Abstract
The purpose of this study was to evaluate normal values for regional and global myocardial wall motion parameters in young and elderly individuals, as detected by navigator gated high temporal resolution tissue phase mapping. Radial, longitudinal and circumferential ventricular wall motion, as well as ventricular torsion and longitudinal strain rates, were assessed in two age groups of volunteers, 23 ± 3 (n = 14) and 66 ± 7 years old (n = 9), respectively. All subjects were healthy, non-smokers without known cardiac disease. An increased global left ventricular (LV) torsion rate (peak systolic torsion rate 20.6 ± 2.0 versus 14.5 ± 1.0°/s/cm, peak diastolic torsion rate −25.2 ± 1.8 versus −14.1 ± 1.3°/s/cm) and a decrease in longitudinal LV motion (peak systolic values at mid-ventricle 5.9 ± 0.5 versus 8.5 ± 0.8 cm/s, peak diastolic values −10.7 ± 0.7 versus −15.2 ± 0.9 cm/s) in the older age group were the most prominent findings. Lower peak diastolic radial velocities with a longer time-to-peak values, most pronounced at the apex, are consistent with reduced diastolic function with ageing. Lower peak clockwise and counter-clockwise velocities at all LV levels revealed limitations in resting LV rotational motions in the older group. Significant changes in the undulating pattern of the rotational motions of the left ventricle were also observed. The results demonstrate distinct changes in regional and global myocardial wall motion in elderly individuals. Increased LV torsion rate and reduced LV longitudinal motion were particularly prominent in the older group. These parameters may have a role in the assessment of global LV contractility and help differentiate age-related changes from cardiac disease.
Keywords: Ventricular wall motion, Cardiac magnetic resonance, Phase contrast velocity mapping
Introduction
Ageing is related to structural and functional alterations in the heart. Whilst cardiac reserve and diastolic filling decrease over time, an increase in left ventricular ejection fraction has been reported in older individuals (Nikitin et al. 2006). The mechanism for these changes remains incompletely understood, even though the alterations in left ventricular physiology with ageing may partially explain the reduced cardiac reserve in response to disease, pharmacological interventions and surgical procedures (Nikitin et al. 2006). Moreover, understanding the physiological remodelling of the left ventricle with ageing may prove important to help differentiate age-related changes from cardiovascular disease. Left ventricular systolic contraction results from a complex interplay of cardiomyocyte aggregates orientated in different directions along and within the heart muscle wall. Cardiac magnetic resonance (MR) studies using navigator gated tissue phase mapping (TPM) techniques have helped our understanding of the complex longitudinal and circumferential motion patterns of the left ventricle (Codreanu et al. 2010). The goal of this study was to evaluate normal values for regional and global myocardial wall motion parameters in young and elderly individuals as detected by TPM.
Methods
Regional ventricular wall motion was assessed in two age groups of healthy volunteers, with an average age of 23 ± 3 and 66 ± 7 years old, respectively. The younger age group included 14 healthy subjects (all males), while the older age group included 9 subjects (6 males, 3 females). All subjects were healthy, non-smokers and underwent a medical assessment prior to acceptance in the study to exclude a history of cardiovascular and metabolic disease. All volunteers had normal ventricular dimensions and ejection fractions, normal LV mass and wall thickness, without evidence of resting left ventricular wall motion abnormalities. The study was conducted according to the principles of the Declaration of Helsinki and was approved by a local Oxfordshire Clinical Research Ethics Committee. Each subject provided written informed consent.
Cardiac MR examinations were performed using a 1.5-Tesla Siemens Sonata clinical scanner (Erlangen, Germany). Cine images for navigator gated high temporal resolution TPM were acquired using a black blood prepared segmented k-space gradient echo sequence (Jung et al. 2006a, b) with a temporal resolution of 13.8 ms (TR = 6.9 ms, flip angle = 15°, bandwidth = 650 Hz/pixel, FOV = 400 × 300 mm, matrix = 256 × 96), as previously described (Codreanu et al. 2010, 2011a, b). Three equidistant short-axis positions along the left ventricle were obtained. The basal slice was positioned parallel to the base of the heart and distal to the left ventricular (LV) outflow tract. Basal, mid-ventricular and apical slices were positioned 15 to 20 mm apart, depending on the heart size. Depending on the navigator efficiency of the respiratory gating, each short axis acquisition took approximately 3–5 min.
TPM analysis was performed with customized software (Matlab, version 6.5; Mathworks, Natick, MA, USA). The endocardial and epicardial borders were contoured manually for base, mid-ventricle and apex in each phase of the cardiac cycle, excluding papillary muscles. End systole for each slice was determined as the time point corresponding to the smallest LV cavity. The cardiac phases were subsequently calculated and normalized for the entire group based on their average duration during a cardiac cycle (Codreanu et al. 2010, 2011a, b). Global ventricular velocity time courses for radial, circumferential and longitudinal motion were calculated for each group by averaging over the entire segmentation mask. Time-to-peak ventricular velocities were calculated. In addition, graphical representations of all ventricular velocities during a cardiac cycle for individual LV segments were obtained. Global ventricular torsion and strain rates were also determined. The variables were tested for normal distribution and, after the assumption was met, a pooled t test was used to compare the values between the two groups. Otherwise, a non-parametric (Mann–Whitney) test was used. A p value of less than 0.05 was considered significant. The reproducibility of the measurements using navigator gated TPM was evaluated in a previous study, with repeated MR scans in a group of healthy volunteers performed 3 weeks apart (Codreanu et al. 2010). The method showed high reproducibility and a coefficient of variation for repeated values between 9.3 % and 11.4 % for calculated parameters.
Results
Radial motion
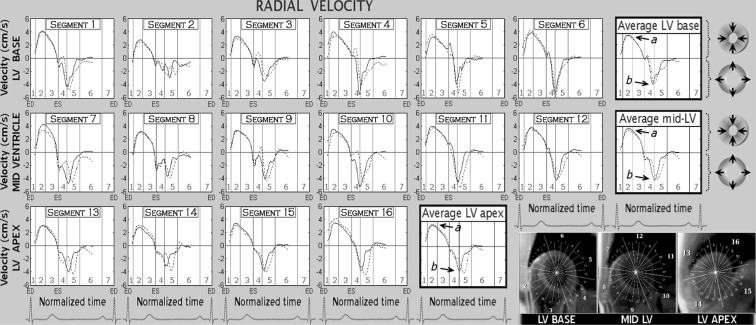
The peak systolic and diastolic radial velocities at the LV base, mid-ventricle and apex, as well as their corresponding peak times in both age groups, are provided in Table 1. Graphical representation of radial velocities for individual LV segments as well as average values for LV base, mid-ventricle and apex is shown in Fig. 1. Positive values reflect LV segmental motion towards the centre of the ventricle, whilst negative values reflect an outward motion.
