Abstract
Neuromusculoskeletal (physical) frailty is an aging-attributable biomedical issue of extremely high import, from both public health and individual perspectives. Yet, it is rarely studied in nonhuman research subjects and very rarely studied in animals with extended longevity. In an effort to address this relatively neglected area, we have conducted a longitudinal investigation of the neuromusculoskeletal healthspan in mice with two senescence-slowing interventions: growth hormone (GH) resistance, produced by GH receptor “knockout” (GHR-KO), and caloric restriction (CR). We report marked improvements in the retention of strength, balance, and motor coordination by the longevity-conferring GHR/BP gene disruption, CR regimen, or a combination of the two. Specifically, GHR-KO mice exhibit superior grip strength, balance, and motor coordination at middle age, and CR mice display superior grip strength at middle age. The advantageous effects established by middle-age are more pronounced in old-age, and these robust alterations are, generally, not gender-specific. Thus, we show that genetic and/or dietary interventions that engender longevity are also beneficial for the retention of neuromusculoskeletal health and functionality. The translational knowledge to be gained from subsequent molecular or histological investigations of these models of preserved functionality and decelerated senescence is potentially relevant to the efforts to reduce the specter of fear, falls, fracture, and frailty in the elderly.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-013-9551-x) contains supplementary material, which is available to authorized users.
Keywords: Healthspan, Longevity, Caloric restriction, Growth hormone/somatotrophin, Neuromusculoskeletal function
Introduction
Frailty is a multi-faceted consequence of the syndrome of senescence, comprising declines in endocrine, skeletal muscle, cardiovascular, pulmonary, immunologic, bone, and neurologic (including cognitive and psychological) systems and resulting in declining physiological reserve (the ability to compensate for aging-induced declines in functionality), increasing dysregulation of homeostasis, and adverse health outcomes (including mortality) (Walston et al. 2006; Levers et al. 2006; Ahmed et al. 2007). Clinically, the phenotype of frailty manifests as multi-system pathologies characterized by unintentional weight loss, low physical activity, global weakness with low muscle strength, facile fatigability/exhaustion, overall slowness (particularly of gait), and cognitive impairment, among others (Fried et al. 2001; Topinková 2008; Avila-Funes et al. 2009).
Increasing physical disability with age is a prevalent and conspicuous hallmark of senescence in organisms ranging from the nematode Caenorhabditis elegans (Herndon et al. 2002; Kirkwood and Finch 2002) to human beings (recently reviewed in Topinková 2008; Lang et al. 2009; Weiss 2011; Ko 2011). This condition is devastating for two major reasons: (1) it reduces the ability to function in the vast majority of occupational settings, ultimately precluding regular employment; (2) it marginalizes the ability to engage in activities of daily living (ADLs) and many recreational activities, thus impinging greatly on quality of life. Therefore, whether for socioeconomic or psychological reasons, this facet of declining healthspan (the period of life during which an organism is functionally able to sustain independent existence and is free from substantial morbidity) is very important to the ever-burgeoning proportion of the senescing population (Kinsella and Wan 2009); any approaches (pharmacological, behavioral, or otherwise) that would facilitate understanding and intervening in this physiological decline and to engender greater retention of physical ability with advancing age are both socioeconomically (Kinsella and Wan 2009) and individually (Strawbridge et al. 2002) crucial.
The growth hormone receptor/binding protein gene-disrupted (“knockout”) (GHR-KO) mouse (Zhou et al. 1997) is a model of, among other human health concerns, decelerated and ameliorated aging due not solely to its markedly enhanced longevity above the already robust life expectancy of its littermate control (Coschigano et al. 2000; Coschigano et al. 2003), but also to its reduced rate of development of neoplasia (Ikeno et al. 2009) and its retention of memory capacity (Kinney et al. 2001; Kinney-Forshee et al. 2004; Bartke 2005). Similarly, animals maintained under dietary conditions of “undernutrition without malnutrition,” in which their caloric intake is restricted while maintaining sufficient macro- and micronutrient intake for optimal physiological functioning, have been extensively studied for manifold healthspan (Moreschi 1909; Rous 1914) and lifespan (McCay et al. 1935; Mercken et al. 2011; Roth and Polotsky 2012) benefits. These calorically restricted animals constitute the preeminent paragon of slow-aging animals.
Most studies of neuromusculoskeletal issues in experimental gerontology deal with the common, well-documented, aging-associated decline in neuromuscular or skeletal strength or performance (Marzetti and Leeuwenburgh 2006; Parks et al. 2012; Graber et al. 2013). Save for studies with calorically restricted animals (Marzetti and Leeuwenburgh 2006; Landi et al. 2010; Mercken et al. 2011) or with exercise (Marzetti and Leeuwenburgh 2006; Liu and Fielding 2011; Mercken et al. 2011), evidence of genetic or environmental factors that might improve physical functioning is limited, and, to the best of our knowledge, no combinatorial analyses of the interaction of two different factors have been reported.
