Abstract
The large burden and coexistence of physical disability, cognitive impairment, and depression in the oldest old makes summary markers of global functioning of great value, allowing for risk stratification. Inflammation may be a common underlying cause or represents a final common pathway. The present study investigated the association between elevated serum inflammatory markers and global functioning and its underlying aspects. A representative sample of 415 community-dwelling elderly subjects participating in the BELFRAIL study, with a mean age of 85 years, was included in the present analysis. Data on physical performance, dependence, and mental aspects of functioning and serum levels of 15 inflammatory proteins, including cytokines, chemokines, and acute-phase proteins, were assessed. Interleukin (IL)-6 was negatively associated with global functioning (odds ratio (OR) 4.35). The odds ratios for C-reactive protein (CRP) (OR 2.37) and the combined score of IL-6 and CRP (OR 2.59) were lower or not significant. IL-6 was significantly associated with physical dependence and cognitive function, and only a highly elevated serum level was associated with physical performance. Physical dependence was associated with a highly elevated CRP serum level. The proportion of functionally impaired older persons with elevated IL-6 was 81.93 %, giving a low positive predictive value (0.38), but a high negative predictive value (0.87). So, IL-6 is strongly associated with global functioning and all of the individual aspects of functioning, except suspected depression, in community-dwelling persons 80 years and older.
Keywords: Aged 80 and over, Chronic inflammation, Immune markers, Functional impairment
Introduction
The number of persons aged 80 years or older is growing worldwide and has been accompanied by a concurrent increase in physical disability, cognitive impairment, and depression. Identifying useful biomarkers to assess global functioning could be of great value in the elderly.
Aging is characterized by a chronic low-grade systemic inflammatory status that is involved in the pathogenesis and course of several age-related disorders, such as atherosclerosis, osteoporosis, and cardiovascular disease (Vasto et al. 2007; Franceschi et al. 2007). First, this upregulation of inflammatory activity at older age, or “inflammaging,” shows an increase in acute phase proteins, such as C-reactive protein (CRP). Second, an increase in cytokines or hormone-like substances released by many cells to mediate the immune system can be observed. Cytokine dysregulation is believed to play a common key role or to be a final common pathway in the remodeling of the immune-inflammatory responses and physical or mental changes accompanying old age (Wilson et al. 2002; Singh and Newman 2011; Krabbe et al. 2004).
Serum interleukin (IL)-6, tumor necrosis factor alpha (TNF-α), and CRP have been identified in epidemiologic studies to be related to or to predict physical disability in aging populations, with IL-6 being the most robust predictor (Singh and Newman 2011). The relationship between immune markers, such as IL-1β, IL-6, IL-8, TNF-α, and CRP, and cognitive functioning has been described in several population studies on older persons (Singh and Newman 2011; Holmes et al. 2003; Baune et al. 2008; Weaver et al. 2002; Bruunsgaard et al. 1999; Dik et al. 2005; van Exel et al. 2003).
Although impairment in one individual aspect of functioning might have different degrees of effect on the global functioning of older people, only a few studies have reported that poorer self-rated global health is associated with elevated inflammatory markers among older adults (Christian et al. 2011; Cohen et al. 1997). To our knowledge, no associations for global functioning with serum inflammatory markers have been described.
Because data regarding cytokine profiling in the elderly are mostly limited to a small subset of cytokines, the present study focuses on an extensive battery of immune markers and investigated whether these circulating marker levels are associated with global functional impairment in a sample of community-dwelling older persons. In addition, the individual aspects of functioning that may underlie this association will be explored (physical dependence, physical performance, and cognitive and suspected depression status).
Methods
Subjects
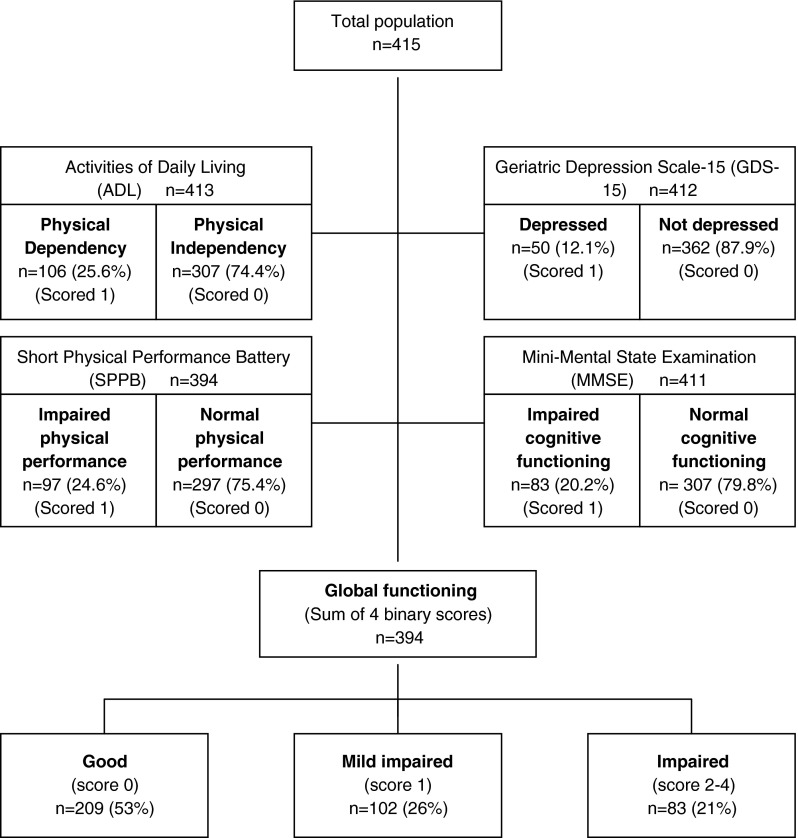
The BELFRAIL study is a prospective, observational, population-based cohort study performed in three areas of Belgium. Details of the study's methods and sampling design have been published elsewhere (Vaes et al. 2010). Between November 2, 2008 and September 15, 2009, 567 subjects (age range 80 to 102 years) were included in the BFC80+ study. Only three exclusion criteria were used: (1) severe dementia reported by the GP, (2) in palliative care, and (3) medical emergency. The GP recorded background variables and medical history. The clinical research assistant performed an extensive examination, including performance testing, questionnaires, and technical examinations. In the present study, 152 of the 567 individuals were excluded because their immune markers could not be determined due to a technical issue. This selection resulted in a final population sample of 415 participants who were highly comparable to the initial sample. In 394 out of the 415 subjects, the global functioning score could be determined, as presented in a flow diagram (Fig. 1).
