ABSTRACT
BACKGROUND
Previous studies found normal weight compared to overweight/obese adults with type 2 diabetes had a higher mortality risk, and body-mass index (BMI)–mortality studies do not typically account for baseline diabetes status.
OBJECTIVE
To determine if diabetes influences the BMI–mortality relationship.
DESIGN
Using a prospective study design, we analyzed data from a nationally representative sample of US adults participating in the National Health Interview Survey from 1997 to 2002, and followed for mortality through 2006.
PARTICIPANTS
Excluding those with heart disease or cancer, our final analytic sample included 74,710 (34,805 never smoker) adults.
MAIN MEASURES
BMI was calculated from self-reported height and weight. Diabetes status was based on self-reported diagnosis from a health professional. We used direct age standardization to calculate all-cause mortality rates and adjusted Cox models for all-cause mortality hazard ratios by BMI quintile; this was done separately for adults with diabetes and without diabetes.
KEY RESULTS
Among never smokers, mean age was 50.1 years and 43 % were men. Mean BMI was 27.4 kg/m2, 26 % were obese, and 2,035 (5 %) reported diagnosed diabetes. After 9 years, there were 4,355 deaths (754 of 4,740 with diabetes; 3,601 of 69,970 without) among 74,710 participants, and 1,238 (247 of 2,035 with diabetes; 991 of 32,770 without) among 34,805 never smokers. We observed a qualitative interaction with diabetes on the BMI–mortality relationship (p = 0.002). Death rates were substantially higher among participants with diabetes compared to those without diabetes across all BMI quintiles. However, death rates in participants with diabetes fell with increasing BMI quintile, while rates followed a J-shaped curve among those without diabetes. In adjusted Cox models, BMI was positively associated with mortality in adults without diabetes, but inversely associated with mortality among participants with diabetes.
CONCLUSIONS
Mortality increased with increasing BMI in adults without diabetes, but decreased with increasing BMI among their counterparts with diabetes. Future studies need to be better designed to answer the question of whether normal weight adults with diabetes have a higher risk of mortality, by minimizing the possibility of reverse causation. Future studies should also account for prevalent diabetes in all investigations of the BMI–mortality relationship.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-013-2553-7) contains supplementary material, which is available to authorized users.
KEY WORDS: diabetes, body mass index, mortality
INTRODUCTION
The prevalence of overweight and obesity in the United States has increased at an alarming rate over the past several decades,1,2 and is considered a public health crisis. Although obesity is a well-established risk factor for a host of serious and costly health conditions, including cardiovascular disease (CVD), type 2 diabetes (T2DM), and certain cancers, the relationship between body-mass index (BMI) and mortality in sub-groups defined by demographics or comorbid disease remains controversial.3–7
T2DM is strongly related to both BMI and mortality risk, but it is not typically taken into account by studies of the BMI–mortality risk relationship. Most studies of BMI and mortality exclude individuals with a history of CVD or cancer, since both conditions lead to both weight loss and mortality. Smokers are excluded for the same reason. In contrast, individuals with diabetes are generally not excluded, under the presumption that the BMI–mortality relationship in people with diabetes parallels the relationship in their counterparts without diabetes. This presumption, however, is not often empirically tested. The importance of demonstrating the threat posed by disease-related weight loss as a source of bias in observational studies of body weight and mortality has recently been underscored.8 Therefore, our objective was to determine the extent to which diabetes influences the BMI–mortality relationship. We hypothesized that diabetes would appreciably modify the relationship between BMI and mortality.
METHODS
The National Health Interview Survey
We used nationally representative survey data from the National Health Interview Survey (NHIS). NHIS uses a three-stage stratified cluster probability sampling design to conduct annual face-to-face health interviews of non-institutionalized US civilians. A complete description of NHIS procedures is available elsewhere.9
The survey interviews were conducted using computer-assisted personal interviewing (CAPI). Within each family in a household, one child (not included in this analysis) and one adult (the focus of this analysis) were randomly selected to provide more extensive health-related information. We used NHIS data pooled from 1997 to 2002 by the Integrated Health Interview Series with links to the National Death Index (NDI) up to 31 December 2006.10 The average response rate for the randomly sampled adults over the study period was 73.7 % (range: 71.1–78.1 %).