Therefore, we decided to test, longitudinally, the effects of GH receptor deletion, 30 % caloric restriction (CR), or both on neuromusculoskeletal performance at middle or old age. We conscientiously chose to employ naturalistic assessments of relative, as contrasted from absolute, functionality as these are the manners that more closely reflect the ADLs that elder humans are challenged by. As physical frailty is a characteristic decrement of aging, we were interested in determining whether the extension of lifespan by the employed genetic and dietary interventions is associated with extended neuromusculoskeletal healthspan in these slow-aging mammals.
Materials and methodologies
Animal husbandry
GHR-KO mice and their littermate controls (derived from 129/Ola founders, provided by Dr. J. J. Kopchick (Ohio University, Athens, OH, USA), and outbred to Balb/c, C57Bl/6 J, and C3H/HeJ strains) were bred in a closed colony, housed under standard conditions (12-h light/12-h dark cycle and 20–23 °C), and fed Lab Diet Formula 5001 (23 % protein, 4.5 % fat, 6 % fiber) (Nestlé Purina, St. Louis, MO, USA). Although lacking the methodological benefits of “reproducible genetic heterogeneity” (Miller et al. 1999), this stock possesses considerably greater genetic variation, which correlates with broad-based health and life expectancy, than an inbred strain. All animals were fed AL for the first ∼ 18–27 weeks of life; thereafter, the mice were either fed AL (AL groups) or 30 % of AL (CR groups). The mice were weighed in the morning after a feeding day, approximately 16–20 h after the CR groups had been fed. Animal protocols were approved by the Animal Care and Use Committee of Southern Illinois University School of Medicine.
Age-grade classification
Age staging was based on a combination of (1) quantitative extrapolation from prior survivorship data (Bonkowski et al. 2006b), (2) presence/appearance of aging-associated wizening (as represented quantitatively by declining body weight), and (3) spontaneous, testing-independent (and thus, presumably) aging-resultant declining vivacity and/or increasing mortality.
Expounding on the chief age staging criterion above, young adulthood is marked by at least 90 % of reproductively competent negative control subjects being alive, middle age is the period between when approximately 90 % of the control subjects are still alive and median survivorship, old age is the period between median survivorship and when approximately 10 % of the subjects are alive, and oldest old age is designated as the period when ≤10 % of the controls remain.
As for the second criterion, young adulthood is characterized by steady weight gain at a rate below that of juveniles, the onset of wizening for the negative control subjects demarks the difference between middle age and old age, and the rate of wizening tends to increase in oldest old age. The third criterion ensures that demographic expectations concur with animal husbandry observations.
Functional observation battery
Mice were evaluated for general health employing a functional observation battery before each set of neuromusculoskeletal tests. First, observation of the mouse in its home cage was used to gauge whether the animal showed signs of illness, such as initial posture, salivation, lacrimation, fur appearance, or vocalization (Supplemental Table 1) (Crawley 2007). Second, 5-min open-field tests were administered to monitor for impaired mobility/gait (Supplemental Table 1) (Crawley 2007). Based on these analyses, only ostensibly healthy subjects were subjected to the neuromusculoskeletal assessments.
Assays of neuromusculoskeletal impairment
Wire hang (grip strength) test
A standard wire cage lid was held horizontally, and a mouse was placed on top of it. The cage lid was then lightly shaken three times, which should cause a standard, healthy mouse to grip the wire. The lid was then rotated 180° along its horizontal axis, turning the mouse completely upside-down, and held approximately 20 cm above the bedding in the cage. Timing with a stopwatch began as soon as the mouse was inverted, to measure how long the mouse maintained its grip, up to 60 s (Supplemental Table 2) (Crawley 2007). Lower neuromusculoskeletal scores mark superior grip strength; testing of relative performance on naturalistic tasks, such as this, result in a perfect score of 1 being the score for a healthy, young-adult mouse (Crawley 2007; Arum et al. 2009).
Inclining rod (balance/motor coordination) test
Mice were placed in the middle of a 1-in-diameter, 40-cm-long metal rod that began at a horizontal start point of 0°. The rod was steadily raised at one end so that it ultimately angled up to 60° to the horizon. Measurements will be made from 10° to 60° in 10° increments (Supplemental Table 2) (Crawley 2007). Lower neuromusculoskeletal scoring indicates enhanced maintenance of equilibrium; testing of relative performance on naturalistic tasks, such as this, result in a perfect score of 1 being the score for a healthy, young-adult mouse (Crawley 2007; Arum et al. 2009).
Inverted screen (motor coordination/agility) test
A 2-ft2 wire mesh screen was held horizontally, a mouse subject was placed at the center of the screen, and the screen was tilted at a 45° angle to the horizon with the subject facing upwards. The screen was then gingerly rotated 180° along its horizontal plane so that the mouse was facing downward at a distance of 20 cm above the cage’s bedding. A physically capable mouse will innately be inclined to turn around 180°, while holding onto the screen, so that it faces upwards, and then to climb upwards. Timing began as soon as the subject was facing downward (Supplemental Table 2) (Crawley 2007). For neuromusculoskeletal scoring on this task, lower scores denote better motor coordination and/or agility; testing of relative performance on naturalistic tasks, such as this, result in a perfect score of 1 being the score for a healthy, young-adult mouse (Crawley 2007; Arum et al. 2009).
