Abstract
Chronic exposure of human skin to solar ultraviolet (UV) irradiation causes premature skin aging, which is characterized by reduced type I collagen production and increased fragmentation of the dermal collagenous extracellular matrix. This imbalance of collagen homeostasis is mediated, in part, by elevated expression of the matricellular protein cysteine-rich protein 61 (CCN1), in dermal fibroblasts, the primary collagen producing cell type in human skin. Here, we report that the actions of CCN1 are mediated by induction of interleukin 1β (IL-1β). CCN1 and IL-1β are strikingly induced by acute UV irradiation, and constitutively elevated in sun-exposed prematurely aged human skin. Elevated CCN1 rapidly induces IL-1β, inhibits type I collagen production, and upregulates matrix metalloproteinase-1, which degrades collagen fibrils. Blockade of IL-1β actions by IL-1 receptor antagonist largely prevents the deleterious effects of CCN1 on collagen homeostasis. Furthermore, knockdown of CCN1 significantly reduces induction of IL-1β by UV irradiation, and thereby partially prevents collagen loss. These data demonstrate that elevated CCN1promotes inflammaging and collagen loss via induction of IL-1β and thereby contributes to the pathophysiology of premature aging in chronically sun-exposed human skin.
Keywords: Inflammaging, CCN1, IL-1β, Collagen homeostasis, Fibroblasts
Introduction
Solar UV irradiation is a common environmental factor that can adversely affect the structure and function of human skin. Acute exposure to excessive UV irradiation leads to sunburn, pigmentation, inflammation, and immune suppression, whereas repeated chronic exposure leads to the accumulation of connective tissue damage and premature aging (solar aging) and cancer (Gilchrest and Yaar 1992; Fisher et al. 1997; Narayanan et al. 2010; Bernstein and Uitto 1996). One important mechanism by which UV irradiation initiates such deleterious alterations in human skin involves induction of proinflammatory cytokines (Kondo 2000; Krutmann 2000; Clydesdale et al. 2001). UV-inducible proinflammatory cytokines not only orchestrate skin defense responses, but also lead to dermal connective tissue damage. Proinflammatory cytokines such as IL-1β negatively regulate collagen homeostasis by inhibiting production and promoting degradation of collagen, and therefore contribute to premature skin aging (Fisher et al. 2002; Honda et al. 2008; Bauge et al. 2007). Accumulating evidence supports a new concept called “inflammaging”, which suggests that low-grade chronic elevation of proinflammatory mediators can be a driving force for long-term tissue damage and aging progression (Franceschi et al. 2007; Goto 2008). Although epidermal keratinocytes and skin leukocytes are a rich source of proinflammatory cytokines following UV irradiation and wounding, recent studies have shown that stromal cells, particularly dermal fibroblasts, also play an active role in the immune system by releasing cytokines, chemokines, and other inflammatory factors in response to tissue injury (Buckley 2011; Fisher et al. 2000). However, little notice has been paid to UV irradiation induction of proinflammatory cytokines in stromal dermal fibroblasts, the primary cells responsible for collagen homeostasis in human skin.
CCN1 is a member of the CCN family, which at present comprises six distinct members: cysteine-rich protein 61 (CCN1), connective tissue growth factor (CCN2), nephroblastoma overexpressed (CCN3), Wnt-inducted secreted protein-1 (CCN4), Wnt-inducted secreted protein-2 (CCN5), and Wnt-inducted secreted protein-3 (CCN6) (Lau and Lam 1999; Perbal 2004; Leask and Abraham 2006). CCN proteins are secreted and associate with the ECM. CCN proteins appear to be important regulators of diverse cellular functions including cell adhesion, proliferation, migration, chemotaxis, apoptosis, motility, and ECM remodeling in wound healing (Chen and Lau 2009; Lau and Lam 1999). We have previously reported that CCN1 is predominantly expressed in dermal fibroblasts and that elevated CCN1 negatively regulates collagen homeostasis by downregulating type I collagen and promoting its degradation in cultured dermal fibroblasts (Quan et al. 2006). However, little is known about the underlying mechanism by which CCN1 negatively regulates collagen homeostasis. Here we report that elevated CCN1 markedly induces IL-1β and thereby promotes inflammaging and collagen loss in chronically sun-exposed prematurely aged human skin.
Experimental procedures
Procurement of human skin samples and ultraviolet irradiation
Human skin punch biopsies were obtained from healthy adult human volunteers, as previously described (Fisher and Voorhees 1998). Sun-protected buttock skin of human volunteers (N = 6) were irradiated with 2 MED (minimum erythema dose) solar-simulated UV (SPEC 450 W xenon arc solar simulator, 0.00006 % UVC, 6.6 % UVB, 16.5 % UVA2, and 76.8 % UVA1) (Quan et al. 2004). 2MED UV irradiation dose was used in current study based on our previous studies (Fisher et al. 1996; Fisher et al. 1997). The UV dose that caused slight skin reddening (minimal erythema dose) was determined for each subject 24 h after UV irradiation. Four-millimeter skin biopsies were taken at 8 and 24 h after UV exposure. Adult human skin punch biopsies of premature aged skin and subject matched sun-protected skin (N = 6) were obtained from the extensor forearm and underarm, respectively. The presence of severely premature aged skin was determined based on clinical criteria, as described previously (Griffiths et al. 1992). The study involving human subjects was approved by the institutional review board at the University of Michigan, and all subjects provided written informed consent before entering the study.
Cell culture and UV irradiation
Adult human primary dermal fibroblasts were prepared from punch biopsies of adult normal buttock skin (aged 21–55 years), as described previously (Fisher et al. 1991). Cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10 % fetal bovine serum (Invitrogen, Carlsbad, CA), penicillin (100 U/ml), and streptomycin (100 μg/ml) in a humidified incubator with 5 % CO2 at 37 °C. Cells were plated at 70–80 % confluence, and used 1 day after plating. Cells were utilized between passages 4 and 10. Independent replicates of studies, indicated by ‘N’ in the figure legends, were performed with cells from different individuals. UV irradiation of human skin primary dermal fibroblasts was performed as described previously (Quan et al. 2001). Briefly, sub-confluent cells were irradiated with UVB (50 mJ/cm2) using an Ultralite Panelite lamp containing six FS24T12 UVB-HO bulbs (47 % UVB, 18 % UVA2, 9 % UVA1, 26 % visible light). UVC irradiation (wavelengths below 290 nm) was removed by a Kodacel TA410/407 filter (Eastman Kodak, Rochester, NY). UVB irradiation dose (50 mJ/cm2) used in the current study was determined based on our previous study (Quan et al. 2010). The intensity of UV irradiation was monitored with an IL400A phototherapy radiometer and a SED240/UVB/W photodetector (International Light, Newbury, MA).
