarian AV. Aging alters regional multichemical profile of the human brain: an in vivo 1H-MRS study of young versus middle-aged subjects. J Neurochem. 2001;77:292–303. doi: 10.1046/j.1471-4159.2001.t01-1-00238.x. [DOI] [PubMed] [Google Scholar]
- Gruber S, Pinker K, Riederer F, Chmelík M, Stadlbauer A, Bittšanský M, Mlynárik V, Frey R, Serles W, Bodamer O, Moser E. Metabolic changes in the normal ageing brain: consistent findings from short and long echo time proton spectroscopy. Eur J Radiol. 2008;68:320–327. doi: 10.1016/j.ejrad.2007.08.038. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Weisdorf SA, Rajanayagan V, Terpstra M, Merkle H, Truwit CL, Garwood M, Nyberg SL, Ugurbil K. Resolution improvements in in vivo 1H NMR spectra with increased magnetic field strength. J Magn Reson. 1998;135:260–264. doi: 10.1006/jmre.1998.1542. [DOI] [PubMed] [Google Scholar]
- Harada M, Miyoshi H, Otsuka H, Nishitani H, Uno M. Multivariate analysis of regional metabolic differences in normal ageing on localised quantitative proton MR spectroscopy. Neuroradiol. 2001;43:448–452. doi: 10.1007/s002340000513. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JDE (2004) Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci 87–96 [DOI] [PubMed]
- Heinzer-Schweizer S, De Zanche N, Pavan M, Mens G, Struzenegger U, Henning A, Boesiger P. In-vivo assessment of tissue metabolite levels using 1H MRS and the Electric REference To access In vivo Concentrations (ERETIC) method. NMR in Biomed. 2010;23:406–413. doi: 10.1002/nbm.1476. [DOI] [PubMed] [Google Scholar]
- Hirono N, Mori E, Ishii K, Ikejiri Y, Imamura T, Shimomura T, Hashimoto M, Yamashita H, Sasaki M. Hypofunction in the posterior cingulate gyrus correlates with disorientation for time and place in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1998;64:552–554. doi: 10.1136/jnnp.64.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono D, O’Brien R, Resnick SM, Zonderman AB, Pletnikova O, Rudow G, An Y, West MJ, Crain B, Troncoso JC. Neuronal hypertrophy in asymptomatic Alzheimer disease. J Neuropathol Exp Neurol. 2008;67:578–89. doi: 10.1097/NEN.0b013e3181772794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura K. Proton MR spectroscopy of the brain with a focus on chemical issues. Magn Reson Med. 2003;2:117–132. doi: 10.2463/mrms.2.117. [DOI] [PubMed] [Google Scholar]
- Jansen JFA, Backes WH, Nicolay K, Kooi ME. 1H MRS of brain absolute quantification of metabolites. Radiology. 2006;240:318–332. doi: 10.1148/radiol.2402050314. [DOI] [PubMed] [Google Scholar]
- Kantarci K, Jack CR, Jr, Xu YC, Campeau NG, O’Brien PC, Smith GE, Ivnik RJ, Boeve BF, Kokmen E, Tangalos EG, Petersen RC. Regional metabolic patterns in mild cognitive impairment and Alzheimer's disease: a 1H MRS study. Neurology. 2000;55:210–217. doi: 10.1212/WNL.55.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis R, Slotboom J, Hofmann L, Boesch C. Integrated data acquisition and processing to determine metabolite contents, relaxation times, and macromolecule baseline in single examinations of individual subjects. Magn Reson Med. 2005;54:761–8. doi: 10.1002/mrm.20673. [DOI] [PubMed] [Google Scholar]
- Leary SM, Brex PA, MacManus DG, Parker GJM, Barker GJ, Miller DH, Thompson AJ. A 1H magnetic resonance spectroscopy study of aging in parietal white matter: implications for trials in multiple sclerosis. Magn Reson Imaging. 2000;18:455–459. doi: 10.1016/S0730-725X(00)00131-4. [DOI] [PubMed] [Google Scholar]
- Liang WS, Reiman EM, Valla J, Dunckley T, Beach TG, Grover A, Niedzielko TL, Schneider LE, Mastroeni D, Caselli R, Kukull W, Morris JC, Hulette CM, Schmechel D, Rogers J, Stephan DA. Alzheimer's disease is associated with reduced expression of energy metabolism genes in posterior cingulate neurons. Proc Natl Acad Sci. 2008;105:4441–4446. doi: 10.1073/pnas.0709259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Xu F, Rodrigue KM, Cheng Y, Flicker B, Hebrank AC, Uh J, Park DC. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb Cortex. 2011;21:1426–1434. doi: 10.1093/cercor/bhq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak HK, Zhang Z, Yau KKW, Zhang L, Chan Q, Chu LW. Efficacy of voxel-based morphometry with DARTEL and standard registration as imaging biomarkers in Alzheimer's patients and cognitively normal older adults at 3.0 Tesla MR imaging. J Alzheimer’s Dis. 2011;23:655–664. doi: 10.3233/JAD-2010-101659. [DOI] [PubMed] [Google Scholar]