Statistical analysis and data presentation
Body weight gain data were contrasted with analysis of variance for repeated measures. Discrete data were compared with analysis of variance, followed by Dunnett’s t test post hoc test, with the littermate controls on AL (N on AL) designated as the reference group (SPSS 17, SPSS, Inc., Chicago, IL, USA). All data were analyzed in a gender-specific fashion. Graphs were generated with Excel (Microsoft, Redmond, WA, USA). All measures of central tendency are arithmetic means, and all depictions of variation (error bars) represent standard deviations (SD), with SD being employed as it is the statistically appropriate method of representing the variation in a dataset (Glantz 2002).
Sixteen groups of animal subjects (male or female, GHR-KO (KO) mice or their littermate controls (GHR-N (N)), fed ad libitum (AL) or on 30 % CR, middle-aged (19 ± 2 months of age) or old (32 ± 2 months of age)) were used to assess naturally occurring, aging-associated declines in various components of neuromusculoskeletal capacity, with emphases on strength, balance, motor coordination, and agility. Of particular note, these tests are designed to test an animal subject’s ability to manipulate its own body under some challenging, yet naturalistic, condition (relative performance), not the subject’s ability to manipulate a foreign object (absolute performance); thus, results of these tests are chiefly independent of the size of the subject.
Results
Physical parameters regarding general health and the response to caloric restriction
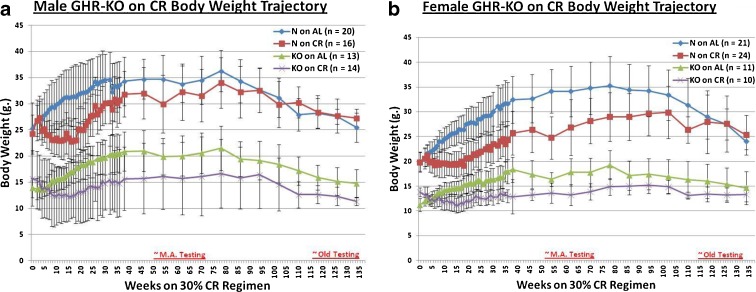
Body weight trajectory plots, from the onset of the restriction to the last testing date, show the expected weight gain-restricting effect of 30 % caloric restriction in both males (mutants and littermate controls) and females (both phenotypes) (p < 0.001 for all four pairwise comparisons of AL mice vs. CR mice, Fig. 1a, b, respectively). Each animal was tested for general health in appearance, behavior, and basic physical functionality prior to each test (Supplemental Table 1). Those showing markers of poor or suspect health were not included in this study.
Fig. 1.
Graphical representation of experimental design, showing body weight trajectories for cohorts assessed longitudinally. a Male N mice on AL diet weigh substantially more than male KO mice on AL, and CR results in an attenuation of body weight gain for each phenotype. b Female N mice on AL weigh significantly more than female KO mice on AL, and CR results in a restraint of body weight gain for each phenotype
Strength
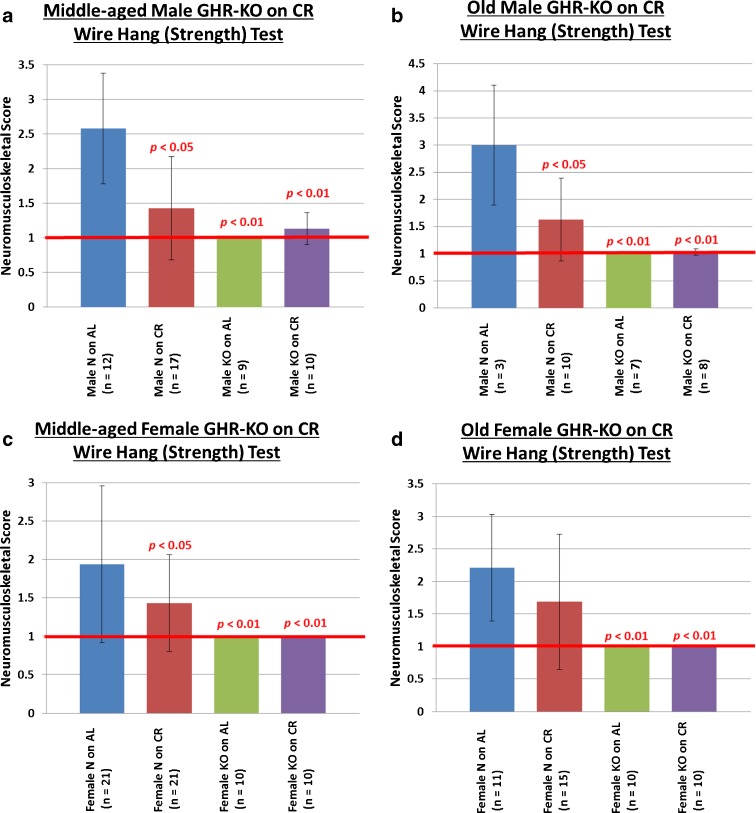
In human beings, higher (manual) grip strength correlates with higher bone mineral density and better general health in multiple clinical studies (Rantanen 2003; Bohannon 2008; Marin et al. 2010). To test basic grip strength, we performed a wire-hang (grip strength) test in which subjects were required to hang from a 0.25-cm-diameter metal rod by their limbs for at least 1 min. For middle-aged mice, this revealed that middle-aged N mice on an AL diet show the expected aging-associated deviation from perfect performance (defined as a score of 1), while N mice on CR have superior strength (p < 0.05 for males and p < 0.05 for females) (Fig. 2a, c, respectively). Middle-aged KO mice on either diet showed little or no impairment for the strength task (Fig. 2a (males): N on AL vs. KO on AL p < 0.01, N on AL vs. KO on CR p < 0.01; Fig. 2c (females): N on AL vs. KO on AL p < 0.01, N on AL vs. KO on CR p < 0.01).