RNA isolation and quantitative real-time RT-PCR
Full-thickness normal human skin samples were physically separated by dissection into epidermis and dermis (Quan et al. 2002). Total RNA was extracted from dermis using a commercial kit (RNeasy mini kit, Qiagen, Chatsworth, CA) and gene expression levels were determined in the dermis. Total RNA (100 ng) was reverse transcribed using a Taqman Reverse Transcription kit (Applied Biosystems, Foster City, CA). Real-time RT-PCR was performed in duplicate with 2 μl of cDNA using the TaqMan Universal PCR Master Mix kit (Applied Biosystems) and a 7300 sequence detector system (Applied Biosystems). PCR procedures were performed using a robotic workstation (Biomek 2000; Beckman Coulter, Inc., Hialeah, FL) to ensure accuracy and reproducibility. PCR primers and probes were obtained from the Applied Biosystems custom oligonucleotide synthesis service. Primers and FAM-labeled probes for real-time PCR were as follows: CCN1 sense primer, 5′-TCAAAGACCTGTGGAACTGGTATC-3′, antisense primer, 5′-CACAAATCCGGGTTTCTTTCA-3′, and probe, 5′-CAATGACAACCCTGAGTG-CCGCCT-3′; IL-1β, sense primer, 5′-TTCTTCGACACATGGGATAACG-3′, anti-sense primer, 5′-TCCCGGAGCGTGCAGTTCA-3′, and probe, 5′-TAACGAGG-CTTATGTGCACGATGCACCTGTACGATCA-3′; matrix metalloproteinase-1 (MMP-1) sense primer, 5′-GGGAGATCATCGGGACAACTC-3′, antisense primer, 5′-AATACCTGGGCCTGG-TTGAAA-3′, and probe, 5′-TGAGCAAGATTTCCTCCAGGTCCATCAA-3′. The type I procollagen and 36B4 primers and probes were as described previously (Quan et al. 2004). Multiplex PCR reactions contained primers and probes for the target gene and 36B4, a ribosomal protein used as an internal normalization control for quantitation. The PCR amplification conditions were as follows: 50 °C, 2 min; 95 °C, 10 min; and 40 cycles of 95 °C, 15 s and 60 °C, 1 min. Target gene levels were normalized to the housekeeping gene 36B4.
Western analysis and ELISA
Dermal fibroblasts were treated with recombinant human CCN1 (10 μg/ml, PeproTech, Rocky Hill, NJ), recombinant human IL-1β (10 ng/ml, R&D Systems, Minneapolis, MN) or IL-1RA (50 ng/ml, R&D Systems, Minneapolis, MN). Whole cell proteins were prepared and equal amounts of protein (∼50 μg/lane) were analyzed for each sample by resolving on 10 % sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The SDS gels were transferred to polyvinylidene difluoride membrane, and the membranes were blocked with PBST (0.1 % Tween 20 in PBS) containing 5 % nonfat milk for 1 h at room temperature. Primary antibodies (Type I collagen, SouthernBiotech, Birmingham, AL; MMP-1 and CCN1, Santa Cruz Biotechnology, Santa Cruz, CA; β-actin, Sigma, St. Louis, MO) were incubated with the polyvinylidene difluoride membrane for 1 h at room temperature, after which the membranes were washed three times with PBST solution and incubated with the appropriate secondary antibody for 1 h at room temperature. After washing three times with PBST, the blots were developed with ECF (Vistra ECF Western blotting system, GE Health Care, Piscataway, NJ) following the manufacturer’s protocol. The membranes were scanned with a STORM PhosphorImager (Molecular Dynamics, Sunnyvale, CA). The intensities of each band were quantified using ImageQuant (GE HealthCare, Piscataway, NJ) and normalized using β-actin as a marker for equal protein loading. IL-1β protein levels were determined by ELISA using a commercial kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s protocol.
Transfection
CCN1 expression vector (Quan et al. 2006) and CCN1 siRNA (Quan et al. 2010) were transiently transfected into human skin fibroblasts by electroporation (Amaxa Nucleofector™, Koeln, Germany), and analysis was performed after 2-days transfection.
Three-dimensional collagen lattice cell cultures
Dermal fibroblasts were cultured in three-dimensional collagen lattices, as previously described (Fisher et al. 2009). Briefly, collagen lattices were prepared by mixing appropriate volume of rat tail type I collagen (BD Biosciences, San Diego, CA) with medium cocktail [DMEM, NaHCO3 (44 mmol/L), l-glutamine (4 mmol/L), folic acid (9 mmol/L), and neutralized with 1 N NaOH to pH 7.2] to yield a final concentration of 2 mg/ml. Fibroblasts (2.5 × 105) were suspended in 2 ml collagen solution in 35-mm culture dish, and placed in an incubator at 37 °C for 30 min to allow polymerization of the collagen. After polymerization, collagen lattices were detached from sides and bottom of the dish, covered with 2 ml of media (DMEM, 10 % fetal bovine serum) and incubated at 37 °C, 5 % CO2. Collagen lattices were washed with PBS and then treated with UV irradiation (UVB, 50 mJ/cm2). To activate secreted MMP-1, collagen lattices were washed extensively with PBS (at least three times for 30 min), and then treated with Trypsin-EDTA (100 ng/ml, Invitrogen) in serum-free media for 24 h (Quan et al. 2012).
Atomic force microscopy imaging
Human skin and three-dimensional collagen lattices were embedded in OCT and 20-μm cryosections were mounted on microscope glass slides (25 × 75 × 1.0 mm, Fisher Scientific, Pittsburgh, PA), and allowed to air dry for at least 48 h before atomic force microscopy (AFM) image analysis (Quan et al. 2012). Collagen fibrils images were obtained by ScanAsyst mode (Dimension Icon, Bruker-AXS, Santa Barbara, CA) in air using a silicon etched cantilever (NSC15/AIBS, MikroMasch, San Jose, CA) with a full tip cone angle ∼40° and the tip radius of curvature ∼10 nm. AFM images were acquired at a scan rate of 0.977 Hz, 512 × 512 pixel resolutions. AFM imaging was conducted at the Electron Microbeam Analysis Laboratory (EMAL), University of Michigan College of Engineering, and analyzed using Nanoscope Analysis software (Nanoscope Analysis v120R1sr3, Bruker-AXS, Santa Barbara, CA).