Table 1.
Peak radial velocities and time-to-peak values in the younger and older age groups
| LV level and myocardial layer | 23 ± 3 years | 66 ± 7 years | p value | 23 ± 3 years | 66 ± 7 years | p value | |
|---|---|---|---|---|---|---|---|
| Peak systolic radial velocity (cm/s) | Time-to-peak values (% of ES) | ||||||
| LV base | Epicardial | 3.0 ± 0.2 | 3.1 ± 0.2 | 0.90 | 40.7 ± 1.6 | 42.7 ± 2.6 | 0.51 |
| Transmural | 3.5 ± 0.2 | 3.5 ± 0.2 | 0.91 | 39.7 ± 1.2 | 42.6 ± 2.6 | 0.39 | |
| Endocardial | 4.0 ± 0.2 | 4.0 ± 0.2 | 0.96 | 39.6 ± 1.2 | 43.6 ± 2.6 | 0.28 | |
| Mid-ventricle | Epicardial | 3.2 ± 0.1 | 3.2 ± 0.3 | 0.98 | 38.9 ± 1.3 | 39.7 ± 1.4 | 0.70 |
| Transmural | 3.7 ± 0.1 | 3.5 ± 0.3 | 0.68 | 39.7 ± 2.4 | 42.7 ± 2.1 | 0.39 | |
| Endocardial | 4.2 ± 0.1 | 4.0 ± 0.3 | 0.41 | 39.7 ± 1.7 | 44.7 ± 1.5 | 0.13 | |
| LV apex | Epicardial | 2.6 ± 0.2 | 3.2 ± 0.2 | 0.02 | 37.7 ± 1.9 | 38.6 ± 1.9 | 0.77 |
| Transmural | 3.1 ± 0.2 | 3.5 ± 0.2 | 0.13 | 38.7 ± 1.4 | 39.1 ± 1.7 | 0.85 | |
| Endocardial | 3.6 ± 0.2 | 3.8 ± 0.2 | 0.55 | 38.5 ± 0.9 | 40.1 ± 1.4 | 0.36 | |
| Peak diastolic radial velocity (cm/s) | Time-to-peak values (% of ED) | ||||||
| LV base | Epicardial | −4.5 ± 0.3 | −4.4 ± 0.4 | 0.96 | 15.6 ± 1.5 | 19.1 ± 1.8 | 0.16 |
| Transmural | −5.5 ± 0.3 | −5.5 ± 0.4 | 0.91 | 15.7 ± 1.5 | 18.8 ± 1.9 | 0.22 | |
| Endocardial | −6.6 ± 0.4 | −6.6 ± 0.5 | 0.88 | 15.6 ± 1.5 | 18.8 ± 1.7 | 0.19 | |
| Mid-ventricle | Epicardial | −4.6 ± 0.2 | −4.3 ± 0.2 | 0.41 | 19.8 ± 1.0 | 23.2 ± 1.2 | 0.06 |
| Transmural | −5.6 ± 0.3 | −5.2 ± 0.2 | 0.29 | 19.0 ± 1.1 | 23.5 ± 1.1 | 0.02 | |
| Endocardial | −6.6 ± 0.3 | −6.1 ± 0.3 | 0.17 | 18.8 ± 1.1 | 23.5 ± 1.1 | 0.01 | |
| LV apex | Epicardial | −4.9 ± 0.3 | −4.2 ± 0.3 | 0.14 | 19.3 ± 1.7 | 27.4 ± 1.4 | <0.01 |
| Transmural | −5.8 ± 0.4 | −4.7 ± 0.3 | 0.04 | 19.0 ± 1.6 | 27.4 ± 1.4 | <0.01 | |
| Endocardial | −6.9 ± 0.5 | −5.2 ± 0.4 | 0.01 | 19.0 ± 1.6 | 27.4 ± 1.4 | <0.01 | |
All values are presented as mean ± SEM
Fig. 1.
Radial velocity graphs for individual left ventricular segments during a cardiac cycle. The graphs represent average myocardial velocities for the two groups of volunteers (solid line, younger age group; dotted line, older age group). Positive values reflect inward motion toward the centre of the ventricle, while negative values show outward expansion. Average velocities for basal, mid and apical slices are shown in bold outline. The arrows show peak systolic (a) and peak diastolic (b) radial velocities. The right lower montage displays left ventricular short axis images of the base, mid ventricle and apex, divided into 16 segments according to American Heart Association segmentation model. ED end diastole, ES end systole, LV left ventricle, 1 isovolumetric contraction, 2 rapid ejection, 3 reduced ejection, 4 isovolumetric relaxation, 5 rapid filling, 6 diastasis, 7 atrial systole
There was no difference in peak systolic radial velocities noted at the LV base and mid-ventricle between the two study groups. At the LV apex, however, a paradoxical increase in peak systolic radial velocities was apparent in the older age group. This difference increased from the endocardium (3.6 cm/s in the younger age group versus 3.8 cm/s in the older age group) towards the epicardium (2.6 cm/s in the younger age group versus 3.2 cm/s in the older age group, p value = 0.02). There were no differences in time-to-peak systolic velocities (Table 1). Contrary to peak systolic values, peak diastolic radial velocities at the LV apex were much higher in the younger age group, the difference increasing towards the endocardial layer (−6.9 cm/s versus −5.2 cm/s, p value = 0.01).
The time-to-peak diastolic radial velocities were longer in the older age group, reaching statistical significance at the mid-ventricle and apex (Table 1). The difference substantially increased towards the LV apex (19 % of end-diastole in younger age group versus 27.4 % of end-diastole in older age group), showing a clear delay in LV radial expansion in older subjects. This delay in radial expansion can be seen in Fig. 1 (dotted graphs, shifted diastolic portion). Segmental graphs similarly showed a shift of the diastolic portion of the radial velocity curves in most ventricular segments, confirming a delay in LV radial expansion in older subjects (Fig. 1). Higher peak systolic values were noted in the older group in segments 14 (apical septal) and 16 (apical lateral), indicating a higher lateral (versus antero-posterior) contractility of the ventricular apex with increasing age.
