Abstract
Age-related skeletal muscle decline is characterized by the modification of sarcolemma ion channels important to sustain fiber excitability and to prevent metabolic dysfunction. Also, calcium homeostasis and contractile function are impaired. In the aim to understand whether these modifications are related to oxidative damage and can be reverted by antioxidant treatment, we examined the effects of in vivo treatment with an waste water polyphenolic mixture (LACHI MIX HT) supplied by LACHIFARMA S.r.l. Italy containing hydroxytirosol (HT), gallic acid, and homovanillic acid on the skeletal muscles of 27-month-old rats. After 6-week treatment, we found an improvement of chloride ClC-1 channel conductance, pivotal for membrane electrical stability, and of ATP-dependent potassium channel activity, important in coupling excitability with fiber metabolism. Both of them were analyzed using electrophysiological techniques. The treatment also restored the resting cytosolic calcium concentration, the sarcoplasmic reticulum calcium release, and the mechanical threshold for contraction, an index of excitation–contraction coupling mechanism. Muscle weight and blood creatine kinase levels were preserved in LACHI MIX HT-treated aged rats. The antioxidant activity of LACHI MIX HT was confirmed by the reduction of malondialdehyde levels in the brain of the LACHI MIX HT-treated aged rats. In comparison, the administration of purified HT was less effective on all the parameters studied. Although muscle function was not completely recovered, the present study provides evidence of the beneficial effects of LACHI MIX HT, a natural compound, to ameliorate skeletal muscle functional decline due to aging-associated oxidative stress.
Keywords: Skeletal muscle, Aging process, Chloride channels, Potassium channels, Calcium homeostasis, Antioxidant therapy
Introduction
Senescence is a complex biological process occurring in live organisms. Variable is the onset and progression of this event, and many causes, either genetic or environmental, may contribute. A leading theory of aging is based on the concept that damage, either due to the toxic products of metabolism or inefficient repair/defensive systems, accumulates throughout the entire life span and gradually leads to cell and organ dysfunction. There is substantial support for a role of reactive oxygen species (ROS) in the aging process. The free radical theory of aging proposed that ROS are responsible for macromolecular damage and for age-related tissue degeneration (Harman 2003; Kregel and Zhang 2007). Skeletal muscle can be strongly affected by aging, with a condition known as sarcopenia, an age-related loss of skeletal muscle mass and function. This is the major healthcare concern for older adults associated with the development of functional disability and may lead to the loss of independence for afflicted individuals (Janssen et al. 2004; Visser et al. 2005). Loss of muscle proteins, resulting from an imbalance between protein synthesis and breakdown, appears to be one of the main contributing factors to sarcopenia (Combaret et al. 2009). Atrophy, together with loss of fast-twitch fibers, contributes to muscle weakness and functional impairment (Larsson et al. 1978; Lenk et al. 2010). Recent findings suggest that ROS are generated in skeletal muscle cells during contractile activity and that this induces the activation of transcription factors that modulate the gene expression of antioxidant and protective proteins (Palomero and Jackson 2010). Several studies indicate that adaptive responses of skeletal muscle activated and regulated by ROS are disrupted during aging and that this might contribute to the loss of muscle mass and function and the progressive deterioration of this organ (Visser et al. 2005; Moylan and Reid 2007).
Our previous findings have demonstrated an important modification of skeletal muscle protein function during aging (Desaphy et al. 1998; Pierno et al. 2003) and that these modifications can be associated with increased ROS levels. During aging, we found a downregulation of the expression of ClC-1, the skeletal muscle chloride channel which is correlated with an abnormal decrease of the resting chloride conductance (gCl; Pierno et al. 1999, 2009). This channel is a key determinant of muscle excitability; indeed, a marked reduction of gCl is characteristic of disabling diseases such as myotonia congenita (Adrian and Bryant 1974; Burge and Hanna 2012; Duran et al. 2010). Thus, this reduction can justify the appearance of skeletal muscle symptoms complained by aged subjects, such as myalgia and muscle weakness. The activity of ClC-1 is normally regulated by protein kinase C (PKC), the activation of which closes the channel (De Luca et al. 1994). This regulation is altered during aging and contributes to gCl reduction (Pierno et al. 1998). A modification of ClC-1 channel expression and function has also been found during muscle disuse due to hindlimb unloading, a condition in which the degree of atrophy was paralleled to the level of oxidative stress (Pellegrino et al. 2011). In this situation, the antioxidant trolox, by neutralizing the oxidative stress in all muscles, prevented the muscle fiber phenotype transition as well as gCl alteration (Desaphy et al. 2010). Oxidation of heterologously expressed ClC-1 protein renders the channel insensitive to intracellular ATP, which may be relevant for the control of muscle fatigability (Zhang et al. 2008). Also, the activity of various types of skeletal muscle potassium channels has been found to be changed by aging. A reduced activity of the ATP-dependent potassium (KATP) channel was observed in aged muscle. This reduction can be detrimental for skeletal muscle function because of their critical role in sensing metabolic changes and in myoprotection. We showed that age-related KATP channel dysfunction can be reversed by the application of antioxidants, such as N-acyl-cysteine and thimerosal, again suggesting the involvement of oxidative stress in skeletal muscle alteration (Tricarico and Conte Camerino 1994). The Ca2+-activated K+ channels (BK) couple cell membrane voltage to intracellular Ca2+ concentration, thereby regulating skeletal muscle contraction. Their occurrence and activity in muscle fibers increase with advanced age (Tricarico et al. 1997). The activity of potassium channels can be directly modified by oxidant molecules. In neurons, KATP channels are directly activated by H2O2, and the oxidation of three intracellular methionines of the BK channel results in increased activity, whereas the oxidation of cysteines is inhibitory (Sesti et al. 2010). It has been proposed that such effects may represent one of the major mechanisms underlying the functional loss of neurons in neurodegenerative diseases and aging.
Previous findings demonstrated that alteration of calcium ion homeostasis may be involved in muscle functional loss occurring with aging. For instance, we found an increase of resting cytosolic Ca2+ concentration in aged rats (Fraysse et al. 2006). Hydrogen peroxide affects the structure and activity of the dihydropyridine and ryanodine receptors and consequently modifies calcium release and muscle contraction (Oba et al. 1996; Espinosa et al. 2006). The oxidation of SH groups alters L-type channel gating with a reduction of calcium currents in cardiac cells (Lacampagne et al. 1995). Other studies showed that ROS target sarcoplasmic reticulum (SR) calcium channels, leading to the release of calcium from the SR into the cytosol. Calcium ions can concentrate in the mitochondria, disrupt the electron transport chain, and lead to the overproduction of ROS. These ROS can further exacerbate SR calcium release, resulting in a vicious cycle leading to toxic oxidative stress (Zhang and Kaufman 2008). Altogether, these data indicate that the overproduction of ROS, possibly occurring in aged skeletal muscles, may alter the expression and function of a number of key proteins involved in the control of muscle excitability and contractility, thereby impairing muscle performance.
As far as the causes of aging were discovered, they become potentially “treatable.” With the aim of finding effective pharmacological compounds able to ameliorate the cell disturbance observed in aged rat skeletal muscle, we have performed a 6- to 8-week chronic treatment of aged animals with a new polyphenolic mixture (LACHI MIX HT) containing hydroxytyrosol (HT), known to have high free radical scavenging ability, and low amounts of other phenolic compounds, such as tyrosol, catechol, gallic acid, homovanillic acid, and caffeic acid. This LACHI MIX HT represents an attractive combination with strong antioxidant potential since the different antioxidant components could act synergistically to ameliorate skeletal muscle function. LACHI MIX HT is a product of green chemistry, being obtained from the olive oil industry wastewaters by means of eco-compatible processes. Thus, the objective of our study was to test the therapeutic potential of LACHI MIX HT in the amelioration of age-induced skeletal muscle alterations. The effects of LACHI MIX HT were examined on oxidative stress levels, chloride and potassium channel conductances and excitability of the sarcolemma, calcium homeostasis and contractile function, muscle atrophy, and muscle force, all of which are sensitive to the activity of ROS. The effects of LACHI MIX HT were compared with those of purified HT, already proposed in different therapies, i.e., anticancer or cardioprotective (Granados-Principal et al. 2010; Warleta et al. 2011; Castañer et al. 2012).
Materials and methods
Exploratory compounds and extraction technique
The pharmacological potential of highly purified HT and of a mixture that contains HT and other phenolic compounds (LACHI MIX HT) was tested in this study. The LACHI MIX HT combination, as measured using HPLC (percent area), contains hydroxytyrosol (47.34), tyrosol (11.96), gallic acid (18.49), homovanillic acid (7.07), catechol (5.80), and caffeic acid (1.66). These phenols are extracted from olive wastewater. The process to treat olive mill wastewater was set up to recover over 95 % of water suitable for civil usage. The extraction methods from the target matrix (more than 60–70 %) include decoction and/or separation method using membrane techniques. The substances extracted were enriched in hydroxytyrosol with a catalytic reaction. Oleuropein was transformed into hydroxytyrosol using an aqueous suspension of Ba(OH)2 under nitrogen stream in reflux conditions. The reaction yield is approximately 65 %. Tyrosol is transformed into hydroxytyrosol by heterogeneous catalytic reaction with H2O2 using methylreniotrioxide as a catalyzer (method patented by Lachifarma IT MI20041627, Villanova et al. 2004; EP 1623960, Villanova et al. 2005; US2006070953, Villanova et al. 2006; US20090023815, Villanova et al. 2009).
In vivo studies
Treatment and animal care
All experiments were performed in accordance with the Italian Guidelines for the use of laboratory animals, which conform with the European Community Directive published in 1986 (86/609/EEC). Adult (5 months old) and aged (27 months old) male Wistar rats (Charles River Laboratories, Italy) were used. At the beginning of the study, the weight of the adult animals was 300–400 g and the weight of the aged animals was 500–600 g. All animals were housed individually in metabolic cages in an environmentally controlled room under veterinary supervision. The animals were randomly assigned to five experimental groups of five to eight animals each: (1) an adult control group, (2) an aged control group treated with H2O vehicle, (3) an aged control group treated with ethanol vehicle, (4) an aged group treated with HT, and (5) an aged group receiving the LACHI MIX HT. Whereas HT was dissolved in water, we used ethanol (<1 %, v/v) to dissolve LACHI MIX HT because it was poorly soluble in water. At the end of the experiments, the results obtained from rats treated with the two different vehicles were very similar, allowing to pool all the data in a unique group named control aged rats. Drugs were orally administrated to rats using an esophageal cannula at a daily dose of 500 μg/kg of body weight for 6–8 weeks. All animals had free access to food (standard rodent diet 4RF21, Charles River Laboratories) and tap water.