Statistical analysis
Data are expressed as the mean ± SEM. Student’s t test was used to evaluate the statistical differences between the groups. All p values are two-tailed, and values less than 0.05 were considered statistically significant.
Results
CCN1 and IL-1β are induced in the dermis of acutely UV-irradiated and chronically sun-exposed, prematurely aged human skin in vivo
We have previously reported that CCN1 is predominantly expressed in the dermis of human skin and elevated CCN1 negatively regulates collagen homeostasis in human dermal fibroblasts (Quan et al. 2006). These data prompted us to explore the possibility that CCN1 is involved in the mechanism that leads to impaired collagen production, which occurs following acute UV irradiation and in chronically sun-exposed, prematurely aged human skin (Fisher et al. 2000; Talwar et al. 1995; Fisher et al. 1997; Fisher et al. 1996). We first investigated regulation of CCN1, type I collagen, and matrix metalloproteinase-1 (MMP-1) by UV irradiation in human dermis in vivo. Human buttocks skin was exposed to solar-simulated UV irradiation and skin samples were obtained 8 and 24 h post-irradiation. Prior to analysis, the epidermis was removed (Quan et al. 2002). We found that CCN1 mRNA was rapidly and markedly induced in the dermis of acutely UV-irradiated human skin (Fig. 1a). CCN1 mRNA was strongly induced at 8 h (19-fold) and further increased at 24 h (33-fold) post-UV irradiation. Coincident with induction of CCN1, mRNA expression of type I collagen, the major structural protein in the human skin, was significantly reduced by acute UV irradiation in the dermis (Fig. 1b). In contrast, mRNA expression of collagen-degrading MMP-1, the primary collagen-degrading enzyme in human skin, was significantly elevated by acute UV irradiation in the dermis (Fig. 1c).
Fig. 1.
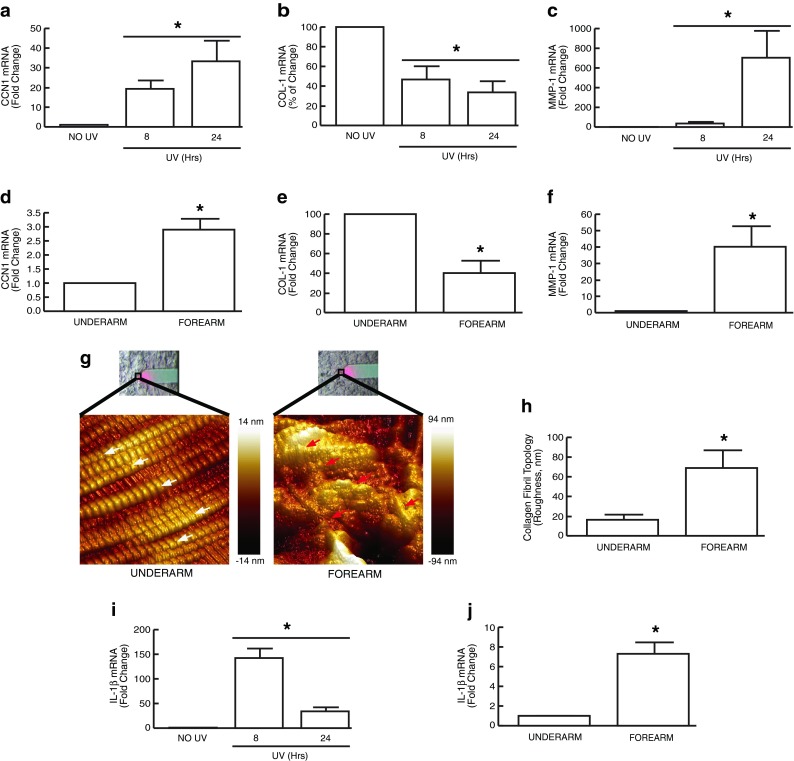
CCN1 and IL-1β are induced by acute UV irradiation and chronically sun-exposed prematurely aged human dermis in vivo. Adult human buttock skin was exposed to solar-simulated UV irradiation (2 MED) and skin samples were obtained at the indicated time points. Sun-protected and chronically sun-exposed prematurely aged skin was obtained from underarm and forearm, respectively. Prior to all analyses, epidermis was removed from dermis by dissection (Quan et al. 2002). mRNA levels were quantified using real-time RT-PCR, and normalized to the housekeeping gene, 36B4 (internal control). CCN1 mRNA is induced by UV irradiation (a); COL-1 mRNA is reduced by UV irradiation (b); MMP-1 mRNA is induced by UV irradiation (c); CCN1 mRNA is constitutively elevated in chronically sun-exposed aged forearm dermis (d); COL-1 mRNA is constitutively reduced in prematurely aged forearm dermis (e); MMP-1 mRNA is constitutively elevated in chronically sun-exposed prematurely aged forearm dermis (f). Representative AFM images of sun-protected underarm dermis (g, left panel) and chronically sun-exposed prematurely aged forearm dermis (g, right panel). The white and red arrows indicate intact and damaged collagen fibrils, respectively. Bright field images showing the AFM cantilever positioned on the dermis are shown above each AFM image. Colors represent topological dispersion of collagen fibrils in the z plane. Color coded scale is shown to the right of each AFM image. Collagen fibril topology, a measure of fibril organization, was quantified using Nanoscope Analysis software (h). IL-1β mRNA is induced by UV irradiation (i) and constitutively elevated in chronically sun-exposed prematurely aged forearm dermis (j). Results are expressed as the mean ± SEM, N = 6, *p < 0.05
We also found that CCN1 was constitutively elevated (3-fold) in the dermis of chronically sun-exposed prematurely aged human skin (Fig. 1d), compared to sun-protected underarm skin. This increase was associated with constitutively reduced type I collagen (Fig. 1e), and constitutively elevated MMP-1 in forearm chronically sun-exposed prematurely aged human skin (Fig. 1f). Hallmarks of skin aging are fragmentation and disorganization of collagen fibrils in the dermis (Fisher et al. 2002, 2008). We used nanoscale atomic force microscopy (AFM) to image and quantify collagen fibril organization in sun-protected and chronically sun-exposed prematurely aged skin. AFM revealed that collagen fibrils in sun-protected underarm dermis were intact, tightly packed, and well organized (Fig. 1g, left panel). In contrast, collagen fibrils in sun-exposed prematurely aged forearm dermis were fragmented, sparse, and disorganized (Fig. 1g, right panel). Quantitation of this disorganization by topographical analysis indicated that the collagen fibrillar network in prematurely aged forearm dermis was 4.2 times more spread out per unit area, than in sun-protected underarm dermis (69 nm vs. 16 nm, Fig. 1h).