Fig. 2.
Beneficial neuromusculoskeletal effects of the GHR/BP gene disruption and/or caloric restriction on grip strength. a Middle-aged male mice enjoy superior grip strength due to either the GHR/BP gene disruption or CR. b Old male mice enjoy superior grip strength due to either the GHR/BP gene disruption or CR. c Middle-aged female KO mice exhibit perfect grip strength, whereas their N counterparts show deficiencies. d Old female KO mice exhibit perfect grip strength, whereas their N counterparts show deficiencies. (All p values are for comparisons between the littermate control on AL (N on AL) group and the group whose bar the probability value is written over. The red horizontal bars at neuromusculoskeletal scores of 1 indicate the performance level of young-adult animals.)
Balance/motor coordination
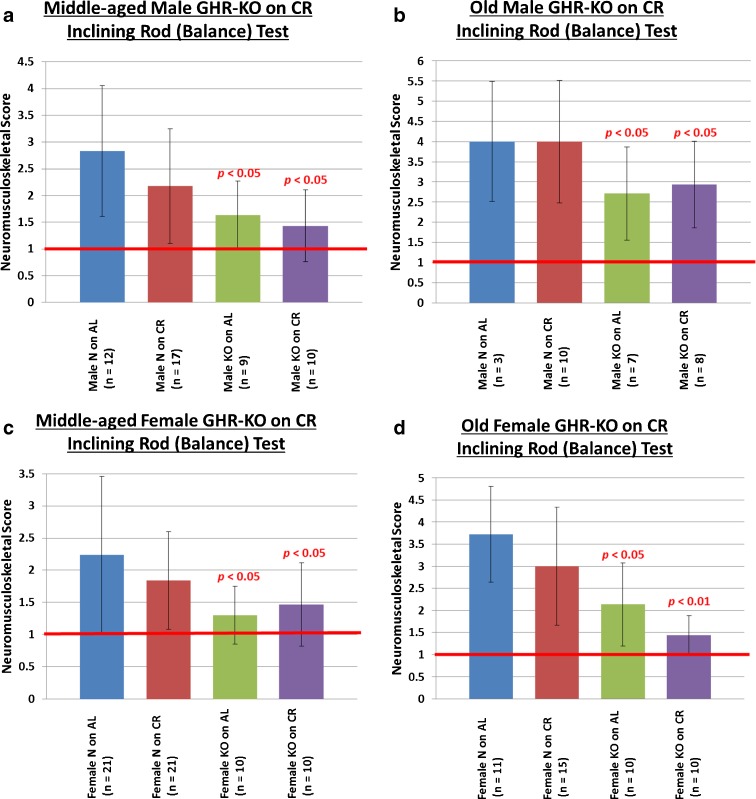
The aging-resultant decline in the physical ability to maintain balance and to prevent falls leads to a very high incidence of vertebral and limb fractures in the elderly (De Laet and Pols 2000), with subsequent pleiotropically negative effects on health and survival (Dennison and Cooper 2000). In studying a more challenging measure of neuromusculoskeletal function, we assessed the ability to maintain equilibrium on a 1-in-diameter, 40-cm-long metal rod that was inclining periodically at 10° per increment. The inclining rod test revealed that middle-aged N mice on an AL diet show the expected aging-associated deviation from perfect performance (defined as a score of 1). Deviating from the results obtained for grip strength, N mice on CR do not have superior balance/motor coordination relative to their AL counterparts (Fig. 3a, c, for males and females, respectively). Middle-aged KO mice on either diet showed little or no impairment in balance/motor coordination, performing superiorly to their littermate controls (Fig. 3a (males): N on AL vs. KO on AL p < 0.05, N on AL vs. KO on CR p < 0.05; Fig. 3b (females): N on AL vs. KO on AL p < 0.05, N on AL vs. KO on CR p < 0.05).
Fig. 3.
Advantageous neuromusculoskeletal effects of the GHR/BP gene disruption on balance/motor coordination. a Middle-aged male KO mice are better able to maintain physical equilibrium than their littermates. b Old male KO mice are better able to maintain physical equilibrium than their littermates. c Middle-aged female KO mice retain balance better than middle-aged female N mice. d Old female KO mice retain balance better than old female N mice. (All p values are for comparisons between the littermate control on AL (N on AL) group and the group whose bar the probability value is written over. The red horizontal bars at neuromusculoskeletal scores of 1 indicate the performance level of young-adult animals.)