Fig. 1.
Flow chart of study population
Laboratory measurements
Blood samples were collected in the morning and stored within 4 h at −80 °C. The analytical process was organized to avoid several freeze-thaw cycles. An extensive panel of inflammatory cytokines and growth factors (IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, TNF-α, IFN-γ, monocyte chemoattractant protein (MCP)-1, IGF-1, VEGF, and EGF) was measured using biochip arrays with the Evidence Investigator Analyzer (Randox Laboratories Limited, Crumlin, UK), which enables the simultaneous analysis of 12 cytokines and growth factors using a limited volume of serum (100 μL) without cross-reactivity. The UniCel® DxC 800 Synchron (Beckman-Coulter, Brea, USA) was used to measure ultrasensitive CRP and prealbumin levels. Details on the analytical methods were previously published (Vaes et al. 2010). The inflammatory markers IL-6 and CRP were divided into tertiles and combined to create an overall measure of pro-inflammatory status. The sum was obtained from the tertiles of the two inflammatory markers, and tertiles of the sum were calculated, leading to three levels of pro-inflammatory status.
Functional measures
Physical functioning
The activities of daily living (ADL) score and short physical performance battery (SPPB) were assessed as a proxy for the subject's physical dependence and performance, respectively. Physical limitations of daily living were assessed by asking the respondent to describe the degree of difficulty they had with six activities of daily living: climbing stairs, walking 5 min outdoors without resting, getting up and sitting down in a chair, dressing and undressing oneself, using own or public transportation, and cutting one's own toenails. The total score was calculated by summing the scores of all of the activities (range 6–30). The lowest gender-adjusted quartiles, of ≤22 or ≤19 for men and women, were used as cutoff, respectively.
The SPPB consists of timed measurements of walking speed, rising from a chair, putting on and taking off a cardigan, and maintaining balance in a tandem stand. The summary performance scale, ranging from 0 to 14, has been used in several studies and has been shown to be a reliable and valid measure of physical functioning (Guralnik et al. 1995; 1994; Puts et al. 2005). The lowest gender-adjusted quartiles, of ≤7 or ≤4 for men and women, were used as cutoff, respectively.
Mental functioning
The mini-mental state examination (MMSE) score and Geriatric Depression Scale (GDS)-15 were reported as a proxy for the subject's cognitive functioning and suspected depression status, respectively. For the MMSE, a cutoff of 24 points (range 6–30) was used for mild to severe cognitive impairment. The GDS-15 has been especially designed to screen for depression in the elderly (Yesavage et al. 1982). A cutoff of 5 of more points was used for a depressed status.
Global functioning
The sum of the binary scores of the four aspects of functioning created an overall score of global functioning. Participants were identified as having good (0), mildly impaired (1), or impaired functioning (2–4) (Fig. 1).
Comorbidity
Non-cardiovascular comorbidities were defined as thyroid problems, anemia, asthma, chronic obstructive pulmonary disease, Parkinson's disease, arthritis, osteoarthritis, documented osteoporosis, malignancy, and renal insufficiency. Cardiovascular comorbidities were defined as hypertension; diabetes mellitus; hyperlipidemia; a history of angina pectoris or myocardial infarction and known cardiomyopathy; a history of transient ischemic attack or cardiovascular accident and peripheral arterial disease; a history of decompensated heart failure, atrial fibrillation, and valvular disease; or a history of edema of the lower extremities.
Statistical analysis
Continuous data are presented as the mean and standard deviation (SD) or median and interquartile range. Categorical data are presented as numbers and frequencies. Comparisons between different categories of subjects were performed using the Kruskal–Wallis test for continuous data and the p for trend test for categorical data; p < 0.05 was considered to be statistically significant.
The relationship between global functioning (dependent variable) and immune markers (independent variable) was assessed with multivariate logistic regression analyses. Only patients with good or impaired global functioning were entered into the multivariate models. No colinearity, interaction, or influential outliers were found with regard to the variables. The associations between the serum levels of the immune markers (independent variables) and individual aspects of physical and mental functioning (dependent variables) were further examined using multivariate logistic regression analyses. The serum levels of the immune markers were entered into the logistic models as tertiles. For all of the analyses, robust estimates were used due to the nonconstant variance (Lumley et al. 2002).
Three consecutive adjusted models were used to assess the relationship with global functional impairment: age, gender, number of cardiovascular comorbidities, and number of non-cardiovascular comorbidities (model 1); adding additional confounders for the outcome variable, including a low level of education, institutionalization, alcohol usage, and widow status (model 2); and adding additional confounders from the independent variable, including BMI, smoking status, and the use of anti-inflammatory medication (model 3). Except for age, BMI, number of cardiovascular comorbidities, and number of non-cardiovascular comorbidities, all of the variables were dichotomized. Model 3 was used in all further multivariate analyses. Potential confounders were based on the bivariate analysis and previous studies of these variables on the exposures or outcome (Singh and Newman 2011). Odds ratios and C statistics of the models were calculated after performing the regression analysis.
For IL-6, the positive predictive value (PPV) and negative predictive value (NPV) were calculated using 2 × 2 tables as a measure of predictive power. The cutoff was defined as the upper limit of the lowest tertile or 1.52 pg/ml because the largest differences in prevalence were observed between the lowest and higher tertiles.