It has been reported that CCN1 is induced by cutaneous wounding (Chen et al. 2001) and modulates immune responses by modulating expression of cytokines and other inflammatory factors (Kular et al. 2011; Bai et al. 2010; Jun and Lau 2010). It is also known that UV irradiation-inducible inflammatory cytokines negatively regulate collagen homeostasis (Fisher et al. 2002; Honda et al. 2008; Krutmann 2000; Fisher and Voorhees 1998). Therefore, we investigated whether CCN1 is involved in UV irradiation induction of inflammatory cytokines. We focused IL-1β, which is one of the primary cytokines induced by UV irradiation in human skin in vivo, and has been shown to negatively regulate collagen production (Faustin and Reed 2008; Feldmeyer et al. 2007; Kondo et al. 1994; Bauge et al. 2007). We found that the basal level of IL-1β in normal human dermis is very low, but markedly increased following acute UV irradiation (Fig. 1i). IL-1β mRNA was strongly induced at 8 h (142-fold) and remained elevated until 24 h (20-fold) post-UV irradiation. Notably, IL-1β, like CCN1, was constitutively elevated in the dermis of forearm premature aged human skin (Fig. 1j, 7-fold).
CCN1 and IL-1β negatively regulate collagen homeostasis in human dermal fibroblasts
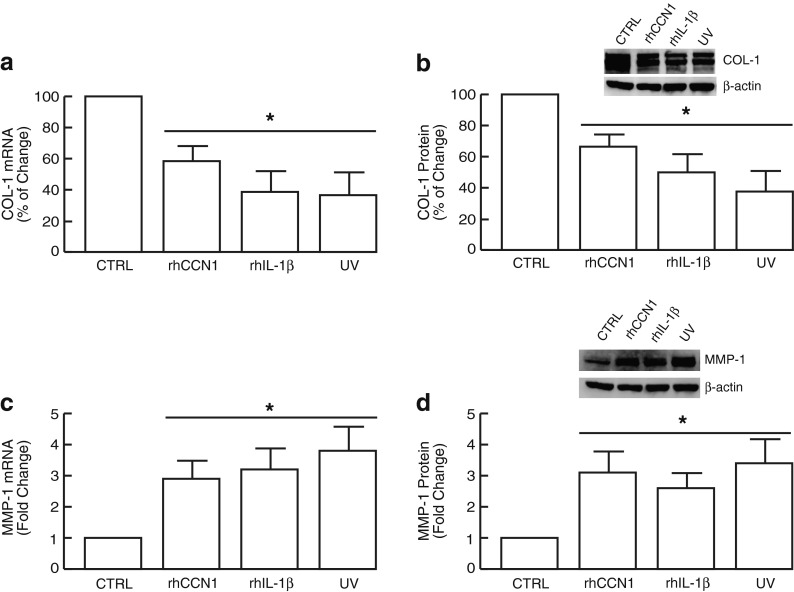
The above data indicate that dermal expression of both CCN1 and IL-1β are markedly induced by acute UV irradiation and constitutively elevated in chronically sun-exposed prematurely aged human skin in vivo. We next investigated the functional consequences of elevated CCN1 and IL-1β in the context of collagen homeostasis in dermal fibroblasts, the primary collagen-producing cells in human skin. Addition of either rhCCN1 or rhIL-1β significantly inhibited type I collagen mRNA and protein expression (Fig. 2a, b) and stimulated MMP-1 mRNA and protein expression (Fig. 2c, d). The magnitude of these alterations was similar to those observed in response to UV irradiation (Fig. 2a-d). These data suggest that elevated CCN1 and IL-1β may contribute to UV irradiation-induced premature skin aging by negatively regulating collagen homeostasis.
Fig. 2.
CCN1 and IL-1β negatively regulate collagen homeostasis in human dermal fibroblasts. Human dermal fibroblasts were treated with rhCCN1 (10 μg/ml), rhIL-1β (10 ng/ml), or UV irradiated (UVB, 50 mJ/cm2) for 24 h. Type I procollagen mRNA (a) and protein (b); MMP-1 mRNA (c) and protein (d) levels were quantified by real-time RT-PCR and Western analysis, respectively. mRNA and protein levels were normalized to the housekeeping gene (36B4, internal control) and β-actin (loading control), respectively. Insets show representative Western blots. Results are expressed as the mean ± SEM, N = 3, *p < 0.05
Induction of IL-1β mediates negative regulation of collagen homeostasis by elevated CCN1 in human dermal fibroblasts
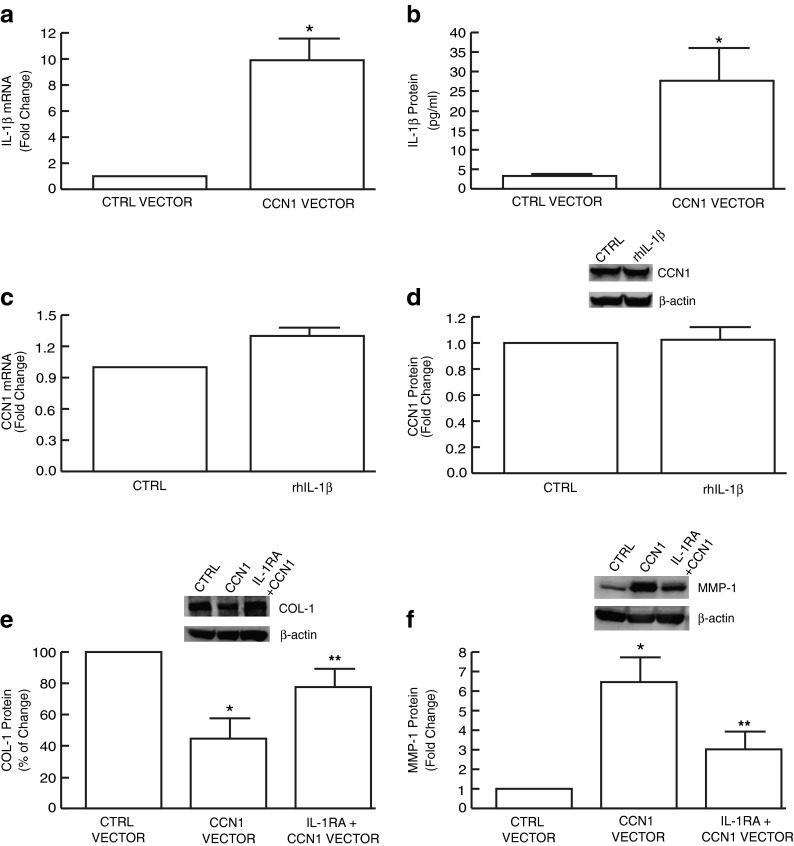
As both IL-1β and CCN1 are markedly upregulated by UV irradiation and negatively regulate collagen homeostasis, we next explored the functional relationship between CCN1 and IL-1β. Elevated expression of CCN1 in dermal fibroblasts resulted in significant upregulation of IL-1β mRNA (Fig. 3a) and protein (Fig. 3b) levels. In contrast, no significant change of CCN1 expression was observed following treatment of fibroblasts with IL-1β (Fig. 3c and d), indicating that CCN1 stimulates IL-1β expression, but not vice versa. These data prompted us to explore whether the ability of CCN1 to regulate collagen homeostasis is dependent on the actions of IL-1β. To investigate this possibility, IL-1β signaling was blocked by addition of naturally occurring IL-1 receptor antagonist, IL-1RA. IL-1RA partially prevented inhibition of type I collagen (Fig. 3e) and upregulation of MMP-1 (Fig. 3f) by elevated CCN1. These data suggest that IL-1β mediates, at least in part, regulation of type I collagen by CCN1.