- Martin WRW. MR spectroscopy in neurodegenerative disease. Mol Imaging Biol. 2007;9:196–203. doi: 10.1007/s11307-007-0087-2. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Frith CD. Aging affects the engagement of the hippocampus during autobiographical memory retrieval. Brain. 2003;126:1511–1523. doi: 10.1093/brain/awg157. [DOI] [PubMed] [Google Scholar]
- Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer's disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, Cohen NJ. Attention control in the aging brain: insights from an fMRI study of the Stroop task. Brain Cogn. 2002;49:277–96. doi: 10.1006/brcg.2001.1501. [DOI] [PubMed] [Google Scholar]
- Mlynarik V, Gruber S, Moser E. Proton T1 and T2 relaxation times of human brain metabolites at 3 Tesla. NMR Biomed. 2001;14:325–331. doi: 10.1002/nbm.713. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–19. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- Mrak RE, Griffin WST. Glia and their cytokines in progression of neurodegeneration. Neurobiol Aging. 2005;26:349–354. doi: 10.1016/j.neurobiolaging.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Douville KL, Seidenberg M, Woodard JL, Miller SK, Franczak M, Antuono P, Rao SM. Age-related functional recruitment for famous name recognition: an event-related fMRI study. Neurobiol Aging. 2006;27:1494–1504. doi: 10.1016/j.neurobiolaging.2005.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L, Crain BJ, Pletnikova O, Rudow G, Iacono D, Riudavets MA, Driscoll I, Price DL, Martin LJ, Troncoso JC. Neuropathologic studies of the Baltimore Longitudinal Study of Aging (BLSA) J Alzheimer’s Dis. 2009;18:665–675. doi: 10.3233/JAD-2009-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JV, Lee JT, Sheikh SA, Surerus-Johnson C, Shah H, Munch KR, Carlis JV, Lewis SM, Kuskowski MA, Dysken MW. Where the brain grows old: decline in anterior cingulated and medial prefrontal function with normal aging. NeuroImage. 2007;35:1231–37. doi: 10.1016/j.neuroimage.2006.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Smith G, Kokman E, Ivnik RJ, Tangalos EG. Memory function in normal aging. Neurology. 1992;42:396–401. doi: 10.1212/WNL.42.2.396. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Spielman D, Sullivan EV, Lim KO. In vivo spectroscopic quantification of the N-acetyl moiety, creatine, and choline from large volumes of brain gray and white matter: effects of normal aging. Magn Reson Med. 1999;41:276–284. doi: 10.1002/(SICI)1522-2594(199902)41:2<276::AID-MRM10>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Raininko R, Mattsson P. Metabolite concentrations in supraventricular white matter from teenage to early old age: a short echo time 1H magnetic resonance spectroscopy (MRS) study. Acta Radiologica. 2010;51:309–315. doi: 10.3109/02841850903476564. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D. Preclinical evidence of Alzheimer's disease in persons homozygous for the epsilon-4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- Restom K, Bangen KJ, Bondi MW, Perthen JE, Liu TT. Cerebral blood flow and BOLD responses to a memory encoding task: a comparison between healthy young and elderly adults. NeuroImage. 2007;37:430–439. doi: 10.1016/j.neuroimage.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Current Directions in Psychological Science. 2008;17:177–182. doi: 10.1111/j.1467-8721.2008.00570.x. [DOI] [Google Scholar]
- Reyngoudt H, De Deene Y, Descamps B, Paemeleire K, Achten E. 1H-MRS of brain metabolites in migraine without aura: absolute quantification using the phantom replacement technique. Magn Reson Mater Phys. 2010;4:227–241. doi: 10.1007/s10334-010-0221-z. [DOI] [PubMed] [Google Scholar]
- Reyngoudt H, Claeys T, Vlerick L, Verleden S, Acou M, Deblaere K, De Deene Y, Audenaert K, Goethals I, Achten E. Age-related differences in metabolites in the posterior cingulate cortex and hippocampus of normal ageing brain: a 1H-MRS study. Eur J Radiol. 2012;81:e223–e231. doi: 10.1016/j.ejrad.2011.01.106. [DOI] [PubMed] [Google Scholar]
- Riudavets MA, Iacono D, Resnick SM, O’Brien R, Zonderman AB, Martin LJ, Rudow G, Pletnikova O, Troncoso JC. Resistance to Alzheimer’s pathology is associated with nuclear hypertrophy in neurons. Neurobiol Aging. 2007;28:1484–1492. doi: 10.1016/j.neurobiolaging.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross BD, Bluml S, Cowan R, Danielsen E, Farrow N, Tan J. In vivo MR spectroscopy of human dementia. Neuroimaging Clin N Am. 1998;8:809–22. [PubMed] [Google Scholar]