Our study was approved by the Institutional Review Board’s Committee on Human Research at the Harvard School of Public Health, and the NHIS received informed consent from each participant.
Study Participants
Our sample included participants who were between 35 and 75 years old and who were Non-Hispanic White or Non-Hispanic Black (henceforth, White or Black), based on self-report. We excluded individuals if they met any of the following conditions: 1) were born outside the US; 2) reported a prior history of cancer and/or heart disease (to minimize the influence of reverse causation); 3) were pregnant at time of interview; 4) had missing data on height, weight, mortality, heart disease, cancer, or smoking status; 5) had an extreme BMI of < 15 or > 55 kg/m2; or 6) had their diabetes diagnosed at < 25 years age and were treated with insulin. After exclusions, our final sample consisted of 74,710 participants (see Supplemental Figure 1, available online). We compared analysis-eligible participants with complete data with their counterparts with missing data, and found no significant differences in age, sex, race, health status, poverty status, or household size.
Measures
All-Cause Mortality
Our main outcome of interest was all-cause mortality. Deaths were identified by linking NHIS to the NDI, and were classified according to International Classification of Disease (ICD)-10 codes.
Body-Mass Index
Using the entire analytic sample of participant-reported heights and weights, we classified participants into BMI quintiles, rounded to the nearest 0.5 BMI unit. Quintile 1 represented BMI 15.0–22.9 kg/m2; quintile 2: BMI 23.0–24.9 kg/m2; quintile 3: BMI 25.0–27.4 kg/m2; quintile 4: BMI 27.5–30.9 kg/m2; and quintile 5: BMI 31.0–55.0 kg/m2. We chose to use both standardized and data-driven categories based on the distribution of our study sample.
Diabetes
Participants were asked, “Have you EVER been told by a doctor or other health professional that you have diabetes or sugar diabetes?” For women, the question was preceded by the phrase, “Other than during pregnancy” to exclude cases of gestational diabetes. Participants categorized as having borderline diabetes were included as not having diabetes (n = 818). Participants ≤ 25 years who reported taking insulin for diabetes were excluded from the analysis to increase the likelihood of including only those with T2DM. Diabetes duration was estimated by subtracting current age from reported age at diabetes diagnosis.
Covariates
Cigarette smoking status was categorized as ever or never, based on whether or not a participant smoked 100 cigarettes over their lifetime, and alcohol consumption was classified as current (1+ drinks in the past year), former (no drinks in the past year) or never (< 12 drinks in life). Leisure-time physical activity was categorized as none/unable, low, or high, based on bouts of how often participants engaged in light/moderate leisure-time or vigorous physical activities for at least 10 min. Activity bouts were classified as either “low” or “high” using the median (i.e. ≥ 9 bouts of moderate and ≥ 5 bouts of vigorous activity) as the cut point. Educational attainment was categorized as < high school (<HS) (no high school diploma), high school (HS) (high school or general equivalency diploma), and > high school (>HS) (any education beyond high school). Marital status was categorized as either married; divorced, widowed, or separated; or never married.
Statistical Analysis
We incorporated sampling weights for all analyses to account for the unequal probabilities of selection resulting from the sample design, from non-response to the NHIS, and from planned oversampling of racial/ethnic minorities and the elderly (≥ 65 years). Standard errors or variance estimations were calculated using Taylor series linearization.11 A two-sided p value < 0.05 was considered statistically significant.
We compared participant sociodemographic characteristics, clinical parameters, and health behaviors overall, according to both diabetes status and BMI quintile. Continuous variables were expressed as means ± their respective standard errors (SE), whereas categorical variables were presented as absolute values with corresponding percentages. To test for differences in pre-specified sociodemographic, clinical, and behavioral characteristics between diabetes status and by BMI quintile, we used the Rao-Scott second-order corrected Pearson statistic.12
We used direct age standardization to calculate all-cause mortality rates in adults with versus without diabetes by BMI quintile. Cox proportional hazard models were used to estimate hazard ratios and corresponding 95 % confidence intervals across BMI quintiles and by diabetes status, with adjustments for age (as the timescale), race, education, marital status, alcohol consumption, leisure-time physical activity, and general health status among those without diabetes.13 We further adjusted for self-reported diabetes medication (i.e. pills, insulin) use and diabetes duration among individuals with diabetes. Follow-up time was counted from age at enrollment to either age of death or, if classified as alive, age at the end of the study period (31 December 2006), or age 75—whichever came first. The proportional hazards assumption of the model, tested using Schoenfield residuals, was met.