Fig. 3.
CCN1 upregulates IL-1β, which mediates CCN1 regulation of i collagen homeostasis in human dermal fibroblasts. Human dermal fibroblasts were transiently transfected with CCN1 expression vector and harvested 48 h later. IL-1β mRNA (a) and protein (b) levels were quantified by real-time RT-PCR and ELISA, respectively. Human skin fibroblasts were treated with rhIL-1β (10 ng/ml) for 24 h and CCN1 mRNA (c) and protein (d) levels were quantified by real-time RT-PCR and Western analysis, respectively. CCN1-inhibition of type I collagen (e) and upregulation of MMP-1 (f) are attenuated by blocking IL-1β signaling. Human skin fibroblasts were transiently transfected with CCN1 expression vector and after 24 h treated with IL-1RA (100 ng/ml) for 24 h. mRNA and protein levels were normalized to the housekeeping gene (36B4, internal control) and β-actin (loading control), respectively. Insets show representative Western blots. Results are expressed as the mean ± SEM, N = 3, *p < 0.05
Negative regulation of collagen homeostasis is mediated by IL-1β in UV-irradiated human dermal fibroblasts
Having shown that CCN1 induces IL-1β, we next explored the functional relationship between IL-1β and CCN1 on collagen homeostasis in UV-irradiated human dermal fibroblasts. UV irradiation markedly induced CCN1 expression, and this induction was significantly reduced by CCN1 siRNA (Fig. 4a). Importantly, knockdown of CCN1 induction significantly reduced UV irradiation induction of IL-1β mRNA (Fig. 4b) and protein (Fig. 4c) levels, indicating that induction of IL-1β is dependent, in part, on induction of CCN1. Furthermore, knockdown of CCN1 induction and/or blocking IL-1β signaling by IL-1RA prevented UV irradiation-induced inhibition of type I collagen (Fig. 4d) and upregulation of MMP-1 (Fig. 4e).
Fig. 4.
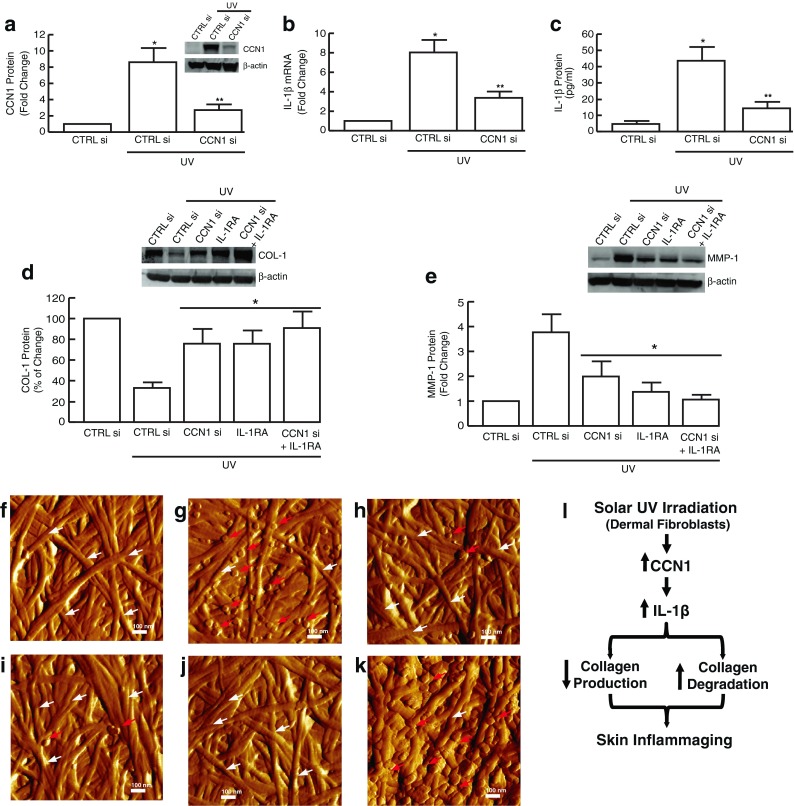
CCN1 induces aberrant collagen homeostasis through upregulation of IL-1β in UV-irradiated human dermal fibroblasts. Human dermal fibroblasts were transfected with CCN1 siRNA, treated with IL-1RA (100 ng/ml), and 24 h exposed to UV irradiation (UVB, 50 mJ/cm2). a CCN1 protein levels were quantified by Western analysis. b IL-1β mRNA and c protein levels were quantified by real-time RT-PCR and ELISA, respectively. d Type I procollagen and e MMP-1 protein levels were quantified by Western analysis. mRNA and protein levels were normalized to the housekeeping gene (36B4, internal control) and β-actin (loading control), respectively. Insets show representative Western blots. Results are expressed as the mean ± SEM, N = 3, *p < 0.05. Dermal fibroblasts were cultured in three-dimensional collagen lattices, treated as described below, and harvested 24 h after UV irradiation (UVB, 50 mJ/cm2). Representative nanoscale AFM images of collagen fibrils are shown (f–k). f Control; g UV irradiation; h CCN1 siRNA transfection followed 24 h later by UV irradiation; i IL-1RA (100 ng/ml) treatment for 24 h followed by UV irradiation; j combined treatment with CCN1 siRNA and IL-1RA for 24 h followed by UV irradiation; k positive control, activated rhMMP-1 (30 ng/ml) for 24 h. The white and red arrows indicate intact and fragmented collagen fibrils, respectively. N = 3. l Proposed model for the role of the CCN1/IL-1β axis promotes inflammaging in chronically sun-exposed premature skin aging (see “Discussion” for details)
Finally, we used nanoscale AFM to examine the impact of CCN1 and IL-1β induction by UV irradiation on collagen fibril structure and organization in three-dimensional collagen lattices. Fibroblasts were cultured in collagen lattices and mock exposed (Fig. 4f) or exposed (Fig. 4g) to UV irradiation alone or in the presence of CCN1 siRNA, IL-1RA, or both. AFM revealed that knockdown of CCN1 expression (Fig. 4h) and/or blocking IL-1β signaling by IL-1RA (Fig. 4i, j) partially prevented UV-induced fragmentation of collagen fibrils. Together, these data indicate that CCN1 regulates collagen homeostasis through upregulation of IL-1β in UV-irradiated human dermal fibroblasts.