Longitudinal motion
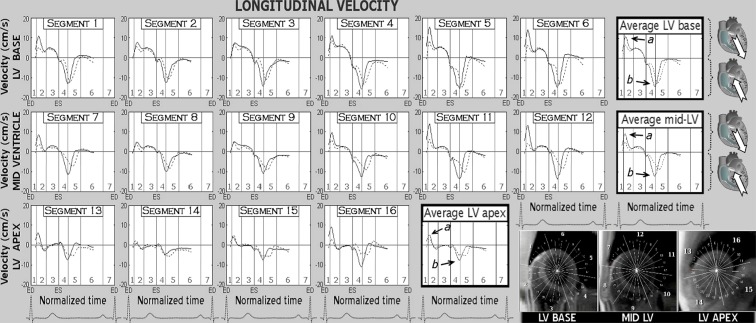
Peak longitudinal velocities with corresponding times-to-peak values for main LV slices are presented in Table 2, while the graphical display for individual LV segments and main LV slices are provided in Fig. 2. Positive values demonstrate downward motion along the longitudinal LV axis (i.e. towards the apex), and negative values show upward motion (i.e. towards the base). Both peak systolic and diastolic longitudinal velocities were significantly lower in the older age group, but the difference decreased towards the apex (Table 2). The time-to-peak diastolic values were longer in older subjects (Table 2), reaching statistical significance at the mid-ventricle (20.6 % versus 24.4 % of end-diastole, p value = 0.04) and LV apex (14.2 % versus 23.0 % of end-diastole, p value < 0.01).
Table 2.
Peak longitudinal velocities and time-to-peak values in the younger and older age groups
| LV level and myocardial layer | 23 ± 3 years | 66 ± 7 years | p value | 23 ± 3 years | 66 ± 7 years | p value | |
|---|---|---|---|---|---|---|---|
| Peak systolic longitudinal velocity (cm/s) | Time-to-peak values (% of ES) | ||||||
| LV base | Epicardial | 10.7 ± 0.9 | 7.8 ± 1.0 | 0.05 | 26.7 ± 0.6 | 26.0 ± 1.2 | 0.61 |
| Transmural | 10.9 ± 0.9 | 7.9 ± 0.9 | 0.04 | 26.6 ± 0.5 | 25.5 ± 1.1 | 0.33 | |
| Endocardial | 11.0 ± 0.9 | 8.0 ± 0.9 | 0.04 | 26.6 ± 0.5 | 25.0 ± 0.9 | 0.12 | |
| Mid-ventricle | Epicardial | 8.6 ± 0.9 | 6.0 ± 0.6 | 0.03 | 26.3 ± 0.6 | 25.1 ± 1.2 | 0.32 |
| Transmural | 8.5 ± 0.8 | 5.9 ± 0.5 | 0.02 | 26.6 ± 0.5 | 25.7 ± 1.3 | 0.46 | |
| Endocardial | 8.4 ± 0.8 | 5.8 ± 0.5 | 0.01 | 27.2 ± 0.6 | 25.7 ± 1.3 | 0.22 | |
| LV apex | Epicardial | 5.1 ± 0.5 | 3.9 ± 0.6 | 0.07 | 23.2 ± 0.6 | 25.8 ± 1.5 | 0.18 |
| Transmural | 4.9 ± 0.5 | 3.7 ± 0.6 | 0.08 | 22.8 ± 0.8 | 25.8 ± 1.5 | 0.13 | |
| Endocardial | 4.8 ± 0.5 | 3.5 ± 0.7 | 0.07 | 22.5 ± 1.0 | 25.8 ± 1.5 | 0.13 | |
| Peak diastolic longitudinal velocity (cm/s) | Time-to-peak values (% of ED) | ||||||
| LV base | Epicardial | −17.7 ± 0.3 | −13.6 ± 1.1 | <0.01 | 17.5 ± 1.7 | 20.9 ± 0.8 | 0.16 |
| Transmural | −18.3 ± 0.4 | −14.1 ± 1.1 | <0.01 | 17.3 ± 1.6 | 20.6 ± 0.8 | 0.17 | |
| Endocardial | −19.0 ± 0.4 | −14.7 ± 1.2 | <0.01 | 17.3 ± 1.6 | 20.1 ± 0.7 | 0.23 | |
| Mid-ventricle | Epicardial | −14.8 ± 0.8 | −10.6 ± 0.7 | <0.01 | 19.1 ± 1.3 | 24.4 ± 0.8 | <0.01 |
| Transmural | −15.2 ± 0.9 | −10.7 ± 0.7 | <0.01 | 20.6 ± 1.6 | 24.4 ± 0.8 | 0.04 | |
| Endocardial | −15.8 ± 1.1 | −10.7 ± 0.7 | <0.01 | 20.4 ± 1.5 | 24.1 ± 0.8 | 0.04 | |
| LV apex | Epicardial | −9.0 ± 1.3 | −7.0 ± 0.6 | 0.22 | 14.2 ± 1.0 | 23.0 ± 2.0 | <0.01 |
| Transmural | −9.1 ± 1.2 | −6.9 ± 0.8 | 0.15 | 14.2 ± 1.0 | 23.0 ± 2.0 | <0.01 | |
| Endocardial | −9.2 ± 1.2 | −6.8 ± 0.7 | 0.11 | 13.4 ± 1.1 | 20.7 ± 2.2 | <0.01 | |
All values are presented as mean ± SEM
Fig. 2.
Longitudinal velocity graphs for individual left ventricular segments during a cardiac cycle. The graphs represent average myocardial velocities for the two groups of volunteers (solid line, younger age group; dotted line, older age group). Positive values correspond to downward motion along the ventricular longitudinal axis (i.e. toward the apex), while negative values reflect upward displacement (i.e. toward the base). Average velocities for basal, mid and apical slices are shown in bold outline. The arrows show peak systolic (a) and peak diastolic (b) longitudinal velocities. The right lower montage displays left ventricular short axis images of the base, mid ventricle and apex, divided into 16 segments according to American Heart Association segmentation model. ED end diastole, ES end systole, LV left ventricle, 1 isovolumetric contraction, 2 rapid ejection, 3 reduced ejection, 4 isovolumetric relaxation, 5 rapid filling, 6 diastasis, 7 atrial systole
Segmental longitudinal velocity graphs similarly showed lower peak systolic and diastolic longitudinal velocities in most individual LV segments in the older age group. A shift in the diastolic portion of the longitudinal graphs was also noted, showing a delay of the longitudinal recoil motion in diastole and a longer time required for reaching peak diastolic longitudinal velocities in older subjects.
