Abstract
We previously reported that moderate calorie restriction (CR) has minimal impact on testicular gene expression in young adult rhesus macaques, and no obvious negative impact on semen quality or plasma testosterone levels. We now extend these findings by examining the influence of CR on various aspects of the reproductive axis of older males, including 24-h circulating testosterone levels, testicular gene expression, and testicular morphology. Young adult and old adult male rhesus macaques were subjected to either 30 % CR for 5–7 years, or were fed a standard control diet. Analysis of the 24-h plasma testosterone profiles revealed a significant age-associated decline, but no evidence for CR-induced suppression in either the young or old males. Similarly, expression profiling of key genes associated with testosterone biosynthesis and Leydig cell maintenance showed no significant CR-induced changes in either the young or old animals. The only evidence for CR-associated negative effects on the testis was detected in the old animals at the histological level; when old CR animals were compared with their age-matched controls, there was a modest decrease in seminiferous tubule diameter and epithelium height, with a concomitant increase in the number of depleted germ cell lines. Reassuringly, data from this study and our previous study suggest that moderate CR does not negatively impact 24-h plasma testosterone profiles or testicular gene expression. Although there appear to be some minor CR-induced effects on testicular morphology in old animals, it is unclear if these would significantly compromise fertility.
Keywords: Calorie restriction, Gene expression, Locomotor activity, Seminiferous tubules, Testis, Testosterone
Introduction
First reported in the mid-1930s (McCay et al. 1935), calorie restriction (CR) remains the only proven nongenetic paradigm for extending lifespan, and it does so by slowing the rate of physiological decline and retarding age-related chronic diseases (Colman et al. 2009; Dhahbi et al. 2004; Gredilla and Barja 2005; Hursting et al. 2003; Koubova and Guarente 2003; Lane et al. 1999b). Moderate CR has demonstrated consistent effects across vertebrate classes with beneficial health effects on many physiological systems. Importantly, there is growing evidence that CR may also be beneficial at combating aging in primates, even though the exact underlying causal mechanism(s) are still poorly understood (Colman et al. 2009; Heilbronn and Ravussin 2003; Ingram et al. 1990; Kemnitz 2011; Mattison et al. 2012; Roth et al. 1999; Weindruch and Walford 1988). Moreover, there is increased public interest in adopting CR as a potential anti-aging remedy. Consequently, it is important to establish if there are any negative side effects of CR on specific aspects of human physiology, especially biological systems such as the hypothalamic–pituitary–gonadal (HPG) axis, which do not immediately and/or obviously impact an individual’s health.
With regard to restriction of calorie intake, distinctions need to be made between moderate and severe CR paradigms and also when the CR was initiated; for example, data from adult female rhesus macaques (Macaca mulatta) suggest that reproductive hormones and normal ovarian cyclicity are not adversely affected by long-term moderate CR (Black et al. 2001; Lane et al. 2001; Mattison et al. 2003). Similarly, ovarian cyclicity does not appear to be perturbed by short-term, moderate CR (Wu 2006). On the other hand, data from male rhesus macaques show that when moderate CR is initiated before puberty, it delays the maturation-related increase in circulating testosterone (T) levels and postpones skeletal growth by approximately 1 year (Lane et al. 1997; Roth et al. 2000). Generally, less is known about post-pubertal implementation of CR in male primates, but recent studies in our laboratory have demonstrated that moderate CR of young adult male macaques has only modest influence on gene expression in the pituitary and testes (Sitzmann et al. 2009) and has no obvious negative effect on semen quality or circulating T levels (Sitzmann et al. 2010). In contrast, there is abundant evidence from both human and nonhuman primate studies showing that short-term fasting (i.e., severe CR) can markedly suppress the release of luteinizing hormone (LH) and T (Cameron 1996; Cameron and Nosbisch 1991; Cameron et al. 1991, 1993; Dubey et al. 1986; Helmreich and Cameron 1992; Schreihofer et al. 1993). Consequently, important questions persist about the possible impact of moderate CR on testicular gene expression, testicular morphology, and T biosynthesis in both young adult and old males.
The HPG axis of male primates has been well studied, and there is clear evidence for an age-related reproductive decline with functional deterioration (Harman et al. 2001; Moffat et al. 2002; Ottinger 1998; Plymate et al. 1989; Schlatt et al. 2008; Sitzmann et al. 2008; Urbanski and Sorwell 2012). This includes decreased hypothalamic gonadotropin-releasing hormone, and decreased gonadotropin output from the pituitary gland, as well as reduced T production and blunted circadian rhythmicity of circulating T and LH levels. The gradual decline of these HPG axis components in the male makes determining the timing of reproductive senescence difficult; furthermore, some level of spermatogenesis can continue well into old age (Buwe et al. 2005; Henkel et al. 2005; Kidd et al. 2001; Plas et al. 2000). This does not mean, however, that there are no physiological ramifications of reproductive aging, especially in humans where decreasing T levels, metabolic and circadian changes, and increasing body weight are generally associated with aging. Body mass index, which typically increases during young and middle age, has been shown to negatively influence reproductive capacity in males (Hammoud et al. 2008; Jensen et al. 2004; Mendiola et al. 2009; Wong et al. 2000).
Because moderate CR is capable of reducing body mass and adiposity and improving general health parameters, it would be of great benefit to know if CR can also maintain reproductive fitness and attenuate age-related changes. For more than 25 years, the National Institute on Aging (NIA) and the University of Wisconsin-Madison have been conducting parallel studies of the effects of CR on general physiology, healthspan, and lifespan in rhesus macaques to determine its potential application in humans (Colman et al. 2009; Ingram et al. 1990; Kemnitz 2011; Mattison et al. 2012). Both of these studies have identified health effects consistent with results observed in other species, including attenuation of age changes in plasma triglycerides, oxidative damage, and glucose regulation and reduced incidence of diabetes, cancer, cardiovascular disease, and brain atrophy; these findings suggest that CR may indeed contribute to healthy aging in primates (Colman et al. 2009; Kemnitz 2011; Lane et al. 1996, 1999a; Mattison et al. 2003, 2012; Roth et al. 2002). For men considering CR as a potential lifestyle choice, the question then becomes whether moderate CR is likely to negatively impact T levels and impair fertility.
The aim of the present study was to help address concerns about potential negative effects of moderate CR on the HPG axis of young and old males. Using the rhesus macaque as a translational animal model, we examined the impact of 5–7 years of moderate CR on 24-h circulating T levels, testicular gene expression, and testicular morphology. Reassuringly, our data show minimal negative impact of moderate CR.
Materials and methods
Animals and diet
All experiments were conducted under approved protocols reviewed by the Institutional Animal Care and Use Committees at the University of Maryland and the Oregon National Primate Research Center (ONPRC). Male rhesus macaques (M. mulatta) were individually housed in a temperature-controlled environment (24 °C) under a fixed 12L/12D photoperiod (lights on 0700–1900 hours) with ad libitum access to drinking water. Individual animals were cared for by the ONPRC in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals (National Research Council 1996), which included daily health checks to ensure normal behavior, food consumption, and waste production. Additionally, routine physical examinations, hematological studies, fecal parasite checks, tuberculin testing, and dental cleaning were performed periodically.
The study consisted of two separate age groups. Group 1 included five young adults, maintained on a control diet (YAC; mean terminal body weight = 10.72 kg) and five young adults that were subjected to CR, beginning during the peripubertal period (Urbanski and Pau 1998) at 4 years and 11 months of age (YACR; mean terminal body weight = 9.19 kg), as previously described (Ingram et al. 1990; Mattison et al. 2003, 2012). Group 2 included six old adults maintained on a control diet (OAC; mean terminal body weight = 6.27 kg) and four calorie-restricted old adults (OACR; mean terminal body weight = 6.69 kg). Differences in mean body mass between the age-matched control and treatment groups were not significant and were consistent with weight and caloric intake changes reported in previous CR studies involving larger cohorts of male rhesus macaques (Mattison et al. 2005a, b).