We calculated adjusted hazard ratios for all-cause mortality by BMI quintile separately in adults with diabetes and without diabetes with a one-year time lag. We tested for an interaction with diabetes and BMI for men and women separately, to determine if the BMI–mortality relationship differed between those with and without diabetes by sex. We also tested for interactions with age for the BMI–mortality relationship among those with and without diabetes in separate models, in addition to a three-way interaction with age, BMI and diabetes. We stratified all mortality analyses by smoking status (ever vs. never), since smoking is well known to increase mortality risk while decreasing body weight. For that reason, we focused our main results on never smokers.
To test the robustness of our results, we conducted several separate pre-specified subgroup analyses, excluding those, at baseline, with: 1) CVD and cancer; 2) CVD only; 3) cancer only; and 4) neither CVD nor cancer. We also conducted an analysis among participants classified as ever smokers.
RESULTS
Characteristics of the Study Population
Sociodemographic, clinical and behavioral characteristics of the 34,805 NHIS study participants who were considered never smokers are shown by diabetes status and BMI quintile in Table 1. The mean age was 50.1 ± 0.1 years, and 43 % were men. Forty-seven percent of all participants were never smokers, 26 % never consumed alcohol, and 33 % reported never engaging in leisure-time physical activity. The mean BMI was 27.4 ± 0.04 kg/m2, 26 % of participants were obese, 25 % had hypertension, 5 % had diabetes, and 8 % (6 % without diabetes; 35 % with diabetes) reported fair or poor general health status. Among those with diabetes, the proportion of Black women, individuals who never married, those who never or were unable to engage in physical activity, and participants with hypertension increased with increasing BMI quintile. Diabetes duration was 8.7 ± 8.2 years, and decreased with increasing BMI quintile. Among those with diabetes, age increased with increasing BMI quintile for individuals 35–49 years old, but decreased with increasing BMI quintile for individuals aged ≥ 65 years.
Table 1.
A Comparison of Sociodemographic Characteristics and Health Behaviors Among Never-Smoking NHIS Participants by Diabetes Status, 1997–2002, Followed Through 2006 (N = 34,805)
| No diabetes | Diabetes | Overall | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | BMI (kg/m2) | ||||||||||
| 15.02–22.83 | 22.84–25.09 | 25.1–27.46 | 27.47–31.02 | 31.03–54.92 | 15.02–22.83 | 22.84–25.09 | 25.1–27.46 | 27.47–31.02 | 31.03–54.92 | ||
| Sample size, n (%) | 6,560(20) | 6,614(21) | 6,698(21) | 6,444(20) | 6,454(19) | 99(5) | 193(9) | 279 (14) | 484 (25) | 980 (48) | 34,805 |
| Age, year ± SE | 47.9 ± 0.15 | 48.9 ± 0.15 | 49.5 ± 0.16 | 49.0 ± 0.15 | 48.4 ± 0.13 | 60.4 ± 1.69 | 61.1 ± 0.86 | 59.3 ± 0.62 | 58.3 ± 0.62 | 55.0 ± 0.40 | 50.1 ± 0.05 |
| Age group, (%) | |||||||||||
