Abstract
Cardiovascular disease (CVD), both clinical and subclinical, has been proposed as one of the mechanisms underlying frailty. However, there is no evidence addressing the relationship between the earliest stage of CVD (endothelial dysfunction) and frailty. The goal of the study was to analyze the association between endothelial dysfunction, evaluated by asymmetric dimethylarginine (ADMA) levels, and frailty. We used data from the Toledo Study for Healthy Aging, a prospective Spanish cohort study. Biological samples were obtained and ADMA levels were determined using an enzyme immunoassay method. Logistic regression was used to estimate the odds ratio (OR) and 95 % confidence intervals of frailty associated with ADMA. Adjustments were made for age, gender, cardiovascular risk factors, and presence of atherosclerotic disease (assessed by ankle–brachial index; ABI). One thousand two hundred eighty-seven community-dwelling elderly were included. One hundred seven (8.3 %) were identified as frail, 542 (42.1 %) as pre-frail, and 638 (49.6 %) as non-frail. ADMA values were higher in frail subjects than in non-frail ones. In addition, an interaction between the presence of atherosclerotic disease and ADMA on the odds of frailty (p = 0.045) was detected. After adjustments for age, classical cardiovascular risk factors, and ABI, the risk of frailty was associated with increasing levels of ADMA in subjects without atherosclerotic disease [OR for 1 standard deviation increase in ADMA = 1.14 (1.01–1.28), p = 0.032] but not in those with atherosclerotic disease. In our study, endothelial dysfunction, assessed by ADMA levels, is associated with frailty. These findings provide additional support for a relevant role of vascular system since its earliest stage in frailty.
Keywords: Frailty, Cardiovascular disease, Endothelial dysfunction, Asymmetric dimethylarginine, ADMA, Aging
Background
In the last decades, the concept of frailty has emerged as an important condition associated with advanced age (Fried et al. 2001). It is a clinically recognizable state characterized by a reduced functional reserve and impaired adaptive capacity across multiple physiologic systems predisposing to falls, fractures, functional impairment, institutionalization, and death (Fried et al. 2001; Rodriguez-Mañas et al. 2012). Gill and colleagues (2006) were the first in suggesting that frailty is a reversible process thus opening new targets in disability prevention and elderly care.
The physiological changes underlying frailty are difficult to identify (Walston et al. 2006). Alterations in several physiological systems have been suggested, including neuroendocrine dysregulation, decreased musculoskeletal functioning, immunological impairment, inflammation, and cardiovascular disease (CVD) (Carcaillon et al. 2012a; Carcaillon et al. 2012b; Barzilay et al. 2007; Newman et al. 2001). The relation between frailty and CVD has been shown not only for clinical diseases like stroke, infarction, angina, or intermittent claudication but also for subclinical manifestations (Newman et al. 2001).
The endothelium plays a crucial role in the vascular physiology and in the mechanisms leading to vascular diseases (Sitia et al. 2010). Previous studies demonstrated that endothelial dysfunction precedes atherosclerotic disease and predicts future cardiovascular events (Sitia et al. 2010; El Assar et al. 2012; Najjar et al. 2005). Among several substances and mediators, nitric oxide (NO) has been extensively studied as one of the most relevant factors released by the endothelium, playing an outstanding role in maintaining the function of the vascular system (El Assar et al. 2012). Reduced NO bioavailability is one of the changes usually observed in endothelial dysfunction and leading to vascular disease (El Assar et al. 2012). Asymmetric dimethylarginine is an endogenous inhibitor of NO synthase (Cooke 2005) commonly used as a marker of endothelial dysfunction (Stuhlinger et al. 2003). Many studies have shown that increased concentrations of asymmetric dimethylarginine (ADMA) was present in several conditions associated to endothelial dysfunction including aging, hypercholesterolemia, hypertension, diabetes mellitus, obesity, hyperhomocysteinemia, and renal failure (Sibal et al. 2010). Furthermore, in prospective studies, increased ADMA concentration has been identified as an independent risk factor for progression of atherosclerosis and cardiovascular death (Meinitzer et al. 2007).
Although the association between CVD (clinical and subclinical) and frailty is well established, there are no studies assessing the relation between endothelial dysfunction (the earliest form of subclinical CVD) and frailty. The goal of this study was to analyze the relationship between endothelial dysfunction, evaluated by ADMA levels, and frailty.
Methods
Study population
The Toledo Study for Healthy Aging (TSHA) is a Spanish longitudinal population-based study, designed for evaluating the determinants of physical frailty in the elderly. The used methodology has been reported previously (Garcia-Garcia et al. 2011). Participants in the TSHA come from two sources. The first one, called “the historic cohort,” is formed by the survivors of a previous study (The Toledo Study), a population of subjects being over 77 years in 2006 (Garcia Garcia et al. 2001). Individuals 65–76 years old in 2006, specially recruited for the TSHA study, form the second one, called “the new cohort.” Subjects from both sources were selected by a two-stage random sampling from the municipal census of Toledo, covering institutionalized and community-dwelling people from rural and urban settings. All subjects underwent the same assessment.
Data were collected between June 2006 and September 2009. Firstly, trained psychologists went to the subjects' house to fill in questionnaires with extensive information on sociodemographic characteristics, social support, dependence in activities of daily living, physical activity, health related quality of life, depressive symptoms, and cognitive function. In addition, data about prevalent disease, including cardiovascular risk factors (hypertension, diabetes, obesity, and hypercholesterolemia) and CVD were collected by self-reporting. Subsequently, enrolled subjects underwent a complete physical exam by a team of nurses. They evaluated the heart rate, blood pressure, anthropometric measures, ankle–brachial index, and physical performance (walk speed, upper and lower extremities strength, balance, and the stand-and-sit from a chair test). Finally, study participants went to their health center to provide a fasting blood sample.
The study protocol was approved by the Clinical Research Ethics Committee of the Complejo Hospitalario de Toledo, Spain. All study participants gave a signed informed consent prior to their inclusion in the cohort.