In both age groups, baseline food intake was established for each animal as characterized by a few uneaten biscuits remaining in their cage at the end of each day. The control animals continued to be fed on this baseline level, which approximated ad libitum feeding, whereas calorie-restricted animals received 30 % less food than their age- and body weight-matched controls. In both groups, food was provided daily at 0800 and 1500 hours and consisted of specially formulated biscuits (Cargill, Minneapolis, MN) supplemented with daily fresh fruits or vegetables (10–40 cal). The composition of the diet was 15 % protein, 5 % fat, and 5 % fiber with a caloric content of ∼3.7 kcal/g. The biscuits included a vitamin/mineral mix that was 40 % higher than the recommended allowance for rhesus macaques by the National Research Council (2003), but were otherwise similar to those used in many laboratory studies of rhesus macaques. This vitamin/mineral supplementation was designed to ensure sufficient availability of essential nutrients to both diet groups. Biochemical assays were performed periodically and with every new shipment to ensure diet content and quality (Black et al. 2001; Mattison et al. 2005b).
Activity data
Actiwatch activity monitors (Philips-Respironics, Bend, OR) were used to record 24-h locomotor activity patterns in each animal (YAC, n = 5; YACR, n = 5; OAC, n = 6; and OACR, n = 4), as previously described (Downs et al. 2007; Haley et al. 2009; Urbanski 2011; Urbanski et al. 2012). These monitors use piezoelectric accelerometers to record the integration of intensity, amount, and duration of movement and thus provide insights about an animal’s daily energy expenditure. In the present study, the monitors were placed inside the pocket of protective jackets worn by the animals during remote blood sampling.
Testosterone measurement
Circulating levels of T vary widely throughout the day because of the underlying episodic and circadian pattern of release (Cameron 1996; Goodman et al. 1974; Urbanski and Sorwell 2012). Therefore, to more accurately assess the impact of moderate CR on hormone secretion, serial blood samples were collected across the day and night from each animal (YAC, n = 5; YACR n = 5; OAC, n = 6; and OACR, n = 4). After 4–5 years of dietary treatment, when animals in groups 1 and 2 were 10.6 ± 0.1 and 26.5 ± 0.7 years of age, respectively; all of the animals were surgically fitted with an indwelling subclavian vein catheter connected to a swivel-tether remote blood sampling system (Downs et al. 2008; Urbanski et al. 1997, 2004; Urbanski 2011). Individuals were allowed a minimum of 2 weeks to become accustomed to wearing a protective jacket prior to catheterization. Serial blood samples (1 ml) were collected remotely, every 30 min over a 24-h period from an adjacent room to avoid disturbing the animals. Samples were collected into EDTA-coated glass tubes, and after centrifugation at 4 °C, plasma supernatant was stored at −20 °C until assay by radioimmunoassay, as previously described (Resko et al. 1973; Urbanski and Pau 1998).
Tissue collection
Animals in group 1 were euthanized when ∼12 years old and after ∼7 years of dietary treatment; animals in group 2 were euthanized when 24–30 years old and after ∼5 years of dietary treatment. The procedure was performed by the ONPRC pathology service and involved ketamine sedation followed by sodium pentobarbital overdose, in accordance with National Research Council (1996) guidelines. Postmortem tissues were made immediately available to various investigators associated with a larger interdisciplinary NIA study. For the present study, a single testis was collected from each animal and weighed. A cross-sectional segment (<1 cm) was dissected from the equatorial region and bath-fixed overnight in Bouin’s aqueous solution for later morphological study, whereas the remainder of the testis was flash-frozen in liquid nitrogen for PCR analysis.
Testicular staining
Bouin’s-fixed testicular tissue from groups 1 (YAC, n = 5 and YACR, n = 5) and 2 (OAC, n = 6 and OACR, n = 4) was rinsed in 70 % ethanol, dehydrated in ethanol, cleared in butanol (Gabe 1968), and embedded in Paraplast Plus (Sigma Chemical Co., St. Louis, MO). Cross sections (5 μm thick) were cut using a Leitz RM 2155 microtome (Leica Microsystems GmbH, Wetzler, Germany), collected at intervals of 500 μm, mounted on albumin-coated microscope slides and then stained with a trichromic stain (Sigma). Briefly, sections were stained 75 s in Harris hematoxylin, rinsed in running tap water for 10 min, and then in distilled water. Slides were stained with 0.5 % erythrosine B (CI, 45,430)–0.5 % orange G (CI, 16,230) for 30 min, rinsed in distilled water, immersed for 10 min in 0.5 % phosphotungstic acid, and rinsed again in distilled water. Finally, sections were stained with 1 % methyl blue (CI, 42780) for 75 s then quickly dehydrated in 95 % ethanol followed by 100 % ethanol. After clearing in xylenes, the slides were coverslipped with Poly-Mount Xylene mounting medium (Polysciences, Inc., Warrington, PA).
Analysis of seminiferous tubules
Stained slides were examined using a bright-field Leitz Orthoplan microscope (Leica) equipped with a CCD Cohu Camera (Cohu, Inc., San Diego, CA) using a ×25 objective. Captured images were digitized using a frame grabber card installed in a Macintosh Power PC (Apple, Cupertino, CA) and analyzed using National Institutes of Health (NIH) Image-J (Bethesda, MD, http://rsb.info.nih.gov/nih-image). Seminiferous tubule (ST) diameter was determined by measuring the smallest axis among the contra-lateral basal membranes in at least 100 circular tubule cross sections. Using the same axis, the height of the epithelium was measured between the base and the relative apex of the Sertolian epithelium.
Analysis of germ line stages
Photomicrographs were obtained with a Zeiss Axioskop 2 bright-field microscope equipped with a Zeiss AxioCam digital camera (Carl Zeiss AG, Oberkochen, Germany) connected to a PC computer utilizing the Zeiss Axiovision Program.
A minimum of 100 ST cross sections for each treatment group were classified qualitatively into two main categories, with multiple subcategories. Designations were made according to what generation of germinal line cells was present (or whether they were partially or totally depleted) and related to the typical germ cell associations that characterize normal stages of the seminiferous epithelium cycle in mature rhesus macaques. Sections were assigned to categories as follows:
- Complete germinal line (CGL)
- CGLNOR: all cell generations are normal and well represented in each cellular association
- CGLABN: partial abnormal depletion in one generation of germinal cells (B spermatogonia, spermatocytes, or spermatids)
- Depleted germinal line (DGL)
- DGL3: a generation of B spermatogonia, spermatocytes or spermatids is absent
- DGL2: all round undifferentiated and elongated differentiated spermatids are absent
- DGL1: spermatids and spermatocytes are absent; Sertoli cells are present
- DGL0: Sertoli and germ cells are absent (fibrosed cords)
RNA extraction
Flash-frozen testis sections from groups 1 and 2 were homogenized with a PowerGen rotor-stator homogenizer (Fisher Scientific, Pittsburgh, PA), and total RNA was isolated using RNeasy Mini Kit according to the manufacturer’s instructions (Qiagen, Valencia, CA). Integrity of RNA in the final samples was assessed by microcapillary electrophoresis (Agilent Bioanalyzer Model 2100; Agilent Technologies, Santa Clara, CA).
Semiquantitative RT-PCR
PrimerExpress® software (Applied Biosystems, Foster City, CA) was used for all primer and probe design (Table 1). Specific primers were designed for each transcript using the human or predicted rhesus macaque mRNA sequences (National Center for Biotechnology Information, Entrez Nucleotide database) and were purchased from Invitrogen (Carlsbad, CA).
Table 1.