| 35–49 | 65 | 61 | 57 | 59 | 61 | 17 | 17 | 19 | 25 | 34 | 55 |
| 50–64 | 24 | 27 | 30 | 29 | 30 | 39 | 36 | 45 | 39 | 42 | 32 |
| ≥ 65 | 11 | 12 | 13 | 12 | 9 | 43 | 47 | 36 | 36 | 24 | 13 |
| Men, % | 20 | 46 | 54 | 54 | 43 | 32 | 43 | 44 | 46 | 36 | 43 |
| Race/Ethnicity, % | |||||||||||
| Non-Hispanic White men | 18 | 43 | 49 | 47 | 37 | 27 | 33 | 37 | 37 | 32 | 39 |
| Non-Hispanic Black men | 2 | 4 | 5 | 7 | 7 | 5 | 11 | 7 | 10 | 5 | 5 |
| Non-Hispanic White women | 75 | 47 | 40 | 38 | 43 | 55 | 45 | 44 | 35 | 42 | 48 |
| Non-Hispanic Black women | 5 | 6 | 6 | 8 | 13 | 13 | 11 | 12 | 18 | 21 | 8 |
| Educational attainment, % | |||||||||||
| < High school | 26 | 30 | 31 | 32 | 35 | 48 | 37 | 35 | 35 | 37 | 31 |
| High school graduate | 5 | 5 | 7 | 8 | 11 | 21 | 20 | 22 | 21 | 22 | 8 |
| Some college | 26 | 26 | 27 | 28 | 30 | 14 | 23 | 22 | 26 | 26 | 28 |
| ≥ College | 43 | 39 | 35 | 31 | 24 | 17 | 20 | 21 | 18 | 15 | 33 |
| Marital Status, % | |||||||||||
| Married | 73 | 73 | 74 | 74 | 67 | 67 | 66 | 68 | 66 | 62 | 72 |
| Divorced/separated/widowed | 17 | 18 | 18 | 17 | 20 | 28 | 30 | 27 | 26 | 28 | 18 |
| Never married | 10 | 9 | 8 | 9 | 13 | 5 | 4 | 5 | 8 | 11 | 10 |
| Living in Poverty, % | 5 | 4 | 5 | 5 | 8 | 11 | 17 | 10 | 11 | 12 | 6 |
| Health Behaviors, % | |||||||||||
| Alcohol consumption | |||||||||||
| Never | 24 | 22 | 24 | 25 | 29 | 55 | 42 | 42 | 43 | 40 | 26 |
| Current | 10 | 12 | 13 | 13 | 15 | 21 | 25 | 25 | 23 | 28 | 13 |
| Former | 66 | 66 | 63 | 62 | 56 | 23 | 33 | 33 | 33 | 32 | 61 |
| Leisure-time physical activity | |||||||||||
| Never/unable | 28 | 29 | 32 | 33 | 42 | 49 | 49 | 41 | 50 | 52 | 33 |
| Low | 35 | 34 | 34 | 35 | 31 | 25 | 24 | 28 | 26 | 23 | 33 |
| High | 38 | 37 | 34 | 2 | 37 | 25 | 27 | 31 | 24 | 24 | 33 |
| Clinical Characteristics | |||||||||||
| BMI (kg/m2 ± SE) | 21.0 ± 0.02 | 24.0 ± 0.01 | 26.4 ± 0.01 | 29.2 ± 0.01 | 35.5 ± 0.06 | 21.1 ± 0.19 | 24.1 ± 0.05 | 26.4 ± 0.04 | 29.3 ± 0.05 | 37.0 ± 0.20 | 27.4 ± 0.04 |
| Hypertension (yes), % | 11 | 16 | 21 | 28 | 39 | 46 | 50 | 56 | 62 | 68 | 25 |
| Diabetes (yes), % | — | — | — | — | — | 11.5 ± 10.3 | 10.5 ± 9.2 | 9.4 ± 7.9 | 8.8 ± 8.1 | 7.6 ± 7.5 | 8.7 ± 8.2 |
| General Health Status, % | |||||||||||
| Excellent/Very Good | 82 | 79 | 76 | 71 | 57 | 35 | 31 | 31 | 29 | 24 | 71 |
| Good | 14 | 17 | 19 | 23 | 31 | 26 | 37 | 41 | 38 | 38 | 21 |
| Fair/Poor | 4 | 4 | 5 | 6 | 12 | 39 | 33 | 28 | 33 | 38 | 8 |
| Region of Country, % | |||||||||||
| Northeast | 20 | 19 | 18 | 17 | 16 | 20 | 17 | 15 | 15 | 13 | 18 |
| Midwest | 25 | 27 | 27 | 29 | 29 | 28 | 21 | 23 | 26 | 27 | 27 |
| South | 37 | 36 | 37 | 38 | 40 | 41 | 41 | 49 | 42 | 48 | 38 |
| West | 19 | 18 | 18 | 16 | 15 | 11 | 21 | 14 | 16 | 12 | 17 |
SE standard error
During the 9-year follow-up, there were 4,355 deaths (754 of 4,740 with diabetes; 3,601 of 69,970 without) among all 74,710 participants, and 1,238 (247 of 2,035 with diabetes; 991 of 32,770 without) among 34,805 never smokers.