The statistical analyses were performed using STATA 11 (StataCorp, College Station, TX).
Results
Identifying potential immune markers and confounders of impaired global functioning
Global functioning was assessed in 394 participants with a mean age of 84.5 years, and 63.4 % of the participants were female. Approximately 10 % of them were institutionalized, 70 % had low education, 6.6 % took anti-inflammatory medication, and 99.3 % reported comorbidities (Table 1). A total of 209 (53 %) participants were identified as having a good functional status; 102 (26 %), as having mild impaired functioning; and 83 (21 %), as having impaired functioning (Fig. 1). Increasing age, institutionalization, low level of education, widow status, unmarried status, low alcohol intake, and a higher number of cardiovascular and non-cardiovascular comorbidities were associated with impaired global functioning. Furthermore, IL-6 and CRP serum levels were significantly higher in patients with an impaired global functional status.
Table 1.
Demographic characteristics and immune parameters according to global functioning
| Total | Good | Mildly impaired | Impaired | p value | |
|---|---|---|---|---|---|
| n = 415 | n = 209 | n = 102 | n = 83 | ||
| Demographic | |||||
| Female gender, n (%) | 262 (63.4) | 127 (60.77) | 71 (69.61) | 51 (61.45) | 0.295 |
| Age (years), mean ± SD | 84.6 ± 3.59 | 83.6 ± 3 | 85.1 ± 3.6 | 86.3 ± 4.5 | <0.001 |
| Institutionalized, n (%) | 43 (10.4) | 8 (3.83) | 10 (9.8) | 19 (22.89) | <0.001 |
| Low level of education, n (%) | 288 (70.8) | 129 (62.32) | 77 (77) | 66 (81.48) | <0.001 |
| Married, n (%) | 173 (42.3) | 104 (50) | 34 (34) | 29 (34.94) | 0.008 |
| Widow/widower, n (%) | 204 (49.9) | 82 (39.42) | 60 (60) | 51 (61.45) | <0.001 |
| Ever smoked, n (%) | 132 (32.3) | 67 (32.37) | 29 (28.71) | 28 (34.15) | 0.711 |
| BMI, mean ± SD | 27.5 ± 4.9 | 27.1 ± 4.42 | 28.3 ± 5.32 | 27.8 ± 4.98 | 0.144 |
| Alcohol unities per day, median (IQR) | 1 (0–7) | 2 (0–7) | 1 (0–7) | 0 (0–2.5) | <0.001 |
| Anti-inflammatory medication, n (%) | 27 (6.6) | 9 (4.33) | 9 (8.91) | 8 (9.64) | 0.123 |
| Number of cardiovascular diseases, median (IQR) | 3 (2–4) | 2 (1–4) | 3 (2–4) | 3 (2–4) | 0.019 |
| Number of non-cardiovascular diseases, median (IQR) | 1 (1–3) | 1 (1–2) | 1 (1–3) | 2 (1–3) | 0.001 |
| Acute phase proteins, median (IQR) | |||||
| Ultrasensitive CRP (mg/dl) | 0.18 (0.08–0.42) | 0.15 (0.06–0.37) | 0.17 (0.1–0.4) | 0.25 (0.09–0.56) | 0.018 |
| Prealbumin (mg/dl) | 25.1 (22.1–29.5) | 25.2 (22.4–29.5) | 25.4 (22–29.5) | 24.3 (21.9–28.2) | 0.499 |
| Cytokines and chemokines, median (IQR) | |||||
| IL-1α (pg/ml) | 0.63 (0–0.88) | 0.66 (0–0.94) | 0.63 (0–0.8) | 0.62 (0–0.85) | 0.502 |
| IL-1β (pg/ml) | 0.99 (0–1.51) | 0.94 (0–1.46) | 0.85 (0–1.51) | 1.04 (0–1.57) | 0.376 |
| IL-2 (pg/ml) | 2.32 (0–3.58) | 2.43 (0–3.58) | 2.25 (0–3.63) | 2.24 (0–3.69) | 0.875 |
| IL-4 (pg/ml) | 1.97 (0–2.81) | 2.04 (0–2.85) | 1.67 (0–2.57) | 2.1 (0–2.99) | 0.237 |
| IL-6 (pg/ml) | 2.06 (1.34–3.78) | 1.63 (1.11–3.01) | 2.45 (1.67–3.75) | 2.36 (1.65–4.86) | <0.001 |
| IL-8 (pg/ml) | 4.07 (2.64–7.03) | 4.01 (2.61–5.89) | 4.52 (2.67–8.46) | 3.87 (2.62–6.37) | 0.1 |
| IL-10 (pg/ml) | 0.77 (0.56–1.06) | 0.75 (0.56–1.05) | 0.82 (0.65–1.05) | 0.74 (0.53–1.14) | 0.337 |
| TNF-α (pg/ml) | 3.68 (2.9–4.76) | 3.6 (2.79–4.38) | 3.83 (2.99–5.06) | 3.86 (3.01–5.15) | 0.177 |
| IFN-γ (pg/ml) | 1.2 (0–1.98) | 1.14 (0–2.19) | 1.26 (0–1.84) | 1.34 (0–1.84) | 0.822 |
| IGF-1 (pg/ml) | 94 (72.3–127.2) | 94.05 (74.02–124.8) | 87.4 (69.6–133.5) | 94.5 (68.2–123.4) | 0.715 |
| EGF (pg/ml) | 3.5 (2.2–7.9) | 3.41 (2.14–7.63) | 3.44 (2.3–7.7) | 3.77 (2.26–8.22) | 0.678 |
| VEGF (pg/ml) | 32.1 (21.7–59.6) | 34.86 (22.43–63.01) | 30.5 (20.3–49.6) | 31.71 (19.77–53.28) | 0.331 |
| MCP-1 (pg/ml) | 191.1 (147.2–254.6) | 187.83 (149.8–259.9) | 191.4 (148–244.4) | 198.81 (142.5–251.2) | 0.853 |
SD standard deviation, IQR interquartile range
Associations between impaired functioning and IL-6 or CRP
The relationship between global functional impairment and IL-6, CRP, or their composite score was further analyzed by calculating odds ratios. Table 2 shows the associations in the unadjusted and adjusted models with increasing C statistics. The highest odds ratios and C statistics were found with model 3. The odds of functional impairment occurring with slightly or highly elevated IL-6 levels were 4.16 (1.6–10.9) or 4.35 (1.7–11.4) times higher, respectively, than the odds for a low IL-6 level. In contrast, the odds ratios for a slightly or highly elevated CRP level were 2.37 (1.0–5.4) or 2.1, the latter being not significant. The odds ratios of the composite score were similar to those of CRP, but significant.