Semiquantitative RT-PCR primers
| Gene name | Primer sequence (5′–3′–) | PCR product (bp) | PCR cycles | Temperature (°C) | GenBank accession ID |
|---|---|---|---|---|---|
| LHCGR | F-AGTGTAGACCATGACCACTGCC | 610 | 26 | 63 | XM_001114090 |
| R-TGAGACAGGGTTCCTACTCACG | |||||
| StAR | F-AACACCACAGAACAAGCAGCG | 281 | 29 | 68 | XM_001090472 |
| R-ATATTGGCCAGGATGGTCTCG | |||||
| CYP17A1 | F-GAGTGGCACCAGCCGGATCAG | 287 | 25 | 65 | NM_001040232 |
| R-CTCCAGGCCTGGCGCACCTTG | |||||
| HSD3β2 | F-CCACACGGTGACATTGTCAAAT | 211 | 28 | 65 | XM_001113717 |
| R-CCCACATGCACATCTCTGTCAT | |||||
| HSD17β3 | F-AGGCCCTGCAAGAGGAATATAGAG | 302 | 25 | 66 | XM_001105829 |
| R-CCTGACCTTGGTGTTGAGCTTC | |||||
| INSL3 | F-CCTCTGTCCCTACTGATTCCTC | 313 | 20 | 63 | NM_005543 |
| R-TGCACATGCAGGGAGCGGAG | |||||
| β-actin | F-CATTGCTCCTCCTGAGCGCAAG | ∼300 | 23 | 65 | NM_001101 |
| R-GGGCCGGACTCGTCATACTCC |
Reference sequences for each of the targeted Macaca mulatta genes can be accessed via GenBank accession ID
LHCGR luteinizing hormone/choriogonadotropin receptor, StAR steroidogenic acute regulatory protein, CYP17A1 family 17, subfamily A, polypeptide 1, HSD3β2 hydroxysteroid 3-beta dehydrogenase 2, HSD17β3 hydroxysteroid 17-beta dehydrogenase 3, INSL3 insulin-like factor 3, β-actin human ACTB
Total RNA (1 μg) was examined in a subgroup of animals from groups 1 (YAC, n = 5 and YACR, n = 5) and 2 (OAC, n = 3 and OACR, n = 3). To synthesize cDNA, we used the Omniscript kit (Qiagen) and oligo d(T)15 primers (Promega Corp, Madison, WI) in 20 μl at 37 °C for 1 h. Semiquantitative RT-PCR amplifications were performed in duplicate using 1 μl cDNA, 0.5 μl deoxynucleotide triphosphates (200 μM final concentration; Promega), 0.5 μl of each primer (0.5 μM final concentration), and 0.15 μl of HotStarTaq® polymerase (2.5 U; Qiagen) in 25 μl with the following thermocycle profile: 95 °C, 15 min; 94 °C, 1 min; specific cycle number and annealing temperature for each primer pair (Table 1), 1 min; and 72 °C, 1 min. Resulting PCR products were resolved by electrophoresis on 2 % agarose gels with ethidium bromide and photographed under ultraviolet light. Subsequent bands were analyzed with NIH Image-J software. A single rectangle was drawn horizontally around all bands in a selected gel image and a plot profile of signal intensities was generated. Area selections were created under the peak for each band using the “straight lines selection” tool; total area under the curves was used for statistical comparisons.
Quantitative real-time RT-PCR
Total RNA (1 μg) was examined in a subgroup of animals from groups 1 (YAC, n = 4 and YACR, n = 4) and 2 (OAC, n = 3 and OACR, n = 3). To synthesize cDNA, we used 200 ng of RNA, the Omniscript kit (Qiagen) and random hexamer primers (Promega); the reactions were diluted 1:100 for subsequent PCR analysis. The PCR mixtures contained 5 μl of Taqman® Universal PCR Master Mix (Applied Biosystems), 0.3 μl of primer (300 nM final concentration; Invitrogen), 0.05 μl of human β-actin primers (50 nM final concentration; Applied Biosystems), 0.25 μl of probe (250 nM final concentration; IDT, Coralville, IA), and 2 μl cDNA. Luteinizing hormone/choriogonadotropin receptor (LHCGR) primers (5′–3′; F-TGCTAAGAAAATGGCAATCCTCAT; R-CTGTGATAAGAGGCGCTTTGAA) and probe (6FAM-TTCACCGATTTCACCTGCATGGCA-TAMRA) were designed using the predicted rhesus macaque sequence available in the NCBI Entrez Nucleotide database (XM_001114090).
Reactions were run in triplicate in an ABI/Prism 7700 Sequences Detector System (Applied Biosystems) with the following thermocycle profile: 50 °C, 2 min; 95 °C, 10 min; and 50 cycles consisting of 95 °C for 15 s and 60 °C annealing for 60 s. Human ACTB (β-actin) endogenous control (Applied Biosystems) was used to generate a standard curve and convert the critical threshold values (i.e., above background) into relative RNA concentrations for each sample, thus compensating for any differences in reverse transcription efficiency.
Amplicon sequencing
Semiquantitative RT-PCR products were purified (QIAquick Gel Extraction Kit, Qiagen) and DNA sequencing performed on an ABI 3130XL Genetic Analyzer using dye terminator sequencing chemistry (Applied Biosystems). Resulting sequences were then BLASTed in the NCBI database (www.ncbi.nlm.nih.gov) to verify primer specificity and proper amplicon production.
Statistical analyses
Group mean hormone values were calculated from the overall average of the individual values spanning the entire 24-h sampling period. Group maximum hormone values were determined by first identifying the maximum value for an individual and then averaging it with two adjacent values on each side of the peak to provide a mean individual maximum value. Similarly, group minimum concentrations were calculated from the mean of the nadir hormone concentration for each individual, averaged with two adjacent values on each side of the nadir.
For each animal, total daily activity was averaged over approximately a 2-week period using Actiware-Sleep version 3.4 software (Cambridge Neurotechnology Ltd., Cambridge, UK), and the animal group means were compared by ANOVA. Similarly, the mean daytime activity (defined as activity during the period between 0700 and 1859 hours), the mean nighttime activity (activity between 1900 and 0659 hours), the mean day/night activity ratio were calculated, and the group means compared by ANOVA.
Statistical comparisons between age and within treatment groups (i.e., plasma T levels, morphological measures, and mRNA expression levels) were conducted using Student’s t test (SPSS Inc., Chicago, IL), or Excel (Microsoft, Redmond, WA), and are expressed as mean ± SEM. For all analyses, the minimum criterion for significance was P < 0.05.
Results
Activity analysis
As expected, the mean 24-h locomotor activity levels of the young animals (group 1) were significantly higher than those of the old animals (group 2), when analyzed without regard to dietary treatment (P < 0.05, Student’s t test); activity levels were especially enhanced during the daytime (P < 0.01), and this was also reflected as a significantly higher day/night activity ratio in the young animals (P < 0.05; data not shown). Conversely, there was no significant (P > 0.05) effect of CR on the level of activity within either age group.