Measure of rat muscle strength with the hanging wire test
The hanging wire test was used to monitor the forelimb strength of the animals. It was performed in all the animal groups at the beginning of the treatment and once a week during all periods of the treatment. For this test, a 55-cm-wide 2-mm-thick metallic wire is tied to two vertical stands. The wire is tightly attached to the frame to avoid vibration or unwanted displacement of the wire while the investigator is handling the animals or during the measurements. The wire is maintained 35 cm above a layer of bedding material to prevent injury to the animal when it falls down. The start position of the rat is with the fore limbs attached to the wire. The time until the rat completely releases the wire and falls down is recorded. For each animal, five trials per session were performed, with a 1-min recovery period between trials.
Electromyographic recordings
Electromyographic (EMG) recordings were performed in vivo to evaluate the presence of muscle electrical abnormalities (Heller et al. 1982). EMG traces were recorded in three to five rats of each experimental condition at the beginning and every 3 weeks until the end of treatment using the Biopack MP100 acquisition system (EMG 100C, Biopack Systems Inc., Santa Barbara, CA). EMG activity was measured by two thin concentric needle electrodes inserted in the gastrocnemius muscle of rats anaesthetized with pentobarbital (60 mg/kg, i.p., supplemented if required). Insertional activity was initiated by slight movement of the electrode within the muscle and the presence of spontaneous activity (unrelated to hindlimb movement) was evaluated. EMG activity was monitored continuously over a 3- to 4-min period starting from the insertion of the electrodes. Rats recovered very well from the anesthesia and were reintroduced in their cage soon after the test. A qualitative estimate of myotonic-like activity was based on various parameters such as voltage excursion and the frequency and duration of the electrical bursts.
Ex vivo studies
Tissue collection and blood creatine kinase levels
At the end of treatment, all the rats were in good health. They were deeply anesthetized with urethane (1.2 g/kg, i.p.) for surgical removal of hindlimb muscles. The tibialis anterior (TA), soleus (Sol), extensor digitorum longus (EDL), and flexor digitorum brevis (FDB) muscles were weighed and promptly used for ex vivo experiments. Blood was collected from a ventricular camera in ethylenediaminetetraacetic acid-rinsed centrifuge tubes. The blood was centrifuged at 3,000×g for 10 min and plasma was separated and stored at −20 °C. Creatine kinase determination was performed with a standard spectrophotometric analysis using a diagnostic kit (Sigma-Aldrich, Milan, Italy). After blood collection, the animals were killed by urethane overdose. Brains were quickly removed for the determination of malondialdehyde (MDA) level and a number of organs were weighed.
Measurement of MDA level in rat brain
The levels of MDA were determined in the brain of adult, aged, LACHI MIX HT-treated, and HT-treated aged rats as a biomarker of oxidative stress. Brains were quickly dissected, weighed, and homogenized in ice-cold Tris–HCl (20 mM) solution. The homogenate was centrifuged at 4,000 rpm at 4 °C for 10 min and the supernatant collected and frozen at −20 °C until the day of assays. The MDA level, which reflects the level of lipid peroxidation, was measured by a fluorescence assay of thiobarbituric acid-reactive substance (TBARS) formation following the indications of a commercial kit (OXItek, ZeptoMetrix Corp., Buffalo, NY). Fluorescence measurements were performed in triplicate using a 96-well plate reader (Victor3V multilabel counter, PerkinElmer).
Resting chloride and potassium conductances and sarcolemma excitability of rat skeletal muscle fibers measured in current-clamp mode by an intracellular microelectrode technique
The fast-twitch EDL muscles dissected from deeply anesthetized rats were immediately placed in a 25-ml muscle bathing chamber maintained at 30 °C. The muscles were perfused with normal or chloride-free physiological solutions (gassed with 95 % O2 and 5 % CO2, pH 7.2–7.3). As previously detailed (Bryant and Conte Camerino 1991), the membrane electrical properties and the component conductances of the sarcolemma were determined in a current-clamp mode by means of the standard two-intracellular-microelectrode technique under computer control. A hyperpolarizing square current pulse (100-ms duration) was passed through one electrode and the membrane voltage response was monitored at two distances from the current electrode (Pierno et al. 1998). In accordance with the infinite linear cable theory, we measured the fiber diameter (dcalc), membrane resistance (Rm), and the total membrane capacitance (Cm). The reciprocal of Rm from each fiber in the normal physiological solution was assumed to be the total membrane conductance (gm), and the same parameter measured in chloride-free solution was considered to be the potassium conductance (gK). The mean chloride conductance (gCl) was estimated as the mean gm minus the mean gK (Bryant and Conte Camerino 1991).
The excitability characteristics of the sampled fibers were determined by recording the intracellular membrane potential response to a square-wave constant (100 ms) current pulse. In each fiber, the membrane potential was set by a steady holding current to −80 mV before passing the depolarizing pulses. By increasing the amplitude of the pulse, we were able to elicit first a single action potential, from which the AP (amplitude of action potential, in millivolts), Ith (threshold current, in nanoamperes), and Lat (latency of action potential, delay from the beginning of the current pulse to the onset of an action potential at threshold, in milliseconds) were calculated. By further increasing current intensity in the same fiber, a train of action potentials (N spikes) was then generated (De Luca et al. 1994).
The acute effects of LACHI MIX HT were evaluated in vitro on the resting gCl and gK of control aged rats. The compound was dissolved at the desired concentration (100–500 μM) in the physiological solution and directly applied to the EDL muscle in the bathing chamber. Electrophysiological recordings started after an appropriate incubation time of 30 min. After recording in the presence of the test compounds, a washout procedure enabled the establishment of the full reversibility of the drug effect.
Mechanical threshold for the contraction of rat skeletal muscle fibers measured in point voltage-clamp mode by the intracellular microelectrode technique
The mechanical threshold (MT) for contraction was determined in the EDL muscle fibers using a computer-assisted two-microelectrode point voltage-clamp method in the presence of 3 μM tetrodotoxin, as described (Pierno et al. 1999). The holding potential was set at −90 mV. Depolarizing current pulses of increasing duration (5–500 ms) were given repetitively (0.3-Hz rate), while the impaled fibers were viewed continuously with a stereomicroscope until visible contraction. The threshold membrane potential was read from a digital voltmeter. The mean threshold membrane potential, V (in millivolts), was plotted as a function of the pulse duration, t (in milliseconds), and the relationship was fitted using the following equation: V(t) = [H − Rexp(t/τ)]/[1 − exp(t/τ)], where H (in millivolts) is the holding potential, R (in millivolts) is the rheobase voltage, and τ (in milliseconds) is the time constant to reach R. The MT values were expressed as the fitted R parameter along with the standard error that was determined from the variance–covariance matrix in the nonlinear squares fitting algorithm (De Luca and Conte Camerino 1992).
Determination of the cytosolic calcium concentration in rat skeletal muscle using FURA-2 imaging technique
The resting cytosolic Ca2+ concentration (restCa) was determined in freshly mechanically dissected EDL muscle fibers using a QuantiCell 900 fluorescence imaging system (Visitech International, Sunderland, UK), as previously described (Fraysse et al. 2006). Briefly, small bundles of five to ten fibers arranged in a single layer were dissected longwise, tendon to tendon, using microscissors. The bundles were incubated with the fluorescent Ca2+ probe FURA-2 for 60–90 min at 30 °C in a physiological (NP) solution containing (in millimolars) 148 NaCl, 4.5 KCl, 2.5 CaCl2, 1 MgCl2, 12 NaHCO3, 0.44 NaH2PO4, and 5.5 glucose. The solution was supplemented with 5 μM of acetoxymethyl ester of the dye mixed with 10 % (v/v) pluronic F-127 (Molecular Probes, Leiden, the Netherlands) and the pH was adjusted to 7.3–7.4 by bubbling the solution with 95 % O2/5 % CO2. After incubation, the cells were washed with NP solution and mounted in a modified RC-27NE recording chamber (Warner Instruments, Hamden, CT). Fiber integrity was verified by assessing visible contractile activity under ×400 magnification in response to a depolarizing solution containing 100 mM K+. Next, the mean sarcomere length was adjusted to ∼2.5 μm by moving the mobile tube fixed to one muscle tendon. The fluorescence ratio was converted off-line to restCa with the calibration parameters determined in each muscle using the equation restCa = (R − Rmin)/(Rmax − R)⋅KD⋅β, where R is the 340:380 nm fluorescence ratio; KD is the affinity constant of fura-2 for Ca2+ given by the manufacturer, i.e., 145 nM (Molecular Probes); and β, Rmin, and Rmax are the calibration parameters determined in ionomycin-permeabilized fibers bathed in NP solution for the calculation of Rmax or in Ca2+-free solution for the calculation of Rmin (Grynkiewicz et al. 1985). The β value was calculated as the ratio of fluorescence intensities emitted by the fibers excited at 380 nm in the Ca2+-free and NP solutions. The cytosolic calcium response of muscle fibers to in vitro application of 40 mM caffeine or 500 nM carbonyl cyanide p-trifluoromethoxy-phenylhydrazone (FCCP) was measured to evaluate calcium release from the sarcoplasmic reticulum or mitochondria, respectively.