Table 2.
Odds ratios of multivariate logistic analysis between global functioning and inflammatory markers/composite score
| Unadjusted model | Model 1a | Model 2b | Model 3c | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (CI) | p value | C stat | OR (CI) | p value | C stat | OR (CI) | p value | C stat | OR (CI) | p value | C stat | |
| IL-6 | ||||||||||||
| L | Ref | Ref | Ref | Ref | ||||||||
| SE | 3.86 (1.91–7.79) | <0.001 | 0.65 | 3.64 (1.65–8.03) | 0.001 | 0.80 | 4.42 (1.83–10.71) | 0.001 | 0.86 | 4.16 (1.60–10.86) | 0.004 | 0.87 |
| HE | 4.28 (2.16–8.49) | <0.001 | 0.65 | 3.26 (1.44–7.40) | 0.005 | 0.80 | 3.94 (1.58–9.82) | 0.003 | 0.86 | 4.35 (1.66–11.43) | 0.003 | 0.87 |
| CRP | ||||||||||||
| L | Ref | Ref | Ref | Ref | ||||||||
| SE | 1.68 (0.88–3.21) | 0.113 | 0.57 | 2.44 (1.16–5.11) | 0.018 | 0.79 | 2.30 (1.04–5.08) | 0.040 | 0.84 | 2.37 (1.04–5.38) | 0.040 | 0.86 |
| HE | 1.83 (0.98–3.44) | 0.059 | 0.57 | 1.95 (0.94–4.06) | 0.073 | 0.79 | 2.01 (0.91–4.42) | 0.084 | 0.84 | 2.10 (0.93–4.73) | 0.074 | 0.86 |
| Composite | ||||||||||||
| L | Ref | Ref | Ref | Ref | ||||||||
| SE | 2.13 (1.08–4.21) | 0.029 | 0.60 | 2.65 (1.25–5.65) | 0.011 | 0.79 | 2.07 (0.92–4.68) | 0.080 | 0.84 | 2.40 (1.04–5.58) | 0.041 | 0.86 |
| HE | 2.45 (1.34–4.48) | 0.004 | 0.60 | 2.15 (1.06–4.36) | 0.035 | 0.79 | 2.34 (1.08–5.05) | 0.031 | 0.84 | 2.59 (1.15–5.84) | 0.022 | 0.86 |
L low tertile, SE slightly elevated tertile, HE highly elevated tertile, OR odds ratio, CI 95 % confidence interval, C stat C statistics, Ref reference value
aModel 1 (controlling for age, gender, number of cardiovascular comorbidity, number of non-cardiovascular comorbidity)
bModel 2 (controlling for age, gender, number of cardiovascular comorbidity, number of non-cardiovascular comorbidity, low level of education, institutionalization, widow status, alcohol usage)
cModel 3 (controlling for age, gender, number of cardiovascular comorbidity, number of non-cardiovascular comorbidity, low level of education, institutionalization, widow status, alcohol usage, BMI, smoking status, use of anti-inflammatory medication)
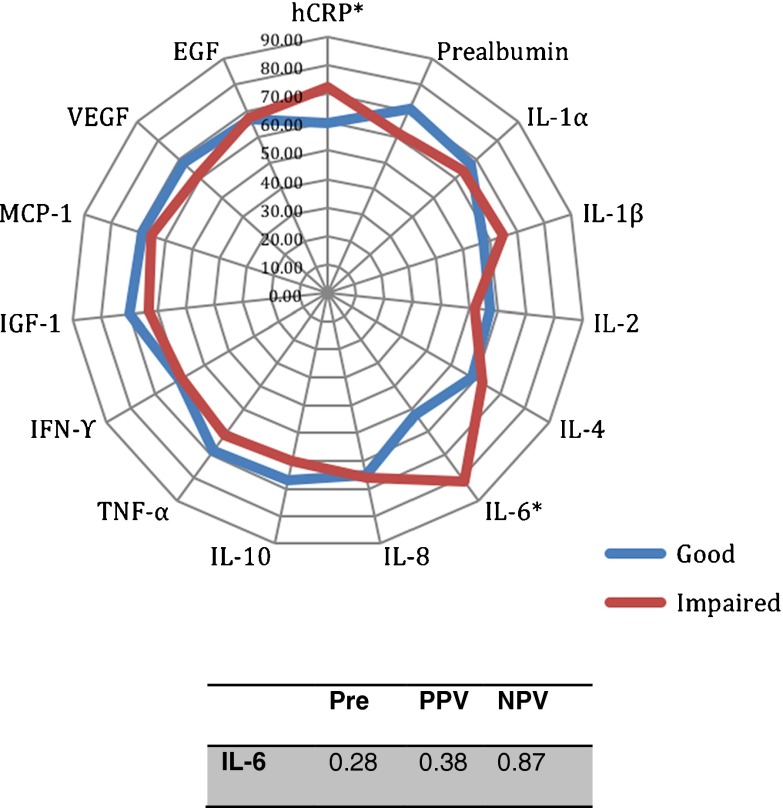
Additionally, the proportion of older persons with an elevated IL-6 or CRP level was 81.93 and 72.29 % in the impaired functioning compared with 52.63 and 59.71 % in those with good functioning, respectively (chi-square test, p < 0.001 and p < 0.045, respectively) (Fig. 2). We found a low PPV for IL-6 compared with the pretest probability, but a high NPV of 0.87 (Fig. 2).