Rotational motion
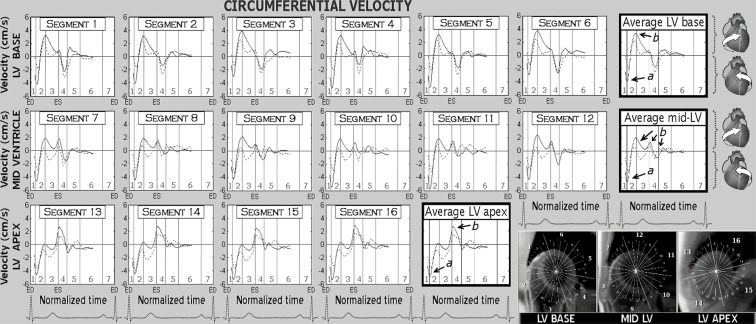
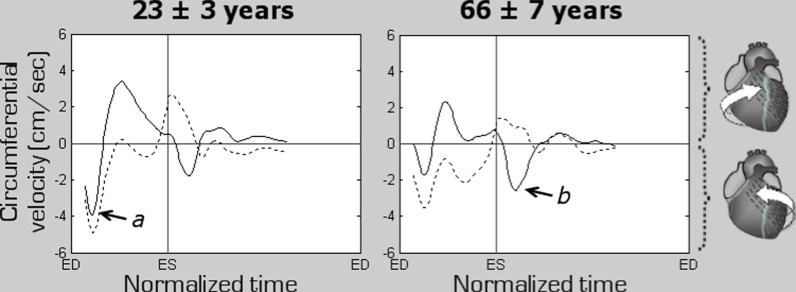
Peak clockwise and counter-clockwise circumferential velocities in both age groups are displayed in Table 3. Positive values indicate clockwise rotation, and negative values reflect counter-clockwise rotation as viewed from the apex. Circumferential velocity graphs for individual LV segments as well as average values for the LV base, mid-ventricle and apex are shown in Fig. 3. Both peak clockwise and counter-clockwise velocities were much lower at all ventricular levels in the older age group (Table 3). In addition, an altered pattern of ventricular motion was noted with increasing age. Thus, in younger subjects, the peak counter-clockwise velocity at the LV base was represented by the initial wave of counter-clockwise rotation of the entire ventricle at the beginning of systole (Fig. 4, arrow a), whilst in the older age group, it was represented by a recoil wave of ventricular untwisting in diastole (Fig. 4, arrow b). This representation of peak counter-clockwise velocities by different waves was also reflected in substantially different time-to-peak values (20.6 % of end-systole in younger subjects and 118.1 % of end-systole in older subjects, Table 3). The time-to-peak counter-clockwise velocities were longer in the older age group, the difference being statistically significant at all LV levels (Table 3).
Table 3.
Peak circumferential velocities and time-to-peak values in the younger and older age groups
| LV level and myocardial layer | 23 ± 3 years | 66 ± 7 years | p value | 23 ± 3 years | 66 ± 7 years | p value | |
|---|---|---|---|---|---|---|---|
| Peak clockwise velocity (cm/s) | Time-to-peak values (% of ES) | ||||||
| LV base | Epicardial | 3.8 ± 0.3 | 2.6 ± 0.3 | <0.01 | 50.7 ± 2.2 | 47.5 ± 1.1 | 0.33 |
| Transmural | 3.7 ± 0.3 | 2.5 ± 0.3 | <0.01 | 50.1 ± 2.1 | 47.5 ± 1.1 | 0.41 | |
| Endocardial | 3.7 ± 0.3 | 2.5 ± 0.3 | <0.01 | 50.0 ± 2.3 | 46.3 ± 1.1 | 0.27 | |
| Mid-ventricle | Epicardial | 2.9 ± 0.3 | 2.0 ± 0.2 | 0.04 | Data were inconsistent due to 3 separate peaks located at about 50–100–150 % of end systole (Fig. 3, arrows b) | ||
| Transmural | 2.6 ± 0.3 | 2.0 ± 0.3 | 0.09 | ||||
| Endocardial | 2.5 ± 0.3 | 2.2 ± 0.3 | 0.20 | ||||
| LV apex | Epicardial | 3.2 ± 0.3 | 2.3 ± 0.3 | 0.02 | 111.1 ± 3.4 | 109.9 ± 4.6 | 0.84 |
| Transmural | 3.2 ± 0.2 | 2.2 ± 0.2 | <0.01 | 110.7 ± 2.4 | 109.9 ± 4.7 | 0.86 | |
| Endocardial | 3.2 ± 0.2 | 2.2 ± 0.2 | <0.01 | 110.4 ± 2.4 | 111.4 ± 5.1 | 0.84 | |
| Peak counter-clockwise velocity (cm/s) | Time-to-peak values (% of ES) | ||||||
| LV base | Epicardial | −4.8 ± 0.5 | −3.3 ± 0.4 | 0.03 | 20.6 ± 0.5 | 117.6 ± 1.5 | <0.01 |
| Transmural | −4.6 ± 0.4 | −3.3 ± 0.4 | 0.04 | 20.6 ± 0.5 | 118.1 ± 1.5 | <0.01 | |
| Endocardial | −4.4 ± 0.4 | −3.4 ± 0.3 | 0.08 | 20.6 ± 0.5 | 118.6 ± 1.7 | <0.01 | |
| Mid-ventricle | Epicardial | −5.0 ± 0.5 | −3.5 ± 0.4 | 0.04 | 20.8 ± 0.7 | 25.3 ± 1.9 | 0.05 |
| Transmural | −4.8 ± 0.4 | −3.5 ± 0.3 | 0.04 | 20.8 ± 0.7 | 25.3 ± 1.9 | 0.05 | |
| Endocardial | −4.6 ± 0.4 | −3.5 ± 0.3 | 0.05 | 20.3 ± 0.6 | 25.3 ± 1.9 | 0.03 | |
| LV apex | Epicardial | −5.1 ± 0.4 | −3.8 ± 0.4 | 0.03 | 22.3 ± 0.8 | 26.2 ± 1.1 | 0.02 |
| Transmural | −4.8 ± 0.4 | −3.7 ± 0.4 | 0.05 | 23.6 ± 0.8 | 26.2 ± 1.1 | 0.05 | |
| Endocardial | −4.4 ± 0.4 | −3.7 ± 0.4 | 0.11 | 23.7 ± 1.1 | 26.7 ± 1.4 | 0.14 | |
All values are presented as mean ± SEM
Fig. 3.