Discussion
Although proinflammatory cytokines produced in the epidermis are believed to mediate UV irradiation-induced skin inflammation, little is known regarding the induction of proinflammatory cytokines in dermal fibroblasts. Recent studies have shown that stromal cells, particularly fibroblasts, are important sentinel cells in the immune system, and play a critical role as a source of immune modulators in chronic inflammation and tissue repair (Buckley et al. 2001; Buckley 2011). The capacity of stromal fibroblasts to produce cytokines, chemokines, and other inflammatory factors suggests that they play an active role in inflammatory diseases. Accumulating evidence suggests that fibroblasts are novel therapeutic targets in chronic inflammation (Buckley 2011; Buckley et al. 2001).
Here, we report that elevated CCN1 upregulates IL-1β in acutely UV-irradiated dermal fibroblasts and that CCN1 and IL-1β are elevated in chronically sun-exposed prematurely aged human dermis. It is noteworthy that both solar UVB and UVA irradiation readily penetrate into the dermis, rendering dermal fibroblasts accessible targets (Herrmann et al. 1993; Wlaschek et al. 2001) Our data suggest that dermal fibroblasts may function to promote immunocompetent cells through CCN1-mediated elevation of IL-1β. Recent evidence indicates that CCN proteins represent a new class of modulators of inflammation (Kular et al. 2011; Jun and Lau 2010; Chen et al. 2007). Consistent with this notion, CCN1 induced by wound healing activates a proinflammatory genetic program in human skin fibroblasts and murine macrophages (Chen et al. 2001; Bai et al. 2010). Evidence also indicates a potential role for CCN1 in chronic inflammatory diseases such as atherosclerosis, rheumatoid arthritis, inflammatory kidney diseases, and neuroinflammatory diseases (Kular et al. 2011).
We demonstrate that IL-1β is constitutively elevated in the dermis of chronically sun-exposed prematurely aged human skin. There is strong association of aging with chronic low-grade inflammatory activity which may progress to long-term tissue damage and systemic chronic inflammation (Buckley et al. 2001; Franceschi et al. 2007; Goto 2008). Accumulating evidence supports a new concept called “inflammaging”, which suggests that low-grade chronic elevation of proinflammatory mediators can be a driving force for the aging progress (Franceschi et al. 2007; Goto 2008). Therefore, it is conceivable that elevated CCN1 promotes inflammaging via sustained elevation of IL-1β, which in turn contributes to progressive skin damage in chronically sun-exposed human skin.
Mechanisms by which UV irradiation induces CCN1 and by which CCN1 upregulates IL-1β in human dermal fibroblasts remain to be determined. UV irradiation generates reactive oxygen species (ROS), which drive many cellular responses (Fisher et al. 2002; Rittie and Fisher 2002). We have found that CCN1 is significantly induced by ROS in dermal fibroblasts, and antioxidant significantly reduced induction of CCN1 by UV irradiation or hydrogen peroxide (unpublished data). Since UV irradiation is known to generate ROS, these data suggest that induction of CCN1 by UV irradiation may be mediated by ROS. We found that oxidative stress increases c-Jun, a critical member of AP-1 complex, and its binding to AP-1 site of the CCN1 promoter.. Functional blocking of c-Jun significantly reduces CCN1 promoter activity and gene expression induced by oxidative stress, suggesting CCN1 is transcriptionally regulated by oxidative stress via transcription factor c-Jun/AP-1. CCN1 is a secreted matricellular protein and is believed to function primarily through interactions with integrins, in a cell-type, and function-specific manner (Lau and Lam 1999; Chen and Lau 2009). Interestingly, CCN1 has also been shown to function as an activator of transcription factors AP-1 and NF-κB signaling, leading to the upregulation of multiple proinflammatory cytokines (Bai et al. 2010; Chen et al. 2001; Jun and Lau 2010; Quan et al. 2006). These data suggest that elevated CCN1 may upregulate IL-1β through activation of multiple pathways involving ROS, AP-1, and NF-κB signaling.
TGF-β pathway is the primary regulator of collagen homeostasis in human dermal fibroblasts, regulating both collagen biosynthesis and degradation (Varga et al. 1987; Chen et al. 2000; Hall et al. 2003; White et al. 2000). It has been reported that IL-1β impairs TGF-β signaling by downregulating the TGF-β type II receptor, resulting in the development of degenerative and inflammatory diseases such as osteoarthritis (Bauge et al. 2007). Consistent with this notion, we have previously reported that CCN1 impairs TGF-β signaling as a result of downregulation of the TGF-β type II receptor (Quan et al. 2006). Therefore, it is conceivable that elevated CCN1 impairs TGF-β signaling through upregulation of IL-1β. Indeed, we have previously reported that the TGF-β pathway is impaired in UV-irradiated and chronically sun-exposed prematurely aged human skin in vivo, largely due to downregulation of the TGF-β type II receptor (Quan et al. 2004; Quan et al. 2001).