With regard to grip strength, balance, and motor coordination in old mice, results similar to those observed at middle age, yet with apparently greater effect sizes, were obtained (Figs. 2b, d and 3b, d).
Agility/motor coordination
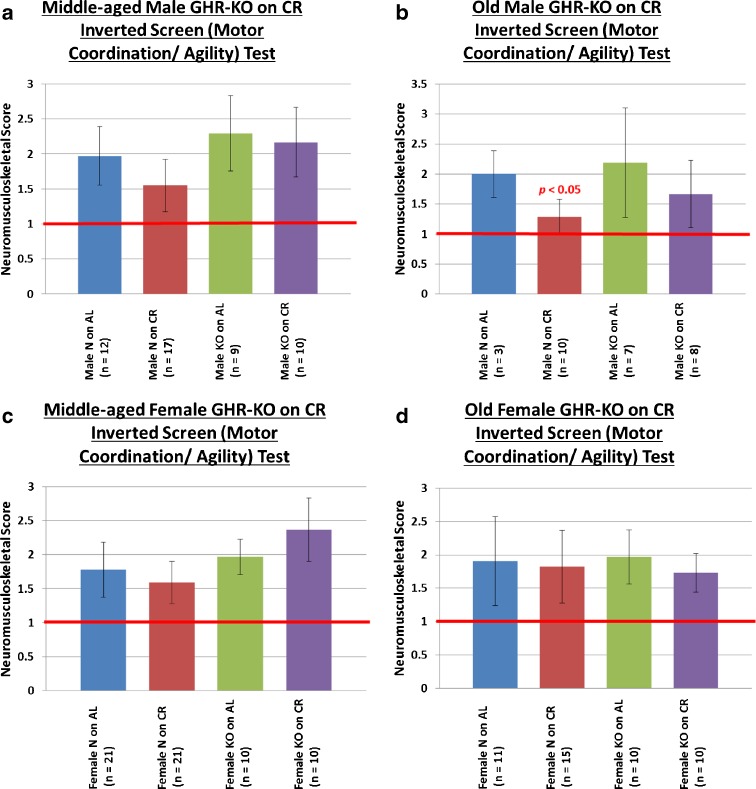
To assess motor coordination under challenging circumstances, we tested agility by orienting the subjects in a presumably uncomfortable position on a wire mesh, namely facing downwards on a wire mesh at a 45° angle. Under these circumstances, mice are innately inclined to invert their positioning and climb upwards. Requiring more than simple balance, this task calls for coordinated motor actions under duress. Curiously, we observed no effect of phenotype at either age for this task (Fig. 4a–d), and the beneficial effect of CR was detected only in old male mice (old males: N on AL vs. N on CR p < 0.05) (Fig. 4b).
Fig. 4.
Limited beneficial neuromusculoskeletal effects of caloric restriction on motor coordination/agility. a Neither CR nor GHR/BP gene disruption affects motor coordination/agility in middle-aged male mice. b CR improves motor coordination/agility in old male mice, but the GHR/BP gene disruption does not. There is no CR or GHR/BP gene disruption effect on motor coordination/agility for either c middle-aged female mice or d old female mice. (The p value is for comparison between the littermate control on AL (N on AL) group and the group whose bar the probability value is written over. The red horizontal bars at neuromusculoskeletal scores of 1 indicate the performance level of young-adult animals.)
Discussion
The key novel finding in this study is that slow-aging GHR-KO mice performed significantly better than normal (littermate control) animals on tests designed to assess strength, balance, and motor coordination. Superior performance of GHR-KO mice was observed in both genders and at both middle age and old age. Further, we show that this mutation that slows aging engenders greater protection from physical decline than the anti-aging dietary intervention. This study establishes further healthspan benefits that coincide with the lifespan benefits not only of the GHR-KO allele or of the dietary restriction independently, but also of both factors in combination (Bonkowski et al. 2006b; Arum et al. 2009). Considering how reliable a marker for general health and a predictor of mortality grip strength is (Rantanen 2003; Bohannon 2008; Marin et al. 2010) and how important manual manipulation of objects is for the execution of activities of daily living, the marked retention of youthful strength engendered by the GHR/BP gene disruption is of considerable potential relevance to devising interventions that might augment human health. Furthermore, accounting for the substantial and lasting disability associated with falls after a loss of balance in aging, the results from the balance assays are similarly promising.
Although the power of the employed tests to assess the performance of GHR-KO mice might have been compromised by the “ceiling effect” of perfect performance (i.e., a score of 1), the results suggest that CR improved strength, balance, and coordination in normal but not in mutant mice. This closely resembles the findings concerning the effects of CR on the longevity of these animals (Bonkowski et al. 2006b). Curiously, neither phenotype nor diet exerted significant effects on performance in a test designed to test agility along with motor coordination, save for a dietary effect on old normal males (Fig. 4b).