Blood collection and measurements
At baseline, blood samples were obtained from all subjects (45 cm3 of blood while fasting). Within 2 h since drawing, samples were taken to the laboratory in containers at a temperature between 2 and 4 °C, and then they were divided in aliquots with EDTA and stored at −80 °C. Asymmetric dimethylarginine was measured in the Research Unit of the Hospital Universitario de Getafe (Madrid, Spain). ADMA levels were determined using a validated enzyme immunoassay method (Schulze et al. 2004) with expected values between 0.4 and 0.75 μmol/l (80–150 ng/ml) and a sensitivity of 0.05 μmol/l. The coefficients of inter-assay variation were 9.8 to 10.3 % for lower levels and 8.3 to 9.4 % for higher levels. The coefficients of intra-assay variation were 5.7 to 6.4 %.
Frailty measure
Frailty was assessed using Fried's criteria (Fried et al. 2001), but using cutoff points for slowness, weakness, and low physical activity adapted to the characteristics of our population (see later). Five items compose this scale: slowness, weakness, weight loss, exhaustion, and low physical activity. The method of measuring every item has been described elsewhere (Garcia-Garcia et al. 2011). Slowness was defined using the 3-m walking speed test; individuals were asked to walk 3 m at their usual pace, following a standardized protocol; sex- and height-adjusted time points were used; the slowest quintile was considered positive. Weakness was measured by grip strength in the dominant hand using a Jamar hydraulic dynamometer; the result was adjusted by the subject's body mass index; those in the bottom quintile were considered positive for this criterion. Weight loss was considered positive for reporting more than 4.5 kg of unintentional weight loss in the previous year. Exhaustion was assessed using two questions (“I felt that anything I did was a big effort” and “I felt that I could not keep on doing things”); answers were scored between 0 and 4 depends on symptoms' frequency; if any question was answered 2 or higher, this criterion was considered positive. Low physical activity was based on the Physical Activity Scale for the Elderly (Schuit et al. 1997); those in the worse quintile of physical activity were considered positive for this item. Subjects were classified as frail if they met three or more of these items, as pre-frail if subjects met one or two criteria, and non-frail or robust if none item was present.
Atherosclerotic disease definition
Atherosclerotic disease was considered as a self-reported history of stroke, myocardial infarction, angina pectoris, or intermittent claudication. Subclinical atherosclerotic disease was assessed by ankle–brachial index (ABI). For this purpose, blood pressure was determined, in all patients, in both arms and ankles (posterior tibial artery and dorsalis pedis artery) with the patient supine for at least 5 min before, using a standard sphygmomanometer and a handheld Doppler ultrasound (Vascular Pocket Doppler Model 841-A; Parks Medical Electronics, Inc, Aloha, OR). A cycle of measurements (right arm, right ankle, left ankle, and left arm) was repeated, and the means of two measurements for each limb were used. Finally, the ratio of the highest systolic pressure in the ankle to the higher of the left or right brachial systolic pressure was used to define the ABI (Espinola-Klein et al. 2008; Hirsch et al. 2006). The participants were classified by their ABI according to the ACCF/AHA 2011 Guidelines (ACCF/AHA 2011) (Table 1).
Table 1.
Subject's characteristics according to Frailty Status (n = 1,287)
| Total | Robust (n = 638) | Pre-frail (n = 542) | Frail (n = 107) | p value | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||||
| Sex | Male | 552 | 275 | 43.1 | 241 | 44.5 | 36 | 33.6 | 0.117 |
| Age groups (years) | |||||||||
| <75 | 685 | 415 | 66.1 | 248 | 45.8 | 22 | 20.6 | <0.0001 | |
| [75–80] | 397 | 169 | 26.5 | 190 | 35.1 | 38 | 35.5 | ||
| ≥80 | 205 | 54 | 8.5 | 104 | 19.2 | 47 | 43.9 | ||
| Educational level | |||||||||
| No formal schooling | 860 | 392 | 61.7 | 391 | 72.1 | 77 | 72.0 | 0.002 | |
| Uncompleted school | 225 | 125 | 19.7 | 81 | 14.9 | 19 | 17.8 | ||
| Primary or secondary school | 199 | 118 | 18.6 | 70 | 12.9 | 11 | 10.3 | ||
| BMI categories (kg/m2) | |||||||||
| <25 | 193 | 77 | 12.1 | 96 | 17.8 | 20 | 18.9 | <0.0001 | |
| [25–30] | 556 | 302 | 47.6 | 218 | 40.5 | 36 | 33.9 | ||
| ≥30 | 530 | 256 | 40.3 | 224 | 41.6 | 50 | 47.2 | ||
| HTA | 665 | 331 | 52.6 | 277 | 51.3 | 57 | 54.3 | 0.817 | |
| Hypercholesterolemia | 491 | 245 | 39.5 | 207 | 38.9 | 39 | 37.5 | 0.927 | |
| Diabetes | 240 | 103 | 16.4 | 113 | 21.0 | 24 | 23.1 | 0.069 | |
| Atherosclerotic disease | 183 | 67 | 10.5 | 86 | 15.9 | 30 | 28.0 | <0.0001 | |
| Ankle–brachial index | |||||||||
| ≤0.9 | 238 | 111 | 18.0 | 99 | 19.0 | 28 | 28.3 | 0.906 | |
| 0.9–1.0 | 322 | 155 | 25.2 | 139 | 26.7 | 28 | 28.3 | ||
| 1.0–1.4 | 647 | 336 | 54.6 | 271 | 52.1 | 40 | 40.4 | ||
| >1.4 | 28 | 14 | 2.3 | 11 | 2.1 | 3 | 3.0 | ||
Statistical analysis
Subject's characteristics according to frailty status were compared using Pearson Chi-square tests. ADMA was log-transformed to normalize its distribution. Levels of ADMA according to subjects' characteristics are consequently displayed as geometric means and interquartile range. GM comparisons were performed using standard Student t tests for dichotomous variables and using ANOVA for variables with more than two categories. Odds ratio (OR) and 95 % confidence interval (95 % CI) of frailty (versus robust and pre-frail) associated with ADMA were estimated using logistic regression. OR were estimated for 1 standard deviation (SD) increased in ADMA as well as for quartiles. Test for linear trend across quartiles are displayed. In addition, deviation from linearity was assessed using appropriate log-likelihood tests. Adjustments were made for classical cardiovascular risk factors (age, sex, hypertension, diabetes, hypercholesterolemia, and BMI), the presence of atherosclerotic disease (clinical and subclinical), and the renal function. Multiplicative interactions between ADMA and adjustment variables were systematically tested before adjusting for this variable. As we detected an interaction between the presence of atherosclerotic disease and ADMA, results are given in both strata separately.