Testosterone analysis
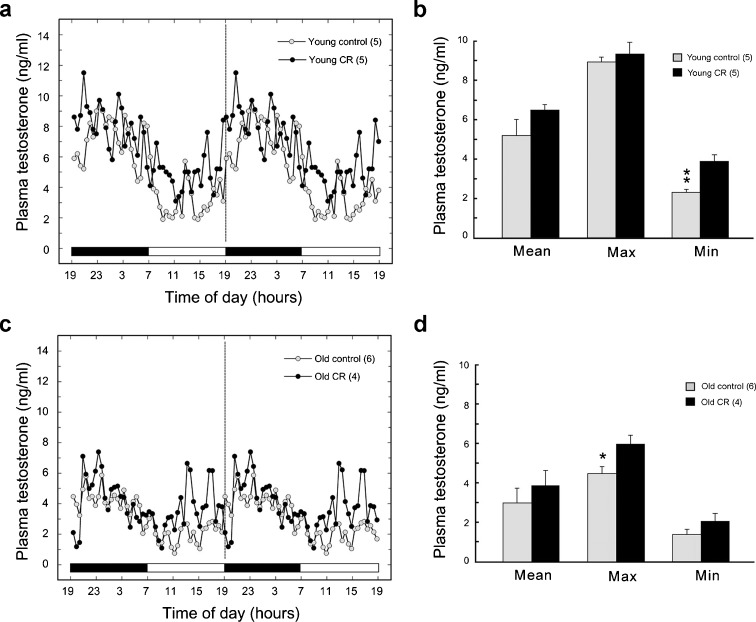
Circulating 24-h plasma T profiles were qualitatively similar in all animals regardless of age or diet; this was characterized by a nocturnal peak and a daytime nadir (Fig. 1a, c). However, there were some obvious quantitative differences. We previously reported that the mean, maximum, and minimum T levels were significantly (P < 0.001) lower in old adults compared with young adults (Urbanski and Sorwell 2012), but in the present study we also observed a subtle effect of diet. Specifically, the YACR had significantly (P < 0.01) enhanced minimum T levels (Fig. 1b), and the OACR had significantly (P < 0.05) increased maximum T levels (Fig. 1d). Importantly, however, the data clearly show that moderate CR did not suppress plasma T levels, either in the young or old animals.
Fig. 1.
Effect of moderate CR (30 %) on daily circulating plasma T concentrations in young (a) and old (c) male rhesus macaques. Data from young adult animals were previously reported (Sitzmann et al. 2010) and show a significant difference (**P < 0.01) in minimum T levels between young controls and young CR animals (b). Differences in the present study were also detected in maximum T concentrations (*P < 0.05) between old controls and old CR animals, with the latter exhibiting higher daily levels (d). Importantly, there was no evidence for a suppressive effect of CR on plasma T levels, either in the young or old animals. (Note: to facilitate visualization of the cyclic expression patterns, SEMs have been omitted and the 24-h hormone profiles have been double plotted, with horizontal bars indicating periods of light and dark)
Testicular morphology
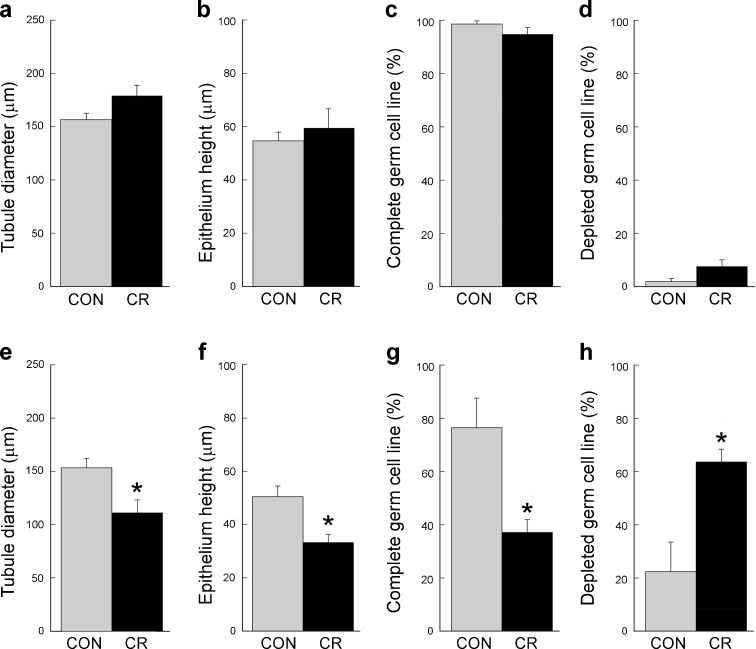
As shown in Table 2, the testis weights of CR animals did not differ significantly from those of their age-matched controls (P > 0.05). However, some differences in testicular morphology were observed (Fig. 2). On the one hand, in the young animals there was no significant effect of CR on ST diameter, epithelium height, or the two main qualitative categories of germ line stages (complete/depleted; Fig. 2a–d). On the other hand, in the old animals CR was associated with a significant (P < 0.05) reduction in diameter, epithelium height, and completion of germ cell line with a concomitant increase in depleted germ cell lines (Fig. 2e–h). To gain additional insight into the nature of these morphological differences, we performed a more detailed analysis of germ cell line production in each of the animal groups. Figure 3 illustrates how the ST were classified using six subcategories of complete and depleted germ line production. Quantitative analysis of ST subtypes is depicted in Fig. 4 and shows that there was no significant difference between control and CR animals in either the young or old age groups.
Table 2.
Mean testicular weights in adult rhesus macaques
| Treatment group | Group mean (g) | SEM |
|---|---|---|
| YAC (n = 5) | 18.6 | 1.9 |
| YACR (n = 5) | 24.0 | 3.1 |
| OAC (n = 3) | 18.8 | 1.2 |
| OACR (n = 3) | 13.4 | 4.1 |
Testes weights of CR animals were not significantly different from their respective age-matched controls (P > 0.05)
Fig. 2.
ST diameter, epithelium height, complete germ cell line percentage, and depleted germ cell line percentage in testicular sections from young adult (a–d) and old adult (e–h) rhesus macaques. Values represent mean ± SEM. Significant effects of CR on all four morphological parameters were detected in the old animals but not in the young adults (*P < 0.05)
Fig. 3.
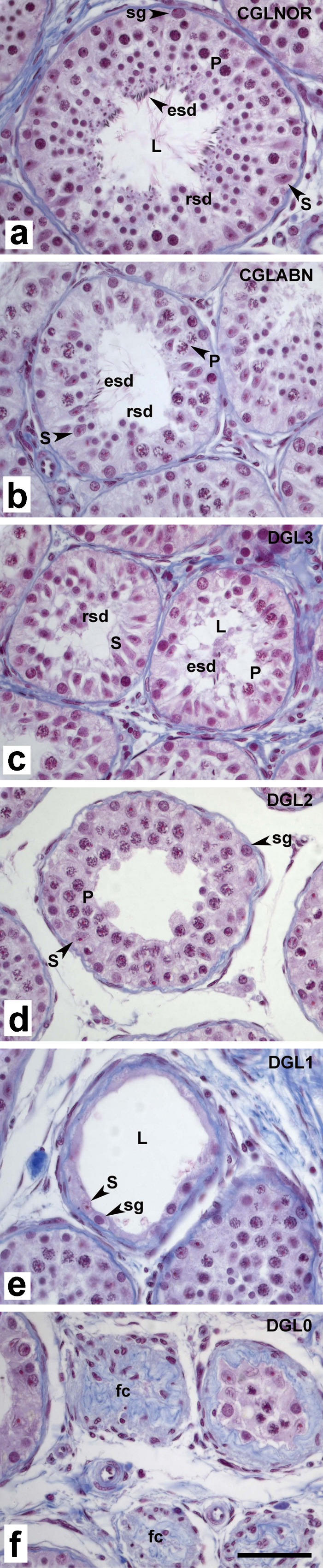
Classification of CGL and DGL, as used in the analysis of rhesus macaque testis. Representative images of spermatogenic stages in ST sections are depicted in (a) to (f). CGLs contain all normal cell generations well represented in each cellular association (CGLNOR; a), whereas an abnormal complete germinal line (CGLABN; b) shows partial depletion in one generation of germ cells, in this case round and elongated spermatids. DGLs occur when one or more generations of germinal cell generations are absent: B spermatogonia, spermatocytes, or spermatids (DGL3; c); all round undifferentiated and elongated differentiated spermatids (DGL2; d); spermatocytes and spermatids but Sertoli cells present (DGL1; e); and germinal and Sertoli cells (fibrosed cords; DGL0; f). Abbreviations: esd elongated-differentiated spermatid, fc fibrous cord, L lumen, P pachytene spermatocyte; rsd round-undifferentiated spermatid, S Sertoli cell, sg spermatogonia. Scale bar = 50 μm
Fig. 4.