Fig. 2.
Proportions of older persons with high inflammatory protein levels. *p < 0.05, significant difference between good and impaired global functioning (chi-square test). Pre pretest probability, PPV positive predictive value, NPV negative predictive value
Inflammatory relationship with specific physical or mental aspects of functioning
Adjusted odds ratios were calculated for all of the immune markers with regard to all of the individual aspects of functioning. A total of 106 (25.6 %) and 97 (24.6 %) subjects were identified as having physical dependence and impaired physical performance, respectively (Fig. 1). A total of 83 (20.2 %) subjects were identified as having cognitive impairment, and 50 (12.1 %) subjects were identified as having a depressed status (Fig. 1). Logistic regression analysis showed IL-6 to be significantly associated with ADL and MMSE, and only a highly elevated serum level was associated with SPPB (Table 3). In contrast, only ADL were associated with a highly elevated CRP serum level (odds ratio (OR) 2.44, 95 % confidence interval 1.24–4.83). No associations were found with GDS-15. Furthermore, the odds of having a low ADL or MMSE score with a highly elevated IL-8 level were 2.19 (1.13–4.22) and 2.29 (1.2–4.37) times higher than the odds for a low IL-8 level, respectively.
Table 3.
Odds ratios of multivariate logistic analysis for the association between inflammatory markers and aspects of functioning
| ADL | SPPB | MMSE | GDS-15 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (CI) | p value | C stat | OR (CI) | p value | C stat | OR (CI) | p value | C stat | OR (CI) | p value | C stat | |
| CRP | ||||||||||||
| L | Ref | Ref | Ref | Ref | ||||||||
| SE | 1.90 (0.94–3.84) | 0.072 | 0.80 | 1.88 (0.96–3.68) | 0.064 | 0.77 | 1.05 (0.53–2.1) | 0.879 | 0.76 | 1.19 (0.55–2.6) | 0.659 | 0.76 |
| HE | 2.44 (1.24–4.83) | 0.010 | 0.80 | 1.87 (0.97–3.61) | 0.062 | 0.77 | 1.92 (0.99–3.72) | 0.052 | 0.76 | 0.86 (0.37–2.01) | 0.722 | 0.76 |
| IL-6 | ||||||||||||
| L | Ref | Ref | Ref | Ref | ||||||||
| SE | 2.9 (1.43–5.89) | 0.003 | 0.81 | 1.77 (0.87–3.63) | 0.117 | 0.77 | 2,57 (1,21–5,44) | 0.014 | 0.77 | 2.06 (0.85–4.97) | 0.110 | 0.76 |
| HE | 2.88 (1.38–6.01) | 0.005 | 0.81 | 2.23 (1,13–4,42) | 0.021 | 0.77 | 2,77 (1,30–5,88) | 0.008 | 0.77 | 1.66 (0.63–4.37) | 0.308 | 0.76 |
| IL-8 | ||||||||||||
| L | Ref | Ref | Ref | Ref | ||||||||
| SE | 1.45 (0.73–2.88) | 0.295 | 0.80 | 1.06 (0.57–1.97) | 0.862 | 0.77 | 0.85 (0.42–1.75) | 0.666 | 0.78 | 1.42 (0.62–3.24) | 0.407 | 0.76 |
| HE | 2.19 (1.13–4.22) | 0.020 | 0.80 | 1.01 (0.53–1.91) | 0.988 | 0.77 | 2,29 (1,20–4,37) | 0.012 | 0.78 | 1.15 nn0.51–2.59) | 0.739 | 0.76 |
L low tertile, SE slightly elevated tertile, HE highly elevated tertile, OR odds ratio, CI 95 % confidence interval, C stat C statistics
Discussion
IL-6 as marker of global functioning in very old persons
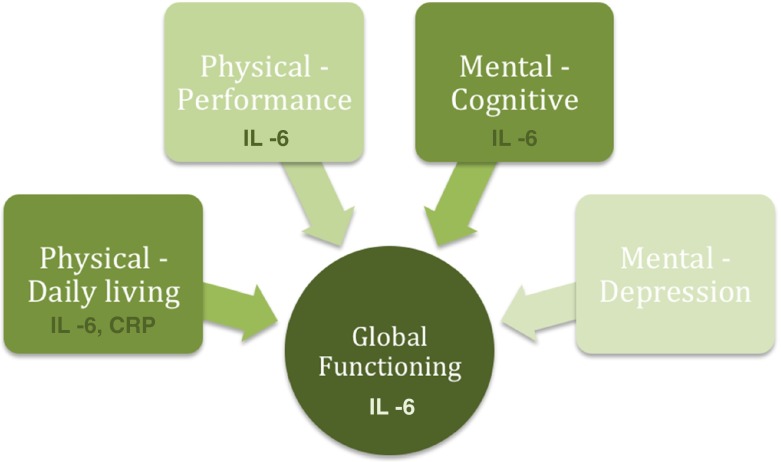
Of the extensive immune markers studied, only IL-6, CRP, and their composite score maintained the associations with global functioning in bivariate and multivariate analyses (Tables 1 and 2). The pro-inflammatory cytokine IL-6, however, is the most robustly associated marker with the global functional status (Fig. 3) in the oldest old, which is consistent with many studies identifying IL-6 as the gerontologists' cytokine (Forsey et al. 2003; Ershler 1993).
Fig. 3.
The most robust markers of inflammation for global functioning and the underlying physical and mental aspects
Previous research from aging population studies reported that IL-6 was associated with physical and cognitive impairment and mortality (Singh and Newman 2011). However, this study is the first to show the additional significance of IL-6 as a global functioning marker in the oldest old. Only a few studies have reported that poorer global self-rated health is associated with elevated inflammatory markers among older adults (Christian et al. 2011; Cohen et al. 1997). Christian et al. (2011) reported IL-6 and CRP to be associated with self-rated health in a relatively healthy study population with caregiving responsibilities with a mean age of 63.8 years, which is in line with our results in the community-dwelling oldest old, even with high comorbidity.
