ABSTRACT
BACKGROUND
Frailty is a multidimensional phenotype that describes declining physical function and a vulnerability to adverse outcomes in the setting of physical stress such as illness or hospitalization. Phase angle is a composite measure of tissue resistance and reactance measured via bioelectrical impedance analysis (BIA). Whether phase angle is associated with frailty and mortality in the general population is unknown.
OBJECTIVE
To evaluate associations among phase angle, frailty and mortality.
DESIGN
Population-based survey.
SETTING
Third National Health and Nutritional Examination Survey (1988–1994).
PARTICIPANTS
In all, 4,667 persons aged 60 and older.
MEASUREMENTS
Frailty was defined according to a set of criteria derived from a definition previously described and validated.
RESULTS
Narrow phase angle (the lowest quintile) was associated with a four-fold higher odds of frailty among women and a three-fold higher odds of frailty among men, adjusted for age, sex, race-ethnicity and comorbidity. Over a 12-year follow-up period, the adjusted relative hazard for mortality associated with narrow phase angle was 2.4 (95 % confidence interval [95 % CI] 1.8 to 3.1) in women and 2.2 (95 % CI 1.7 to 2.9) in men. Narrow phase angle was significantly associated with mortality even among participants with little or no comorbidity.
LIMITATIONS
Analyses of BIA and frailty were cross-sectional; BIA was not measured serially and incident frailty during follow-up was not assessed. Participants examined at home were excluded from analysis because they did not undergo BIA.
CONCLUSIONS
Narrow phase angle is associated with frailty and mortality independent of age and comorbidity.
KEY WORDS: aging, frailty, body composition, bioelectrical impedance analysis
INTRODUCTION
Frailty is a multidimensional phenotype that describes declining physical function and a vulnerability to adverse health outcomes in the setting of physical stress.1–5 Multiple instruments to operationalize a definition of frailty have been developed and validated.1 One index, proposed by Fried, defines frailty as the presence of three or more of five criteria: unintentional weight loss, exhaustion, weakness, slow walking speed, and low physical activity.4 Using these criteria, estimates of the prevalence of frailty among independently living adults vary from 7 % of persons older than 65 years to 40 % of persons older than 80 years; prevalence estimates are higher among persons with chronic disease.2–4,6 Frailty, using this definition, is associated with increased risk of falls, hospitalization, disability and death.4 However, diagnosing frailty in the clinical setting can be cumbersome, because most indices of frailty require a combination of anthropometry and physical function testing.
One technique to aid clinicians in the identification of frail persons is bioelectrical impedance analysis (BIA), a non-invasive test performed by placing an electrode on each of two body segments (e.g., arm or leg) and taking a measurement of the voltage drop across these segments. BIA was pioneered in the 1950s to estimate total body water, but recently has been used to estimate other body compartments, and forms the basis of commercial instruments to estimate percent body fat.7–9 Phase angle, a value calculated from BIA measurements and described in greater detail below, tends to decline with age, relates to the distribution of intracellular and extracellular fluid, and varies with the lipid content of cell membranes. For these reasons, it has been suggested that phase angle could reflect the overall integrity or vitality of living tissue.10–12 Moreover, phase angle appears to reflect prognosis in populations with chronic disease.13–18 Whether phase angle is associated with frailty and mortality in the general population is unknown.
We hypothesized that narrow phase angle would be associated with frailty and mortality in older adults, independent of the associations seen among frailty, advanced age and chronic health conditions.
METHODS
Data Source
We obtained individual-level data from the Third National Health and Nutrition Examination Survey (NHANES III), a nationally representative survey of the health status of US residents collected between 1988 and 1994. NHANES III is a cross-sectional, multistage, stratified, clustered probability sample of the US civilian non-institutionalized population conducted by the National Center for Health Statistics.19 The first of multiple planned mortality linkages was conducted in 2004, using a probabilistic matching algorithm linking NHANES III participants to the National Death Index. A public use version of the mortality linkage data became available in 2006, and contains months of follow-up and vital status on all NHANES III participants older than 17 years for whom sufficient data were available. Discussion of the specific methodology for probabilistic matching is published elsewhere.20 The Institutional Review Board for the Centers for Disease Control and Prevention (CDC) approved NHANES III, and all participants provided written consent. This study was granted exempt status by the Institutional Review Board of Stanford University School of Medicine.