Patch-clamp measurement of potassium channel activity in rat skeletal muscle fibers
Patch-clamp experiments were performed at 20–22 °C on freshly enzymatically dissociated FDB muscle fibers in inside-out configurations using the standard patch-clamp technique, as previously described (Tricarico et al. 2006). The ATP-sensitive potassium (KATP) channel currents were recorded immediately after excision during voltage steps from 0 to −60 mV with 150 mM KCl on both sides of the membrane patches in the absence (control) or the presence of ATP in the muscle bath (Tricarico et al. 2006). The calcium-activated potassium (BK) currents were recorded in the presence of 150 mM KCl on both sides of the membrane during voltage steps of 10- to 20-s durations going from a holding potential of 0 to −60 mV (Vm) and +30 mV (Vm) in the presence of 10 μM concentration of free Ca2+ ions in the bath solution (Tricarico et al. 2004). The currents were recorded at a 1-kHz sampling rate (filter = 0.2 kHz) using an Axopatch-1D amplifier equipped with a CV-4 head stage (Axon Instruments, Union City, CA). Macropatches having an average pipette area of 11.3 ± 1 μm2 were used to measure the mean KATP currents, which were calculated by subtracting the baseline level from the open-channel level of each current trace and then digitally averaging all generated files using CLAMPFIT (Axon Instruments). The baseline level for the KATP current was measured in the presence of internal ATP (5 × 10−3 M; Tricarico et al. 2006). The BK currents were calculated by digital subtraction of the leak currents measured for each patch in the absence of internal Ca2+ ions from the maximal open-channel currents recorded in the presence of internal 10 μM concentrations of free Ca2+ ions (Tricarico et al. 2004). Current amplitude was measured using CLAMPFIT. No correction was made for liquid junction potential, which was <2 mV in our experimental conditions.
Statistical analysis
All data are expressed as the mean ± SEM. Statistical analysis for direct comparison between two groups of data means was performed using unpaired Student’s t test, while multiple statistical comparison between groups was performed using ANOVA test, with Bonferroni’s t test post hoc correction for allowing a better evaluation of intra- and inter-group variability.
Results
In vivo studies
Effects of LACHI MIX HT and HT on rat health and body weight in aged treated and age-matched controls
All the animals were examined daily over the entire period of the treatment for behavior, cleanliness, aspect of hairs and eyes, and food and water consumption. No signs of adverse effects were observed. No variation in daily water consumption and daily urine production was found between the beginning and the end of treatment for each experimental group. Adult animals showed a physiological body weight gain within the 6-week treatment, whereas aged animals showed significant weight loss (Table 1). Both LACHI MIX HT and HT treatments insignificantly reduced body weight in aged rats (Table 1). The mean daily food consumption calculated at the end of the treatment with respect to the beginning was insignificantly decreased in control aged rats from 19.3 ± 1.5 to 17.6 ± 1.6 g/day (n = 5). In LACHI MIX HT and HT-treated rats, no significant change in food consumption was observed at the end of the treatment in comparison with the initial value (from 16.7 ± 0.7 to 16.5 ± 0.4 g/day, n = 5, and from 19.4 ± 1.4 to 19.7 ± 0.7 g/day, n = 5, respectively).
Table 1.
Effects of LACHI MIX HT and HT treatment on body and muscle weight and plasma creatine kinase of aged rats
| Adult | Aged | HT-treated aged | LACHI MIX HT- treated aged | ANOVA (F) | |
|---|---|---|---|---|---|
| n | 5 | 8 | 5 | 5 | |
| % BW change | +28 ± 5.4 | −8.4 ± 1.5a | −4.1 ± 3.6a | −5.9 ± 2.1a | 28.3 (P < 0.001) |
| EDL muscle (g) | 0.27 ± 0.01 | 0.20 ± 0.01a | 0.23 ± 0.02a | 0.24 ± 0.01b | 8.4 (P < 0.001) |
| TA (g) | 0.96 ± 0.02 | 0.75 ± 0.03a | 0.80 ± 0.05a | 0.96 ± 0.06b | 7.5 (P < 0.005) |
| Sol (g) | 0.22 ± 0.01 | 0.18 ± 0.01a | 0.20 ± 0.01 | 0.21 ± 0.01b | 3.1 (n.s.) |
| Plasma CK (U/l) | 347 ± 101 | 3201 ± 1348 | 1606 ± 1350 | 1442 ± 55 | 1.2 (n.s.) |
Each value represents the mean ± SEM from n rats. % BW change indicates the mean variation of body weights between the end of and the beginning of treatment. The weights of the different muscles were measured at the end of the treatment. The mean values of plasma CK were determined at the end of the treatment. Statistical analysis was performed using ANOVA followed by Bonferroni’s t test
EDL extensor digitorum longus, TA tibialis anterior, Sol soleus, CK creatine kinase, n.s. not significant
aAt least P < 0.05 with respect to adult
bAt least P < 0.05 with respect to aged animals
Effects of LACHI MIX HT and HT on rat muscle strength measured by the hanging wire test
The hanging wire test was used to monitor muscle strength. The hanging time before falling off a wire upon exhaustion was measured in each rat once a week. Within each experimental group, no significant variation in hanging time was found along the treatment period (not shown). At the end of the treatment period, the mean hanging time of adult rats was 41.7 ± 5.5 s (n = 5 rats). The hanging time of aged rats was greatly (P < 0.001) reduced to 9.5 ± 1.9 s (n = 8). Chronic treatment with LACHI MIX HT or HT did not significantly ameliorate this parameter, being 12.2 ± 2.5 s (n = 8) and 10.4 ± 2.0 s (n = 5), respectively.
Effects of LACHI MIX HT and HT treatment on EMG activity in the gastrocnemius muscle of aged rats
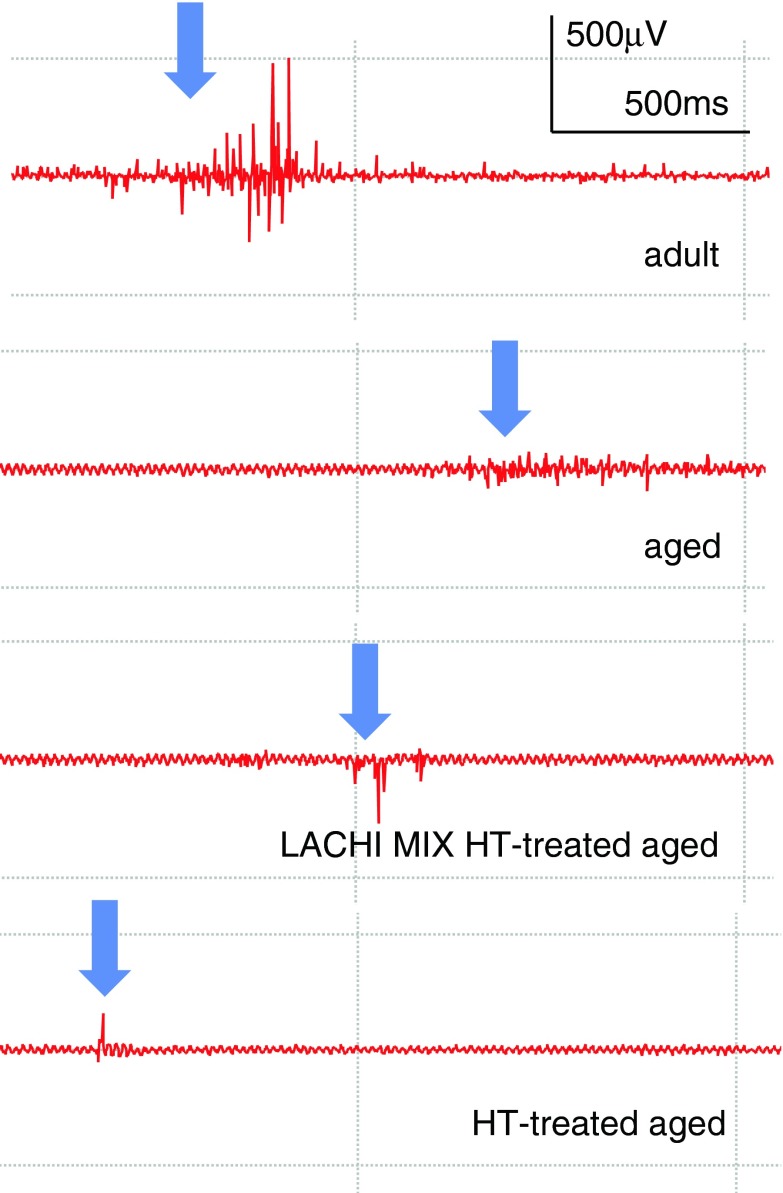
Electromyography performed in vivo on the gastrocnemius muscle of five adult rats showed normal electrical activity characterized by rapid and brief bursts of action potential (AP) occurring during voluntary movements and followed by a long period of rest during muscle inactivity. Representative traces of EMG recordings are shown in Fig. 1. In the five aged rats tested, the EMG recordings were normal in terms of frequency and duration of the burst, although the AP was shorter if compared to that of the adults, an effect which can be a sign of a minor excitability. Both LACHI MIX HT treated (four animals tested) and HT-treated aged animals (three animals tested) showed similar traces with respect to age-matched controls (Fig. 1). Moreover, no sign of myotonic-like activity was found in any animals examined because of the absence of long-lasting and spontaneous repetitive discharges, typical of myotonia.
Fig. 1.
Representative example of the electromyographic (EMG) activity recorded in the gastrocnemius muscle of adult, aged, and LACHI MIX HT and HT-treated aged rats. Normal activity in terms of repetitive discharges was apparent and persisted for a few milliseconds after needle insertion (indicated by the arrows). These bursts were followed by a period of rest during muscle inactivity. The electromyograms showed lower activity in aged rats with respect to the adults, as indicated by the shorter peaks. LACHI MIX HT and HT-treated aged rats showed similar traces with respect to that of aged rats. Calibration bar, 500 μV, 500 ms
Ex vivo studies
Effects of LACHI MIX HT and HT treatment on organ and muscle weights and plasma creatine kinase
Different organs such as the brain, heart, liver, and kidney were weighed at the end of the treatment. No differences in organ weight were found between the treated and untreated aged rats (not shown). In contrast, the weight of the hindlimb muscles, including EDL, TA, and Sol, was significantly reduced in the aged animals as compared to the adults (Table 1). In the HT-treated aged animals, the weights of EDL and TA were still significantly different with respect to those of the adults, whereas the weights of these muscles were significantly ameliorated toward the adult value in the LACHI MIX HT treated aged animals. At the end of treatment, creatine kinase (CK) level was measured in the plasma to evaluate muscle damage. CK was markedly increased in aged rats with respect to the adult ones, although the difference was not significant due to the high inter-individual variability. Interestingly, both treatments partially restored the CK level in aged rats toward the adult value (Table 1).