Inflammatory associations with individual aspects of functioning
A negative association was found between IL-6 and the physical dependence aspect (ADL). Our results are in line with Christian et al. (2011), who reported similar results with the only significant self-rated health component, physical functioning. Moreover, a recent review by Singh and Newman (2011), based on the results from major epidemiologic studies, showed IL-6 to be robustly associated with physical functioning in older adults. This association may be attributable to a direct effect of IL-6 on muscle atrophy and/or to the pathophysiologic role of IL-6 in specific diseases. However, we could only partially confirm strong associations of IL-6 with physical performance (SPPB) in aged populations (Cesari et al. 2004; Cohen et al. 1997; Ferrucci et al. 2002; Penninx et al. 2004; Tiainen et al. 2010). Additionally, no association with IL-6 was found with the age-related loss of muscle strength and function in our community-dwelling oldest old (data not shown).
IL-6 was also negatively associated with cognitive performance (MMSE), which is consistent with a growing body of literature that has shown a correlation of IL-6 with cognitive impairment or decline and dementia in aging populations (Singh and Newman 2011; Ravaglia et al. 2007; Weaver et al. 2002; Yaffe et al. 2003). These results suggest that IL-6 could play a role in complex processes, such as neurogenesis and synaptic plasticity (McAfoose and Baune 2009).
Depression is the most common comorbidity in the elderly, and it is a major determinant or consequence of functional disability. It has become clear that the inflammatory immune system is altered during the course of clinical depression. Paralleling these findings, population studies of depression in younger populations have implicated several peripheral cytokines, most notably IL-6 and TNF-α (Dowlati et al. 2010; Penninx et al. 2003). Our study could not confirm the relationship between IL-6 or TNF-α and suspected depression, which is inconsistent with a recent meta-analysis of smaller population studies in the middle-aged (Dowlati et al. 2010) and studies in older adults (Baune et al. 2012; Dimopoulos et al. 2008; Tiemeier et al. 2003). These apparently contradictory results may reflect a different immune phenotype or survival effect because the current study population was considerably older than the populations of older adults in those previous studies. The BELFRAIL population may thus be a selected population of older individuals being less susceptible to long-term deleterious effects of inflammaging and survivors of the Second World War (Mathei et al. 2011).
In line with previous studies, a logistic association between physical or cognitive impairment and IL-8 was reported (Baune et al. 2008; Kim et al. 2011). IL-8, a chemokine, has a number of pro-inflammatory effects, persists for days and even weeks (compared with most other cytokines), and can cross the BBB. Our findings support the hypothesis of a pro-inflammatory status in the impaired, and that IL-8 might be involved in cognitive processes, such as memory, perceptual speed, and motor function (McAfoose and Baune 2009; Wilson et al. 2002; Franciosi et al. 2005).
Clinical marker for identifying functional impairment
The large burden and coexistence of physical and mental impairment in the oldest old population make summary markers of the global functioning of great value, allowing risk stratification to identify people who need additional care or attention. In contrast to the odds ratios of the individual aspects of functional impairment, the higher odds ratios for global functioning suggest that elevated serum IL-6 could be a future potential candidate as a measure for global functional burden because inflammation may be a common underlying cause of physical and mental impairment or a final common pathway. In our study, IL-6 had an unsatisfactory PPV, but a satisfactory NPV for identifying those with a healthy global functional status (Fig. 2). Thus, despite the strong association, IL-6 does not necessarily qualify for use in screening or primary diagnosis, but it could be explored to exclude global functional impairment in the community-dwelling oldest old. Our unique cohort, however, does not allow us to be certain of the causal relationship due to the cross-sectional design of the study, and future prospective studies are needed to confirm the potential predictive value. Future prospective follow-up data should elucidate whether IL-6 is a risk factor of rapid functional decline. In this respect, Taaffe et al. (2000) reported that lL-6 predicted the onset of disability in older persons and was associated with mortality risk, but IL-6 did not predict a change in performance 7 years later in a high-functioning subset of older adults, except for walking measures and grip strength at baseline. Future studies should elucidate the mechanisms underlying the common inflammatory pathway because global functioning is undoubtedly influenced by multiple factors. Although high C statistics have been reached in our models, improving the power of the association, predictive models should be assessed to evaluate the predictive value of IL-6. Thus, the current evidence is not sufficient to allow or rule out the routine use of inflammatory markers in the elderly because there are few studies in this age range, and most of these studies are short-term and analyzed a small number of inflammatory markers.
Benchmark chronic marker in the oldest old
CRP has been used in adults as the benchmark chronic inflammatory marker and has been shown to be highly associated with individual aspects of functioning in older persons (Singh and Newman 2011). However, in this study, ultrasensitive CRP was only significant in the highest tertile with the single aspect of physical dependence. Furthermore, a bivariate association was observed between CRP and global functioning, but this association only persisted to a certain degree after adjusting for confounders, suggesting IL-6 as the benchmark chronic inflammatory marker in the oldest old for functional impairment. This result may only apply to this age category because IL-6 concentrations may only reach significant measurable levels in individuals aged 75 and over, causing an increased sensitivity due to larger differences (Ershler 1993; Cohen et al. 1997). This tremendous age-related increase was also observed in our cohort in 5-year age categories (p = 0.001, data not shown), making interindividual differences in IL-6 of great value for functioning or even morbidity, but it remains unclear how this pleiotropic cytokine may be involved in impaired functioning.