Study Sample
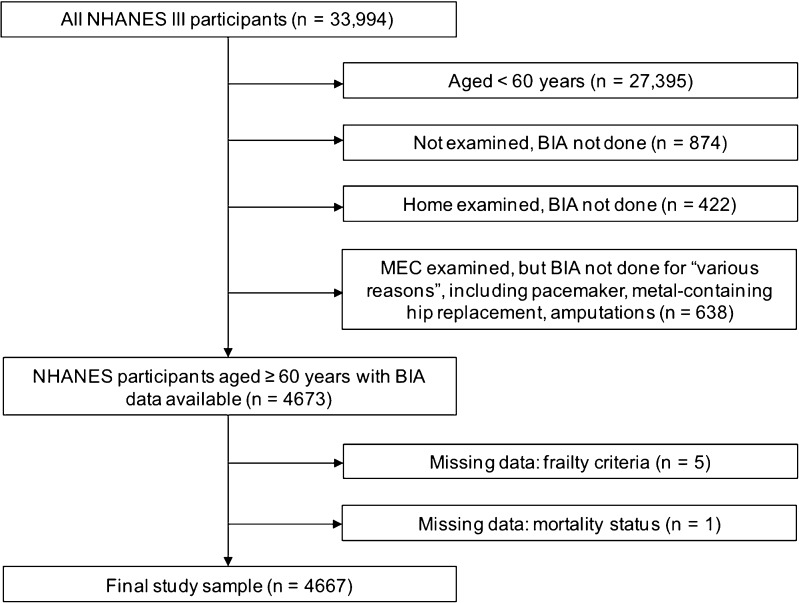
We identified all participants who completed an NHANES interview between 1988 and 1994 (n = 33,198). We limited the study population to persons aged 60 years or older at the time of their NHANES examination with BIA data available (n = 4,673). We excluded persons for whom sufficient data to assess frailty (described below) and mortality were unavailable (n = 6). Persons who chose to be examined in their homes rather than in the mobile examination center did not undergo BIA examination. Excluded home-examined participants (n = 422) were older (mean age 77.8 years), more likely to report non-Hispanic white ethnicity and more likely to suffer from multiple comorbidities than those examined in the mobile examination center. The analytic sample consisted of the remaining 4,667 participants (Fig. 1).
Figure 1.
Study sample.
Frailty
We defined frailty based on a modification of the Fried criteria.4 Our definition adheres to the five frailty domains previously established, but customizes the criteria for application to NHANES III data.
Low body weight for height, defined as BMI ≤ 18.5 kg/m2
Slow walking, defined as the slowest quintile adjusted for sex, in a timed eight-foot walk
Weakness, defined as present if participants answered “some difficulty”, “much difficulty”, or “unable to do” when asked how much difficulty they have “lifting or carrying something as heavy as ten pounds (like a sack of potatoes or rice)”
Exhaustion, defined as present if participants answered “some difficulty”, “much difficulty”, or “unable to do” when asked how much difficulty they have “walking from one room to the other on the same level”
Low physical activity, defined as present if participants answered “less active” when asked “Compared with most (men/women) your age, would you say that you are more active, less active or about the same?”
Persons with available data for three or more frailty domains were included in our analysis.
Bioelectrical Impedance Analysis
The Valhalla Scientific Body Composition Analyzer 1990 B was used to introduce a low amplitude (50 kHz) current across electrodes placed on the right hand and foot of participants, and to measure the resulting voltage drop across electrodes. From the voltage drop, impedance was calculated. Impedance is the frequency-dependent opposition to an alternating current. In biological systems, impedance is the vector sum of its component values, resistance (R) and reactance (Xc), both expressed in ohms (Ω). Resistance is directly proportional to the length of the measured body segment; resistance is inversely proportional to water and electrolyte concentration and the cross sectional area of the measured body segment. Reactance is inversely related to capacitance, which is the storage of charge by a circuit. In the body, capacitance results when regions of high conductivity (e.g., extracellular and intracellular water) are separated by regions of low conductivity (e.g., cell membranes). A body segment that contains a large number of cell membranes that charge and discharge in response to an alternating current will have higher reactance when compared to a body segment with fewer cell membranes capable of holding charge.
Phase angle was calculated as the arc tangent of the reactance to resistance ratio. Phase angle can range in theory from 0 to 90°: 0° if the circuit is only resistive (a system with no cell membranes) and 90° if the circuit is only capacitive (a system of membranes with no fluid).