These assessments of physical performance are considered “neuro-musculo-skeletal” because all three organ systems (nervous, muscular, and skeletal) are directly necessary for the execution of these tasks; thus, declines in any combination of the three systems may be responsible for the inferior performance of the animals (Kandel et al. 1991). Due to their primary deficiency in GH signaling, GHR-KO mice have increased subcutaneous adipose depots, relative to their littermate controls, and thus have reduced lean muscle/body weight ratios; these differences persist throughout the lives of the mice (Berryman et al. 2010). Conversely, calorically restricted mice are lean, relative to their AL counterparts, by virtue of their diet being low in excess calories that can be stored as or converted into fatty acids; this affords them an increased lean muscle/body weight ratio that persists until death. Based on this simplistic anatomical evaluation, it seems unlikely that muscular superiority is necessary or likely to account for the healthspan benefits observed. Yet, this does not invalidate the possibility that the muscle fibers (possibly at the sarcomeric unit level) remain more healthy, efficient, and/or youthful in GHR-KO and/or CR mice.
It is interesting that somatotrophic signaling-deficient mice, whether growth hormone-resistant GHR-KO mice or CR mice with reduced insulin-like growth factor 1 (IGF-1) levels, exhibit superior performance in tests ostensibly favoring the growth of musculature and bone density. The solution to this conundrum may be that, while short-term exposure to growth factors would be beneficial for these processes, long-term exposure may make such contributions to senescence as to undermine any short-term benefits (Thorner (2009). Additionally or alternatively, the relative (as opposed to absolute) nature of these assessments potentially undermines the initial presumption; that is, with regard to manipulating one’s frame within space, the bulk and density that would be beneficial for manipulating a foreign object is of trifling consequence.
Further studies will be necessary to identify morphological and functional differences in the brain, peripheral nerves, musculature, and skeleton between the GHR-KO and normal littermate mice that may represent mechanisms of the observed differences in physical performance. It is interesting to note that, in contrast to the profoundly suppressed peripheral IGF-1 levels, IGF-1 expression in the brain of GH-deficient Ames dwarfs is not impaired (Lupu et al. 2001) and that the GHR-KO mouse’s bone mineral density is low but protected from age-related decline (Bonkowski et al. 2006a). The potential role of systemic or local effects of altered levels of insulin (Abbatecola and Paolisso 2008), as well as pro- and anti-inflammatory cytokines (Ferrucci et al. 2002; Kanapuru and Ershler 2009), also remains to be assessed.
Finally, it should not be overlooked that the assays conducted herein could be used for the assessment of putative aging-slowing, or simply life-extending, interventions. Genetic and environmental mammalian models of longevity have shown that healthspan correlates well with lifespan; therefore, extended healthspan might be a surrogate for extended longevity. Thus, the healthspan benefits conferred by the aging-slowing GHR-KO gene disruption or by CR, as reported in this article, underscore the potentially massive benefits of interventions discovered in basic gerontological investigations of long-lived animals for the amelioration of aging-associated decline and/or disorders (Olshansky et al. 2007; Warner and Sierra 2009; Miller 2009; Kenyon 2010).
Electronic supplementary material
(DOC 51 kb)
(DOC 42 kb)
Acknowledgments
We thank Lisa Cox for the technical assistance, Steve Sandstrom for the copyediting, and Ravneet Boparai, Ph.D., for the photographic assistance. This work was supported by the National Institute on Aging Grants AG19899, U19 AG023122, and 3R01AG019899-07S1, as well as a Senior Scholar Award in Aging from The Ellison Medical Foundation, and The Glenn Foundation for Medical Research; this work was vitally supported by a grant from the Center for Alzheimer’s Disease and Related Disorders at The Southern Illinois University, and the authors would especially like to thank Thomas A. Ala, M.D., and Robert G. Struble, Ph.D.
Contributor Information
Oge Arum, Phone: +1-217-6522904, Email: oge.arum@gmail.com.
Dustin J. Rickman, Email: dustin1988123@gmail.com
John J. Kopchick, Email: kopchick@ohio.edu
Andrzej Bartke, Email: abartke@siumed.edu.