Impact of Diabetes on BMI–Mortality Relationship
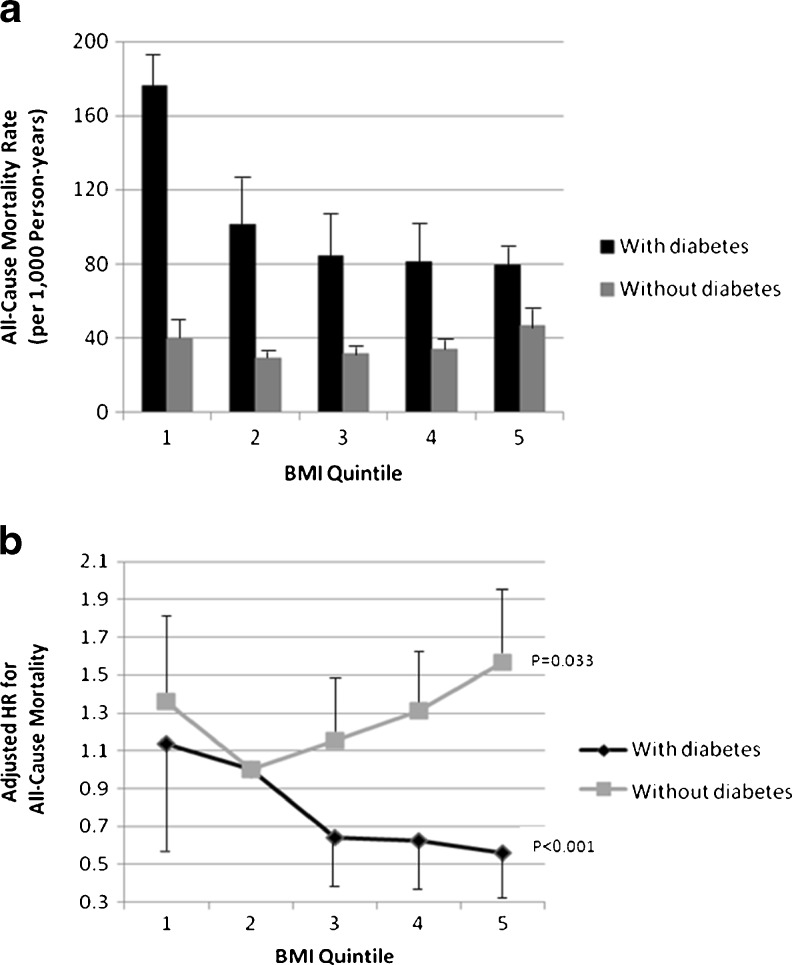
We observed qualitative interaction (p = 0.002) where both the magnitude and direction of the BMI–mortality relationship depended on diabetes, indicating that interaction was present on both the additive and multiplicative scales. Death rates were substantially higher among participants with diabetes compared to those without diabetes across all BMI quintiles. Death rates in participants with diabetes, however, fell with increasing BMI quintile, while rates followed a J-shaped curve among those without diabetes (Fig. 1a). Death rates (per 1,000 person years) by BMI quintiles among participants with diabetes were 176.2 for quintile 1, 101.3 for quintile 2, 84.1 for quintile 3, 81.3 for quintile 4, and 79.3 for quintile 5; while death rates among individuals without diabetes were 39.3 for quintile 1, 28.9 for quintile 2, 31.3 for quintile 3, 33.7 for quintile 4, and 46.8 for quintile 5.