Other Explanatory Variables
We ascertained participant race-ethnicity based on self-report (non-Hispanic white, non-Hispanic black, Mexican American and other). We considered participants to have diabetes if a physician had informed them that they had “diabetes” or if they recorded a hemoglobin A1c ≥ 6 %. We considered participants to have evidence of liver disease if they recorded an aspartate aminotransferase (AST) > 37 U/L or alanine aminotransferase (ALT) > 40 U/L for men and either AST or ALT > 31 U/L for women. We defined chronic kidney disease as either prevalent microalbuminuria in the presence of a normal estimated glomerular filtration rate (eGFR, calculated using the Mayo quadratic equation) or an eGFR < 60 mL/min/1.73 m2.21,22 We considered participants to have peripheral arterial disease if they reported calf pain with activity that was relieved with rest. Similarly, we considered participants to have coronary artery disease if they reported chest pain with activity that was relieved with rest, or if they reported a prior myocardial infarction. We identified participants with arthritis, cancer, chronic lung disease, congestive heart failure or history of stroke based on self-reported physician diagnosis. Questions used to identify these conditions are described in detail in a separate publication.23 We considered the presence of one or more of peripheral arterial disease, coronary artery disease, heart failure or stroke as “overt cardiovascular disease.” Blood pressure was measured according to a protocol described elsewhere24; hypertension was defined as a blood pressure > 140/90, in accord with the guidelines established in the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.25
Statistical Analysis
We conducted data analysis with SAS, accounting for oversampling, stratification and clustering.26 Because body composition differs widely by sex, we conducted sex stratified analyses for frailty and mortality. We ranked phase angle stratified by sex into quintiles, with the upper three quintiles serving as reference. We also examined phase angle as a continuous variable using a linear spline with a single knot at five and six degrees, for men and women, respectively. Results from this method were similar to the analysis by quintiles, so they are not presented here. We controlled for eight comorbidities (diabetes, history of non-skin cancer, chronic lung disease, chronic liver disease, chronic kidney disease, overt cardiovascular disease, arthritis, and hypertension); however, only five (diabetes, chronic lung disease, chronic kidney disease, cardiovascular disease and arthritis) were significantly associated with frailty and remained in our model. For the discrete frailty outcome, we used multivariable logistic regression. In logistic regression models, discrimination was assessed using the area under the receiver operating characteristic (ROC) curve. Calibration was assessed using the Hosmer-Lemeshow goodness-of-fit test.
To determine whether narrow phase angle was independently associated with mortality, we performed a proportional hazards (“Cox”) regression analysis, accounting for the complex survey design and sample weights (SUDAAN version 10, Cary, NC). We created two models, one for women and one for men, that adjusted for age and race-ethnicity as well as the five comorbidities noted above. To determine whether phase angle was associated with mortality among persons with limited comorbidity, we created two additional models: one restricted to participants with no more than one of hypertension, diabetes and arthritis and none of the other comorbid conditions (reflecting organ system failure) considered in our original models (n = 1,517), and another restricted to participants with none of the comorbid conditions (n = 414). Plots of log (−log [survival rate]) against log (survival time) were performed to establish the validity of the proportionality assumption. Since we looked at two outcomes (frailty and mortality) in sex-stratified analyses, we considered p values < 0.0125 (0.05/4) statistically significant.
RESULTS
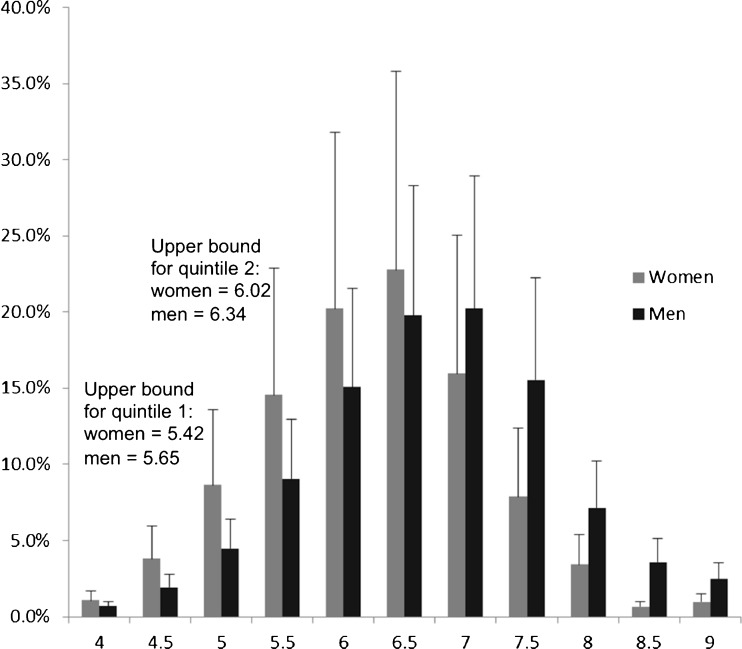
Of the 33,994 NHANES III participants, 4,667 participants met inclusion criteria (Table 1). Basic anthropomorphic and comorbidity data for this cohort have been published elsewhere.23 Among 2,379 women, the prevalence of frailty was 4.91 % (standard error of the percent 0.63), mean reactance was 64.2 Ω (standard error 0.6 Ω) and mean resistance was 584.4 Ω (standard error 4.8 Ω). Among 2,288 men, the prevalence of frailty was 2.66 % (standard error of the percent 0.46), mean reactance and mean resistance were 56.2 Ω (standard error 0.5 Ω) and 479.0 Ω (standard error 3.8 Ω), respectively. Calculated from these values, mean phase angle for women was 6.3° (standard error 0.05°); mean phase angle among men was 6.7° (standard error 0.05°). Distribution of phase angle among women and men is shown in Figure 2.