References
- Abbatecola AM, Paolisso G. Is there a relationship between insulin resistance and frailty syndrome? Curr Pharm Des. 2008;14(4):405–410. doi: 10.2174/138161208783497750. [DOI] [PubMed] [Google Scholar]
- Ahmed N, Mandel R, Fain MJ. Frailty: an emerging geriatric syndrome. Am J Med. 2007;120(9):748–753. doi: 10.1016/j.amjmed.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Arum O, Bonkowski MS, Rocha JS, Bartke A. The growth hormone receptor gene-disrupted mouse fails to respond to an intermittent fasting diet. Aging Cell. 2009;8(6):756–760. doi: 10.1111/j.1474-9726.2009.00520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila-Funes JA, Amieva H, Barberger-Gateau P, Le Goff M, Raoux N, Ritchie K, Carrière I, Tavernier B, Tzourio C, Gutiérrez-Robledo LM, Dartigues JF. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc. 2009;57(3):453–461. doi: 10.1111/j.1532-5415.2008.02136.x. [DOI] [PubMed] [Google Scholar]
- Bartke A. Insulin resistance and cognitive aging in long-lived and short-lived mice. J Gerontol A Biol Sci Med Sci. 2005;60(1):133–134. doi: 10.1093/gerona/60.1.133. [DOI] [PubMed] [Google Scholar]
- Berryman DE, List EO, Palmer AJ, Chung MY, Wright-Piekarski J, Lubbers E, O'Connor P, Okada S, Kopchick JJ. Two-year body composition analyses of long-lived GHR null mice. J Gerontol A Biol Sci Med Sci. 2010;65(1):31–40. doi: 10.1093/gerona/glp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohannon RW. Hand-grip dynamometry predicts future outcomes in aging adults. J Geriatr Phys Ther. 2008;31(1):3–10. doi: 10.1519/00139143-200831010-00002. [DOI] [PubMed] [Google Scholar]
- Bonkowski MS, Pamenter RW, Rocha JS, Masternak MM, Panici JA, Bartke A. Long-lived growth hormone receptor knockout mice show a delay in age-related changes of body composition and bone characteristics. J Gerontol A Biol Sci Med Sci. 2006;61(6):562–567. doi: 10.1093/gerona/61.6.562. [DOI] [PubMed] [Google Scholar]
- Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci USA. 2006;103(20):7901–7905. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141(7):2608–2613. doi: 10.1210/en.141.7.2608. [DOI] [PubMed] [Google Scholar]
- Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144(9):3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- Crawley JN. What’s wrong with my mouse? Behavioral phenotyping of transgenic and knockout mice. 2. Hoboken: Wiley; 2007. [Google Scholar]
- De Laet CE, Pols HA. Fractures in the elderly: epidemiology and demography. Baillieres Best Pract Res Clin Endocrinol Metab. 2000;14(2):171–179. doi: 10.1053/beem.2000.0067. [DOI] [PubMed] [Google Scholar]
- Dennison E, Cooper C. Epidemiology of osteoporotic fractures. Horm Res. 2000;54(Suppl 1):58–63. doi: 10.1159/000063449. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Cavazzini C, Corsi A, Bartali B, Russo CR, Lauretani F, Ferrucci L, Cavazzini C, Corsi AM, Bartali B, Russo CR, Lauretani F, Bandinelli S, Bandinelli S, Guralnik JM. Biomarkers of frailty in older persons. J Endocrinol Invest. 2002;25(10 Suppl):10–15. [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- Glantz SA. How to summarize data. In: Primer of biostatistics. 5. New York: McGraw-Hill; 2002. [Google Scholar]
- Graber TG, Ferguson-Stegall L, Kim JH, Thompson LV (2013) C57BL/6 neuromuscular healthspan scoring system. J Gerontol A Biol Sci Med Sci, in press [DOI] [PMC free article] [PubMed]
- Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, Paupard MC, Hall DH, Driscoll M. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419(6909):808–814. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- Ikeno Y, Hubbard GB, Lee S, Cortez LA, Lew CM, Webb CR, Berryman DE, List EO, Kopchick JJ, Bartke A. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2009;64(5):522–529. doi: 10.1093/gerona/glp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanapuru B, Ershler WB. Inflammation, coagulation, and the pathway to frailty. Am J Med. 2009;122(7):605–613. doi: 10.1016/j.amjmed.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM (1991) Aging of the brain: dementia. In: Principles of neural science, 3rd edn. Appleton & Lange, Norwalk, p 974–983
- Kenyon CJ. The genetics of ageing. Nature. 2010;464(7288):504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Kinney BA, Coschigano KT, Kopchick JJ, Steger RW, Bartke A. Evidence that age-induced decline in memory retention is delayed in growth hormone resistant GH-R-KO (Laron) mice. Physiol Behav. 2001;72(5):653–660. doi: 10.1016/S0031-9384(01)00423-1. [DOI] [PubMed] [Google Scholar]
- Kinney-Forshee BA, Kinney NE, Steger RW, Bartke A. Could a deficiency in growth hormone signaling be beneficial to the aging brain? Physiol Behav. 2004;80(5):589–594. doi: 10.1016/j.physbeh.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Kinsella K, Wan H (2009) U.S. Census Bureau, International Population Reports, P95/09-1, An aging world: 2008. U.S. Government Printing Office, Washington, DC. (http://www.census.gov/prod/2009pubs/p95-09-1.pdf). Accessed June 2009