Composite indicators of inflammation were adopted to increase specificity for ongoing inflammation, because of the influential role of IL-6 in CRP regulation and the generally low levels of circulating cytokines, in previous population studies investigating the association between inflammation and mobility limitation (Penninx et al. 2004; Reuben et al. 2002) or mortality (Reuben et al. 2002; Harris et al. 1999; Giovannini et al. 2011; Jylhä et al. 2007). In contrast, our results do not support this theory regarding the association with global functioning in a representative sample of community-dwelling elderly persons. A combination of sample size, wide age range, poorly selected donors, and variability in assays has continued to fuel the argument of composite indicators (Penninx et al. 2004; Giovannini et al. 2011; Jylhä et al. 2007; Reuben et al. 2002).
A few limitations should be considered. First, we have chosen for a non-validated score to define global functioning. Second, the differing analytical methods and measures of functioning can be seen as a limitation because they could partly lead to inconsistent results across the research area and studies. Third, a reduction in power could have occurred, as the mild impaired group was excluded from the multivariate analyses to have an idea on markers for global functioning. This was justified due to the fact that having impairment in one aspect does not necessarily imply an impaired global functioning.
Conclusion
Our study is the first to demonstrate that in a representative sample of community-dwelling persons of 80 years and older, IL-6 is strongly associated with global functioning and could be used to exclude a global impaired functional status in these oldest old, most likely due to its higher levels than CRP in this specific age category. Furthermore, a pro-inflammatory status, especially IL-6, was associated with all of the individual aspects of functioning, except suspected depression. More research should be performed to understand the factors influencing this association or elucidating the common inflammatory pathway.
Acknowledgements
The BELFRAIL study (B40320084685) was supported by an unconditional grant from Fondation Louvain, Brussels, Belgium. Fondation Louvain is the support unit of Université Catholique de Louvain and is charged with developing the educational and research projects of the university by collecting gifts from corporations, foundations, and alumni.
References
- Baune BT, Ponath G, Golledge J, Varga G, Arolt V, Rothermundt M, Berger K. Association between IL-8 cytokine and cognitive performance in an elderly general population—the MEMO-Study. Neurobiol Aging. 2008;29(6):937–944. doi: 10.1016/j.neurobiolaging.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Baune BT, Smith E, Reppermund S, Air T, Samaras K, Lux O, Brodaty H, Sachdev P, Trollor JN. Inflammatory biomarkers predict depressive, but not anxiety symptoms during aging: the prospective Sydney Memory and Aging Study. Psychoneuroendocrinology. 2012;37(9):1521–30. doi: 10.1016/j.psyneuen.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, Andersen-Ranberg K, Jeune B, Pedersen A, Skinhøj P, Pedersen B. A high plasma concentration of TNF-alpha is associated with dementia in centenarians. J Gerontol A Biol Sci Med Sci. 1999;54(7):M357–364. doi: 10.1093/gerona/54.7.M357. [DOI] [PubMed] [Google Scholar]
- Cesari M, Penninx B, Pahor M, Lauretani F, Corsi A, Rhys Williams G, Guralnik J, Ferrucci L. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59(3):242–248. doi: 10.1093/gerona/59.3.M242. [DOI] [PubMed] [Google Scholar]
- Christian LM, Glaser R, Porter K, Malarkey WB, Beversdorf D, Kiecolt-Glaser JK. Poorer self-rated health is associated with elevated inflammatory markers among older adults. Psychoneuroendocrinology. 2011;36(10):1495–1504. doi: 10.1016/j.psyneuen.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Pieper C, Harris T, Rao K, Currie M. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J Gerontol A Biol Sci Med Sci. 1997;52(4):M201–208. doi: 10.1093/gerona/52A.4.M201. [DOI] [PubMed] [Google Scholar]
- Dik M, Jonker C, Hack C, Smit J, Comijs H, Eikelenboom P. Serum inflammatory proteins and cognitive decline in older persons. Neurology. 2005;64(8):1371–1377. doi: 10.1212/01.WNL.0000158281.08946.68. [DOI] [PubMed] [Google Scholar]
- Dimopoulos N, Piperi C, Psarra V, Lea RW, Kalofoutis A. Increased plasma levels of 8-iso-PGF2alpha and IL-6 in an elderly population with depression. Psychiatry Res. 2008;161(1):59–66. doi: 10.1016/j.psychres.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Ershler W. Interleukin-6: a cytokine for gerontologists. J Am Geriatr Soc. 1993;41(2):176–181. doi: 10.1111/j.1532-5415.1993.tb02054.x. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Penninx B, Volpato S, Harris T, Bandeen-Roche K, Balfour J, Leveille S, Fried L, Guralnik J. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50:1947–1954. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- Forsey RJ, Thompson JM, Ernerudh J, Hurst TL, Strindhall J, Johansson B, Nilsson BO, Wikby A. Plasma cytokine profiles in elderly humans. Mech Ageing Dev. 2003;124(4):487–493. doi: 10.1016/S0047-6374(03)00025-3. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia M, Invidia L, Celani L, Scurti M, Cevenini E, Castellani G, Salvioli S. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128(1):92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Franciosi S, Choi HB, Kim SU, McLarnon JG. IL-8 enhancement of amyloid-beta (Abeta 1–42)-induced expression and production of pro-inflammatory cytokines and COX-2 in cultured human microglia. J Neuroimmunol. 2005;159(1–2):66–74. doi: 10.1016/j.jneuroim.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Giovannini S, Onder G, Liperoti R, Russo A, Carter C, Capoluongo E, Pahor M, Bernabei R, Landi F. Interleukin-6, C-reactive protein, and tumor necrosis factor-alpha as predictors of mortality in frail, community-living elderly individuals. J Am Geriatr Soc. 2011;59(9):1679–1685. doi: 10.1111/j.1532-5415.2011.03570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):85–94. doi: 10.1093/geronj/49.2.M85. [DOI] [PubMed] [Google Scholar]