Table 1.
Study Population Characteristics
| Phase angle by quintile, % (s.e.) | |||
|---|---|---|---|
| 1–2 (2.66–6.02) | 3–5 (6.02–12.64) | p value | |
| Women n = 2,379 | |||
| Demography | |||
| Aged 60–69 years (n = 1,028) | 26.2 (3.3) | 63.7 (3.1) | < 0.0001* |
| Aged 70–79 years (n = 820) | 40.9 (3.1) | 30.2 (3.0) | |
| Aged > = 80 years (n = 528) | 32.9 (3.7) | 6.2 (1.2) | |
| Non-Hispanic white (n = 1,401) | 90.0 (1.5) | 80.1 (2.6) | < 0.0001† |
| Non-Hispanic black (n = 465) | 5.6 (1.1) | 10.4 (1.4) | |
| Mexican American (n = 419) | 1.1 (0.4) | 2.3 (0.4) | |
| Other race/ethnicity (n = 91) | 2.8 (1.0) | 7.2 (2.1) | |
| Comorbidities | |||
| Diabetes (n = 680) | 21.9 (2.3) | 20.8 (2.2) | 0.05§ |
| Cardiovascular disease (n = 526) | 24.5 (2.6) | 17.5 (2.3) | < 0.0001§ |
| Chronic kidney disease (n = 517) | 27.9 (3.2) | 15.6 (5.9) | < 0.0001§ |
| Chronic lung disease (n = 250) | 13.3 (1.6) | 11.9 (2.0) | 0.15§ |
| Chronic liver disease (n = 157) | 7.0 (2.0) | 6.3 (1.3) | 0.05§ |
| Cancer, non-skin (n = 201) | 11.9 (1.6) | 8.2 (1.8) | 0.006§ |
| Arthritis (n = 1,228) | 55.1 (2.8) | 46.1 (3.3) | 0.001§ |
| Hypertension (n = 1,720) | 74.5 (2.6) | 62.2 (3.5) | < 0.0001§ |
| Men n = 2,288 | |||
| Demography | |||
| Aged 60–69 years (n = 1,047) | 27.3 (3.6) | 67.4 (2.9) | < 0.0001* |
| Aged 70–79 years (n = 730) | 45.4 (3.3) | 28.3 (2.7) | |
| Aged > = 80 years (n = 508) | 27.5 (3.2) | 4.3 (0.9) | |
| Non-Hispanic white (n = 1,304) | 89.0 (2.1) | 82.7 (2.5) | 0.004† |
| Non-Hispanic black (n = 454) | 6.4 (1.1) | 8.1 (1.2) | |
| Hispanic (n = 467) | 1.8 (0.4) | 2.8 (0.3) | |
| Other race/ethnicity (n = 60) | 2.8 (1.4) | 6.4 (2.1) | |
| Comorbidities | |||
| Diabetes (n = 646) | 26.0 (3.0) | 24.0 (2.9) | 0.96§ |
| Cardiovascular disease (n = 627) | 30.5 (2.9) | 22.8 (2.9) | 0.0017§ |
| Chronic kidney disease (n = 651) | 33.8 (3.2) | 21.7 (2.6) | < 0.0001§ |
| Chronic lung disease (n = 253) | 15.3 (2.5) | 11.1 (2.1) | 0.0017§ |
| Chronic liver disease (n = 100) | 4.0 (1.3) | 4.1 (1.2) | 0.05§ |
| Cancer, non-skin (n = 180) | 12.4 (2.1) | 5.1 (1.3) | < 0.0001§ |
| Arthritis (n = 830) | 40.2 (3.2) | 34.2 (3.1) | 0.0001§ |
| Hypertension (n = 1,591) | 70.9 (3.4) | 63.8 (3.5) | 0.0018§ |
Figure 2.