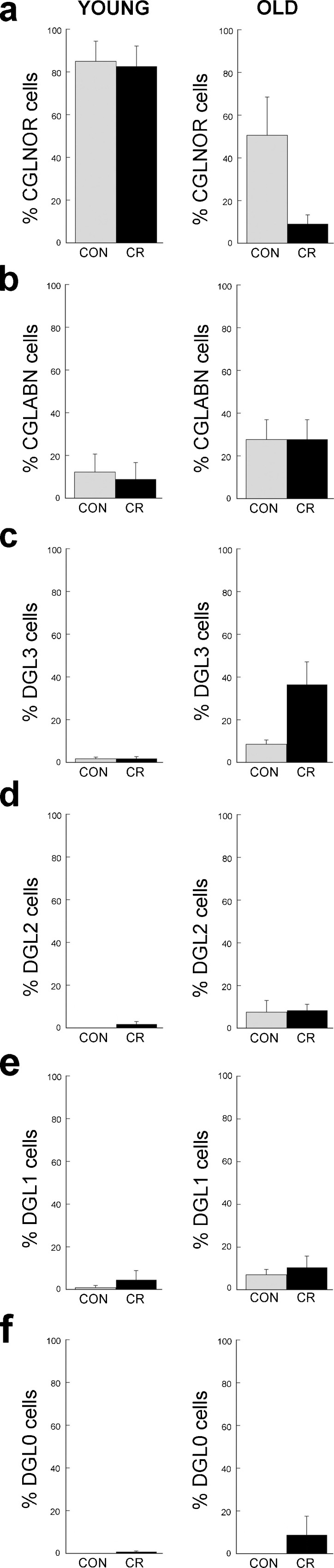
Analysis of ST from calorie restricted (CR) and age-matched control (CON) rhesus macaques. Subcategories of complete germinal line (CGL) and depleted germinal line (DGL), as defined in Fig. 3, are depicted as percentages in (a) to (f)
Semiquantitative and quantitative real-time RT-PCR
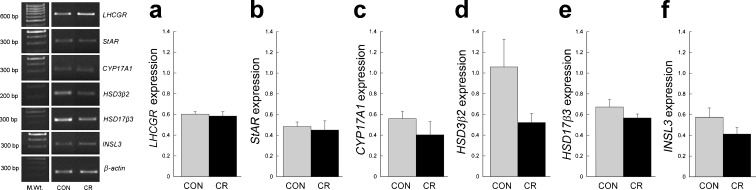
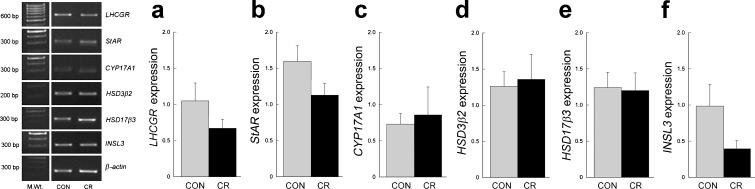
Amplicons were detected between 20 and 29 cycles at 62–68 °C (Table 1). Representative gel images of semiquantitative RT-PCR products and expression levels for genes related to T biosynthesis or Leydig cell maintenance are presented in Fig. 5 (group 1: young adult) and Fig. 6 (group 2: old adult). Overall, the gene expression levels showed no significant diet-induced effect either in the young or in the old animals. Although the insulin-like factor 3 (INSL3) mRNA expression appeared to differ between CON and CR in old males, this did not prove to be statistically significant due to the small group sizes (n = 3); more power would potentially reveal a difference with CR in the direction of expression observed in young males. Quantitative real-time RT-PCR measurements of LHCGR mRNA expression showed no significant age or diet-induced changes (YAC, 5.21 ± 1.44; YACR, 3.73 ± 0.87; OAC, 5.85 ± 2.18; and OACR, 4.53 ± 0.87).
Fig. 5.
Representative semiquantitative RT-PCR gel image demonstrating expression of testicular genes in calorie restricted (CR; n = 5) or control (CON; n = 5) young adult (∼12 years old) rhesus macaques. The housekeeping gene β-actin was used as a positive control and for normalizing images for analysis. No significant differences (mean ± SEM; P > 0.05) in gene expression were observed between the treatment groups
Fig. 6.
Representative semiquantitative RT-PCR gel image demonstrating expression of testicular genes in calorie restricted (CR; n = 3) or control (CON; n = 3) old adult (24–30 years old) rhesus macaques. The housekeeping gene β-actin was used as a positive control and for normalizing images for analysis. No significant differences (mean ± SEM; P > 0.05) in gene expression were observed between the treatment groups
DNA sequencing
Amplicon sequences were BLASTed in the NCBI database to determine the sequence of best fit. In each instance, the experimentally-derived sequence returned a best fit for at least one, and usually both, of the primer pairs for the human or predicted rhesus macaque mRNA sequence that had been used for primer design (data not shown).
Discussion
As expected, aging was found to be associated with an overall reduction in 24-h locomotor activity. Within each age group, however, the activity patterns of CR and control animals were very similar. This suggests that the metabolic expenditures were most likely similar in the CR and age-matched control animals.
Similarly, there was no obvious detrimental effect of moderate CR on circulating T levels, even though T levels were significantly lower in the older animals. T is a critical feedback component of the HPG axis and essential for production of spermatozoa. Although the impact of T decline on eventual reproductive senescence is not understood, there is evidence that declining T levels are associated with many subtle changes that affect reproductive function and fertility. The Brown Norway rat, for example, experiences a significant age-related decline in T production as a result of Leydig cell dysfunction rather than a reduction in cell numbers (Chen and Zirkin 1999; Zirkin and Chen 2000). Overall LHCG receptor numbers and their affinity for LH also decline as a function of age in rats (Chen et al. 2002; Zirkin and Chen 2000). In humans, some investigators reported no effect of age per se when excluding those with severe co-morbidity (Sartorius et al. 2012), whereas many others documented a clear decline in T production beginning after 30 years of age and continuing gradually into old age (Hardy and Schlegel 2004; Henkel et al. 2005; Wang et al. 2005). Consequently, there is potential for a cascade effect within the entire reproductive system as a result of decreased circulating T levels. Testosterone decline, especially during aging, can result in weakening muscle function, bone density and alteration of other physiological parameters related to overall aging (Bremner et al. 1983; Feldman et al. 2002; Harman et al. 2001; Moffat et al. 2002; Sitzmann et al. 2008). Also of importance is the neurological impact of T loss. Some studies have reported that men with Alzheimer’s disease (AD) have significantly lower T levels than aged men without AD (Moffat et al. 2002; Rosario et al. 2006). Significantly, T depletion appears to occur well before clinical and pathological diagnosis of AD, suggesting that low T levels may contribute to AD pathogenesis rather than results from it (Rosario et al. 2006).
As previously reported, we did not detect any suppressive influence of CR on mean, maximum, or minimum circulating T levels in young adult rhesus macaques (Sitzmann et al. 2010). In the present study we expand and extend these previous finding by also analyzing the 24-h T profiles from ten old adult males. Taken together, the data show that in male rhesus macaques, daily circulating plasma T levels have a well-defined 24-h pattern which persists even into old age. Importantly, however, the data also emphasize that T levels are significantly lower in older animals and that CR has no negative impact on mean, maximum, or minimum circulating T levels. Surprisingly, maximum circulating T levels were significantly higher in old CR males than in their age-matched controls. Whether this apparent increase is physiologically significant is unclear, but when considered together with our previous finding that CR enhanced the daily minimum T levels in young males, it may be indicative of a subtle improvement of physiological efficiency elicited by moderate CR. By decreasing the daily swing between maximum and minimum levels and maintaining tighter control over T release, younger CR animals may be able to divert energy toward more critical functions of growth and life maintenance (Roth et al. 2004). Moreover, findings in the old adults may be an indication that CR can contribute to the maintenance of elevated T levels during old age, which would be of great physiological benefit. Although we cannot rule out the possibility that the subtle enhancement of circulating T levels stems from nontesticular origin, in normal rhesus macaques it is clear that androgens of adrenal origin do not contribute significantly to the 24-h pattern of T in the circulation (Goodman et al. 1974). Alternatively, it is also plausible that CR exerts a subtle suppressive effect on the clearance of T by the liver. Regardless of the source of T, our data clearly demonstrate that moderate CR does not perturb the 24-h plasma T profile of young or old males, and it does not diminish the levels below that of age-matched controls; if anything, it enhances them.