Effects of LACHI MIX HT and HT treatment on brain lipid peroxidation
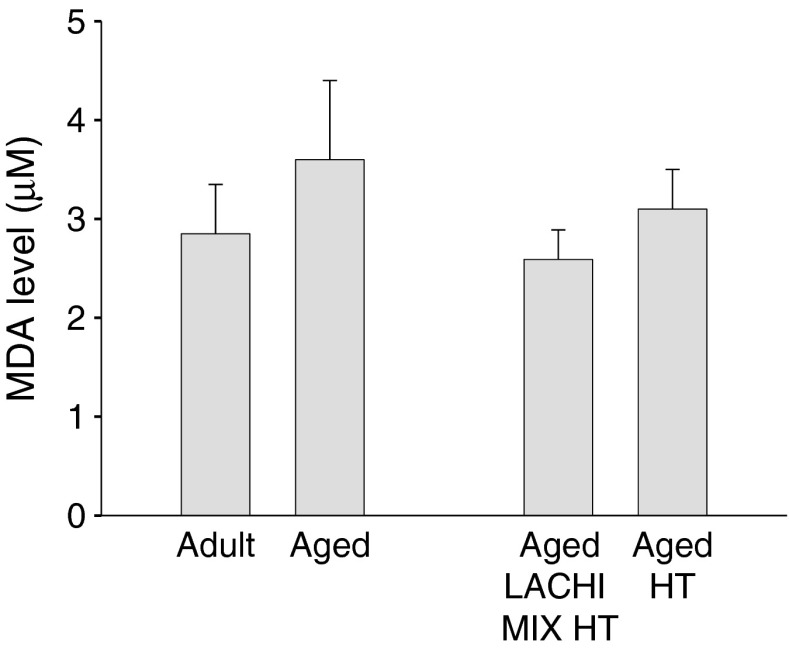
The effects of LACHI MIX HT and HT treatments on free radical production were evaluated using a TBARS assay kit in the brain, a tissue which is notoriously affected by ROS increase during aging. The level of malondialdehyde, as an oxidative stress marker, was increased, although insignificantly, in the brain of five aged rats with respect to the five adults and was reduced in the five LACHI MIX HT treated aged animals toward the adult value (Fig. 2). Minor effects were observed in the brain of HT-treated aged animals (Fig. 2).
Fig. 2.
Level of malondialdehyde (MDA) in the brain of adult and aged rats and effects of LACHI MIX HT and HT treatment in aged animals. The MDA levels were measured using a commercial kit as a marker of oxidative stress in rat brain. Each bar represents the mean value ± SEM from five animals of each group
Effects of LACHI MIX HT and HT treatment on the macroscopic ionic conductances and membrane excitability of EDL muscle
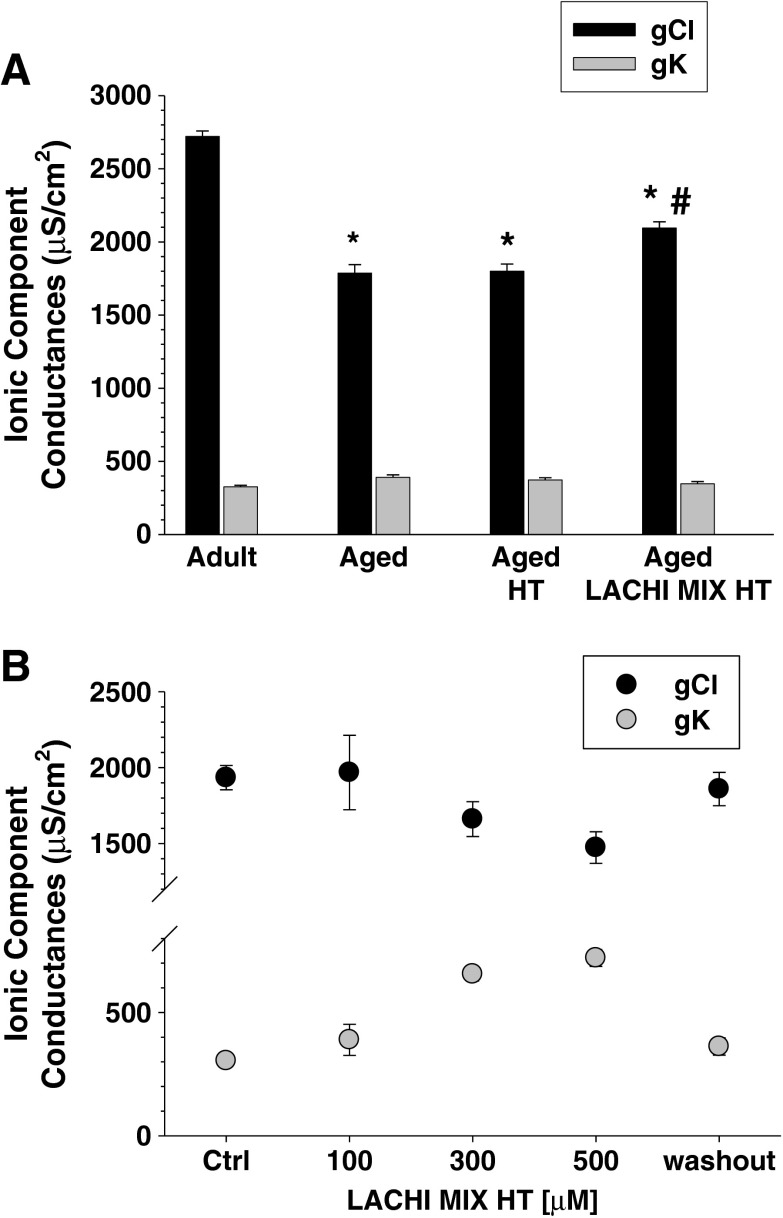
As already shown (Pierno et al. 1999), a significant reduction of the component chloride conductance of the sarcolemma (gCl) was found in the EDL muscle fibers of aged rats with respect to the adults (−34.3 ± 2.0 %; Fig. 3a). The value of gCl measured in the EDL of both HT- and LACHI MIX HT-treated animals was still significantly different with respect to the adult value, being reduced by −33.8 ± 1.7 and −23.0 ± 1.5 %, respectively. Interestingly, the value of gCl found in the LACHI MIX HT-treated rats was also significantly different with respect to the value found in the aged rats and shifted toward the adult value, suggesting a beneficial effect of LACHI MIX HT on this parameter (Fig. 3a). Also, macroscopic potassium conductance (gK) was significantly increased in aged animals with respect to the adults (by 20.0 ± 4.7 %). Again, resting gK was ameliorated only in the LACHI MIX HT treated aged animals (Fig. 3a). Membrane excitability, sustained by the activity of different channels, such as Cl−, K+, and Na+, was measured in EDL muscle fibers. In aged rats, the excitability parameters were modified, showing a significant increase of latency of action potential and a decrease of the maximum number of spikes (N; Table 2). A non-significant decrease of the threshold current (Ith) to evoke the first AP was also observed. These parameters were not ameliorated by either HT or LACHI MIX HT treatment (Table 2).
Fig. 3.
Effect of LACHI MIX HT and HT on the sarcolemma chloride conductance (g Cl) and potassium conductance (g K) obtained in the EDL muscle of aged rats. a Effects of chronic treatment. Each bar represents the mean value ± SEM of g Cl (black) and g K (gray) measured, respectively, in 36–41 and 26–50 muscle fibers from adult, aged, and HT- and LACHI MIX HT-treated aged rats. Statistical analysis was performed using ANOVA followed by Bonferroni’s t test. Significant differences were found for resting g Cl between aged, HT-treated aged, LACHI MIX HT-treated aged, and adult rats (F = 80; *P < 0.001 vs. adult, by ANOVA/Bonferroni). In addition, the value found in LACHI MIX HT-treated animals was significantly different with respect to the aged and HT-treated aged rats (F = 80; # P < 0.001 vs. aged and HT-treated aged, by ANOVA/ Bonferroni). Resting g K was significantly different between adult and aged and between adult and HT-treated aged rats (F = 2.9; § P < 0.05 vs. adult, by ANOVA/Bonferroni). b Acute effects of in vitro application. Each point represents the mean value ± SEM of g Cl (black) and g K (gray) measured, respectively, in 7–38 EDL muscle fibers from two to six aged rats after in vitro application of the LACHI MIX HT at different concentrations, as indicated. Ctrl is the value of g Cl measured in the absence of LACHI MIX HT. Statistical analysis was performed using unpaired Student’s t test (at least P < 0.005)
Table 2.
Effects of LACHI MIX HT and HT treatment on the membrane excitability parameters of the extensor digitorum longus muscle fibers of aged rats
| Adult | Aged | HT-treated aged | LACHI MIX HT-treated aged | ANOVA (F) | |
|---|---|---|---|---|---|
| N rats/n fibers | 8/51 | 4/10 | 5/17 | 5/20 | |
| AP (mV) | 101.0 ± 1.3 | 98.0 ± 2.5 | 96.6 ± 2.4 | 91.3 ± 2.3a | 5.20 (P < 0.005) |
| I th (nA) | 188 ± 7 | 169 ± 16 | 165 ± 12 | 165 ± 10 | 1.68 (n.s.) |
| Lat (ms) | 5.6 ± 0.2 | 9.5 ± 0.4a | 9.1 ± 0.5a | 9.7 ± 0.7a | 31.5 (P < 0.001) |
| N spikes | 7.0 ± 0.3 | 5.2 ± 0.7a | 6.0 ± 0.7 | 4.7 ± 0.4a | 5.8 (P < 0.005) |
Membrane excitability was determined in EDL muscle fibers in current-clamp mode using the two-intracellular microelectrode technique. The excitability parameters are the amplitude of the first AP, the threshold current (I th, minimum current intensity able to elicit a single action potential), the latency of the first action potential (Lat, maximal delay from the beginning of the current pulse to the onset of the spike), and the maximum number of action potentials elicited by raising the intensity of a 100-ms duration pulse in the same fiber (N spikes). Values are given as the mean ± SEM from n fibers and N rats. Statistical analysis was performed using ANOVA followed by Bonferroni’s t test
aAt least P < 0.001 with respect to adults
Because of the effects of treatments on sarcolemma ion conductances in vivo, we examined the effects of LACHI MIX HT on cable parameters when applied in vitro to isolated EDL muscles of aged rats (Fig 3b). This compound dose-dependently reduced resting gCl, with a significant 24 ± 4.4 % of reduction at the higher dose. We also observed a significant and marked 115 ± 16 and 137 ± 17 % increases of gK with the application of the higher doses (300 and 500 μM) of LACHI MIX HT, respectively. These effects were reversed after LACHI MIX HT washout.