Proportion of persons in each phase angle range, adjusted for survey design. Range is centered around value shown (i.e., “4.5” shows a range from 4.25 to 4.75). Error bar shows standard error.
Phase Angle and Frailty
Phase angle was associated with frailty in both women and men. In women, the first (range 2.655 to 5.419°) quintile for phase angle was associated with a significantly higher odds of frailty, even after adjustment for age (stratified by decade), race-ethnicity and comorbidities (Table 2). The second quintile (range 5.423 to 6.020°) for phase angle in women was marginally associated with a higher odds of frailty, but did not meet our stringent significance requirement (p value 0.049). Results for men were consistent with the results for women. In men, the first quintile of phase angle (range 3.070 to 5.646°) was associated with a significantly higher odds of frailty (Table 3). The models exhibited good discrimination and were well calibrated.
Table 2.
Odds of Frailty, Women Older Than 60 Years
| Odds ratio | 95 % CI | ||
|---|---|---|---|
| Phase angle | |||
| Quintiles three through five (n = 1,430) | Reference | — | |
| Second quintile (n = 476) | 1.9 | 1.0 | 3.5 |
| Lowest quintile (n = 476) | 4.4 | 2.6 | 7.7 |
| Age | |||
| 60–70 years (n = 1,141) | Reference | — | |
| 70–80 years (n = 929) | 1.3 | 0.6 | 2.6 |
| Greater than 80 years (n = 649) | 1.6 | 0.7 | 3.4 |
| Race/ethnicity | |||
| Non-hispanic White (n = 1,581) | Reference | — | |
| Non-hispanic Black (n = 547) | 2.4 | 1.3 | 4.3 |
| Mexican American (n = 495) | 2.8 | 1.3 | 6.0 |
| Other race and ethnicity (n = 96) | 1.8 | 0.6 | 5.4 |
| Comorbidities | |||
| Diabetes (n = 803) | 2.0 | 1.2 | 3.4 |
| Chronic lung disease (n = 291) | 2.5 | 1.2 | 4.9 |
| Arthritis (n = 1,434) | 3.0 | 1.7 | 5.4 |
| Cardiovascular disease (n = 658) | 1.8 | 1.1 | 2.9 |
| Chronic kidney disease (n = 625) | 2.5 | 1.5 | 4.4 |
Area under the Receiver Operator Curve (c statistic) = 0.78
p value for Hosmer Lemeshaw Goodness-of-Fit test was nonsignificant
Table 3.
Odds of Frailty, Men Older Than 60 Years
| Odds ratio | 95 % CI | ||
|---|---|---|---|
| Phase angle | |||
| Quintiles three through five (n = 1,375) | Reference | — | |
| Second quintile (n = 458) | 0.8 | 0.3 | 2.0 |
| Lowest quintile (n = 458) | 3.1 | 1.2 | 7.9 |
| Age | |||
| 60–70 years (n = 1,162) | Reference | — | |
| 70–80 years (n = 829) | 1.9 | 0.7 | 4.7 |
| Greater than 80 years (n = 601) | 1.6 | 0.5 | 4.7 |
| Race/ethnicity | |||
| Non-hispanic White (n = 1,466) | Reference | — | |
| Non-hispanic Black (n = 528) | 2.7 | 1.5 | 4.9 |
| Mexican American (n = 534) | 1.7 | 1.0 | 3.0 |
| Other race and ethnicity (n = 64) | 4.6 | 1.2 | 17.6 |
| Comorbidities | |||
| Diabetes (n = 738) | 1.0 | 0.5 | 2.2 |
| Chronic lung disease (n = 288) | 1.0 | 0.5 | 2.4 |
| Arthritis (n = 955) | 4.9 | 2.6 | 9.4 |
| Cardiovascular disease (n = 742) | 2.3 | 1.3 | 4.0 |
| Chronic kidney disease (n = 757) | 2.6 | 1.2 | 5.8 |
Area under the Receiver Operator Curve (c statistic) = 0.77
p value for Hosmer Lemeshaw Goodness-of-Fit test was nonsignificant
Phase Angle and Mortality
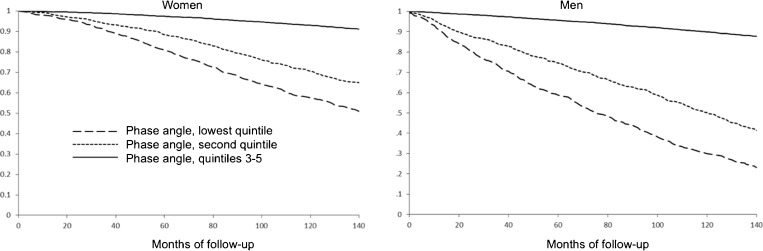
Narrow phase angle was associated with mortality in both women and men (Fig. 3). These findings were independent of age, race-ethnicity, comorbidity and the presence or absence of frailty (data not shown). Over a 12-year follow-up period, the adjusted mortality hazard ratio associated with narrow phase angle was 2.4 (95 % confidence interval [95 % CI] 1.8 to 3.1) in women and 2.2 (95 % CI 1.7 to 2.9) in men.