- Ross AJ, Sachdev PS. Magnetic resonance spectroscopy in cognitive research. Brain Research Reviews. 2004;44:83–102. doi: 10.1016/j.brainresrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Saunders DE, Howe FA, van den Boogaart A, Griffiths JR, Brown MM. Aging of the adult human brain: in vivo quantitation of metabolite content with proton magnetic resonance spectroscopy. J Magn Reson Imaging. 1999;9:711–16. doi: 10.1002/(SICI)1522-2586(199905)9:5<711::AID-JMRI14>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Schuff N, Ezekiel F, Gamst AC, Amend DL, Capizzano AA, Maudsley AA, Weiner MW. Region and tissue differences of metabolites in normally aged brain using multislice 1H magnetic resonance spectroscopic imaging. Magn Reson Med. 2001;45:899–907. doi: 10.1002/mrm.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinno H, Inagaki T, Miyaoka T, Okazaki S, Kawamukai T, Utani E, Inami Y, Horiguchi J. A decrease in N-acetylaspartate and an increase in myoinositol in the anterior cingulate gyrus are associated with behavioral and psychological symptoms in Alzheimer's disease. J Neurol Sci. 2007;260:132–138. doi: 10.1016/j.jns.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Smith CD, Landrum W, Carney JM, Landfield PW, Avison MJ. Brain creatine kinase with aging in F-344 rats: analysis by saturation transfer magnetic spectroscopy. Neurobiol Aging. 1997;18:617–22. doi: 10.1016/S0197-4580(97)00156-5. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Chua EF, Cocchiarella AJ, Rentz DM, Rosen BR, Schacter DL, Albert MS. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003;74:44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan D, Di Cesare F, Andrasescu A, Popa E, Lazariev A, Vescovo E, Strbak O, Williams S, Starcuk Z, Cabanas M, van Ormondt D, Graveron-Demilly D. Quantitation of magnetic resonance spectroscopy signals: the jMRUI software package. Meas Sci Technol. 2009;20:104035. doi: 10.1088/0957-0233/20/10/104035. [DOI] [Google Scholar]
- Terry RD, DeTteresa R, Hansen LA. Neocortical cell counts in normal human adult aging. Ann Neurol. 1987;21:530–539. doi: 10.1002/ana.410210603. [DOI] [PubMed] [Google Scholar]
- Träber F, Block W, Lamerichs R, Gieseke J, Schild HH. 1H metabolite relaxation times at 3.0 Tesla: measurements of T1 and T2 values in normal brain and determination of regional differences in transverse relaxation. Magn Reson Imaging. 2004;19:537–545. doi: 10.1002/jmri.20053. [DOI] [PubMed] [Google Scholar]
- Urenjak J, Williams SR, Gadian DG, Noble M. Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci. 1993;13:981–89. doi: 10.1523/JNEUROSCI.13-03-00981.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurbil K, Adriany G, Andersen P, Chen W, Gruetter R, Hu X, Merkle H, Kim DS, Kim SG, Strupp J, Zhu XH, Ogawa S. Magnetic resonance studies of brain function and neurochemistry. Annu Rev Biomed Eng. 2000;2:633–660. doi: 10.1146/annurev.bioeng.2.1.633. [DOI] [PubMed] [Google Scholar]
- Vehmas AK, Kawas CH, Stewart WF, Troncoso JC. Immune reactive cells in senile plaques and cognitive decline in Alzheimer’s disease. Neurobiol Aging. 2003;24:321–31. doi: 10.1016/S0197-4580(02)00090-8. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhao C, Yu L, Zhou W, Li K. Regional metabolic changes in the hippocampus and posterior cingulate area detected with 3-Tesla magnetic resonance spectroscopy in patients with mild cognitive impairment and Alzheimer disease. Acta Radiologica. 2009;50:312–319. doi: 10.1080/02841850802709219. [DOI] [PubMed] [Google Scholar]
- Weschler DA. Weschler Memory Scale-III. New York: Psychological Corp; 1997. [Google Scholar]
- West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet. 1994;344:769–772. doi: 10.1016/S0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- Yankner B, Lu T, Loerch P. The aging brain. Annul Rev Pathol Mech Dis. 2008;3:41–66. doi: 10.1146/annurev.pathmechdis.2.010506.092044. [DOI] [PubMed] [Google Scholar]
- Yeo RA, Hill D, Campbell R, Vigil J, Brooks WM. Developmental instability and working memory ability in children: a magnetic resonance spectroscopy investigation. Developmental Neuropsychology. 2000;17:143–59. doi: 10.1207/S15326942DN1702_01. [DOI] [PubMed] [Google Scholar]