Effects of LACHI MIX HT and HT treatment on the mechanical threshold for the contraction of EDL muscle fibers
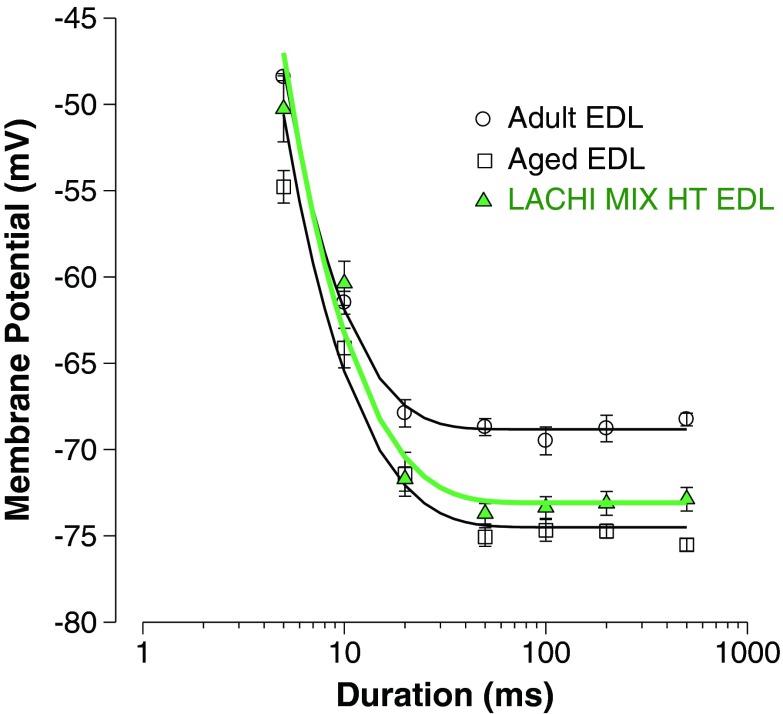
The MT for contraction is an index of excitation–contraction coupling and represents a measure of skeletal muscle contractility. This parameter takes into account the contractile response originated from an intact single muscle fiber comprehensive of its whole contractile apparatus. The threshold potential needed to elicit contraction was determined by applying pulse current of various durations (5–500 ms) and showed typical dependence on command pulse duration, being more negative the longer the duration of the pulse. As previously observed (De Luca and Conte Camerino 1992), the fibers of the EDL muscle of aged rats needed significantly less depolarization to contract at each pulse duration; consequently, the threshold potential–duration relationship was shifted toward more negative potentials in the EDL muscle of aged rats as compared with adults (Fig 4). The rheobase (R) voltage, which is the minimum voltage able to induce fiber contraction, was measured from the fit of the experimental points and was significantly more negative in aged animals with respect to the adult ones. LACHI MIX HT in vivo treatment was able to significantly ameliorate the R value, which was significantly different with respect to aged rats (Table 3). In contrast, HT was ineffective on this parameter (Table 3).
Fig. 4.
Strength–duration curves of the threshold potential for mechanical activation (mechanical threshold, MT). MT was measured in EDL muscle fibers from adult (n = 5 rats), aged (n = 5 rats), and LACHI MIX HT-treated aged rats (n = 5 rats). The threshold voltage/duration relationship was obtained by plotting the values of threshold potential (in millivolts) needed to obtain myofiber contraction as a function of the voltage pulse duration (in milliseconds). Each point is expressed as the mean value ± SE of 14–26 fibers. The curves fitting the experimental points have been obtained using the equation described in “Materials and methods.” Statistical differences were evaluated using ANOVA followed by Bonferroni’s t test. All the threshold potential values measured in aged animals were significantly more negative with respect to those of adults (P < 0.001). The values measured in LACHI MIX HT-treated aged muscle fibers were significantly different with respect to those of aged rats at 5, 10, 50, 200, and 500 ms (P < 0.05 or less), being more similar to the adult value
Table 3.
Effects of LACHI MIX HT and HT treatment on the rheobase voltage of the extensor digitorum longus muscle fibers of aged rats
| Adult | Aged | HT-treated aged | LACHI MIX HT-treated aged | ANOVA (F) | |
|---|---|---|---|---|---|
| N rats/n fibers | 5/26 | 5/14 | 5/22 | 5/17 | |
| Rheobase (mV) | −67.2 ± 0.2 | −75.5 ± 0.4a | −74.9 ± 0.4a | −72.9 ± 0.7a,b | 95 (P < 0.001) |
The mechanical threshold, an index of excitation–contraction coupling, was measured in EDL muscle fibers using the point voltage-clamp method. The values of rheobase, which is the lowest voltage able to induce fiber contraction and measured from the fit of the experimental points, are given as the mean ± SEM from n fibers and N rats. Statistical analysis was performed using ANOVA followed by Bonferroni’s t test
aSignificantly different with respect to adult (P < 0.001)
bSignificantly different with respect to aged rats (P < 0.001)
Effects of LACHI MIX HT and HT treatment on the calcium homeostasis of EDL muscle fibers
Using the FURA-2 cytofluorimetric technique, we evaluated the effects of LACHI MIX HT and HT treatment on the calcium homeostasis of the EDL muscle fibers of aged rats (Fig. 5). In accord with previous studies (Fraysse et al. 2006), aged fast-twitch muscle fibers showed a significant increase of resting cytosolic calcium (restCa) with respect to the adults (Fig. 5a). Indeed, in the EDL muscle, the restCa was increased in the EDL of aged rats by 80 ± 11 % with respect to the adults. Whereas HT treatment of aged rats had no significant effect on restCa, LACHI MIX HT treatment significantly reduced restCa by 24 ± 5 % toward the adult value (Fig. 5a). We evaluated the effects of in vitro application of 40 mM caffeine, an agonist of ryanodine receptor calcium channel, on EDL muscle fibers (Fig. 5b). The muscle fibers of control and HT-treated aged rats were less sensitive to caffeine, showing a minor calcium transient in the cytosol with respect to the adult ones. In contrast, total restoration of the amplitude of calcium transient induced by caffeine was found in LACHI MIX HT-treated rats toward the typical values of adult rats (Fig. 5b). We also applied 500-nM FCCP in vitro to EDL muscle fibers. This compound is an oxidative phosphorylation uncoupler that is useful in evaluating the mitochondrial calcium content (Smaili and Russell 1999). The calcium release induced by FCCP was greater in the muscle fibers from aged rats with respect to adults, suggesting greater calcium content in aged mitochondria. Neither LACHI MIX HT nor HT treatment was able to ameliorate the FCCP responsiveness (Fig. 5c, d).
Fig. 5.