Figure 3.
Survival curves, stratified for gender, by phase angle quintile.
Of the 4,667 persons included in the study sample, 1,517 met inclusion criteria for the limited comorbidity subpopulation. Of those, 414 were free of any of the eight comorbidities we identified, and 1,103 reported only one of: hypertension (n = 753), arthritis (n = 262) or diabetes (n = 88). Narrow phase angle was significantly associated with mortality in women and men who had limited or no comorbidity. Among women, phase angle in the first quintile conferred a relative hazard for mortality of 2.6 (95 % CI 1.5 to 4.7); among men, phase angle in the first quintile conferred a relative hazard of 2.4 (95 % CI 1.4 to 4.0). Among the 414 participants with no comorbidity, relative hazards for mortality among women and men with phase angles in the lowest quintiles were 5.9 (95 % CI 2.4 to 14.3) and 3.8 (95 % CI 1.4 to 10.3) respectively. Thus, even in the absence of comorbid conditions, narrow phase angle was associated with an increased risk of mortality.
DISCUSSION
Using data from a nationally representative sample of older adults, we found phase angle to be associated with frailty in both women and men. Among women, study participants with phase angle in the first quintile demonstrated a more than four-fold higher odds of frailty; for women with phase angle in the second quintile, the odds of frailty were nearly two-fold higher than for women with wider phase angles (above 6.0°). Among men, the significantly increased odds of frailty was limited to phase angles in the first quintile (below 5.7°), and was roughly three-fold higher than for men with wider phase angles.
Narrow phase angle was significantly associated with mortality, even after accounting for age, race-ethnicity and comorbidity. Given the high degree of comorbidity among frail study participants, it is not surprising that a measure associated with frailty (phase angle) predicts mortality in this group. However, phase angle also provided prognostic value among persons with limited comorbidity. Among the groups with limited or no comorbidity, persons with narrow phase angle demonstrated a higher risk of mortality over the follow-up period, suggesting that measurement of phase angle could help to risk stratify otherwise healthy older adults.
The association between narrow phase angle and mortality in populations with a variety of disabling chronic diseases has been well documented. For example, among persons with advanced HIV infection, phase angle was a more potent predictor of survival than CD4 cell count.17 Phase angle has also been associated with survival among persons with dialysis-requiring chronic kidney disease,13,16,18,27,28 lung cancer,29 colorectal and pancreatic cancer,14,15 and liver cirrhosis.8 Despite these associations, the precise clinical interpretation of phase angle remains elusive. Phase angle could reflect a measure of cellular integrity. Several studies have shown a direct correlation among phase angle and nutritional status (e.g., serum albumin), and an inverse correlation with markers of inflammation or disease activity.30,31
We propose that phase angle can be interpreted as a global marker of health in aging, and that older adults with narrower phase angles are at increased risk for two of the principal hallmarks of unhealthy aging: frailty and mortality, whether in the presence or absence of comorbidity.
Experienced clinicians can readily identify older persons at risk for frailty among those with various comorbidities such as cardiovascular disease and impaired kidney or liver function. In addition, BIA may enable clinicians to identify apparently healthy older adults at risk for death and adverse events. While efforts intended to increase phase angle have not been undertaken, nor has evidence accumulated that a change in phase angle can modify risk, apparently healthy older persons with narrow phase angles would appear to be ideal candidates for closer observation by their primary providers and/or for referral to physical or occupational therapy, physiatry and/or geriatric medicine, for focused assessment and intervention.
Our study’s strengths include analysis of a nationally representative sample of US adults with robust recruitment of persons older than 80 years. Follow-up, while limited to mortality, was exceptionally complete. Furthermore, our study utilizes an examination technique, BIA, that is known for ease of use and reproducibility; our analysis relies on phase angle, which is calculated directly. Other studies that report BIA data often use regression equations that incorporate measured resistance and reactance with patient characteristics to estimate body compartments. These equations are limited in that they depend on the population in which they were derived, and errors may be introduced when these equations are applied to non-source populations. Our use of the phase angle circumvents the problems inherent in the use of regression equations to estimate body composition in a population for which those equations have not been validated.