Results
Study population
One thousand two hundred eighty-seven persons (552 men and 735 women) composed our study population. The mean age was 74.4 (5.4) years. Table 1 displays the sample characteristics according to frailty status and the Table 2 shows the ADMA levels according to subject characteristics. Overall, 18.8 % of the subjects were diabetic, 52.1 % had hypertension, 39.0 % had hypercholesterolemia, and 41.4 % were obese. Regarding clinical atherosclerotic diseases, 401 diagnoses of angor, myocardial infarction, stroke, or intermittent claudication, either isolated or in combination, were collected. Women had more comorbidities than men 1.7 (SD = 1.1) versus 1.4 (SD = 1.1), p < 0.0001. Frailty was associated with older age (p < 0.0001), lower educational level (p = 0.002), BMI (p < 0.0001), and presence of atherosclerotic disease (p < 0.0001).
Table 2.
Levels of ADMA according to subject's characteristics
| n | % | GM | IQR | p valuea | |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 552 | 42.8 | 0.77 | (0.63;0.92) | 0.025 |
| Female | 735 | 57.1 | 0.79 | (0.65;0.98) | |
| Age groups (years) | |||||
| <75 | 685 | 53.2 | 0.75 | (0.63;0.92) | <0.0001 |
| [75–80] | 397 | 30.8 | 0.80 | (0.65;0.97) | |
| ≥80 | 205 | 15.9 | 0.85 | (0.69;1.02) | |
| Educational level | |||||
| No formal schooling | 860 | 66.9 | 0.79 | (0.67;0.96) | 0.022 |
| Uncompleted school | 225 | 17.5 | 0.74 | (0.61;0.92) | |
| Primary or secondary school | 199 | 15.4 | 0.77 | (0.63;0.96) | |
| BMI categories (kg/m2) | |||||
| <25 | 193 | 15.0 | 0.77 | (0.67;0.91) | 0.469 |
| [25–30] | 556 | 43.4 | 0.79 | (0.65;0.97) | |
| ≥30 | 530 | 41.4 | 0.77 | (0.65;0.95) | |
| HTA | |||||
| No | 609 | 47.8 | 0.78 | (0.65;0.95) | 0.520 |
| Yes | 665 | 52.1 | 0.77 | (0.65;0.94) | |
| Hypercholesterolemia | |||||
| No | 766 | 60.9 | 0.79 | (0.65;0.97) | 0.080 |
| Yes | 491 | 39.0 | 0.77 | (0.65;0.93) | |
| Diabetes | |||||
| No | 1,031 | 81.1 | 0.78 | (0.65;0.96) | 0.102 |
| Yes | 240 | 18.8 | 0.76 | (0.63;0.91) | |
| Atherosclerotic disease | |||||
| No | 1,104 | 85.8 | 0.79 | (0.65;0.95) | 0.940 |
| Yes | 183 | 14.2 | 0.79 | (0.65;0.98) | |
| Ankle–brachial index | |||||
| ≤0.9 | 238 | 19.3 | 0.78 | (0.65;0.95) | 0.906 |
| 0.9–1.0 | 322 | 26.1 | 0.79 | (0.65;0.98) | |
| 1.0–1.4 | 647 | 52.4 | 0.78 | (0.65;0.95) | |
| >1.4 | 28 | 2.2 | 0.79 | (0.63;0.90) | |
| Frailty | |||||
| No frail | 638 | 49.5 | 0.78 | (0.65;0.94) | 0.045 |
| Pre-frail | 542 | 42.1 | 0.79 | (0.64;0.95) | |
| Frail | 107 | 8.3 | 0.84 | (0.70;1.02) | |
GM Geometric mean, IQR Inter Quartile Range
aEstimated from linear regression with the log transformation of ADMA as the dependent variable.
When we assessed levels of ADMA according to subject's characteristics (Table 2), we found differences in ADMA values according to the frailty status (p = 0.045) as well as according to age, sex, and education level. There was no association with classical risk factors neither with clinical CVD.
Effect of the endothelial dysfunction on the relationship between ADMA and frailty
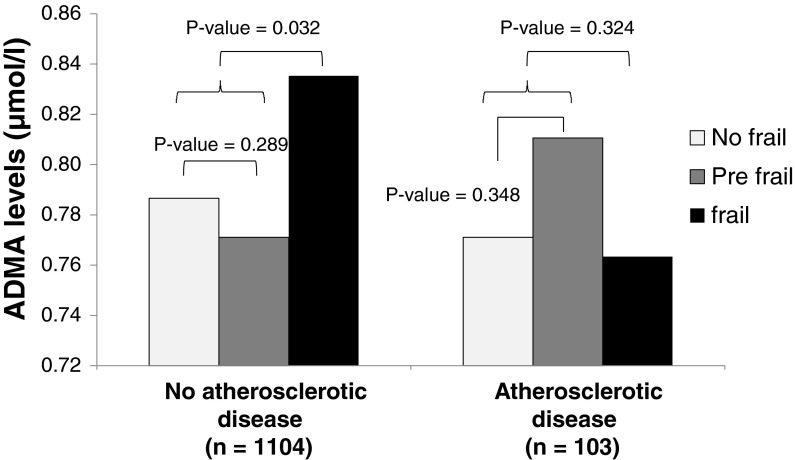
There was an interaction between ADMA levels and atherosclerotic disease on the odds of frailty (p = 0.045). In subjects without clinical atherosclerotic disease, the mean ADMA levels were significantly higher in frail than in pre-frail or non-frail subjects [M = 0.83 (SD = 0.26), M = 0.77 (SD = 0.26), and M = 0.78 (SD = 0.23) respectively, p for difference = 0.032]. In contrast, we did not find any statistically significant relationship in subjects with clinical atherosclerotic disease (p = 0.324) (Fig. 1).