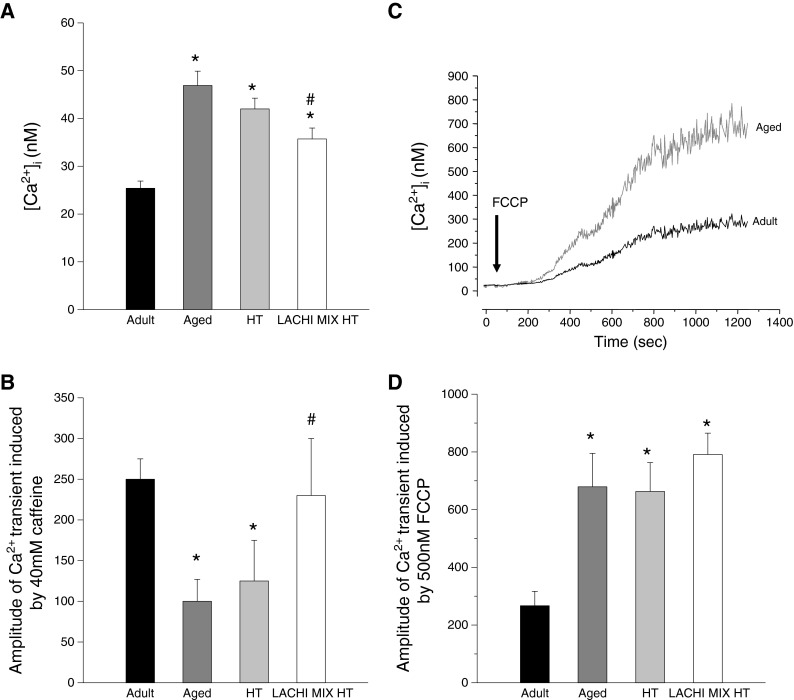
Effects of LACHI MIX HT and HT treatment on the calcium homeostasis in EDL muscle fibers during aging. a Resting [Ca2+]i measured by FURA-2 fluorescence imaging in single fibers mechanically dissociated from the EDL muscle of adult (n = 5), aged (n = 5), and HT- (n = 5) and LACHI MIX HT-treated rats (n = 5). Each bar is the mean ± SEM from 35–71 fibers. Statistical analysis performed using Student’s t test showed significant differences: *P < 0.05 or less (vs. adult); # P < 0.05 or less (vs. aged). b Caffeine responsiveness determined by evaluating the amplitude of the calcium transient after in vitro application of 40 mM caffeine for each animal group. Each bar represents the mean amplitude ± SEM from four to ten fibers. Statistical analysis performed using Student’s t test showed significant differences: *P < 0.05 or less (vs. adult); # P < 0.05 or less (vs. aged). c Example of traces reproducing the effect of the in vitro application of carbonyl cyanide p-trifluoromethoxy-phenylhydrazone (FCCP), an oxidative phosphorylation uncoupler, in the muscle fibers of adult and aged rats. d FCCP responsiveness determined by evaluating the amplitude of the calcium transient after in vitro application of 500 nM FCCP in each animal group. Each bar represents the mean value ± SEM from 5–11 fibers. Statistical analysis performed using Student’s t test showed significant differences: *P < 0.05 or less (vs. adult)
Effects of LACHI MIX HT and HT treatment on potassium channel activity in FDB muscle fibers
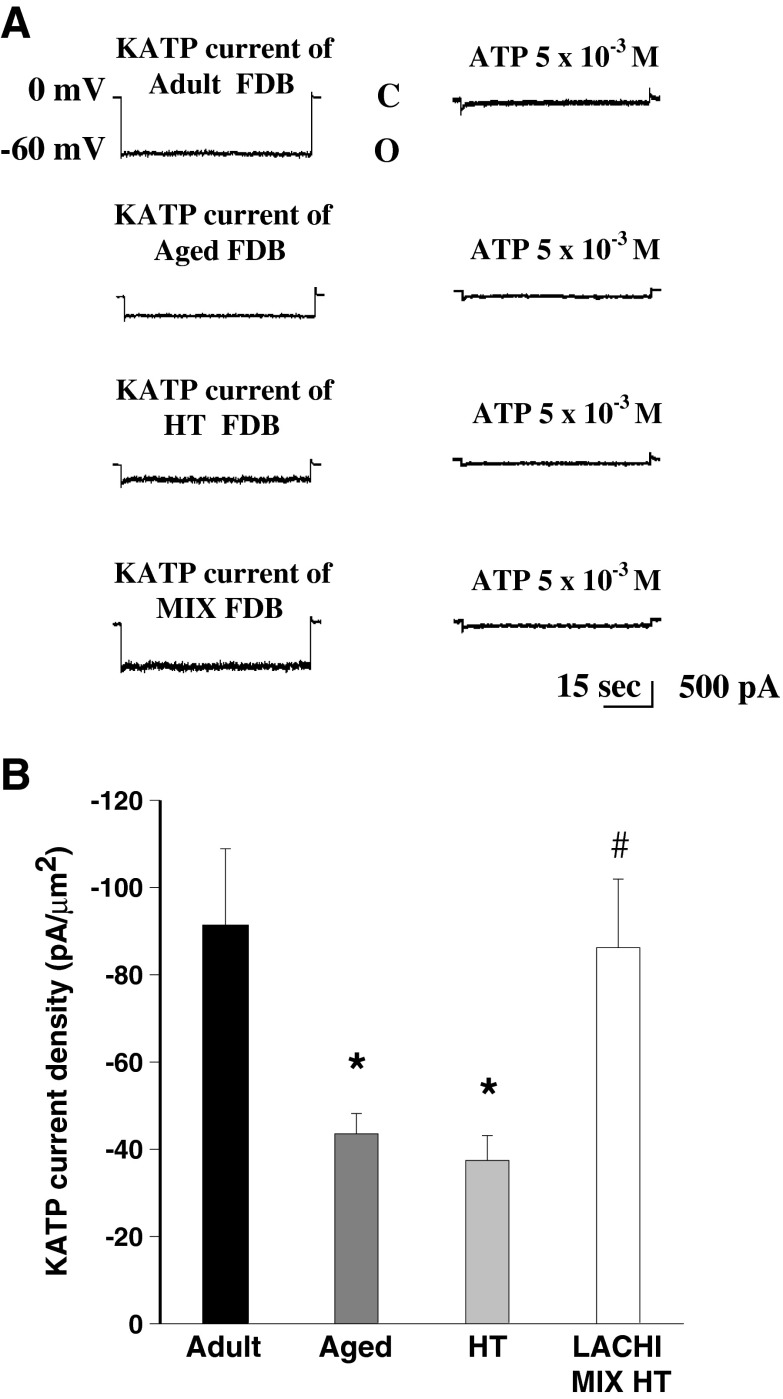
Patch-clamp experiments were performed on fast-twitch FDB muscle fibers to test whether the antioxidant compounds LACHI MIX HT and HT can have beneficial effects on the activity of KATP and BK channels, molecular sensors of the metabolic state of the cell which are known to be altered during aging. As previously demonstrated (Tricarico and Conte Camerino 1994), the amplitude of the KATP channel current was reduced in the FDB muscle of aged rats with respect to the adults (Fig. 6). Exposure of macropatches to intracellular ATP (5 × 10−3 M) greatly reduced the current amplitude, confirming that these currents flowed through KATP channels (Fig. 6a). The mean amplitudes of KATP currents were −1036.3 ± 12 pA (n = 35 patches from five muscles), −459.4 ± 52 pA (n = 12 patches from five muscles), −439.6 ± 27 pA (n = 12 patches from five muscles), and −982.0 ± 15 pA (n = 16 patches from four muscles) in adult, aged, and HT- and LACHI MIX HT-treated rats, respectively. From these current values, the current densities were calculated through normalization by patch area (Fig. 6b). Chronic treatment with LACHI MIX HT significantly restored KATP current density toward the adult value. No beneficial effect was found in HT-treated animals. Also, the activity of the Ca-dependent K+ channels (BK), known to regulate muscle activation by sensing calcium influx, was modified during aging. Indeed, as previously demonstrated (Tricarico et al. 1997), it was increased in the fast-twitch FDB muscle of aged rats with respect to the adults. No significant effects of LACHI MIX HT or HT were found in the FDB of all the aged treated rats with respect to the controls (data not shown).
Fig. 6.
Effect of LACHI MIX HT and HT treatments on the potassium channel activity in flexor digitorum brevis (FDB) muscle of aged rats. a Sample KATP channel current traces of patches excised from FDB muscle fibers of adult, aged, and HT- and MIX-treated rats. The currents flowing through macropatches were recorded during voltage steps going from 0 to −60 mV (V m) in the presence of 150 × 10−3 M KCl on both sides of the membrane. ATP applied on the intracellular side of the patches inhibited the currents in all patches. O open-channel levels, C closed-channel levels. b KATP channel current densities recorded from FDB muscles of adult, aged, and HT- and LACHI MIX HT-treated rats. *Significantly different with respect to the adult animals (P < 0.05, by Student’s t test). #Significantly different with respect to the aged animals (P < 0.05, by Student’s t test)
Discussion
With the growing interest for medical uses of naturally occurring antioxidants, scientific attention toward dietary phenolic compounds is tremendously increasing. In light of scientific evidence that the consumption of olive oil contained in the Mediterranean diet reduces the incidence of cardiovascular disease and tumor, we wondered whether a phenol mixture (LACHI MIX HT) derived from the olive oil industry with high antioxidant potential may prove beneficial in the prevention of muscle impairment induced by aging. The LACHI MIX HT combination mainly contains hydroxytyrosol (HT) and lower amounts of other phenols. Bioavailability studies have demonstrated that HT and tyrosol are dose-dependently absorbed in animals and humans after olive oil ingestion and accumulated in the body, where they can exert positive biological effects (Visioli et al. 2000; Weinbrenner et al. 2004; Tuck et al. 2001). In addition, these molecules do not show appreciable toxicity up to 2 g/kg when orally administered to rats (D’Angelo et al. 2001).
We observed that chronic treatment in aged rats with LACHI MIX HT exerted protective effects on a number of muscle molecular parameters altered by aging. In particular, we demonstrated its beneficial effect in preventing the reduction of resting gCl, observed in aged animals, by shifting the value toward that occurring in adults. The muscle gCl is mainly due to the activity of ClC-1 chloride channels. We previously demonstrated that the reduction of gCl in aged rat muscles is due to a reduction of ClC-1 expression levels and the increased activity of PKC able to close the channel (De Luca et al. 1994; Pierno et al. 1999, 2007). Interestingly, it has been described that mitochondrial dysfunction and the consequent increase of ROS during aging produce an intracellular accumulation of diacylglycerol and the activation of different PKC isoforms, which may contribute to aging-related insulin resistance (Kim et al. 2008). Thus, we can hypothesize that LACHI MIX HT, through its antioxidant activity, may ameliorate the gCl in aged rat muscle through the removal of PKC overactivity on the ClC-1 channel. Accordingly, we previously found that treatment of aged rats with taurine, a sulfonic amino acid with antioxidant properties, increases the resting gCl in EDL muscle, likely via PKC inhibition (Pierno et al. 1998). However, the recovery of gCl with LACHI MIX HT treatment was not complete, suggesting a limited effect on ClC-1 channel expression. It is also worth noting that the ClC-1 chloride channel is directly sensitive to the redox state. In heterologous systems of expression, the oxidation of ClC-1 renders the channel insensitive to the inhibitory action of intracellular ATP, which may have critical relevance for muscle fatigue (Zhang et al. 2008). Accordingly, we observed that the application of LACHI MIX HT in vitro reduced the gCl in aged rat muscle, maybe by increasing the sensitivity of the ClC-1 channel to ATP. Nevertheless, the acute inhibiting effects of LACHI MIX HT on the gCl cannot account for the recovery of gCl exerted in vivo.
In skeletal muscle, an increase of the potassium current generated by the calcium-activated potassium (BK) channels and a reduction of the activity of ATP-dependent potassium (KATP) channels contribute to the metabolic dysfunction described during aging (Tricarico and Conte Camerino 1994). According to these observations, the macroscopic potassium conductance, resulting from the activity of the aforementioned and other potassium channels, including the inward rectifier potassium channels, was slightly increased in aged animals. Previous studies have already demonstrated that different antioxidant compounds, such as N-acetyl-cysteine and thimerosal, applied in vitro, counteract the alteration of KATP function observed in aged skeletal muscle (Tricarico and Conte Camerino 1994). This observation supports the hypothesis that oxidative stress can be directly involved in the alteration of these channels and that antioxidant treatment may prove useful. Accordingly, our results showed a beneficial effect of LACHI MIX HT in increasing KATP current toward the adult values. No effect of LACHI MIX HT or HT treatment was found on the BK activity of aged muscles. In this regard, it can be hypothesized that the modifications observed in the BK channels during aging, in terms of an increased number of functioning channels (Tricarico et al. 1997), are not related to oxidative stress. Moreover, it has been described that in skeletal muscle tissue, the application of mannitol, a ROS scavenger, does not protect the BK channel from copper inhibition, suggesting that the effect did not occur through oxidation mediated by ROS (Morera et al. 2003).