Our study also carries a few important limitations. First, NHANES III data are primarily cross-sectional. However, the NHANES III data do offer the first wave of mortality data to be published for an NHANES data set and this follow-up allows for estimation of mortality risk in subgroups of NHANES III participants. Second, home examined survey participants did not undergo BIA, and so could not be included in the analysis. Since these participants tended to have more comorbidity than other participants, we have probably underestimated the prevalence of frailty. Finally, while we adjusted for comorbidity, there is likely confounding of the phase angle-frailty and phase angle-mortality associations by other unmeasured characteristics or conditions. However, the association between phase angle and frailty remained strong among persons with no or limited comorbidity, which lessens the risk that the association is due in large part to unaccounted for confounding. Additionally, if phase angle can capture some of the more subtle factors leading to frailty and mortality, application of the technology, given its safety and low cost, may prove worthwhile.
The intersection of body composition analysis and aging is an important area of research with much to be studied. Future research should focus on longitudinal data that demonstrate how phase angle changes over time in adults who are aging well and poorly. Also, interventional studies should be undertaken to demonstrate which interventions, if any, might increase (widen) phase angle in individuals. It remains to be demonstrated if widening the phase angle could lower the risks of frailty, mortality or other complications.
Acknowledgments
Support for Dr. Wilhelm-Leen was provided by a Medical Scholars grant from the Stanford University School of Medicine. Dr. Chertow was supported by K24 DK085446.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
REFERENCES
- 1.Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc. 2004;52(11):1929–1933. doi: 10.1111/j.1532-5415.2004.52521.x. [DOI] [PubMed] [Google Scholar]
- 2.Morley JE, Haren MT, Rolland Y, Kim MJ. Frailty. Med Clin North Am. 2006;90(5):837–847. doi: 10.1016/j.mcna.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Slaets JP. Vulnerability in the elderly: frailty. Med Clin North Am. 2006;90(4):593–601. doi: 10.1016/j.mcna.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 5.Hoogerduijn JG, Schuurmans MJ, Duijnstee MS, de Rooij SE, Grypdonck MF. A systematic review of predictors and screening instruments to identify older hospitalized patients at risk for functional decline. J Clin Nurs. 2007;16(1):46–57. doi: 10.1111/j.1365-2702.2006.01579.x. [DOI] [PubMed] [Google Scholar]
- 6.Morley JE. Diabetes, sarcopenia, and frailty. Clin Geriatr Med. 2008;24(3):455–469. doi: 10.1016/j.cger.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Buchholz AC, Bartok C, Schoeller DA. The validity of bioelectrical impedance models in clinical populations. Nutr Clin Pract. 2004;19(5):433–446. doi: 10.1177/0115426504019005433. [DOI] [PubMed] [Google Scholar]
- 8.Selberg O, Selberg D. Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. Eur J Appl Physiol. 2002;86(6):509–516. doi: 10.1007/s00421-001-0570-4. [DOI] [PubMed] [Google Scholar]
- 9.Nyboer J, Kreider MM, Hannapel L. Quantitative studies of electrical conductivity of the peripheral body segments; basic and practical considerations. Ann West Med Surg. 1951;5(1):11–20. [PubMed] [Google Scholar]
- 10.Bellizzi V, Scalfi L, Terracciano V, Marra M, Di Iorio B. The prediction of single-frequency BIA variables from individual characteristics. Acta Diabetol. 2003;40(Suppl 1):S233–235. doi: 10.1007/s00592-003-0073-3. [DOI] [PubMed] [Google Scholar]
- 11.Dittmar M. Reliability and variability of bioimpedance measures in normal adults: effects of age, gender, and body mass. Am J Phys Anthropol. 2003;122(4):361–370. doi: 10.1002/ajpa.10301. [DOI] [PubMed] [Google Scholar]