Circumferential velocity graphs for individual left ventricular segments during a cardiac cycle. The graphs represent average myocardial velocities for the two groups of volunteers (solid line, younger age group; dotted line, older age group). Positive values correspond to clockwise rotation of the left ventricle as viewed from the apex, while negative values reflect counter-clockwise motion. Average velocities for basal, mid and apical slices are shown in bold outline. The right lower montage displays left ventricular short axis images of the base, mid ventricle and apex, divided into 16 segments according to American Heart Association segmentation model. The arrows show peak counter-clockwise (a) and peak clockwise (b) velocities. ED end diastole, ES end systole, LV left ventricle, 1 isovolumetric contraction, 2 rapid ejection, 3 reduced ejection, 4 isovolumetric relaxation, 5 rapid filling, 6 diastasis, 7 atrial systole
Fig. 4.
Changes in the rotational motion pattern of the left ventricular (LV) apex and base in the two age groups. Positive values correspond to clockwise rotation as viewed from the apex, while negative values reflect counter-clockwise motion. The graphs represent average myocardial velocities for the two groups of volunteers. Solid line shows rotational motion of the LV base, while dotted line shows rotational motion of the LV apex. The arrows point towards the peak counter-clockwise velocity of the ventricular base, which in younger subjects was represented by the initial wave of counter-clockwise rotation of the entire ventricle at the commencement of systole (a, solid graph), whilst in the older age group, it was represented by a recoil wave of ventricular untwisting in diastole (b, solid graph). ED end diastole, ES end systole
Segmental circumferential velocity graphs showed lower amplitudes of rotational motion with lower peak clockwise and counter-clockwise velocities in all LV segments with increasing age. An altered pattern of rotational motion was also observed in all segments (Figs. 3 and 4).
Global ventricular torsion rate and longitudinal strain rate
Peak ventricular torsion rates and peak longitudinal strain rates in both age groups are provided in Table 4. An increase in peak systolic torsion rate from 14.5 to 20.6°/cm/s (p value < 0.01) and in peak diastolic torsion rate from −14.1 to −25.2°/cm/s (p value < 0.01) was noted in older subjects. Graphical display of the global ventricular torsion rate and its variation during a cardiac cycle for both age groups is provided in Fig. 5. No statistical significance between longitudinal strain rates were noted in the two age groups (Table 4).
Table 4.
Peak torsion rate and peak longitudinal strain rate in the two age groups
| Young age, 23 ± 3 years | Older age, 66 ± 7 years | p value | |
|---|---|---|---|
| Peak systolic torsion rate (degrees/s/cm) | 14.5 ± 1.0 | 20.6 ± 2.0 | <0.01 |
| Peak diastolic torsion rate (degrees/s/cm) | −14.1 ± 1.3 | −25.2 ± 1.8 | <0.01 |
| Peak systolic longitudinal strain rate (s−1) | −2.0 ± 0.2 | −1.8 ± 0.2 | 0.50 |
| Peak diastolic longitudinal strain rate (s−1) | 3.3 ± 0.3 | 2.7 ± 0.2 | 0.11 |
All values are presented as mean ± SEM and are compared using paired t test
Fig. 5.
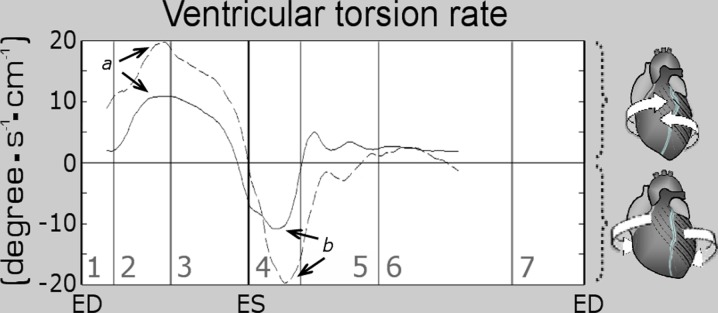
Changes in global left ventricular (LV) torsion rate in the two age groups. The graphs represent average values for the two groups of volunteers (solid line, younger age group; dashed line, older age group). Systolic torsion, expressed in positive values, results from a clockwise rotation of LV base and a simultaneous counter-clockwise rotation of the LV apex, while torsion rate represents the speed at which the twisting motion occurs. Subsequent recoil of twist deformation following ventricular repolarization is associated with a clockwise rotation of LV apex and a simultaneous counter-clockwise rotation of the LV base (reversed motions), translating into negative values of torsion rate. The arrows show peak systolic (a) and peak diastolic (b) torsion rates. ED end diastole, ES end systole, 1 isovolumetric contraction, 2 rapid ejection, 3 reduced ejection, 4 isovolumetric relaxation, 5 rapid filling, 6 diastasis, 7 atrial systole
Discussion
The results obtained using cardiac MR TPM demonstrated significant differences in radial, circumferential and longitudinal motions of the left ventricle in young and elderly individuals. These alterations may help explain the reduced diastolic function and augmented ejection fraction seen with ageing.