Based on previous findings, we were expecting to find subtle, but nonsignificant, age-related declines in spermatogenic parameters (Kidd et al. 2001; Plas et al. 2000). Indeed, comparison of testicular morphology within age groups revealed some significant effects of diet. First, ST diameters were significantly reduced in OACR animals compared with their age-matched OAC controls. Although diameter measurements are themselves considered rough metrics of ST health, they do permit some detection of spermatogenic depression, which correlates to ST activity (Russell et al. 1990; Sinha Hikim et al. 1989). Moreover, when tubule diameter was paired with measurements of epithelium height and semiquantitative histological determination of CGL percentages, a better picture of testicular functionality emerged. In the case of our study subjects, epithelium height and CGL percentages were significantly reduced in OACR animals relative to OAC controls. The mixture of focal and regional damage (Chen and Zirkin 1999), where many stages of the spermatogenic cycle were affected to varying degrees, could signal a decrease in ST function within our older treatment group, possibly due to CR. Upon more detailed analysis of germ line stages, however, the differences became less clear and not statistically different. With larger group sizes these subtle differences may have become more evident. Although OACR animals exhibited decreased complete germinal line production, it is unclear if this would necessarily correlate to a significant physiological impact on fertility.
Previous gene profiling studies in our laboratory using Affymetrix GeneChip® Rhesus Macaque Genome Arrays revealed high expression of steroidogenic enzyme encoding genes in young adult rhesus macaque testis but failed to disclose any effect of moderate CR (Sitzmann et al. 2009). In the present study, we use semiquantitative and quantitative real-time RT-PCR to further examine the influence of CR on testicular gene expression in greater detail. Specifically, we were interested in genes related to the T biosynthesis pathway and Leydig cell maintenance. With the exception of the cytochrome P450, family 11, subfamily A, polypeptide 1, microarray analysis included all components of the T biosynthesis pathway (luteinizing hormone/choriogonadotropin receptor (LHCGR); steroidogenic acute regulatory protein; cytochrome P450, family 17, subfamily A, polypeptide 1; hydroxysteroid 3-beta dehydrogenase 2; and hydroxysteroid 17-beta dehydrogenase 3) as well as the peptide hormone, INSL3, an indicator of Leydig cell health. No significant differences in mRNA expression were detected within either the young or old age groups, indicating that CR does not exert any obvious negative impact on the expression of key testicular genes. Although INSL3 gene expression did not differ significantly in old males due to the small group sizes, it appears that the CR males had levels of expression that more closely resembled that of young males (Figs. 5 and 6). This would be in line with the observed positive effects of CR on circulating T levels and suggestive of an effect of CR on Leydig cells.
In summary, the present study used a nonhuman primate model to determine whether long-term moderate CR is likely to negatively impact T levels and impair fertility in human males. Clinically, this is an important issue because fasting, even short term, has been shown to rapidly suppress the primate reproductive axis (Cameron 1996; Cameron and Nosbisch 1991; Cameron et al. 1991, 1993; Dubey et al. 1986; Helmreich and Cameron 1992; Schreihofer et al. 1993). Although availability of old male rhesus macaques was a limiting factor, we nevertheless were able to establish that moderate CR has no negative impact on circulating T measurements and testicular mRNA expression, and minimal impact on testis morphology. Overall, these findings should be particularly reassuring to men who are using CR as a potential means of combating negative physiological aspects of aging. Even though there was some indication of modest spermatogenesis impairment in the old CR animals, it should be emphasized that similar findings were not evident in young adults, and it is unclear if such subtle changes in testicular morphology would significantly impair fertility. The most important finding, however, is that moderate CR does not suppress T production or disrupt the 24-h pattern of T in the circulation; if anything, it may even enhance circulating T levels. Consequently, CR is unlikely to negatively impact androgen-mediated nonreproductive functions, and may exert additional beneficial effects.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants AG-019914, AG-036670, and OD-011092. Additional support was provided by the Intramural Research Program of the NIH, and the Department of Animal and Avian Sciences at the University of Maryland. The authors would like to thank the members of the Oregon National Primate Research Center for their technical help: Drs. Dario Lemos, Jodi Downs, Yibing Jia, Betsy Ferguson, Eliot Spindel, and the animal care staff, including the late Dr. John Fanton. Statistical support graciously provided by Erin Hoerl Leone at the Florida Fish and Wildlife Research Institute.
References
- Black A, Allison DB, Shapses SA, Tilmont EM, Handy AM, Ingram DK, Roth GS, Lane MA. Calorie restriction and skeletal mass in rhesus monkeys (Macaca mulatta): evidence for an effect mediated through changes in body size. J Gerontol Ser A Biol Sci Med Sci. 2001;56:B98–B107. doi: 10.1093/gerona/56.3.B98. [DOI] [PubMed] [Google Scholar]
- Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56:1278–1281. doi: 10.1210/jcem-56-6-1278. [DOI] [PubMed] [Google Scholar]
- Buwe A, Guttenbach M, Schmid M. Effect of paternal age on the frequency of cytogenetic abnormalities in human spermatozoa. Cytogenet Genome Res. 2005;111:213–228. doi: 10.1159/000086892. [DOI] [PubMed] [Google Scholar]
- Cameron JL. Regulation of reproductive hormone secretion in primates by short-term changes in nutrition. Rev Reprod. 1996;1:117–126. doi: 10.1530/ror.0.0010117. [DOI] [PubMed] [Google Scholar]
- Cameron JL, Nosbisch C. Suppression of pulsatile luteinizing hormone and testosterone secretion during short term food restriction in the adult male rhesus monkey (Macaca mulatta) Endocrinology. 1991;128:1532–1540. doi: 10.1210/endo-128-3-1532. [DOI] [PubMed] [Google Scholar]
- Cameron JL, Weltzin TE, McConaha C, Helmreich DL, Kaye WH. Slowing of pulsatile luteinizing hormone secretion in men after forty-eight hours of fasting. J Clin Endocrinol Metab. 1991;73:35–41. doi: 10.1210/jcem-73-1-35. [DOI] [PubMed] [Google Scholar]
- Cameron JL, Helmreich DL, Schreihofer DA. Modulation of reproductive hormone secretion by nutritional intake: stress signals versus metabolic signals. Hum Reprod Suppl. 1993;2:162–167. doi: 10.1093/humrep/8.suppl_2.162. [DOI] [PubMed] [Google Scholar]