Fig. 1.
Age-adjusted geometric means of ADMA in frail, pre-frail, and non-frail subjects with and without atherosclerotic disease
To further evaluate the relationship between endothelial dysfunction and frailty, multivariate analyses were performed separately in subjects with and without clinical CVD. After adjustment for classical cardiovascular risk factors, there was a significantly increased risk of frailty associated with increased ADMA levels (p for trend = 0.032) in subjects without clinical CVD, but not in those with clinical CVD (Table 3). Further adjustment for ABI, an objective measurement of subclinical atherosclerotic disease, did not substantially modify the results. The risk of being frail increased as did the concentration of ADMA, being doubled for the subjects in the highest quartile [2.09, 95 % CI (0.95–4.61), p for trend = 0.018] (Table 3). Finally, as ADMA levels can be modified by the presence of kidney dysfunction, we make a final adjustment by creatinine levels. This last adjustment did not produce any significant change in the association trend [2.05, 95 % CI (0.92–4.51), p for trend = 0.021] (Table 3). Again, no relationship was found in subjects with clinical CVD. We did not identify any significant interaction between other subjects' characteristics and ADMA on the odds of frailty.
Table 3.
Odds (95 % CI) of frailty associated with ADMA by atherosclerotic diseases status
| Atherosclerotic diseases | ||||||
|---|---|---|---|---|---|---|
| No (n = 1,104) | p value | Yes (n = 183) | p value | |||
| OR | 95 % CI | OR | 95 % CI | |||
| Model 1a | ||||||
| For 1 SD increase | 1.18 | (1.07–1.31) | 0.001 | 0.97 | (0.83–1.14) | 0.735 |
| Quartiles | Ref | Ref | ||||
| 1.27 | (0.58–2.79) | 0.551 | 1.26 | (0.42–3.73) | 0.679 | |
| 1.70 | (0.79–3.65) | 0.173 | 0.81 | (0.27–2.45) | 0.713 | |
| 2.85 | (1.40–5.83) | 0.004 | 0.80 | (0.28–2.58) | 0.778 | |
| p for trend | 0.001 | 0.619 | ||||
| Model 2b | ||||||
| For 1 SD increase | 1.13 | (1.01–1.26) | 0.032 | 0.91 | (0.76–1.10) | 0.324 |
| Quartiles | Ref | Ref | ||||
| 1.00 | (0.44–2.26) | 0.995 | 1.28 | (0.41–4.02) | 0.668 | |
| 1.26 | (0.57–2.78) | 0.564 | 0.69 | (0.22–2.20) | 0.529 | |
| 2.07 | (0.99–4.33) | 0.055 | 0.66 | (0.21–2.15) | 0.494 | |
| p for trend | 0.022 | 0.342 | ||||
| Model 3c | ||||||
| For 1 SD increase | 1.12 | (1.00–1.25) | 0.049 | 0.89 | (0.73–1.10) | 0.286 |
| Quartiles | Ref | Ref | ||||
| 1.08 | (0.47–2.51) | 0.859 | 1.72 | (0.49–6.06) | 0.402 | |
| 1.46 | (0.64–3.31) | 0.368 | 0.62 | (0.17–2.29) | 0.475 | |
| 2.06 | (0.94–4.49) | 0.070 | 0.65 | (0.18–2.40) | 0.519 | |
| p for trend | 0.032 | 0.342 | ||||
| Model 4d | ||||||
| For 1 SD increase | 1.14 | (1.01–1.28) | 0.032 | 0.84 | (0.67–1.04) | 0.110 |
| Quartiles | Ref | Ref | ||||
| 0.88 | (0.37–2.13) | 0.782 | 1.90 | (0.51–7.11) | 0.341 | |
| 1.51 | (0.66–3.44) | 0.328 | 0.56 | (0.14–2.28) | 0.422 | |
| 2.09 | (0.95–4.61) | 0.067 | 0.39 | (0.09–1.69) | 0.207 | |
| p for trend | 0.018 | 0.104 | ||||
| Model 5e | ||||||
| For 1 SD increase | 1.35 | (1.02–1.79) | 0.035 | 0.64 | (0.38–1.10) | 0.105 |
| Quartiles | Ref | Ref | ||||
| 0.87 | (0.36–2.09) | 0.749 | 2.07 | (0.55–7.71) | 0.280 | |
| 1.45 | (0.63–3.31) | 0.382 | 0.49 | (0.12–1.99) | 0.315 | |
| 2.05 | (0.92–4.51) | 0.076 | 0.39 | (0.09–1.70) | 0.210 | |
| p for trend | 0.021 | 0.098 | ||||
aCrude
bAdjusted for age
cAdjusted for age, hypertension, hypercholesterolemia, diabetes, sex, and BMI categories
dAdjusted for age, hypertension, hypercholesterolemia, diabetes, sex, BMI categories, and ABI categories
eAdjusted for age, hypertension, hypercholesterolemia, diabetes, sex, BMI categories, ABI categories, and renal function
Discussion
In the present paper, we show for the first time an association between frailty and endothelial dysfunction. These findings not only reinforce the known relationship between frailty and CVD (clinical and subclinical) but also support that this relation exists since a very early stage when only the endothelial dysfunction is present.
The existence of a relationship between the vascular system and frailty has been claimed since more than a decade. However, the precise stage of the vascular disease from which this association is present remains unclear although it may be of great clinical relevance as to target populations suitable for intervention and prevention. The first data showing an association between frailty and CVD were published in a secondary analysis of the Zutphen Elderly Men's Study in 1999 [OR for CVD in frail men = 4.1, 95 % CI (1.8–9.3)] (Chin et al. 1999). Later, in 2001, data from the Cardiovascular Health Study confirmed this relationship [OR = 2.79, 95 % CI (2.12–3.67)] (Newman et al. 2001). The Women's Health Initiative Observational Study was the first and largest study to find that CVD was a risk factor for developing incident frailty (Woods et al. 2005). Furthermore, epidemiological data reinforce these findings in different settings and with different frailty scores (Newman et al. 2006; Purser et al. 2006). Only one previous study (the Cardiovascular Health Study (Newman et al. 2001)), reported an association between subclinical CVD and frailty. In this study, the presence of different biomarkers of CVD in absence of clinical manifestations was associated with frailty.