Radial motion
Concomitant changes in ventricular wall thickness occurring with radial motion are expected to cause the endocardial layer to travel a relatively higher distance towards the virtual centre of the left ventricle compared to the epicardial layer (Fig. 6). This likely accounts for higher systolic and diastolic radial velocities recorded at the endocardial compared to epicardial layer in both age groups (Table 1). The increase in peak systolic radial velocities at the LV apex in the older age group may appear paradoxical, though the findings are in agreement with previously reported increases in LV ejection fraction with ageing (Bauer et al. 1988; Klein et al. 1999; Nikitin et al. 2006). The lower peak diastolic radial velocities, with a longer time-to-peak values (Table 1), most pronounced at the apex, are consistent with reduced diastolic function with ageing. The progressive reduction of myocardial relaxation with ageing was firstly described by experimental studies (Kaufman et al. 1990) and subsequently confirmed by invasive (Hirota 1980) and non-invasive reports in humans (Spirito et al. 1986; Sagie et al. 1993). Similar changes, showing significantly delayed and reduced myocardial relaxation in older healthy individuals, have been reported using MR tagging (Oxenham et al. 2003; Foll et al. 2009). The findings may reflect a reduction of myocardial compliance with increasing age (Bauer et al. 1988), commonly attributed to ultrastructural modification of cardiomyocytes, involving calcium cellular transport by the sarcoplasmic reticulum (Kaufman et al. 1990). Shifted curves on the radial velocity graphs in diastole (Fig. 1) are also consistent with delayed radial expansion and reduced myocardial compliance in the older age group. Further studies are needed to provide a mechanism for these changes, though age-related decrease in Ca2+ uptake by the sarcoplasmic reticulum (Pugh and Wei 2001) and an increase in myocardial stiffness (Villari et al. 1997) are potential hypotheses to explain why myocardial relaxation is slower in the elderly.
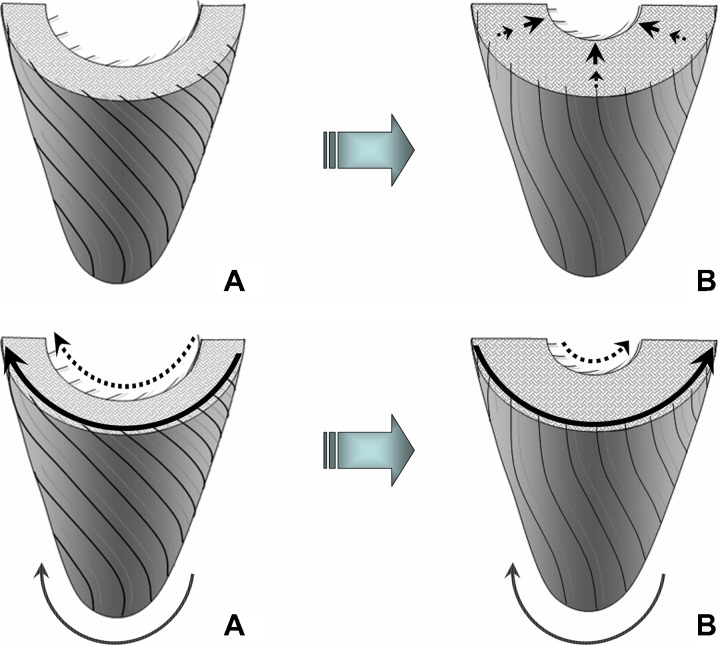
Fig. 6.
Schematic representation of radial and rotational LV motions during systole: (A) beginning of systole and (B) mid systole. The endocardial points are expected to travel a higher distance per time unit during their radial motion due to concomitant changes in LV wall thickness (upper panel), while the epicardial points are expected to travel a higher distance per time unit during their circumferential motion due to a higher rotational orbit (lower panel). This may explain the higher systolic and diastolic radial velocities, but slightly lower rotational velocities for the endocardial compared to epicardial layer noted in both age groups (Tables 1 and 3). Of note is that at the beginning of systole, the entire LV rotates counter-clockwise as viewed from the apex (A, lower panel). Subsequently, the LV apex continues its counter-clockwise motion, while the LV base rotates in an opposite (clockwise) direction, resulting in LV twisting (B, lower panel)
Longitudinal motion
A significant difference between the two age groups was found in ventricular longitudinal motion, suggesting longitudinal velocities may be a sensitive marker for the evaluation of myocardial contractility with ageing. The velocity of the downward displacement of the left ventricle along the longitudinal LV axis during systole, when the entire ventricle is pushed in an opposite direction to the ejected blood, is expected to be proportional to the overall force of ejection, including the force of radial motion and LV twisting. Subsequently, this directly affects the force of the recoil motion in diastole, i.e. peak diastolic longitudinal velocity, when the entire ventricle is being pulled back to its initial location by the stretched great vessels and elastic structures that attach the heart to mediastinum. Consequently, peak diastolic longitudinal velocity may serve as a measure of global ventricular contractility, the resultant recoil in diastole being proportional to the sum of all applied forces during systole (Codreanu et al. 2010). These findings are consistent with previous studies reporting similar differences in longitudinal LV motion in older patients as well as in patients who might have higher risk of developing heart failure with normal ejection fraction (Nikitin et al. 2006; Innelli et al. 2008; Tan et al. 2009).
The age-dependent deterioration in longitudinal systolic function, observed in healthy subjects, has also been ascribed to the smaller left ventricular volumes and higher wall thickness occurring with ageing (Nikitin et al. 2006). Since the LV apex shows little motion along the longitudinal axis due to concomitant LV shortening/twisting in systole and elongation/untwisting in diastole (Codreanu et al. 2010), the biggest difference in longitudinal motion can be expected at the LV base (Table 2).
Rotational motion
The lower peak clockwise and counter-clockwise velocities involving all ventricular segments in the older age group showed limitations in LV rotational motions in the elderly. These findings were confirmed by much lower amplitudes of rotational motion on all circumferential velocity graphs. Of particular note, the peak counter-clockwise velocity at the LV base in younger subjects was represented by an initial wave of counter-clockwise rotation of the entire ventricle at the commencement of systole (Fig. 4, arrow a), whilst in the older age group, it was represented by a recoil wave of ventricular untwisting in diastole (Fig. 4, arrow b). LV apical rotation in the older age group was affected even to a higher degree, altering also the undulating pattern of recoil motions in diastole. These changes translated into an overall altered pattern of rotational motions of the LV apex and base against each other, affecting both ventricular twisting and untwisting, as shown in Fig. 4. Since the rotational motion for the epicardial layer occurs at a slightly higher orbit compared to endocardial layer, the epicardial points are expected to travel a relatively higher distance per time unit during their circumferential movement (Fig. 6). This likely accounts for the slightly higher rotational velocities recorded at the epicardial compared to endocardial layer in both age groups (Table 3).