Figure 1.
a) Age standardized death rates and b) adjusted hazard ratios for mortality among never smoking adults with and without diabetes by BMI quintile, National Health Interview Survey, 1997–2002, followed through 2006. BMI quintiles: 1) 15.02–22.83 kg/m2; 2) 22.84–25.09 kg/m2; 3) 25.1–27.46 kg/m2; 4) 27.47–31.02 kg/m2; 5) 31.03–54.92 kg/m2; Reference: Quintile 2. a) Age-standardization by the direct method with 2000 US Census as the standard population; error bars represent upper confidence intervals. b) HR = hazard ratio. Adjusted for age, race, education, marital status, alcohol consumption, leisure-time physical activity, as well as diabetes medications and duration among participants with diabetes; error bars represent upper and lower confidence intervals; p value indicates p for trend from BMI quintiles 2 to 5.
In adjusted Cox models, BMI was positively associated with mortality in adults without diabetes, but inversely associated among participants with diabetes (Fig. 1b). Among individuals with diabetes, the hazard ratios (HR) for all-cause mortality using BMI quintile 2 as the reference category were as follows: HR = 1.09 (95 % Confidence Interval [CI]: 0.52–2.27) for BMI quintile 1; HR = 0.63 (95 % CI: 0.33–1.20) for BMI quintile 3; HR = 0.58 (95 % CI: 0.33–1.01) for BMI quintile 4; and HR = 0.52 (95 % CI: 0.31–0.86) for BMI quintile 5. There was, however, a positive association between increasing BMI and mortality among individuals without diagnosed diabetes.
Our subgroup analyses, excluding participants at baseline with: 1) CVD or cancer, 2) CVD only, 3) cancer only, and 4) neither CVD nor cancer, yielded the same inferences as our main results in that BMI remained positively associated with mortality in adults without diabetes, but inversely associated in individuals with diabetes (see Table 2). The positive association between BMI and mortality among those without diabetes was stronger for individuals < 65 years compared to those ≥ 65 years. The inverse relationship for those with diabetes was weaker for individuals ≥ 65 years. Age interactions were significant for the BMI–mortality relationship among both individuals without (β = 0.9969; p < 0.0001) and with diabetes (β = 0.9986; p = 0.006). The three-way interaction between continuous age, BMI and diabetes was also significant (β = 0.9992; p < 0.0001) (data not shown).
Table 2.
Adjusted Hazard Ratios (95 % CIs) for All-Cause Mortality by Diabetes Status and BMI Quintile, National Health Interview Survey, 1997–2002, Followed Through 2006
| BMI (kg/m2) | ||||||
|---|---|---|---|---|---|---|
| 15.02–22.83 | 22.84–25.09 | 25.1–27.46 | 27.47–31.02 | 31.03–54.92 | ||
| Participants, N † | 6,659 | 6,807 | 6,977 | 6,928 | 7,434 | |
| All-Cause Mortality† | 221 | 207 | 227 | 251 | 332 | |
| Diabetes Cases† | 99 | 193 | 279 | 484 | 980 | |
| All-Cause Mortality, Diabetes† | 23 | 34 | 33 | 55 | 102 | |
| All Never Smokers* | N | |||||
| No Diabetes | 32,770 | 1.32 (0.97–1.79) | 1 (ref) | 1.11 (0.83–1.48) | 1.26 (0.97–1.64) | 1.48 (1.15–1.92) |
| Diabetes | 2,035 | 1.09 (0.52–2.27) | 1 (ref) | 0.63 (0.33–1.20) | 0.58 (0.33–1.01) | 0.52 (0.31–0.86) |
| Never Smoking Whites* | ||||||
| No Diabetes | 25,581 | 1.34 (0.95–1.88) | 1 (ref) | 1.27 (0.92–1.77) | 1.40 (1.05–1.86) | 1.77 (1.32–2.37) |
| Diabetes | 1,208 | 1.03 (0.45–2.39) | 1 (ref) | 0.76 (0.37–1.56) | 0.61 (0.32–1.16) | 0.56 (0.32–1.16) |
| Never Smoking Blacks* | ||||||
| No Diabetes | 5,115 | 1.39 (0.72–1.88) | 1 (ref) | 0.56 (0.30–1.05) | 0.80 (0.44–1.47) | 0.79 (0.51–1.22) |