- Harris T, Ferrucci L, Tracy R, Corti M, Wacholder S, Ettinger W, Heimovitz H, Cohen H, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/S0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Holmes C, El-Okl M, Williams A, Cunningham C, Wilcockson D, Perry V. Systemic infection, interleukin 1b, and cognitive decline in Alzheimer's disease. J Neurol Neurosurg Pyschiatry. 2003;74:788–789. doi: 10.1136/jnnp.74.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jylhä M, Paavilainen P, Lehtimäki T, Goebeler S, Karhunen P, Hervonen A, Hurme M. Interleukin-1 receptor antagonist, interleukin-6, and C-reactive protein as predictors of mortality in nonagenarians: the vitality 90+ study. J Gerontol A Biol Sci Med Sci. 2007;62(9):1016–1021. doi: 10.1093/gerona/62.9.1016. [DOI] [PubMed] [Google Scholar]
- Kim SM, Song J, Kim S, Han C, Park MH, Koh Y, Jo SA, Kim YY. Identification of peripheral inflammatory markers between normal control and Alzheimer's disease. BMC Neurol. 2011;11:51. doi: 10.1186/1471-2377-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krabbe K, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39(5):687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Lumley T, Diehr P, Emerson S, Chen L. The importance of the normality assumption in large public health data sets. Annu Rev Public Health. 2002;23:151–169. doi: 10.1146/annurev.publhealth.23.100901.140546. [DOI] [PubMed] [Google Scholar]
- Mathei C, Vaes B, Wallemacq P, Degryse J. Associations between cytomegalovirus infection and functional impairment and frailty in the BELFRAIL Cohort. J Am Geriatr Soc. 2011;59(12):2201–2208. doi: 10.1111/j.1532-5415.2011.03719.x. [DOI] [PubMed] [Google Scholar]
- McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 2009;33(3):355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Penninx B, Kritchevsky S, Newman AB, Nicklas BJ, Simonsick E, Rubin S, Nevitt M, Visser M, Harris T, Pahor M. Inflammatory markers and incident mobility limitation in the elderly. J Am Geriatr Soc. 2004;52:1105–1113. doi: 10.1111/j.1532-5415.2004.52308.x. [DOI] [PubMed] [Google Scholar]
- Penninx B, Kritchevsky S, Yaffe K, Newman AB, Simonsick E, Rubin S, Ferrucci L, Harris T, Pahor M. Inflammatory markers and depressed mood in older persons: results from the health, aging and body composition study. Biol Psychiatry. 2003;54:566–572. doi: 10.1016/S0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- Puts MT, Lips P, Deeg DJ. Static and dynamic measures of frailty predicted decline in performance-based and self-reported physical functioning. J Clin Epidemiol. 2005;58(11):1188–1198. doi: 10.1016/j.jclinepi.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Ravaglia G, Forti P, Maioli F, Chiappelli M, Montesi F, Tumini E, Mariani E, Licastro F, Patterson C. Blood inflammatory markers and risk of dementia: the Conselice Study of Brain Aging. Neurobiol Aging. 2007;28(12):1810–1820. doi: 10.1016/j.neurobiolaging.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Reuben D, Cheh A, Harris T, Ferrucci L, Rowe J, Tracy R, Seeman T. Peripheral blood markers of inflammation predict mortality and functional decline in high-functioning community-dwelling older persons. J Am Geriatr Soc. 2002;50:638–644. doi: 10.1046/j.1532-5415.2002.50157.x. [DOI] [PubMed] [Google Scholar]
- Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10(3):319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taaffe D, Harris T, Ferrucci L, Rowe J, Seeman T. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci. 2000;55(12):M709–715. doi: 10.1093/gerona/55.12.M709. [DOI] [PubMed] [Google Scholar]
- Tiainen K, Hurme M, Hervonen A, Luukkaala T, Jylha M. Inflammatory markers and physical performance among nonagenarians. J Gerontol A Biol Sci Med Sci. 2010;65(6):658–663. doi: 10.1093/gerona/glq056. [DOI] [PubMed] [Google Scholar]
- Tiemeier H, Hofman A, van Tuijl H, Kiliaan A, Meijer J, Breteler M. Inflammatory proteins and depression in the elderly. Epidemiology. 2003;14(1):103–107. doi: 10.1097/00001648-200301000-00025. [DOI] [PubMed] [Google Scholar]
- Vaes B, Pasquet A, Wallemacq P, Rezzoug N, Mekouar H, Olivier PA, Legrand D, Mathei C, Van Pottelbergh G, Degryse J. The BELFRAIL (BFC80+) study: a population-based prospective cohort study of the very elderly in Belgium. BMC Geriatr. 2010;10:39. doi: 10.1186/1471-2318-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Exel E, de Craen A, Remarque E, Gussekloo J, Houx P, Bootsma-van der Wiel A, Frölich M, Macfarlane P, Blauw G, Westendorp R. Interaction of atherosclerosis and inflammation in elderly subjects with poor cognitive function. Neurology. 2003;61(12):1695–1701. doi: 10.1212/01.WNL.0000098877.07653.7C. [DOI] [PubMed] [Google Scholar]
- Vasto S, Candore G, Balistreri C, Caruso M, Colonna-Romano G, Grimaldi M, Listi F, Nuzzo D, Lio D, Caruso C. Inflammatory networks in ageing, age-related diseases and longevity. Mech Ageing Dev. 2007;128(1):83–91. doi: 10.1016/j.mad.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Weaver J, Huang M, Albert M, Harris T, Rowe J, Seeman T. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59(3):371–378. doi: 10.1212/WNL.59.3.371. [DOI] [PubMed] [Google Scholar]
- Wilson C, Finch C, Cohen H. Cytokines and cognition—the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50(12):2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Penninx B, Simonsick E, Pahor M, Kritchevsky S, Launer L, Kuller L, Rubin S, Harris T. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61(1):76–80. doi: 10.1212/01.WNL.0000073620.42047.D7. [DOI] [PubMed] [Google Scholar]
- Yesavage J, Brink T, Rose T, Lum O, Huang V, Adey M, Leirer V. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]