We previously demonstrated that the resting cytosolic calcium concentration is abnormally increased in fast- or in slow-twitch muscle fibers of aged rats (Fraysse et al. 2006), an effect which may contribute to the activation of Ca-dependent proteases and the consequent sarcopenia. We also found that the increase of intracellular calcium is likely not attributable to enhanced Ca2+ influx (Fraysse et al. 2006), suggesting that intracellular compartments are involved in cytosolic calcium increase. Various studies have shown that ROS may modify calcium homeostasis and contractility (Annunziato et al. 2002). It has been shown that the oxidation of ryanodine receptor causes intracellular calcium leak that may contribute to muscle weakness in aging (Damiani et al. 1996; Andersson et al. 2011; Sun et al. 2011). Accordingly, the smaller calcium transient we observed in aged muscle in response to caffeine stimulation suggests a reduced SR calcium content. Importantly, LACHI MIX HT treatment was able to restore caffeine response and cytosolic calcium levels in aged EDL muscle. Such effects allowed a partial restoration of the mechanical threshold, a measure of the excitation–contraction coupling. In addition, the decrease of cytosolic calcium by LACHI MIX HT may contribute to the restoration of gCl by reducing the activity of Ca-dependent PKC. On an other hand, the alteration of mitochondrial calcium content in aged muscles and its insensitivity to LACHI MIX HT treatment agree with the widely accepted concept that mitochondrial dysfunction may represent an early event in the aging process (Calvani et al. 2013). Our results suggest that calcium increase in the aged mitochondria may occur independently of ROS production, or that oxidative damage of the aged mitochondria is too much severe to be counteracted by antioxidants. Importantly, calcium overload may act synergistically with oxidative stress to open the mitochondrial permeability transition pore, resulting in oxidative phosphorylation uncoupling, release of cytochrome c in the cytosol, and consequent apoptosis that may contribute to muscle atrophy (Zhang and Kaufman 2008; Paradies et al. 2013). Interestingly, LACHI MIX HT treatment allowed recovering muscle weight and blood creatine kinase, suggesting important benefits in maintaining muscle structural integrity.
It is worth noting that LACHI MIX HT was more effective than purified HT in ameliorating most of the parameters altered by aging in skeletal muscle. Most probably, the efficacy of LACHI MIX HT relates to the synergic activity of its various components. Indeed, it has been shown that gallic acid and catechol have strong radical scavenger abilities (Umadevi et al. 2012). Homovanillic acid has an important role, as HT, in the protection against hydrogen peroxide-induced cellular injury, although at higher concentrations (Incani et al. 2010). Accordingly, we observed a major antioxidant effect of LACHI MIX HT compared to HT in the brains of aged rats.
In conclusion, LACHI MIX HT treatment showed a clear ability to counteract, at least partially, some of the alterations typically observed in skeletal muscle during aging, including the resting ion conductances of the sarcolemma, KATP channel activity, SR calcium release, resting cytosolic calcium concentration, mechanical threshold, muscle weight, and blood creatine kinase. All the effects are likely related to the antioxidant activity of LACHI MIX HT, although we cannot definitely exclude some specific actions at the molecular level. Such result confirms the usefulness of antioxidants in the protection of aging-induced muscle impairment. Muscle recovery was incomplete since mitochondrial calcium overload, excitability parameters, electrical activity, and muscle strength were not ameliorated by LACHI MIX HT treatment. Nevertheless, in light of the positive results, it would be of interest to verify whether a longer and/or earlier treatment or higher LACHI MIX HT doses may increase efficiency. Because of their relative safety, antioxidants like LACHI MIX HT may also be considered as an adjuvant therapy to increase efficacy and limit the toxicity of conventional specific anti-aging therapies. Last but not least, one additional interest of LACHI MIX HT holds on its provenience from green chemistry of agro-industrial wastes, thereby fully espousing the general principles of human health protection.
Acknowledgments
Dedicated to the memory of Dr. Luigi Villanova, brilliant scientist and great friend. This work has been supported by Regione Puglia Project PE-004.
References
- Adrian RH, Bryant SH. On the repetitive discharge in myotonic muscle fibres. J Physiol. 1974;240:505–515. doi: 10.1113/jphysiol.1974.sp010620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson DC, Betzenhauser MJ, Reiken S, Meli AC, Umanskaya A, Xie W, Shiomi T, Zalk R, Lacampagne A, Marks AR. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab. 2011;14:196–207. doi: 10.1016/j.cmet.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziato L, Pannaccione A, Cataldi M, Secondo A, Castaldo P, Di Renzo G, Taglialatela M. Modulation of ion channels by reactive oxygen and nitrogen species: a pathophysiological role in brain aging? Neurobiol Aging. 2002;23:819–834. doi: 10.1016/S0197-4580(02)00069-6. [DOI] [PubMed] [Google Scholar]
- Bryant SH, Conte Camerino D. Chloride channel regulation in the skeletal muscle of normal and myotonic goats. Pflugers Arch. 1991;417:605–610. doi: 10.1007/BF00372958. [DOI] [PubMed] [Google Scholar]
- Burge JA, Hanna MG. Novel insights into the pathomechanisms of skeletal muscle channelopathies. Curr Neurol Neurosci Rep. 2012;12:62–69. doi: 10.1007/s11910-011-0238-3. [DOI] [PubMed] [Google Scholar]
- Calvani R, Joseph A-M, Adhihetty PJ, Miccheli A, Bossola M, Leeuwenburgh C, Bernabei R, Marzetti E. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol Chem. 2013;394(3):393–414. doi: 10.1515/hsz-2012-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castañer O, Covas MI, Khymenets O, Nyyssonen K, Konstantinidou V, Zunft HF, de la Torre R, Muñoz-Aguayo D, Vila J, Fitó M. Protection of LDL from oxidation by olive oil polyphenols is associated with a downregulation of CD40-ligand expression and its downstream products in vivo in humans. Am J Clin Nutr. 2012;95:1238–1244. doi: 10.3945/ajcn.111.029207. [DOI] [PubMed] [Google Scholar]
- Combaret L, Rieu I, Magne H, Savary-Auzeloux I, Averous J, Bos C, Peyron MA, Dardevet D. Reduction of low grade inflammation restores blunting of postprandial muscle anabolism and limits sarcopenia in old rats. J Physiol. 2009;587:5483–5492. doi: 10.1113/jphysiol.2009.178319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani E, Larsson L, Margreth A. Age-related abnormalities in regulation of the ryanodine receptor in rat fast-twitch muscle. Cell Calcium. 1996;19:15–27. doi: 10.1016/S0143-4160(96)90010-X. [DOI] [PubMed] [Google Scholar]
- D’Angelo S, Manna C, Migliardi V, Mazzoni O, Morrica P, Capasso G, Pontoni G, Galletti P, Zappia V. Pharmacokinetics and metabolism of hydroxytyrosol, a natural antioxidant from olive oil. Drug Metab Dispos. 2001;29:1492–1498. [PubMed] [Google Scholar]
- De Luca A, Conte Camerino D. Effects of aging on the mechanical threshold of rat skeletal muscle fibers. Pflugers Arch. 1992;420:407–409. doi: 10.1007/BF00374477. [DOI] [PubMed] [Google Scholar]
- De Luca A, Tricarico D, Pierno S, Conte Camerino D. Aging and chloride channel regulation in rat fast-twitch muscle fibres. Pflugers Arch. 1994;427:80–85. doi: 10.1007/BF00585945. [DOI] [PubMed] [Google Scholar]
- Desaphy J-F, De Luca A, Pierno S, Imbrici P, Camerino DC. Partial recovery of skeletal muscle sodium channel properties in aged rats chronically treated with growth hormone or the GH-secretagogue hexarelin. J Pharmacol Exp Ther. 1998;286:903–912. [PubMed] [Google Scholar]
- Desaphy J-F, Pierno S, Liantonio A, Giannuzzi V, Digennaro C, Dinardo MM, Camerino GM, Ricciuti P, Brocca L, Pellegrino MA, Bottinelli R, Conte Camerino D. Antioxidant treatment of hindlimb-unloaded mouse counteracts fiber type transition but not atrophy of disused muscles. Pharmacol Res. 2010;61:553–563. doi: 10.1016/j.phrs.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Duran C, Thompson CH, Xiao Q, Hartzell HC. Chloride channels: often enigmatic, rarely predictable. Annu Rev Physiol. 2010;72:95–121. doi: 10.1146/annurev-physiol-021909-135811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa A, Leiva A, Peña M, Müller M, Debandi A, Hidalgo C, Carrasco MA, Jaimovich E. Myotube depolarization generates reactive oxygen species through NAD(P)H oxidase; ROS-elicited Ca2+ stimulates ERK, CREB, early genes. J Cell Physiol. 2006;209:379–388. doi: 10.1002/jcp.20745. [DOI] [PubMed] [Google Scholar]
- Fraysse B, Desaphy JF, Rolland JF, Pierno S, Liantonio A, Giannuzzi V, Camerino C, Didonna MP, Cocchi D, De Luca A, Conte Camerino D. Fiber type-related changes in rat skeletal muscle calcium homeostasis during aging and restoration by growth hormone. Neurobiol Dis. 2006;21:372–380. doi: 10.1016/j.nbd.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Granados-Principal S, Quiles JL, Ramirez-Tortosa CL, Sanchez-Rovira P, Ramirez-Tortosa MC. Hydroxytyrosol: from laboratory investigations to future clinical trials. Nutr Rev. 2010;68:191–206. doi: 10.1111/j.1753-4887.2010.00278.x. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Harman D. The free radical theory of aging. Antioxid Redox Signal. 2003;5:557–561. doi: 10.1089/152308603770310202. [DOI] [PubMed] [Google Scholar]