- Kirkwood TB, Finch CE. Ageing: the old worm turns more slowly. Nature. 2002;419(6909):794–795. doi: 10.1038/419794a. [DOI] [PubMed] [Google Scholar]
- Ko FC. The clinical care of frail, older adults. Clin Geriatr Med. 2011;27(1):89–100. doi: 10.1016/j.cger.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Landi F, Abbatecola AM, Provinciali M, Corsonello A, Bustacchini S, Manigrasso L, Cherubini A, Bernabei R, Lattanzio F. Moving against frailty: does physical activity matter? Biogerontology. 2010;11(5):537–545. doi: 10.1007/s10522-010-9296-1. [DOI] [PubMed] [Google Scholar]
- Lang PO, Michel JP, Zekry D. Frailty syndrome: a transitional state in a dynamic process. Gerontology. 2009;55(5):539–549. doi: 10.1159/000211949. [DOI] [PubMed] [Google Scholar]
- Levers MJ, Estabrooks CA, Ross Kerr JC. Factors contributing to frailty: literature review. J Adv Nurs. 2006;56(3):282–291. doi: 10.1111/j.1365-2648.2006.04021.x. [DOI] [PubMed] [Google Scholar]
- Liu CK, Fielding RA. Exercise as an intervention for frailty. Clin Geriatr Med. 2011;27(1):101–110. doi: 10.1016/j.cger.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev Biol. 2001;229(1):141–162. doi: 10.1006/dbio.2000.9975. [DOI] [PubMed] [Google Scholar]
- Marin RV, Pedrosa MA, Moreira-Pfrimer LD, Matsudo SM, Lazaretti-Castro M. Association between lean mass and handgrip strength with bone mineral density in physically active postmenopausal women. J Clin Densitom. 2010;13(1):96–101. doi: 10.1016/j.jocd.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Marzetti E, Leeuwenburgh C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol. 2006;41(12):1234–1238. doi: 10.1016/j.exger.2006.08.011. [DOI] [PubMed] [Google Scholar]
- McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. Nutrition. 1935;5(3):155–171. [PubMed] [Google Scholar]
- Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R. Of mice and men: the benefits of caloric restriction, exercise, and mimetics. Ageing Res Rev. 2011;11:390–398. doi: 10.1016/j.arr.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA. “Dividends” from research on aging—can biogerontologists, at long last, find something useful to do? J Gerontol A Biol Sci Med Sci. 2009;64(2):157–160. doi: 10.1093/gerona/gln062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Burke D, Nadon N. Announcement: four-way cross mouse stocks: a new, genetically heterogeneous resource for aging research. J Gerontol A Biol Sci Med Sci. 1999;54(8):B358–B360. doi: 10.1093/gerona/54.8.B358. [DOI] [PubMed] [Google Scholar]
- Moreschi C. Ztschr. f. Immunitätsforsch. u. exper. Therap. 1909;2:651. [Google Scholar]
- Olshansky SJ, Perry D, Miller RA, Butler RN. Pursuing the longevity dividend: scientific goals for an aging world. Ann N Y Acad Sci. 2007;1114:11–13. doi: 10.1196/annals.1396.050. [DOI] [PubMed] [Google Scholar]
- Parks RJ, Fares E, Macdonald JK, Ernst MC, Sinal CJ, Rockwood K, Howlett SE. A procedure for creating a frailty index based on deficit accumulation in aging mice. J Gerontol A Biol Sci Med Sci. 2012;67(3):217–227. doi: 10.1093/gerona/glr193. [DOI] [PubMed] [Google Scholar]
- Rantanen T. Muscle strength, disability and mortality. Scand J Med Sci Sports. 2003;13(1):3–8. doi: 10.1034/j.1600-0838.2003.00298.x. [DOI] [PubMed] [Google Scholar]
- Roth LW, Polotsky AJ. Can we live longer by eating less? A review of caloric restriction and longevity. Maturitas. 2012;71(4):315–319. doi: 10.1016/j.maturitas.2011.12.017. [DOI] [PubMed] [Google Scholar]
- Rous P. The influence of diet on transplanted and spontaneous mouse tumors. J Exp Med. 1914;20(5):433–451. doi: 10.1084/jem.20.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawbridge WJ, Wallhagen MI, Cohen RD. Successful aging and well-being: self-rated compared with Rowe and Kahn. Gerontologist. 2002;42(6):727–733. doi: 10.1093/geront/42.6.727. [DOI] [PubMed] [Google Scholar]
- Thorner MO. Statement by the Growth Hormone Research Society on the GH/IGF-I axis in extending health span. J Gerontol A Biol Sci Med Sci. 2009;64(10):1039–1044. doi: 10.1093/gerona/glp091. [DOI] [PubMed] [Google Scholar]
- Topinková E. Aging, disability and frailty. Ann Nutr Metab. 2008;52(Suppl 1):6–11. doi: 10.1159/000115340. [DOI] [PubMed] [Google Scholar]
- Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, Ershler WB, Harris T, Fried LP. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54(6):991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- Warner HR, Sierra F. The longevity dividend: why invest in basic aging research? Can J Aging. 2009;28(4):391–394. doi: 10.1017/S0714980809990286. [DOI] [PubMed] [Google Scholar]
- Weiss CO. Frailty and chronic diseases in older adults. Clin Geriatr Med. 2011;27(1):39–52. doi: 10.1016/j.cger.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, Okada S, Cataldo L, Coschigamo K, Wagner TE, Baumann G, Kopchick JJ. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse) Proc Natl Acad Sci USA. 1997;94(24):13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 51 kb)
(DOC 42 kb)