In summary, we report here that CCN1 is markedly upregulated in the dermis of human skin in vivo by acute UV irradiation and constitutively elevated in chronically sun-exposed prematurely aged human skin. Elevated CCN1 in dermal fibroblasts upregulates IL-1β, which in turn inhibits collagen production and promotes collagen degradation. Therefore, we propose that the CCN1/IL-1β axis plays an important role in deleterious alterations to the dermal collagen network observed in chronically sun-exposed prematurely aged human dermis (Fig. 4i). Understanding molecular mechanisms of CCN1 actions may provide novel targets for therapeutic interventions to mitigate inflammation that deleteriously effects the dermal collagenous extracellular matrix in sun-exposed human skin.
Acknowledgments
We thank Suzan Rehbine for the procurement of tissue specimens, and Diane Fiolek and Patrick Robichaud for administrative assistance. This work was supported by the National Institute of Health (RO1 ES014697 and ES014697 30S1 to T. Quan).
Abbreviations
- UV
Ultraviolet
- CCN1
CCN family member 1
- CCN family
Cysteine-rich protein 61, connective tissue growth factor, nephroblastoma overexpressed
- IL-1β
Interleukin 1β
- COL-1
Type I collagen
- MMP-1
Matrix metalloproteinase-1
- ECM
Extracellular matrix
- AFM
Atomic force microscopy
- AP-1
Activator protein-1
- NF-κB
Nuclear factor kappa B
References
- Bai T, Chen CC, Lau LF. Matricellular protein CCN1 activates a proinflammatory genetic program in murine macrophages. J Immunol. 2010;184(6):3223–3232. doi: 10.4049/jimmunol.0902792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauge C, Legendre F, Leclercq S, Elissalde JM, Pujol JP, Galera P, Boumediene K. Interleukin-1beta impairment of transforming growth factor beta1 signaling by down-regulation of transforming growth factor beta receptor type II and up-regulation of Smad7 in human articular chondrocytes. Arthritis Rheum. 2007;56(9):3020–3032. doi: 10.1002/art.22840. [DOI] [PubMed] [Google Scholar]
- Bernstein EF, Uitto J. The effect of photodamage on dermal extracellular matrix. Clin Dermatol. 1996;14(2):143–151. doi: 10.1016/0738-081X(95)00149-A. [DOI] [PubMed] [Google Scholar]
- Buckley CD. Why does chronic inflammation persist: an unexpected role for fibroblasts. Immunol Lett. 2011;138(1):12–14. doi: 10.1016/j.imlet.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley CD, Pilling D, Lord JM, Akbar AN, Scheel-Toellner D, Salmon M. Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 2001;22(4):199–204. doi: 10.1016/S1471-4906(01)01863-4. [DOI] [PubMed] [Google Scholar]
- Chen CC, Lau LF. Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell Biol. 2009;41(4):771–783. doi: 10.1016/j.biocel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SJ, Yuan W, Lo S, Trojanowska M, Varga J. Interaction of smad3 with a proximal smad-binding element of the human alpha2(I) procollagen gene promoter required for transcriptional activation by TGF-beta. J Cell Physiol. 2000;183(3):381–392. doi: 10.1002/(SICI)1097-4652(200006)183:3<381::AID-JCP11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Chen CC, Mo FE, Lau LF. The angiogenic factor Cyr61 activates a genetic program for wound healing in human skin fibroblasts. J Biol Chem. 2001;276(50):47329–47337. doi: 10.1074/jbc.M107666200. [DOI] [PubMed] [Google Scholar]
- Chen CC, Young JL, Monzon RI, Chen N, Todorovic V, Lau LF. Cytotoxicity of TNFalpha is regulated by integrin-mediated matrix signaling. EMBO J. 2007;26(5):1257–1267. doi: 10.1038/sj.emboj.7601596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clydesdale GJ, Dandie GW, Muller HK. Ultraviolet light induced injury: immunological and inflammatory effects. Immunol Cell Biol. 2001;79(6):547–568. doi: 10.1046/j.1440-1711.2001.01047.x. [DOI] [PubMed] [Google Scholar]
- Faustin B, Reed JC. Sunburned skin activates inflammasomes. Trends Cell Biol. 2008;18(1):4–8. doi: 10.1016/j.tcb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Feldmeyer L, Keller M, Niklaus G, Hohl D, Werner S, Beer HD. The inflammasome mediates UVB-induced activation and secretion of interleukin-1beta by keratinocytes. Curr Biol. 2007;17(13):1140–1145. doi: 10.1016/j.cub.2007.05.074. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Voorhees JJ. Molecular mechanisms of photoaging and its prevention by retinoic acid: ultraviolet irradiation induces MAP kinase signal transduction cascades that induce Ap-1-regulated matrix metalloproteinases that degrade human skin in vivo. J Investig Dermatol Symp Proc. 1998;3(1):61–68. [PubMed] [Google Scholar]
- Fisher GJ, Esmann J, Griffiths CE, Talwar HS, Duell EA, Hammerberg C, Elder JT, Finkel LJ, Karabin GD, Nickoloff BJ, et al. Cellular, immunologic and biochemical characterization of topical retinoic acid-treated human skin. J Invest Dermatol. 1991;96(5):699–707. doi: 10.1111/1523-1747.ep12470632. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, Voorhees JJ. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379(6563):335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337(20):1419–1428. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Datta S, Wang Z, Li XY, Quan T, Chung JH, Kang S, Voorhees JJ. c-Jun-dependent inhibition of cutaneous procollagen transcription following ultraviolet irradiation is reversed by all-trans retinoic acid. J Clin Invest. 2000;106(5):663–670. doi: 10.1172/JCI9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, Voorhees JJ. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138(11):1462–1470. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Varani J, Voorhees JJ. Looking older: fibroblast collapse and therapeutic implications. Arch Dermatol. 2008;144(5):666–672. doi: 10.1001/archderm.144.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GJ, Quan T, Purohit T, Shao Y, Cho MK, He T, Varani J, Kang S, Voorhees JJ. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am J Pathol. 2009;174(1):101–114. doi: 10.2353/ajpath.2009.080599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128(1):92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Gilchrest BA, Yaar M. Ageing and photoageing of the skin: observations at the cellular and molecular level. Br J Dermatol. 1992;127(Suppl 41):25–30. doi: 10.1111/j.1365-2133.1992.tb16984.x. [DOI] [PubMed] [Google Scholar]