ADMA reduces NO production by a competitive inhibition of endothelial nitric oxide synthase (eNOS) (Cooke 2005). NO induces vasodilatation and inhibits platelets aggregation, adhesion of monocytes and leukocytes to the endothelium, smooth muscle cell proliferation, oxidation of LDL, and vascular inflammation by suppressing the expression and activity of adhesion molecules and chemokines, protecting the vascular wall from different stresses and injuries (Sibal et al. 2010). As a consequence, when ADMA levels increases, endothelial dysfunction appears and, if it is maintained along the time, subsequent atherosclerosis develops (Cooke 2005). Several studies done in patients with hypertension and hypercholesterolemia showed ADMA levels inversely correlated to the endothelial dependent vasodilatation, measured by brachial artery flow (Achan et al. 2003; Perticone et al. 2005). All these studies have reported ADMA as a novel marker of endothelial dysfunction. Furthermore, in prospective studies, increased ADMA levels were strong predictors of cardiovascular events and mortality not just in patients with cardiovascular risk factors and CVD but also in healthy people (Schulze et al. 2005).
Increased concentration of ADMA by age and impaired renal function are usual findings (El Assar et al. 2012; Xiao et al. 2001; Kielstein et al. 2003). However, they do not seem to account for our findings since both variables have been included in the adjusted multivariant models, without changing the association between ADMA and frailty in older people without CVD.
Moreover, although not directly accounting for this relationship, some common mechanisms may explain, at least partially, the direct relationship between ADMA levels and frailty. One of the most constant findings in frail people is an increase in inflammation and in oxidative stress (Mulero et al. 2011). And these two same mechanisms have been related to a decrease in the vascular level of dimethylarginine dimethylaminohydrolase (DDAH), the key enzyme for ADMA metabolism (Teerlink 2005).
It has been demonstrated that increased ADMA concentration is associated with reduction of DDAH expression in some cardiovascular diseases such as atherosclerosis and hypertension (Chen et al. 2013). DDAH exists in two isoforms DDAH-1 and DDAH-2 with distinct tissue-relevant distribution. DDAH-1 is predominantly expressed in tissues expressing neuronal nitric oxide synthase, whereas DDAH-2 is located mainly in vasculature tissues containing the eNOS isoform. It was shown that DDAH-2 is inhibited by reactive oxygen species leading to ADMA accumulation (Ito et al. 1999). Furthermore, the expression of DDAH has been shown to be reduced in endothelial cells pretreated with TNF-α (Ito et al. 1999).
The association between endothelial dysfunction and frailty strengthen the role of vascular disease in frailty, suggesting that it would be relevant since the very early stages of vascular dysfunction when only functional impairment (endothelial dysfunction) is apparent. This fact opens new perspectives in the field of frailty. First, these results offer new views to the interpretation of some research results regarding frailty. Until now, different observational studies have shown a relationship between immunological and thrombosis biomarkers (factor VIII, D-dimer, C-reactive protein, low hemoglobin, high leukocytes, high fibrinogen…) with both CVD and frailty showing possible mechanistic links between them (Phan et al. 2008; Walston et al. 2002). However, many of these factors are also related to endothelial dysfunction, thus supporting a potential role for endothelial dysfunction in the pathophysiology of frailty. In this regard, it is interesting to note that aging per se induces endothelial dysfunction, in absence of cardiovascular risk factor and CVD (Rodriguez-Mañas et al. 2009; Angulo et al. 2012). The mechanism of this age-associated endothelial dysfunction has two complementary sources: an increased oxidative stress and a pro-inflammatory profile (Rodriguez-Mañas et al. 2009). As it has been previously stated, these two mechanisms are also related to both increased plasma levels of ADMA and frailty, suggesting a common underlying mechanism. Unfortunately, we did not measure these biomarkers that could be of utility to support this pathophysiological link. Second, our results suggest that ADMA, in addition to be a risk marker of endothelial dysfunction and a strong predictor of CVD, might be a novel marker of frailty in patients without CVD. This opens new alternatives for elderly care, including both the treatment and prevention of frail older people. In this regard, a necessity to identify potential biomarkers of frailty useful to improve the diagnosis and prognosis of this syndrome has been claimed by different groups of experts. In addition, recent literature proposes ADMA as a target for pharmacotherapy in diseases and conditions that are different from CVD but in which endothelial dysfunction may be involved (Sibal et al. 2010; Beltowski and Kedra 2006). The fact that we did not find any association between ADMA and frailty among subjects with clinical atherosclerotic disease may be due to the weight of clinical CVD in determining frailty, making undetectable the effect of the endothelial dysfunction on frailty when the symptomatic disease is present. Regarding subclinical CVD, the association between endothelial dysfunction and frailty is independent of its presence, as it remains after adjusting for this factor. As a whole, this picture draws a scenario where pathological changes in the cardiovascular system are involved in mechanisms leading to frailty since their earliest stages, with the heaviest role for clinical CVD but with relevant roles for both subclinical CVD and endothelial dysfunction.