| Diabetes | 580 | 1.20 (0.26–5.42) | 1 (ref) | 0.17 (0.02–1.36) | 0.49 (0.16–1.48) | 0.41 (0.17–0.97) |
| Never Smokers, < 65 Years Old* | ||||||
| No Diabetes | 28,199 | 1.18 (0.74–1.88) | 1 (ref) | 1.28 (0.83–1.97) | 1.48 (1.01–2.15) | 1.73 (1.20–2.50) |
| Diabetes | 1,318 | 1.22 (0.33–4.50) | 1 (ref) | 0.45 (0.15–1.39) | 0.46 (0.17–1.22) | 0.42 (0.17–1.01) |
| Never Smokers, Age 65 + * | ||||||
| No Diabetes | 4,571 | 1.42 (0.99–2.04) | 1 (ref) | 1.00 (0.68–1.38) | 1.08 (0.75–1.57) | 1.27 (0.87–1.86) |
| Diabetes | 717 | 1.17 (0.49–2.77) | 1 (ref) | 0.78 (0.34–1.78) | 0.59 (0.29–1.21) | 0.53 (0.28–1.00) |
| Never Smokers with Diabetes < 10 Years* | ||||||
| Diabetes | 1,226 | 1.28 (0.32–5.01) | 1 (ref) | 0.78 (0.27–2.24) | 0.59 (0.23–1.46) | 0.55 (0.22–1.37) |
| Never Smokers with Diabetes 10+ Years* | ||||||
| Diabetes | 782 | 1.05 (0.44–2.49) | 1 (ref) | 0.58 (0.26–1.28) | 0.61 (0.30–1.25) | 0.53 (0.29–0.96) |
| Never Smokers with Diabetes Using No Pills/Insulin* | ||||||
| Diabetes | -- | -- | 1 (ref) | -- | -- | -- |
| Never Smokers with Diabetes Using Pills Only* | ||||||
| Diabetes | 1,158 | 0.98 (0.37–2.61) | 1 (ref) | 0.70 (0.32–1.55) | 0.52 (0.26–1.06) | 0.39 (0.19–0.79) |
| Never Smokers with Diabetes Using Insulin* | ||||||
| Diabetes | 577 | 1.25 (0.36–4.39) | 1 (ref) | 0.62 (0.20–1.88) | 0.56 (0.20–1.61) | 0.61 (0.23–1.60) |
| All Ever Smokers* | ||||||
| No Diabetes | 32,200 | 1.27 (1.12–1.46) | 1 (ref) | 0.90 (0.78–1.04) | 0.80 (0.69–0.93) | 0.85 (0.72–1.00) |
| Diabetes | 2,705 | 1.16 (0.76–1.78) | 1 (ref) | 0.56 (0.36–0.86) | 0.52 (0.36–0.74) | 0.51 (0.36–0.73) |
| Never Smokers, No Fair/Poor Health* | ||||||
| No Diabetes | 63,106 | 1.13 (0.96–1.32) | 1 (ref) | 0.90 (0.75–1.06) | 0.79 (0.67–0.94) | 0.83 (0.68–1.01) |
| Diabetes | 2,845 | 1.69 (0.76–1.78) | 1 (ref) | 0.99 (0.56–1.77) | 0.88 (0.49–1.60) | 1.00 (0.56–1.78) |
| Never Smokers, No Respiratory or Neurodegenerative Mortality* | ||||||
| No Diabetes | 69,916 | 1.25 (1.09–1.43) | 1 (ref) | 0.91 (0.79–1.06) | 0.80 (0.69–0.93) | 0.87 (0.73–1.02) |
| Diabetes | 4,728 | 1.41 (0.90–2.20) | 1 (ref) | 0.68 (0.45–1.05) | 0.71 (0.49–1.03) | 0.79 (0.55–1.14) |
| Never Smokers, No Exclusion for CVD or Cancer | ||||||
| No Diabetes | 97,915 | 1.09 (0.86–1.38) | 1 (ref) | 0.94 (0.76–1.17) | 1.16 (0.95–1.40) | 1.26 (1.05–1.53) |
| Diabetes | 5,447 | 1.78 (1.05–3.04) | 1 (ref) | 1.05 (0.64–1.72) | 0.82 (0.52–1.28) | 1.00 (0.64–1.55) |
| Never Smokers, No CVD (Cancer Included) | ||||||
| No Diabetes | 93,532 | 1.16 (0.90–1.49) | 1 (ref) | 0.94 (0.75–1.19) | 1.20 (0.98–1.48) | 1.37 (1.12–1.68) |
| Diabetes | 4,924 | 2.19 (1.22–3.94) | 1 (ref) | 1.08 (0.62–1.87) | 0.82 (0.51–1.34) | 1.06 (0.66–1.70) |
| Never Smokers, No Cancer (CVD Included) | ||||||
| No Diabetes | 92,797 | 1.06 (0.82–1.37) | 1 (ref) | 0.91 (0.72–1.15) | 1.20 (0.98–1.47) | 1.27 (1.04–1.56) |
| Diabetes | 4,619 | 1.41 (0.78–2.55) | 1 (ref) | 0.99 (0.57–1.71) | 0.82 (0.49–1.36) | 0.79 (0.49–1.27) |
Adjusted model: age (four categories), marital status, smoking status, leisure-time physical activity, alcohol consumption, poor income, region of country, and self-reported general health status
† = among never smokers only
*Models exclude individuals with heart disease and cancer
CIs confidence intervals; ref reference; CVD cardiovascular disease
Although the sample sizes in the lowest and highest categories were too low to provide stable estimates, the use of standard BMI categories did not alter the interpretation of results (see Supplemental Table 3, available online).