- 12.VanderJagt DJ, Trujillo MR, Bode-Thomas F, Huang YS, Chuang LT, Glew RH. Phase angle correlates with n-3 fatty acids and cholesterol in red cells of Nigerian children with sickle cell disease. Lipids Health Dis. 2003;2:2. doi: 10.1186/1476-511X-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chertow GM, Jacobs DO, Lazarus JM, Lew NL, Lowrie EG. Phase Angle Predicts Survival in Hemodialysis Patients. J Renal Nutrition. 1997;7(4):204–207. doi: 10.1016/S1051-2276(97)90020-0. [DOI] [Google Scholar]
- 14.Gupta D, Lammersfeld CA, Burrows JL, et al. Bioelectrical impedance phase angle in clinical practice: implications for prognosis in advanced colorectal cancer. Am J Clin Nutr. 2004;80(6):1634–1638. doi: 10.1093/ajcn/80.6.1634. [DOI] [PubMed] [Google Scholar]
- 15.Gupta D, Lis CG, Dahlk SL, Vashi PG, Grutsch JF, Lammersfeld CA. Bioelectrical impedance phase angle as a prognostic indicator in advanced pancreatic cancer. Br J Nutr. 2004;92(6):957–962. doi: 10.1079/BJN20041292. [DOI] [PubMed] [Google Scholar]
- 16.Maggiore Q, Nigrelli S, Ciccarelli C, Grimaldi C, Rossi GA, Michelassi C. Nutritional and prognostic correlates of bioimpedance indexes in hemodialysis patients. Kidney Int. 1996;50(6):2103–2108. doi: 10.1038/ki.1996.535. [DOI] [PubMed] [Google Scholar]
- 17.Ott M, Fischer H, Polat H, et al. Bioelectrical impedance analysis as a predictor of survival in patients with human immunodeficiency virus infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9(1):20–25. doi: 10.1097/00042560-199505010-00003. [DOI] [PubMed] [Google Scholar]
- 18.Mushnick R, Fein PA, Mittman N, Goel N, Chattopadhyay J, Avram MM. Relationship of bioelectrical impedance parameters to nutrition and survival in peritoneal dialysis patients. Kidney Int Suppl. 2003;87:S53–56. doi: 10.1046/j.1523-1755.64.s87.22.x. [DOI] [PubMed] [Google Scholar]
- 19.Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Series 1: programs and collection procedures. Vital Health Stat 1. 1994;(32):1–407. [PubMed]
- 20.National Center for Health Statistics. Office of Analysis and Epidemiology, The Third National Health and Nutrition Examination Survey (NHANES III) Linked Mortality File, Mortality Follow-up Through 2006: Matching Methodology. May 2009. Hyattsville, Maryland.
- 21.Rule AD, Gussak HM, Pond GR, et al. Measured and estimated GFR in healthy potential kidney donors. Am J Kidney Dis. 2004;43(1):112–119. doi: 10.1053/j.ajkd.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 22.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141(12):929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 23.Wilhelm-Leen ER, Hall YN, K Tamura M, Chertow GM. Frailty and chronic kidney disease: the Third National Health and Nutrition Evaluation Survey. Am J Med. 2009;122(7)):664–671. doi: 10.1016/j.amjmed.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reference Manual: Physician Examiner’s Training Manual. Rockville, MD: National Center for Health Statistics;1991. http://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/phys.pdf
- 25.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 26.Introduction to Survey Sampling and Analysis Procedures. SAS OnlineDoc 9.1.3. Cary, NC: SAS Institute; 2002–2008.
- 27.Chertow GM, Lazarus JM, Lew NL, Ma L, Lowrie EG. Bioimpedance norms for the hemodialysis population. Kidney Int. 1997;52(6):1617–1621. doi: 10.1038/ki.1997.493. [DOI] [PubMed] [Google Scholar]
- 28.Chertow GM, Lowrie EG, Wilmore DW, et al. Nutritional assessment with bioelectrical impedance analysis in maintenance hemodialysis patients. J Am Soc Nephrol. 1995;6(1):75–81. doi: 10.1681/ASN.V6175. [DOI] [PubMed] [Google Scholar]
- 29.Toso S, Piccoli A, Gusella M, et al. Altered tissue electric properties in lung cancer patients as detected by bioelectric impedance vector analysis. Nutrition. 2000;16(2):120–124. doi: 10.1016/S0899-9007(99)00230-0. [DOI] [PubMed] [Google Scholar]
- 30.Johansen KL, Kaysen GA, Young BS, Hung AM, da Silva M, Chertow GM. Longitudinal study of nutritional status, body composition, and physical function in hemodialysis patients. Am J Clin Nutr. 2003;77(4):842–846. doi: 10.1093/ajcn/77.4.842. [DOI] [PubMed] [Google Scholar]
- 31.Fein PA, Gundumalla G, Jorden A, Matza B, Chattopadhyay J, Avram MM. Usefulness of bioelectrical impedance analysis in monitoring nutrition status and survival of peritoneal dialysis patients. Adv Perit Dial. 2002;18:195–199. [PubMed] [Google Scholar]