Our study presents some strengths and limitations. As strengths, we have evaluated this relationship in the TSHA cohort. This study is being carried out on a large population-based sample of subjects (Garcia-Garcia et al. 2011). Frailty was measured using Fried's clinical criteria, one of the most validated frailty scales, but fitted to the profile of our population. This adjustment of the cutoff point for meeting the diagnostic criteria is important when you are working on populations phenotypically different from the ones where frailty criteria were originally described. There is some literature where it was found that the prevalence of frailty was really discordant between different countries using the same cutoff points as those used for the US population (Santos-Eggimann et al. 2009). For avoiding these differences, the main studies that assess the frailty phenotype in different settings, which is with different populations, use the LP Fried criteria but standardizing them to their own populations (continuous variables are thus considered positive when the subject belongs to the lowest quintile in its own population not in the US population) (Espinoza et al. 2010). Otherwise, the risk for misestimating frailty is really high (Rodriguez-Mañas et al. 2012; Bergman et al. 2007; Santos-Eggimann et al. 2009). To avoid misdiagnosis of frailty, we adapted the cutoff points for the three items highly dependent on the characteristics of the individuals (gait velocity, grip strength, and physical activity) to our population, maintaining the criteria to meet each one of the items (Percentile 20 of the distribution) as originally described. The measurements were performed by skilled, trained, certified researchers. We used ADMA as a marker of endothelial dysfunction. Although a direct assessment of endothelial function is the “gold standard,” its use in epidemiological studies with many participants presents relevant challenges that can be overcome by the use of biomarkers like ADMA. ADMA has been used in several recent studies (not only epidemiological but also in little cohorts of individuals or in case–control studies) as a reliable biomarker of endothelial function (Hsu et al. 2012; Rentoukas et al. 2012; Dogru et al. 2012). ADMA determinations were performed using a validated quantitative determination by enzyme immunoassay (Schulze et al. 2004). All determinations were performed twice to reduce the risk of error variation.
The two main limitations of our results are its cross-sectional design, which does not allow us to conclude in terms of causality and the self-reported nature of the information regarding the presence of CVD. Nonetheless, self-report data have been extensively used in epidemiological studies although some authors have claimed that they underestimated the true prevalence of disease (Espelt et al. 2012). A bias of the results based on the differential misclassification of the subjects according to their cardiovascular status is unlikely so this issue may only lead to underestimate the association of ADMA with frailty among subjects without atherosclerosis. Moreover, the tendency observed in the group of self-reported atherosclerotic disease goes in the opposite direction, decreasing the OR as ADMA increases and, anyway, far from the statistical significance. In addition, we further adjusted our model for a robust surrogate of the presence of atherosclerosis (ABI), which remains as a good biomarker of subclinical atherosclerosis (Berni et al. 2011; Lim et al. 2013; Zhang et al. 2013) and the renal function, estimated by the creatinine levels, because of the kidney metabolism of the ADMA. The possible remaining variability due to these factors has been taken into account in the last models, showing no differences in the association.
In conclusion, we show for the first time a relationship between endothelial dysfunction and frailty in older people. These findings provide additional support for a relevant role of vascular system in frailty since the early stages of vascular disease that, if confirmed, should raise new targets for detection, intervention, and prevention of frailty.
Acknowledgments
This work was supported by grants from Ministerio de Economía y Competitividad [Instituto de Salud Carlos III, PI08/1649, PI11/01068, RETICEF RD06/0013], Spanish Government.
References
- ACCF/AHA Focused update of the guideline for the management of patients with peripheral artery disease (Updating the 2005 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines (2011) Circulation. 2011;124(18):2020–2045. doi: 10.1161/CIR.0b013e31822e80c3. [DOI] [PubMed] [Google Scholar]
- Achan V, Broadhead M, Malaki M, Whitley G, Leiper J, MacAllister R, Vallance P. Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler Thromb Vasc Biol. 2003;23(8):1455–1459. doi: 10.1161/01.ATV.0000081742.92006.59. [DOI] [PubMed] [Google Scholar]
- Angulo J, Vallejo S, El Assar M, Garcia-Septiem J, Sanchez-Ferrer CF, Rodriguez-Manas L. Age-related differences in the effects of alpha and gamma peroxisome proliferator-activated receptor subtype agonists on endothelial vasodilation in human microvessels. Exp Gerontol. 2012 doi: 10.1016/j.exger.2012.06.014. [DOI] [PubMed] [Google Scholar]
- Barzilay JI, Blaum C, Moore T, Xue QL, Hirsch CH, Walston JD, Fried LP. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Arch Intern Med. 2007;167(7):635–641. doi: 10.1001/archinte.167.7.635. [DOI] [PubMed] [Google Scholar]
- Beltowski J, Kedra A. Asymmetric dimethylarginine (ADMA) as a target for pharmacotherapy. Pharmacol Rep. 2006;58(2):159–178. [PubMed] [Google Scholar]
- Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, Karunananthan S, Wolfson C. Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62(7):731–737. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berni A, Giuliani A, Tartaglia F, Tromba L, Sgueglia M, Blasi S, Russo G (2011) Effect of vascular risk factors on increase in carotid and femoral intima-media thickness: identification of a risk scale. Atheroscler 216(1):109–114. doi:10.1016/j.atherosclerosis.2011.01.034 [DOI] [PubMed]