Global torsion and longitudinal strain rates
The higher peak systolic and diastolic torsion rates obtained in the older age group are in agreement with previous tissue Doppler, speckle-tracking echocardiography (Nakai et al. 2006; Takeuchi et al. 2006; van Dalen et al. 2008) and cardiac MR tagging studies (Matter et al. 1996; Fonseca et al. 2003; Oxenham et al. 2003). Greater peak torsional shear strains and greater ventricular torsion rates in the older group are frequently explained by an increase in concentric hypertrophy (mass-to-volume ratio) since LV torsion is known to be increased with hypertrophy (Young et al. 1994; Stuber et al. 1999; Fonseca et al. 2003). Another cause for the augmented LV torsion with ageing is commonly attributed to subendocardial underperfusion, which results from an increased LV end diastolic pressure or subendocardial fibrosis. This, in turn, diminishes the counteractive motion of the subendocardial fibres against the subepicardial fibres (Lumens et al. 2006). The slightly lower values for longitudinal strain rates in the older age group (Table 4) are consistent with reports of systolic and diastolic longitudinal strain rates decreasing with age (Fonseca et al. 2003; Kuznetsova et al. 2008; Zghal et al. 2011). In the current study, however, the difference did not reach statistical significance (p values of 0.11 and 0.50). Peak rates of relaxation for both circumferential and longitudinal strains have been inversely correlated with systolic and diastolic blood pressure values and with the ratio of LV mass to end diastolic volume (EDV), suggesting that relaxation rates are influenced by concentric hypertrophy (Fonseca et al. 2003). Consequently, it has been suggested that reduced relaxation rate is directly related to the reduced diastolic function observed in the normal ageing process (Fonseca et al. 2003).
Overall, our findings are in agreement with recent results obtained using TPM technique by Foll et al. (2009). Additional correlations with gender, LV geometry, global function, heart rate or blood pressure have already been reported by Foll et al. (2009) and were beyond the scope of this study.
Study limitations
Only two age groups were included in this one time point study. A longitudinal study examining the same patients over an extended period of time is required to definitively examine age-related changes in cardiac physiology. The cardiac phases were calculated and normalized for the entire group based on their average duration during systole and diastole and not on the timing of cardiac valves closure and opening, which was not recorded. The phases are provided for general orientation and even when slightly displaced would not affect data interpretation.
Conclusions
Navigator gated TPM has allowed a detailed assessment of baseline parameters reflecting global and regional ventricular wall motion in young and elderly individuals. An increased global LV torsion rate and a significant decrease in longitudinal motion were the most prominent findings in elderly individuals. A paradoxical increase in peak radial velocities at the LV apex is consistent with prior reports of increased ejection fraction with ageing. Decreased circumferential velocities and an altered pattern of rotational motion are suggestive of limitations in LV twisting and untwisting with increasing age. Our data suggest that longitudinal motion may represent a sensitive parameter in evaluating global LV contractility, which is consistent with prior reports that the effect of ageing on left ventricular longitudinal myocardial function may precede the age-related effects on LV chamber systolic function (Innelli et al. 2008). These results highlight the importance of understanding normal age-related changes in LV motion, and these cardiac MR parameters may be important to help differentiate the effect of ageing from cardiac pathology.
Acknowledgments
This research was funded by the British Heart Foundation and was supported by the Oxford Partnership Comprehensive Biomedical Research Centre, with funding from the Department of Health's National Institute for Health Research Biomedical Research Centre. This work was additionally supported by the Australian Heart Foundation.
References
- Bauer R, Busch U, van de Flierdt E, Stettmeier H, Raab W, Langhammer HR, Pabst HW. Age-related heart function in patients with healthy hearts. Z Kardiol. 1988;77(10):632–641. [PubMed] [Google Scholar]
- Codreanu I, Robson MD, Golding SJ, Jung BA, Clarke K, Holloway CJ. Longitudinally and circumferentially directed movements of the left ventricle studied by cardiovascular magnetic resonance phase contrast velocity mapping. J Cardiovasc Magn Reson. 2010;12(1):48. doi: 10.1186/1532-429X-12-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codreanu I, Pegg TJ, Selvanayagam JB, Robson MD, Rider OJ, Dasanu CA, Jung BA, Taggart DP, Clarke K, Holloway CJ. Details of left ventricular remodeling and the mechanism of paradoxical ventricular septal motion after coronary artery bypass graft surgery. J Invasive Cardiol. 2011;23(7):276–282. [PubMed] [Google Scholar]
- Codreanu I, Robson MD, Rider OJ, Pegg TJ, Jung BA, Dasanu CA, Clarke K, Holloway CJ. Chasing the reflected wave back into the heart: a new hypothesis while the jury is still out. Vasc Health Risk Manag. 2011;7:365–373. doi: 10.2147/VHRM.S20845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foll D, Jung B, Schilli E, Staehle F, Geibel A, Hennig J, Bode C, Markl M. Magnetic resonance tissue phase mapping of myocardial motion: new insight in age and gender. Circ Cardiovasc Imaging. 2009;3(1):54–64. doi: 10.1161/CIRCIMAGING.108.813857. [DOI] [PubMed] [Google Scholar]
- Fonseca CG, Oxenham HC, Cowan BR, Occleshaw CJ, Young AA. Aging alters patterns of regional nonuniformity in LV strain relaxation: a 3-D MR tissue tagging study. Am J Physiol Heart Circ Physiol. 2003;285(2):H621–H630. doi: 10.1152/ajpheart.01063.2002. [DOI] [PubMed] [Google Scholar]