- Chen HL, Zirkin BR. Long-term suppression of Leydig cell steroidogenesis prevents Leydig cell aging. Proc Natl Acad Sci U S A. 1999;96:14877–14881. doi: 10.1073/pnas.96.26.14877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HL, Hardy MP, Zirkin BR. Age-related decreases in Leydig cell testosterone production are not restored by exposure to LH in vitro. Endocrinology. 2002;143:1637–1642. doi: 10.1210/en.143.5.1637. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhahbi JM, Kim HJ, Mote PL, Beaver RJ, Spindler SR. Temporal linkage between the phenotypic and genomic responses to caloric restriction. Proc Natl Acad Sci U S A. 2004;101:5524–5529. doi: 10.1073/pnas.0305300101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs JL, Dunn MR, Borok E, Shanabrough M, Horvath TL, Kohama SG, Urbanski HF. Orexin neuronal changes in the locus coeruleus of the aging rhesus macaque. Neurobiol Aging. 2007;28:1286–1295. doi: 10.1016/j.neurobiolaging.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Downs JL, Mattison JA, Ingram DK, Urbanski HF. Effect of age and caloric restriction on circadian adrenal steroid rhythms in rhesus macaques. Neurobiol Aging. 2008;29:1412–1422. doi: 10.1016/j.neurobiolaging.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey AK, Cameron JL, Steiner RA, Plant TM. Inhibition of gonadotropin secretion in castrated male rhesus monkeys (Macaca mulatta) induced by dietary restriction: analogy with the prepubertal hiatus of gonadotropin release. Endocrinology. 1986;118:518–525. doi: 10.1210/endo-118-2-518. [DOI] [PubMed] [Google Scholar]
- Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 2002;87:589–598. doi: 10.1210/jc.87.2.589. [DOI] [PubMed] [Google Scholar]
- Gabe M. Techniques histologiques. Paris: Masson et Cie; 1968. [Google Scholar]
- Goodman RL, Hotchkiss J, Karsch FJ, Knobil E. Diurnal variations in serum testosterone concentrations in the adult male rhesus monkey. Biol Reprod. 1974;11:624–630. doi: 10.1095/biolreprod11.5.624. [DOI] [PubMed] [Google Scholar]
- Gredilla R, Barja G. Minireview: the role of oxidative stress in relation to caloric restriction and longevity. Endocrinology. 2005;146:3713–3717. doi: 10.1210/en.2005-0378. [DOI] [PubMed] [Google Scholar]
- Haley GE, Landauer N, Renner L, Hooper K, Urbanski HF, Kohama SG, Neuringer M, Raber J. Circadian activity associated with spatial learning and memory in aging rhesus monkeys. Exp Neurol. 2009;217:55–62. doi: 10.1016/j.expneurol.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammoud AO, Gibson M, Peterson CM, Meikle AW, Carrell DT. Impact of male obesity on infertility: a critical review of the current literature. Fertil Steril. 2008;90:897–904. doi: 10.1016/j.fertnstert.2008.08.026. [DOI] [PubMed] [Google Scholar]
- Hardy MP, Schlegel PN. Testosterone production in the aging male: where does the slowdown occur? Endocrinology. 2004;145:4439–4440. doi: 10.1210/en.2004-0888. [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jc.86.2.724. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr. 2003;78:361–369. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- Helmreich DL, Cameron JL. Suppression of luteinizing hormone secretion during food restriction in male rhesus monkeys (Macaca mulatta): failure of naloxone to restore normal pulsatility. Neuroendocrinology. 1992;56:464–473. doi: 10.1159/000126263. [DOI] [PubMed] [Google Scholar]
- Henkel R, Maass G, Schuppe HC, Jung A, Schubert J, Schill WB. Molecular aspects of declining sperm motility in older men. Fertil Steril. 2005;84:1430–1437. doi: 10.1016/j.fertnstert.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC. Calorie restriction, aging, and cancer prevention: mechanisms of action and a applicability to humans. Annu Rev Med. 2003;54:131–152. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Cutler RG, Weindruch R, Renquist DM, Knapka JJ, April M, Belcher CT, Clark MA, Hatcherson CD, Marriott BM, Roth GS. Dietary restriction and aging—the initiation of a primate study. J Gerontol. 1990;45:B148–B163. doi: 10.1093/geronj/45.5.B148. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Andersson AM, Jorgensen N, Andersen AG, Carlsen E, Petersen JH, Skakkebaek NE. Body mass index in relation to semen quality and reproductive hormones among 1,558 Danish men. Fertil Steril. 2004;82:863–870. doi: 10.1016/j.fertnstert.2004.03.056. [DOI] [PubMed] [Google Scholar]
- Kemnitz JW. Calorie restriction and aging in nonhuman primates. ILAR J. 2011;52:66–77. doi: 10.1093/ilar.52.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd SA, Eskenazi B, Wyrobek AJ. Effects of male age on semen quality and fertility: a review of the literature. Fertil Steril. 2001;75:237–248. doi: 10.1016/S0015-0282(00)01679-4. [DOI] [PubMed] [Google Scholar]
- Koubova J, Guarente L. How does calorie restriction work? Genes Dev. 2003;17:313–321. doi: 10.1101/gad.1052903. [DOI] [PubMed] [Google Scholar]
- Lane MA, Baer DJ, Rumpler WV, Weindruch R, Ingram DK, Tilmont EM, Cutler RG, Roth GS. Calorie restriction lowers body temperature in rhesus monkeys, consistent with a postulated anti-aging mechanism in rodents. Proc Natl Acad Sci U S A. 1996;93:4159–4164. doi: 10.1073/pnas.93.9.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Ingram DK, Roth GS. Beyond the rodent model: calorie restriction in rhesus monkeys. Age. 1997;20:45–56. doi: 10.1007/s11357-997-0004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Ingram DK, Roth GS. Calorie restriction in nonhuman primates: effects on diabetes and cardiovascular disease risk. Toxicol Sci. 1999;52:41–48. doi: 10.1093/toxsci/52.2.41. [DOI] [PubMed] [Google Scholar]
- Lane MA, Tilmont EM, De Angelis H, Handy A, Ingram DK, Kemnitz JW, Roth GS. Short-term calorie restriction improves disease-related markers in older male rhesus monkeys (Macaca mulatta) Mech Ageing Dev. 1999;112:185–196. doi: 10.1016/S0047-6374(99)00087-1. [DOI] [PubMed] [Google Scholar]
- Lane MA, Black A, Handy AM, Shapses SA, Tilmont EM, Kiefer TL, Ingram DK, Roth GS. Energy restriction does not alter bone mineral metabolism or reproductive cycling and hormones in female rhesus monkeys. J Nutr. 2001;131:820–827. doi: 10.1093/jn/131.3.820. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Lane MA, Roth GS, Ingram DK. Calorie restriction in rhesus monkeys. Exp Gerontol. 2003;38:35–46. doi: 10.1016/S0531-5565(02)00146-8. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Black A, Huck J, Moscrip T, Handy A, Tilmont E, Roth GS, Lane MA, Ingram DK. Age-related decline in caloric intake and motivation for food in rhesus monkeys. Neurobiol Aging. 2005;26:1117–1127. doi: 10.1016/j.neurobiolaging.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Croft MA, Dahl DB, Roth GS, Lane MA, Ingram DK, Kaufman PL. Accommodative function in rhesus monkeys: effects of aging and calorie restriction. Age. 2005;27:59–67. doi: 10.1007/s11357-005-4005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Alison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of the life span and upon the ultimate body size. J Nutr. 1935;10:63–70. [PubMed] [Google Scholar]
- Mendiola J, Torres-Cantero AM, Moreno-Grau JM, Ten J, Roca M, Moreno-Grau S, Bernabeu R. Food intake and its relationship with semen quality: a case–control study. Fert Steril. 2009;91:812–818. doi: 10.1016/j.fertnstert.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Metter EJ, Blackman MR, Harman SM, Resnick SM. Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. J Clin Endocrinol Metab. 2002;87:5001–5007. doi: 10.1210/jc.2002-020419. [DOI] [PubMed] [Google Scholar]