- Goto M. Inflammaging (inflammation + aging): A driving force for human aging based on an evolutionarily antagonistic pleiotropy theory? Biosci Trends. 2008;2(6):218–230. [PubMed] [Google Scholar]
- Griffiths CE, Wang TS, Hamilton TA, Voorhees JJ, Ellis CN. A photonumeric scale for the assessment of cutaneous photodamage. Arch Dermatol. 1992;128(3):347–351. doi: 10.1001/archderm.1992.01680130061006. [DOI] [PubMed] [Google Scholar]
- Hall MC, Young DA, Waters JG, Rowan AD, Chantry A, Edwards DR, Clark IM. The comparative role of activator protein 1 and Smad factors in the regulation of Timp-1 and MMP-1 gene expression by transforming growth factor-beta 1. J Biol Chem. 2003;278(12):10304–10313. doi: 10.1074/jbc.M212334200. [DOI] [PubMed] [Google Scholar]
- Herrmann G, Wlaschek M, Lange TS, Prenzel K, Goerz G, Scharffetter-Kochanek K. UVA irradiation stimulates the synthesis of various matrix-metalloproteinases (MMPs) in cultured human fibroblasts. Exp Dermatol. 1993;2(2):92–97. doi: 10.1111/j.1600-0625.1993.tb00015.x. [DOI] [PubMed] [Google Scholar]
- Honda A, Abe R, Makino T, Norisugi O, Fujita Y, Watanabe H, Nishihira J, Iwakura Y, Yamagishi S, Shimizu H, Shimizu T. Interleukin-1beta and macrophage migration inhibitory factor (MIF) in dermal fibroblasts mediate UVA-induced matrix metalloproteinase-1 expression. J Dermatol Sci. 2008;49(1):63–72. doi: 10.1016/j.jdermsci.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol. 2010;12(7):676–685. doi: 10.1038/ncb2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S. The roles of cytokines in photoaging. J Dermatol Sci. 2000;23(Suppl 1):S30–S36. doi: 10.1016/S0923-1811(99)00076-6. [DOI] [PubMed] [Google Scholar]
- Kondo S, Sauder DN, Kono T, Galley KA, McKenzie RC. Differential modulation of interleukin-1 alpha (IL-1 alpha) and interleukin-1 beta (IL-1 beta) in human epidermal keratinocytes by UVB. Exp Dermatol. 1994;3(1):29–39. doi: 10.1111/j.1600-0625.1994.tb00263.x. [DOI] [PubMed] [Google Scholar]
- Krutmann J. Ultraviolet A radiation-induced biological effects in human skin: relevance for photoaging and photodermatosis. J Dermatol Sci. 2000;23(Suppl 1):S22–S26. doi: 10.1016/S0923-1811(99)00077-8. [DOI] [PubMed] [Google Scholar]
- Kular L, Pakradouni J, Kitabgi P, Laurent M, Martinerie C. The CCN family: a new class of inflammation modulators? Biochimie. 2011;93(3):377–388. doi: 10.1016/j.biochi.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Lau LF, Lam SC. The CCN family of angiogenic regulators: the integrin connection. Exp Cell Res. 1999;248(1):44–57. doi: 10.1006/excr.1999.4456. [DOI] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119(Pt 23):4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- Narayanan DL, Saladi RN, Fox JL. Ultraviolet radiation and skin cancer. Int J Dermatol. 2010;49(9):978–986. doi: 10.1111/j.1365-4632.2010.04474.x. [DOI] [PubMed] [Google Scholar]
- Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004;363(9402):62–64. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- Quan T, He T, Voorhees JJ, Fisher GJ. Ultraviolet irradiation blocks cellular responses to transforming growth factor-beta by down-regulating its type-II receptor and inducing Smad7. J Biol Chem. 2001;276(28):26349–26356. doi: 10.1074/jbc.M010835200. [DOI] [PubMed] [Google Scholar]
- Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Connective tissue growth factor: expression in human skin in vivo and inhibition by ultraviolet irradiation. J Invest Dermatol. 2002;118(3):402–408. doi: 10.1046/j.0022-202x.2001.01678.x. [DOI] [PubMed] [Google Scholar]
- Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-beta type II receptor/Smad signaling. Am J Pathol. 2004;165(3):741–751. doi: 10.1016/S0002-9440(10)63337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, He T, Shao Y, Lin L, Kang S, Voorhees JJ, Fisher GJ. Elevated cysteine-rich 61 mediates aberrant collagen homeostasis in chronologically aged and photoaged human skin. Am J Pathol. 2006;169(2):482–490. doi: 10.2353/ajpath.2006.060128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, Qin Z, Xu Y, He T, Kang S, Voorhees JJ, Fisher GJ. Ultraviolet irradiation induces CYR61/CCN1, a mediator of collagen homeostasis, through activation of transcription factor AP-1 in human skin fibroblasts. J Invest Dermatol. 2010;130(6):1697–1706. doi: 10.1038/jid.2010.29. [DOI] [PubMed] [Google Scholar]
- Quan T, Qin Z, Voorhees JJ, Fisher GJ. Cysteine-rich protein 61 (CCN1) mediates replicative senescence-associated aberrant collagen homeostasis in human skin fibroblasts. J Cell Biochem. 2012;113(9):3011–3018. doi: 10.1002/jcb.24179. [DOI] [PubMed] [Google Scholar]
- Rittie L, Fisher GJ. UV-light-induced signal cascades and skin aging. Ageing Res Rev. 2002;1(4):705–720. doi: 10.1016/S1568-1637(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Talwar HS, Griffiths CE, Fisher GJ, Hamilton TA, Voorhees JJ. Reduced type I and type III procollagens in photodamaged adult human skin. J Invest Dermatol. 1995;105(2):285–290. doi: 10.1111/1523-1747.ep12318471. [DOI] [PubMed] [Google Scholar]
- Varga J, Rosenbloom J, Jimenez SA. Transforming growth factor beta (TGF beta) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem J. 1987;247(3):597–604. doi: 10.1042/bj2470597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LA, Mitchell TI, Brinckerhoff CE. Transforming growth factor beta inhibitory element in the rabbit matrix metalloproteinase-1 (collagenase-1) gene functions as a repressor of constitutive transcription. Biochim Biophys Acta. 2000;1490(3):259–268. doi: 10.1016/S0167-4781(00)00002-6. [DOI] [PubMed] [Google Scholar]
- Wlaschek M, Tantcheva-Poor I, Naderi L, Ma W, Schneider LA, Razi-Wolf Z, Schuller J, Scharffetter-Kochanek K. Solar UV irradiation and dermal photoaging. J Photochem Photobiol B. 2001;63(1–3):41–51. doi: 10.1016/S1011-1344(01)00201-9. [DOI] [PubMed] [Google Scholar]