- Hirota Y. A clinical study of left ventricular relaxation. Circulation. 1980;62(4):756–763. doi: 10.1161/01.CIR.62.4.756. [DOI] [PubMed] [Google Scholar]
- Innelli P, Sanchez R, Marra F, Esposito R, Galderisi M. The impact of aging on left ventricular longitudinal function in healthy subjects: a pulsed tissue Doppler study. Eur J Echocardiogr. 2008;9(2):241–249. doi: 10.1016/j.euje.2007.03.044. [DOI] [PubMed] [Google Scholar]
- Jung B, Foll D, Bottler P, Petersen S, Hennig J, Markl M. Detailed analysis of myocardial motion in volunteers and patients using high-temporal-resolution MR tissue phase mapping. J Magn Reson Imaging. 2006;24(5):1033–1039. doi: 10.1002/jmri.20703. [DOI] [PubMed] [Google Scholar]
- Jung B, Markl M, Foll D, Hennig J. Investigating myocardial motion by MRI using tissue phase mapping. Eur J Cardiothorac Surg. 2006;29(Suppl 1):S150–S157. doi: 10.1016/j.ejcts.2006.02.066. [DOI] [PubMed] [Google Scholar]
- Kaufman TM, Horton JW, White DJ, Mahony L. Age-related changes in myocardial relaxation and sarcoplasmic reticulum function. Am J Physiol. 1990;259(2 Pt 2):H309–H316. doi: 10.1152/ajpheart.1990.259.2.H309. [DOI] [PubMed] [Google Scholar]
- Klein AL, Leung DY, Murray RD, Urban LH, Bailey KR, Tajik AJ. Effects of age and physiologic variables on right ventricular filling dynamics in normal subjects. Am J Cardiol. 1999;84(4):440–448. doi: 10.1016/S0002-9149(99)00330-6. [DOI] [PubMed] [Google Scholar]
- Kuznetsova T, Herbots L, Richart T, D'Hooge J, Thijs L, Fagard RH, Herregods MC, Staessen JA. Left ventricular strain and strain rate in a general population. Eur Heart J. 2008;29(16):2014–2023. doi: 10.1093/eurheartj/ehn280. [DOI] [PubMed] [Google Scholar]
- Lumens J, Delhaas T, Arts T, Cowan BR, Young AA. Impaired subendocardial contractile myofiber function in asymptomatic aged humans, as detected using MRI. Am J Physiol Heart Circ Physiol. 2006;291(4):H1573–H1579. doi: 10.1152/ajpheart.00074.2006. [DOI] [PubMed] [Google Scholar]
- Matter C, Nagel E, Stuber M, Boesiger P, Hess OM. Assessment of systolic and diastolic LV function by MR myocardial tagging. Basic Res Cardiol. 1996;91(Suppl 2):23–28. doi: 10.1007/BF00795358. [DOI] [PubMed] [Google Scholar]
- Nakai H, Takeuchi M, Nishikage T, Kokumai M, Otani S, Lang RM. Effect of aging on twist-displacement loop by 2-dimensional speckle tracking imaging. J Am Soc Echocardiogr. 2006;19(7):880–885. doi: 10.1016/j.echo.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Nikitin NP, Loh PH, de Silva R, Witte KK, Lukaschuk EI, Parker A, Farnsworth TA, Alamgir FM, Clark AL, Cleland JG. Left ventricular morphology, global and longitudinal function in normal older individuals: a cardiac magnetic resonance study. Int J Cardiol. 2006;108(1):76–83. doi: 10.1016/j.ijcard.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Oxenham HC, Young AA, Cowan BR, Gentles TL, Occleshaw CJ, Fonseca CG, Doughty RN, Sharpe N. Age-related changes in myocardial relaxation using three-dimensional tagged magnetic resonance imaging. J Cardiovasc Magn Reson. 2003;5(3):421–430. doi: 10.1081/JCMR-120022258. [DOI] [PubMed] [Google Scholar]
- Pugh KG, Wei JY. Clinical implications of physiological changes in the aging heart. Drugs Aging. 2001;18(4):263–276. doi: 10.2165/00002512-200118040-00004. [DOI] [PubMed] [Google Scholar]
- Sagie A, Benjamin EJ, Galderisi M, Larson MG, Evans JC, Fuller DL, Lehman B, Levy D. Reference values for Doppler indexes of left ventricular diastolic filling in the elderly. J Am Soc Echocardiogr. 1993;6(6):570–576. doi: 10.1016/s0894-7317(14)80174-0. [DOI] [PubMed] [Google Scholar]
- Spirito P, Maron BJ, Bonow RO. Noninvasive assessment of left ventricular diastolic function: comparative analysis of Doppler echocardiographic and radionuclide angiographic techniques. J Am Coll Cardiol. 1986;7(3):518–526. doi: 10.1016/S0735-1097(86)80461-2. [DOI] [PubMed] [Google Scholar]
- Stuber M, Scheidegger MB, Fischer SE, Nagel E, Steinemann F, Hess OM, Boesiger P. Alterations in the local myocardial motion pattern in patients suffering from pressure overload due to aortic stenosis. Circulation. 1999;100(4):361–368. doi: 10.1161/01.CIR.100.4.361. [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Nakai H, Kokumai M, Nishikage T, Otani S, Lang RM. Age-related changes in left ventricular twist assessed by two-dimensional speckle-tracking imaging. J Am Soc Echocardiogr. 2006;19(9):1077–1084. doi: 10.1016/j.echo.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Tan YT, Wenzelburger F, Lee E, Heatlie G, Leyva F, Patel K, Frenneaux M, Sanderson JE. The pathophysiology of heart failure with normal ejection fraction: exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J Am Coll Cardiol. 2009;54(1):36–46. doi: 10.1016/j.jacc.2009.03.037. [DOI] [PubMed] [Google Scholar]
- van Dalen BM, Soliman OI, Vletter WB, ten Cate FJ, Geleijnse ML. Age-related changes in the biomechanics of left ventricular twist measured by speckle tracking echocardiography. Am J Physiol Heart Circ Physiol. 2008;295(4):H1705–H1711. doi: 10.1152/ajpheart.00513.2008. [DOI] [PubMed] [Google Scholar]
- Villari B, Vassalli G, Schneider J, Chiariello M, Hess OM. Age dependency of left ventricular diastolic function in pressure overload hypertrophy. J Am Coll Cardiol. 1997;29(1):181–186. doi: 10.1016/S0735-1097(96)00440-8. [DOI] [PubMed] [Google Scholar]
- Young AA, Kramer CM, Ferrari VA, Axel L, Reichek N. Three-dimensional left ventricular deformation in hypertrophic cardiomyopathy. Circulation. 1994;90(2):854–867. doi: 10.1161/01.CIR.90.2.854. [DOI] [PubMed] [Google Scholar]
- Zghal F, Bougteb H, Réant P, Lafitte S, Roudaut R. Assessing global and regional left ventricular myocardial function in elderly patients using the bidimensional strain method. Echocardiography. 2011;28(9):978–982. doi: 10.1111/j.1540-8175.2011.01476.x. [DOI] [PubMed] [Google Scholar]