- Guide for the care and use of laboratory animals. Washington, DC: The National Academies Press; 1996. [PubMed] [Google Scholar]
- Nutrient requirements of nonhuman primates, 2nd revised edn. Washington, DC: The National Academies Press; 2003. [Google Scholar]
- Ottinger MA. Male reproduction: testosterone, gonadotropins, and aging. In: Mobbs CV, Hof PR, editors. Interdisciplinary topics in gerontology: Functional endocrinology of aging. New York: S. Karger AG; 1998. pp. 105–126. [Google Scholar]
- Plas E, Berger P, Hermann M, Pfluger H. Effects of aging on male fertility. Exp Gerontol. 2000;35:543–551. doi: 10.1016/S0531-5565(00)00120-0. [DOI] [PubMed] [Google Scholar]
- Plymate SR, Tenover JS, Bremner WJ. Circadian variation in testosterone, sex hormone-binding globulin, and calculated non-sex hormone-binding globulin bound testosterone in healthy young and elderly men. J Androl. 1989;10:366–371. doi: 10.1002/j.1939-4640.1989.tb00120.x. [DOI] [PubMed] [Google Scholar]
- Resko JA, Malley A, Begley D, Hess DL. Radioimmunoassay of testosterone during fetal development of the rhesus monkey. Endocrinology. 1973;93:156–161. doi: 10.1210/endo-93-1-156. [DOI] [PubMed] [Google Scholar]
- Rosario ER, Carroll JC, Oddo S, LaFerla FM, Pike CJ. Androgens regulate the development of neuropathology in a triple transgenic mouse model of Alzheimer’s disease. J Neurosci. 2006;26:13384–13389. doi: 10.1523/JNEUROSCI.2514-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth GS, Ingram DK, Lane MA. Calorie restriction in primates: will it work and how will we know? J Am Geriatr Soc. 1999;47:896–903. doi: 10.1111/j.1532-5415.1999.tb03851.x. [DOI] [PubMed] [Google Scholar]
- Roth GS, Ingram DK, Black A, Lane MA. Effects of reduced energy intake on the biology of aging: the primate model. Eur J Clin Nutr. 2000;54:S15–S20. doi: 10.1038/sj.ejcn.1601020. [DOI] [PubMed] [Google Scholar]
- Roth GS, Lane MA, Ingram DK, Mattison JA, Elahi D, Tobin JD, Muller D, Metter EJ. Biomarkers of caloric restriction may predict longevity in humans. Science. 2002;297:811. doi: 10.1126/science.1071851. [DOI] [PubMed] [Google Scholar]
- Roth GS, Mattison JA, Ottinger MA, Chachich ME, Lane MA, Ingram DK. Aging in rhesus monkeys: Relevance to human health interventions. Science. 2004;305:1423–1426. doi: 10.1126/science.1102541. [DOI] [PubMed] [Google Scholar]
- Russell LD, Ettlin RA, Sinha Hikim AP, Clegg ED. Histopathology of the testis. In: Russell LD, Ettlin RA, Sinha Hikim AP, Clegg ED, editors. Histological and histopathological evaluation of the testis. Clearwater, FL: Cache River Press; 1990. pp. 210–266. [Google Scholar]
- Sartorius G, Spasevska S, Idan A, Turner L, Forbes E, Zamojska A, Allan CA, Ly LP, Conway AJ, McLachlan RI, Handelsman DJ. Serum testosterone, dihydrotestosterone and estradiol concentrations in older men self-reporting very good health: the healthy man study. Clin Endocrinol. 2012;77:755–763. doi: 10.1111/j.1365-2265.2012.04432.x. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Pohl CR, Ehmcke J, Ramaswamy S. Age-related changes in diurnal rhythms and levels of gonadotropins, testosterone, and inhibin B in male rhesus monkeys (Macaca mulatta) Biol Reprod. 2008;79:93–99. doi: 10.1095/biolreprod.107.066126. [DOI] [PubMed] [Google Scholar]
- Schreihofer DA, Parfitt DB, Cameron JL. Suppression of luteinizing hormone secretion during short-term fasting in male rhesus monkeys. Role Metab Versus Stress Signals Endocrinol. 1993;132:1881–1889. doi: 10.1210/endo.132.5.8477641. [DOI] [PubMed] [Google Scholar]
- Sinha Hikim AP, Amador AG, Klemcke HG, Bartke A, Russell LD. Correlative morphology and endocrinology of Sertoli cells in hamster testes in active and inactive states of spermatogenesis. Endocrinology. 1989;125:1829–1843. doi: 10.1210/endo-125-4-1829. [DOI] [PubMed] [Google Scholar]
- Sitzmann BD, Urbanski HF, Ottinger MA. Aging in male primates: reproductive decline, effects of calorie restriction and future research potential. Age. 2008;30:157–168. doi: 10.1007/s11357-008-9065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitzmann BD, Mattison JA, Ingram DK, Roth GS, Ottinger MA, Urbanski HF. Impact of moderate calorie restriction on the reproductive neuroendocrine axis of male rhesus macaques. Open Longev Sci. 2009;3:38–47. doi: 10.2174/1876326X00903010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitzmann BD, Leone EH, Mattison JA, Ingram DK, Roth GS, Urbanski HF, Zelinski MB, Ottinger MA. Effects of moderate calorie restriction on testosterone production and semen characteristics in young rhesus macaques (Macaca mulatta) Biol Reprod. 2010;83:635–640. doi: 10.1095/biolreprod.110.084186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski HF. Circadian variation in the physiology and behavior of humans and nonhuman primates. In: Raber J, editor. Animal models of behavioral analysis. New York: Humana Press; 2011. pp. 217–235. [Google Scholar]
- Urbanski HF, Pau FK-Y. A biphasic developmental pattern of circulating leptin in the male rhesus macaque (Macaca mulatta) Endocrinology. 1998;139:2284–2286. doi: 10.1210/en.139.5.2284. [DOI] [PubMed] [Google Scholar]
- Urbanski HF, Sorwell KG. Age-related changes in neuroendocrine rhythmic function in the rhesus macaque. Age. 2012;34:1111–1121. doi: 10.1007/s11357-011-9352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski HF, Garyfallou VT, Kohama SG, Hess DL. α-adrenergic receptor antagonism and N-methyl-d-aspartate (NMDA) induced luteinizing hormone release in female rhesus macaques. Brain Res. 1997;744:96–104. doi: 10.1016/S0006-8993(96)01083-9. [DOI] [PubMed] [Google Scholar]
- Urbanski HF, Downs JL, Garyfallou VT, Mattison JA, Lane MA, Roth GS, Ingram DK. Effect of caloric restriction on the 24-hour plasma DHEAS and cortisol profiles of young and old male rhesus macaques. Ann NY Acad Sci. 2004;1019:443–447. doi: 10.1196/annals.1297.081. [DOI] [PubMed] [Google Scholar]
- Urbanski HF, Kohama SG, West GA, Glynn C, Williams-Karnesky RL, Earl E, Neuringer MN, Renner L, Weiss A, Stenzel-Poore M, Bahjat FR. Changes in spontaneous activity assessed by accelerometry correlate with extent of cerebral ischemia–reperfusion injury in the nonhuman primate. Transl Stroke Res. 2012;3:442–451. doi: 10.1007/s12975-012-0191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Shen CL, Dyson MT, Eimerl S, Orly J, Hutson JC, Stocco DM. Cyclooxygenase-2 regulation of the age-related decline in testosterone biosynthesis. Endocrinology. 2005;146:4202–4208. doi: 10.1210/en.2005-0298. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford RL. The retardation of aging and disease by dietary restriction. Springfield, IL: Charles C. Thomas; 1988. [Google Scholar]
- Wong WY, Thomas CMG, Merkus JMWM, Zielhuis GA, Steegers-Theunissen RPM. Male factor subfertility: possible causes and the impact of nutritional factors. Fertil Steril. 2000;73:435–442. doi: 10.1016/S0015-0282(99)00551-8. [DOI] [PubMed] [Google Scholar]
- Wu JM. Effects of moderate calorie restriction on ovarian function and decline in rhesus monkeys. Dissertation: University of Maryland; 2006. [Google Scholar]
- Zirkin BR, Chen HL. Regulation of Leydig cell steroidogenic function during aging. Biol Reprod. 2000;63:977–981. doi: 10.1095/biolreprod63.4.977. [DOI] [PubMed] [Google Scholar]