- Carcaillon L, Blanco C, Alonso-Bouzon C, Alfaro-Acha A, Garcia-Garcia FJ, Rodriguez-Manas L. Sex differences in the association between serum levels of testosterone and frailty in an elderly population: the Toledo Study for Healthy Aging. PLoS One. 2012;7(3):e32401. doi: 10.1371/journal.pone.0032401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcaillon L, Garcia-Garcia FJ, Tresguerres JA, Gutierrez Avila G, Kireev R, Rodriguez-Manas L. Higher levels of endogenous estradiol are associated with frailty in postmenopausal women from the Toledo Study for Healthy Aging. J Clin Endocrinol Metab. 2012 doi: 10.1210/jc.2012-1271. [DOI] [PubMed] [Google Scholar]
- Cooke JP. ADMA: its role in vascular disease. Vasc Med. 2005;10(Suppl 1):S11–17. doi: 10.1177/1358836X0501000103. [DOI] [PubMed] [Google Scholar]
- Chen XM, Xia J, Zhou T, Yuan Q, Zhang WF, Hu CP, Li YJ, Jiang JL. Involvement of DDAH/ADMA pathway in the pathogenesis of rheumatoid arthritis in rats. Int Immunopharmacol. 2013;16(2):322–331. doi: 10.1016/j.intimp.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Chin APMJ, Dekker JM, Feskens EJ, Schouten EG, Kromhout D. How to select a frail elderly population? A comparison of three working definitions. J Clin Epidemiol. 1999;52(11):1015–1021. doi: 10.1016/S0895-4356(99)00077-3. [DOI] [PubMed] [Google Scholar]
- Dogru T, Genc H, Tapan S, Ercin CN, Ors F, Aslan F, Kara M, Sertoglu E, Bagci S, Kurt I, Sonmez A. Elevated asymmetric dimethylarginine in plasma: an early marker for endothelial dysfunction in non-alcoholic fatty liver disease? Diabetes Res Clin Pract. 2012;96(1):47–52. doi: 10.1016/j.diabres.2011.11.022. [DOI] [PubMed] [Google Scholar]
- El Assar M, Angulo J, Vallejo S, Peiro C, Sanchez-Ferrer CF, Rodriguez-Manas L. Mechanisms involved in the aging-induced vascular dysfunction. Front Physiol. 2012;3:132. doi: 10.3389/fphys.2012.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espelt A, Goday A, Franch J, Borrell C. Validity of self-reported diabetes in health interview surveys for measuring social inequalities in the prevalence of diabetes. J Epidemiol Community Health. 2012;66(7):e15. doi: 10.1136/jech.2010.112698. [DOI] [PubMed] [Google Scholar]
- Espinola-Klein C, Rupprecht HJ, Bickel C, Lackner K, Savvidis S, Messow CM, Munzel T, Blankenberg S. Different calculations of ankle–brachial index and their impact on cardiovascular risk prediction. Circulation. 2008;118(9):961–967. doi: 10.1161/CIRCULATIONAHA.107.763227. [DOI] [PubMed] [Google Scholar]
- Espinoza SE, Jung I, Hazuda H. Lower frailty incidence in older Mexican Americans than in older European Americans: the San Antonio Longitudinal Study of Aging. J Am Geriatr Soc. 2010;58(11):2142–2148. doi: 10.1111/j.1532-5415.2010.03153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia FJ, Gutierrez Avila G, Alfaro-Acha A, Amor Andres MS, De Los Angeles De La Torre Lanza M, Escribano Aparicio MV, Humanes Aparicio S, Larrion Zugasti JL, Gomez-Serranillo Reus M, Rodriguez-Artalejo F, Rodriguez-Manas L. The prevalence of frailty syndrome in an older population from Spain. The Toledo Study for Healthy Aging. J Nutr Health Aging. 2011;15(10):852–856. doi: 10.1007/s12603-011-0075-8. [DOI] [PubMed] [Google Scholar]
- Garcia Garcia FJ, Sanchez Ayala MI, Perez Martin A, Martin Correa E, Marsal Alonso C, Rodriguez Ferrer G, Garcia Colmenero C, Romero Rizos L, Rodriguez Barqueroa MJ, Gutierrez Avila G. The prevalence of dementia and its main subtypes in subjects older than 65 years: impact of occupation and education. The Toledo Study. Med Clin (Barc) 2001;116(11):401–407. doi: 10.1016/S0025-7753(01)71849-0. [DOI] [PubMed] [Google Scholar]
- Gill TM, Gahbauer EA, Allore HG, Han L. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166(4):418–423. doi: 10.1001/archinte.166.4.418. [DOI] [PubMed] [Google Scholar]
- Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47(6):1239–1312. doi: 10.1016/j.jacc.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Hsu CP, Lin SJ, Chung MY, Lu TM. Asymmetric dimethylarginine predicts clinical outcomes in ischemic chronic heart failure. Atherosclerosis. 2012;225(2):504–510. doi: 10.1016/j.atherosclerosis.2012.09.040. [DOI] [PubMed] [Google Scholar]
- Ito A, Tsao PS, Adimoolam S, Kimoto M, Ogawa T, Cooke JP. Novel mechanism for endothelial dysfunction: dysregulation of dimethylarginine dimethylaminohydrolase. Circulation. 1999;99(24):3092–3095. doi: 10.1161/01.CIR.99.24.3092. [DOI] [PubMed] [Google Scholar]
- Kielstein JT, Bode-Boger SM, Frolich JC, Ritz E, Haller H, Fliser D. Asymmetric dimethylarginine, blood pressure, and renal perfusion in elderly subjects. Circulation. 2003;107(14):1891–1895. doi: 10.1161/01.CIR.0000060496.23144.A7. [DOI] [PubMed] [Google Scholar]
- Lim S, Hong J, Liu CT, Hivert MF, White CC, Murabito JM, O'Donnell CJ, Dupuis J, Florez JC, Meigs JB (2013) Common variants in and near IRS1 and subclinical cardiovascular disease in the Framingham Heart Study. Atheroscler 229(1):149–154. doi:10.1016/j.atherosclerosis.2013.03.037 [DOI] [PMC free article] [PubMed]
- Meinitzer A, Seelhorst U, Wellnitz B, Halwachs-Baumann G, Boehm BO, Winkelmann BR, Marz W. Asymmetrical dimethylarginine independently predicts total and cardiovascular mortality in individuals with angiographic coronary artery disease (the Ludwigshafen Risk and Cardiovascular Health study) Clin Chem. 2007;53(2):273–283. doi: 10.1373/clinchem.2006.076711. [DOI] [PubMed] [Google Scholar]
- Mulero J, Zafrilla P, Martinez-Cacha A. Oxidative stress, frailty and cognitive decline. J Nutr Health Aging. 2011;15(9):756–760. doi: 10.1007/s12603-011-0130-5. [DOI] [PubMed] [Google Scholar]
- Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005;46(3):454–462. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- Newman AB, Gottdiener JS, McBurnie MA, Hirsch CH, Kop WJ, Tracy R, Walston JD, Fried LP. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56(3):M158–166. doi: 10.1093/gerona/56.3.M158. [DOI] [PubMed] [Google Scholar]
- Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, Pahor M, Satterfield S, Brach JS, Studenski SA, Harris TB. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295(17):2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- Perticone F, Sciacqua A, Maio R, Perticone M, Maas R, Boger RH, Tripepi G, Sesti G, Zoccali C. Asymmetric dimethylarginine, l-arginine, and endothelial dysfunction in essential hypertension. J Am Coll Cardiol. 2005;46(3):518–523. doi: 10.1016/j.jacc.2005.04.040. [DOI] [PubMed] [Google Scholar]
- Phan HM, Alpert JS, Fain M. Frailty, inflammation, and cardiovascular disease: evidence of a connection. Am J Geriatr Cardiol. 2008;17(2):101–107. [PubMed] [Google Scholar]
- Purser JL, Kuchibhatla MN, Fillenbaum GG, Harding T, Peterson ED, Alexander KP. Identifying frailty in hospitalized older adults with significant coronary artery disease. J Am Geriatr Soc. 2006;54(11):1674–1681. doi: 10.1111/j.1532-5415.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- Rentoukas E, Tsarouhas K, Kaplanis I, Korou E, Nikolaou M, Marathonitis G, Kokkinou S, Haliassos A, Mamalaki A, Kouretas D, Tsitsimpikou C. Connection between telomerase activity in PBMC and markers of inflammation and endothelial dysfunction in patients with metabolic syndrome. PLoS One. 2012;7(4):e35739. doi: 10.1371/journal.pone.0035739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Mañas L, El-Assar M, Vallejo S, Lopez-Doriga P, Solis J, Petidier R, Montes M, Nevado J, Castro M, Gomez-Guerrero C, Peiro C, Sanchez-Ferrer CF. Endothelial dysfunction in aged humans is related with oxidative stress and vascular inflammation. Aging Cell. 2009;8(3):226–238. doi: 10.1111/j.1474-9726.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Mañas L, Feart C, Mann G, Vina J, Chatterji S, Chodzko-Zajko W, Gonzalez-Colaco Harmand M, Bergman H, Carcaillon L, Nicholson C, Scuteri A, Sinclair A, Pelaez M, Van der Cammen T, Beland F, Bickenbach J, Delamarche P, Ferrucci L, Fried LP, Gutierrez-Robledo LM, Rockwood K, Rodriguez Artalejo F, Serviddio G, Vega E. Searching for an operational definition of frailty: A Delphi method based consensus statement.The frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci. 2012 doi: 10.1093/gerona/gls119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Eggimann B, Cuenoud P, Spagnoli J, Junod J. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J Gerontol A Biol Sci Med Sci. 2009;64(6):675–681. doi: 10.1093/gerona/glp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuit AJ, Schouten EG, Westerterp KR, Saris WH. Validity of the Physical Activity Scale for the Elderly (PASE): according to energy expenditure assessed by the doubly labeled water method. J Clin Epidemiol. 1997;50(5):541–546. doi: 10.1016/S0895-4356(97)00010-3. [DOI] [PubMed] [Google Scholar]
- Schulze F, Maas R, Freese R, Schwedhelm E, Silberhorn E, Boger RH. Determination of a reference value for N(G), N(G)-dimethyl-l-arginine in 500 subjects. Eur J Clin Invest. 2005;35(10):622–626. doi: 10.1111/j.1365-2362.2005.01561.x. [DOI] [PubMed] [Google Scholar]
- Schulze F, Wesemann R, Schwedhelm E, Sydow K, Albsmeier J, Cooke JP, Boger RH. Determination of asymmetric dimethylarginine (ADMA) using a novel ELISA assay. Clin Chem Lab Med. 2004;42(12):1377–1383. doi: 10.1515/CCLM.2004.257. [DOI] [PubMed] [Google Scholar]
- Sibal L, Agarwal SC, Home PD, Boger RH. The role of asymmetric dimethylarginine (ADMA) in endothelial dysfunction and cardiovascular disease. Curr Cardiol Rev. 2010;6(2):82–90. doi: 10.2174/157340310791162659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitia S, Tomasoni L, Atzeni F, Ambrosio G, Cordiano C, Catapano A, Tramontana S, Perticone F, Naccarato P, Camici P, Picano E, Cortigiani L, Bevilacqua M, Milazzo L, Cusi D, Barlassina C, Sarzi-Puttini P, Turiel M. From endothelial dysfunction to atherosclerosis. Autoimmun Rev. 2010;9(12):830–834. doi: 10.1016/j.autrev.2010.07.016. [DOI] [PubMed] [Google Scholar]
- Stuhlinger MC, Oka RK, Graf EE, Schmolzer I, Upson BM, Kapoor O, Szuba A, Malinow MR, Wascher TC, Pachinger O, Cooke JP. Endothelial dysfunction induced by hyperhomocyst(e)inemia: role of asymmetric dimethylarginine. Circulation. 2003;108(8):933–938. doi: 10.1161/01.CIR.0000085067.55901.89. [DOI] [PubMed] [Google Scholar]
- Teerlink T. ADMA metabolism and clearance. Vasc Med. 2005;10(Suppl 1):S73–81. doi: 10.1177/1358836X0501000111. [DOI] [PubMed] [Google Scholar]
- Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, Ershler WB, Harris T, Fried LP. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54(6):991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, Gottdiener J, Fried LP. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162(20):2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, Masaki K, Murray A, Newman AB. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc. 2005;53(8):1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- Xiao S, Wagner L, Schmidt RJ, Baylis C. Circulating endothelial nitric oxide synthase inhibitory factor in some patients with chronic renal disease. Kidney Int. 2001;59(4):1466–1472. doi: 10.1046/j.1523-1755.2001.0590041466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Buzkova P, Wassel CL, Roman MJ, North KE, Crawford DC, Boston J, Brown-Gentry KD, Cole SA, Deelman E, Goodloe R, Wilson S, Heiss G, Jenny NS, Jorgensen NW, Matise TC, McClellan BE Jr, Nato AQ Jr, Ritchie MD, Franceschini N, Kao WH (2013) Lack of associations of ten candidate coronary heart disease risk genetic variants and subclinical atherosclerosis in four U.S. populations: the Population Architecture using Genomics and Epidemiology (PAGE) study. Atheroscler 228(2):390–399. doi:10.1016/j.atherosclerosis.2013.02.038 [DOI] [PMC free article] [PubMed]