- Heller AH, Eicher EM, Hallett M, Sidman RL. Myotonia, a new inherited muscle disease in mice. J Neurosci. 1982;2:924–933. doi: 10.1523/JNEUROSCI.02-07-00924.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incani A, Deiana M, Corona G, Vafeiadou K, Vauzour D, Dessì MA, Spencer JP. Involvement of ERK, Akt and JNK signalling in H2O2-induced cell injury and protection by hydroxytyrosol and its metabolite homovanillic alcohol. Mol Nutr Food Res. 2010;54:788–796. doi: 10.1002/mnfr.200900098. [DOI] [PubMed] [Google Scholar]
- Janssen I, Lee SJ, Heymsfield SB, Ross R. Relation between whole-body and regional measures of human skeletal muscle. Am J Clin Nutr. 2004;80:1215–1221. doi: 10.1093/ajcn/80.5.1215. [DOI] [PubMed] [Google Scholar]
- Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008;102:401–414. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007;292:R18–R36. doi: 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- Lacampagne A, Duittoz A, Bolaños P, Peineau N, Argibay JA. Effect of sulfhydryl oxidation on ionic and gating currents associated with L-type calcium channels in isolated guinea-pig ventricular myocytes. Cardiovasc Res. 1995;30:799–806. [PubMed] [Google Scholar]
- Larsson L, Orlander J, Kiessling KH, Karlsson J, Aniansson A. Skeletal muscle metabolism and ultrastructure in relation to age in sedentary men. Acta Physiol Scand. 1978;104:249–261. doi: 10.1111/j.1748-1716.1978.tb06277.x. [DOI] [PubMed] [Google Scholar]
- Lenk K, Schuler G, Adams V. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachex Sarcopenia Muscle. 2010;1:9–21. doi: 10.1007/s13539-010-0007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morera FJ, Wolff D, Vergara C. External copper inhibits the activity of the large-conductance calcium- and voltage-sensitive potassium channel from skeletal muscle. J Membr Biol. 2003;192:65–72. doi: 10.1007/s00232-002-1064-y. [DOI] [PubMed] [Google Scholar]
- Moylan JS, Reid MB. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve. 2007;35:411–429. doi: 10.1002/mus.20743. [DOI] [PubMed] [Google Scholar]
- Oba T, Koshita M, Yamaguchi M. H2O2 modulates twitch tension and increases Po of Ca2+ release channel in frog skeletal muscle. Am J Physiol. 1996;271:810–818. doi: 10.1152/ajpcell.1996.271.3.C810. [DOI] [PubMed] [Google Scholar]
- Palomero J, Jackson MJ. Redox regulation in skeletal muscle during contractile activity and aging. J Anim Sci. 2010;88:1307–1313. doi: 10.2527/jas.2009-2436. [DOI] [PubMed] [Google Scholar]
- Paradies G, Paradies V, Ruggiero FM, Petrosillo G. Changes in the mitochondrial permeability transition pore in aging and age-associated diseases. Mech Ageing Dev. 2013;134(1–2):1–9. doi: 10.1016/j.mad.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Pellegrino MA, Desaphy JF, Brocca L, Pierno S, Camerino DC, Bottinelli R. Redox homeostasis, oxidative stress and disuse muscle atrophy. J Physiol. 2011;589:2147–2160. doi: 10.1113/jphysiol.2010.203232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierno S, De Luca A, Camerino C, Huxtable RJ, Conte Camerino D. Chronic administration of taurine to aged rats improves the electrical and contractile properties of skeletal muscle fibers. J Pharmacol Exp Ther. 1998;286:1183–1190. [PubMed] [Google Scholar]
- Pierno S, De Luca A, Beck CL, George AL, Jr, Conte Camerino D. Aging-associated down-regulation of ClC-1 expression in skeletal muscle: phenotypic-independent relation to the decrease of chloride conductance. FEBS Lett. 1999;449:12–16. doi: 10.1016/S0014-5793(99)00202-1. [DOI] [PubMed] [Google Scholar]
- Pierno S, De Luca A, Desaphy JF, Fraysse B, Liantonio A, Didonna MP, Lograno M, Cocchi D, Smith RG, Camerino DC. Growth hormone secretagogues modulate the electrical and contractile properties of rat skeletal muscle through a ghrelin-specific receptor. Br J Pharmacol. 2003;139:575–584. doi: 10.1038/sj.bjp.0705284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierno S, Desaphy J-F, Liantonio A, De Luca A, Zarrilli A, Mastrofrancesco L, Procino G, Valenti G, Conte Camerino D. Disuse of rat muscle in vivo reduces protein kinase C activity controlling the sarcolemma chloride conductance. J Physiol. 2007;584:983–995. doi: 10.1113/jphysiol.2007.141358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierno S, Camerino GM, Cippone V, Rolland JF, Desaphy JF, De Luca A, Liantonio A, Bianco G, Kunic JD, George AL, Jr, Conte Camerino D. Statins and fenofibrate affect skeletal muscle chloride conductance in rats by differently impairing ClC-1 channel regulation and expression. Br J Pharmacol. 2009;156:1206–1215. doi: 10.1111/j.1476-5381.2008.00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesti F, Liu S, Cai SQ. Oxidation of potassium channels by ROS: a general mechanism of aging and neurodegeneration? Trends Cell Biol. 2010;20:45–51. doi: 10.1016/j.tcb.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Smaili SS, Russell JT. Permeability transition pore regulates both mitochondrial membrane potential and agonist-evoked Ca2+ signals in oligodendrocyte progenitors. Cell Calcium. 1999;26:121–130. doi: 10.1054/ceca.1999.0061. [DOI] [PubMed] [Google Scholar]
- Sun QA, Hess DT, Nogueira L, Yong S, Bowles DE, Eu J, Laurita KR, Meissner G, Stamler JS. Oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor-Ca2+ release channel by NADPH oxidase 4. Proc Natl Acad Sci U S A. 2011;108:16098–16103. doi: 10.1073/pnas.1109546108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricarico D, Conte Camerino D. ATP-sensitive K+ channels of skeletal muscle fibers from young adult and aged rats: possible involvement of thiol-dependent redox mechanisms in the age-related modifications of their biophysical and pharmacological properties. Mol Pharmacol. 1994;46:754–761. [PubMed] [Google Scholar]
- Tricarico D, Petruzzi R, Conte Camerino D. Changes of the biophysical properties of calcium-activated potassium channels of rat skeletal muscle fibres during aging. Pflugers Arch. 1997;434:822–829. doi: 10.1007/s004240050471. [DOI] [PubMed] [Google Scholar]
- Tricarico D, Barbieri M, Mele A, Carbonara G, Conte Camerino D. Carbonic anhydrase inhibitors are specific openers of skeletal muscle BK channel of K+-deficient rats. FASEB J. 2004;18:760–761. doi: 10.1096/fj.03-0722fje. [DOI] [PubMed] [Google Scholar]
- Tricarico D, Mele A, Lundquist AL, Desai RR, Jr GA, Conte Camerino D. Hybrid assemblies of ATP-sensitive K+ channels determine their muscle-type-dependent biophysical and pharmacological properties. Proc Natl Acad Sci U S A. 2006;103:1118–1123. doi: 10.1073/pnas.0505974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuck KL, Freeman MP, Hayball PJ, Stretch GL, Stupans I. The in vivo fate of hydroxytyrosol and tyrosol, antioxidant phenolic constituents of olive oil, after intravenous and oral dosing of labeled compounds to rats. J Nutr. 2001;131:1993–1996. doi: 10.1093/jn/131.7.1993. [DOI] [PubMed] [Google Scholar]
- Umadevi S, Gopi V, Simna SP, Parthasarathy A, Yousuf SM, Elangovan V (2012) Studies on the cardio-protective role of gallic acid against AGE-induced cell proliferation and oxidative stress in H9C2 (2-1) cells. Cardiovasc Toxicol 12:304–311 [DOI] [PubMed]
- Villanova L, Villanova L, Fasiello G, Merendino A (2004) Processo per il recupero di Tirosolo, Idrossitirosolo e altri composti fenolici da acque di vegetazione e metodo di ossidazione catalitica di tirosolo a idrossitirosolo, Italian Patent Application Publication IT MI20041627
- Villanova L, Villanova L, Fasiello G, Merendino A (2005) Process for the recovery of tyrosol and hydroxytyrosol from oil mill wastewaters and catalytic oxidation method in order to convert tyrosol in hydroxytyrosol. European Patent Application Publication EP 1623960
- Villanova L, Villanova L, Fasiello G, Merendino A (2006) Process for the recovery of tyrosol and hydroxytyrosol from oil mill wastewaters and catalytic oxidation method in order to convert tyrosol in hydroxytyrosol. United States Patent Application Publication US2006070953
- Villanova L, Villanova L, Fasiello G, Merendino A (2009) Process for the recovery of tyrosol and hydroxytyrosol from oil mill wastewaters and catalytic oxidation method in order to convert tyrosol in hydroxytyrosol. United States Patent Application Publication US20090023815
- Visioli F, Galli C, Bornet F, Mattei A, Patelli R, Galli G, Caruso D. Olive oil phenolics are dose-dependently absorbed in humans. FEBS Lett. 2000;468:159–160. doi: 10.1016/S0014-5793(00)01216-3. [DOI] [PubMed] [Google Scholar]
- Visser M, Newman AB, Lee SJ, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Nevitt M, Harris TB. Weight change and the conservation of lean mass in old age: the health, aging and body composition study. Am J Clin Nutr. 2005;82:872–878. doi: 10.1093/ajcn/82.4.872. [DOI] [PubMed] [Google Scholar]
- Warleta F, Quesada CS, Campos M, Allouche Y, Beltrán G, Gaforio JJ. Hydroxytyrosol protects against oxidative DNA damage in human breast cells. Nutrients. 2011;3:839–857. doi: 10.3390/nu3100839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbrenner T, Fitó M, Farré Albaladejo M, Saez GT, Rijken P, Tormos C, Coolen S, De La Torre R, Covas MI. Bioavailability of phenolic compounds from olive oil and oxidative/antioxidant status at postprandial state in healthy humans. Drugs Exp Clin Res. 2004;30:207–212. [PubMed] [Google Scholar]
- Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XD, Tseng PY, Chen TY. ATP inhibition of CLC-1 is controlled by oxidation and reduction. J Gen Physiol. 2008;132:421–428. doi: 10.1085/jgp.200810023. [DOI] [PMC free article] [PubMed] [Google Scholar]