DISCUSSION
In our study, the BMI–mortality relationship was found to be substantially different in adults with versus those without prevalent diabetes. In adults with diagnosed diabetes, the relationship of BMI to mortality appeared to be inverse. This inverse relationship was strong, fairly graded, and independent of smoking, CVD, cancer, and a range of other potential confounders. BMI remained positively associated with mortality in adults without diabetes, but inversely associated in their counterparts with diabetes. This qualitative interaction indicates that prevalent diabetes modifies the BMI–mortality relationship on both the additive and multiplicative scales. As the additive scale is most relevant to public health, clinicians and investigators should keep in mind that individuals with diabetes have a higher risk of mortality at all levels of BMI compared to their counterparts without diagnosed diabetes.
Excess adiposity (especially beyond a BMI of 35) in the general, healthy population is an established risk factor for mortality,14 but its relationship to mortality is unclear in diseased populations like those with diabetes, as recent data suggest individuals who have a normal weight at diabetes diagnosis may be at an increased mortality risk compared to their overweight/obese counterparts.15 Investigators increasingly reference an obesity paradox among individuals with chronic diseases, whereby obese persons have higher survival rates compared to non-obese individuals.16–24 Mechanisms for this obesity paradox are not fully understood, but proposed explanations include: 1) sick individuals lose weight and become frail as a result of underlying illnesses that cause wasting;25 2) obese, sick individuals may have greater cardiorespiratory fitness (a proxy for physical wellness) than non-obese individuals who are also sick, but more likely to be frail and unfit,26 which is supported by a “surplus calorie theory” hypothesizing that the obese have increased calorie reserves resulting in higher serum lipid levels, less protein/muscle breakdown and less uremia;27 3) healthy individuals without major disease or injuries experience monotonically increasing mortality risk from excess adiposity, and individuals with major diseases or injuries experience monotonically decreasing mortality risk from excess adiposity; 28,29 4) older obese individuals receive more aggressive treatment that may lead to a potentially false appearance that obesity is protective; and 5) methodological concerns related to residual confounding, survivor bias, and lead-time bias.25,30–32
Regarding diabetes, it is widely acknowledged that body weight can be influenced by disease duration and weight loss (both intentional and unintentional) following diabetes development/diagnosis. Other common comorbidities such as CVD and cancer may also result in unhealthy weight loss and increased risk of mortality. Depletion of individuals most susceptible to the ill effects of diabetes may also contribute to these findings, as the most unhealthy obese individuals with diabetes may have died before becoming eligible to participate in research studies.
Many prior studies included individuals with prevalent diabetes33–36 and did not take baseline diabetes status into account.5,37–41 Numerous previous studies, therefore, should be interpreted with caution, because adiposity can be affected by the disease state and may lead to reverse causation. Individuals with poorly controlled, advanced prevalent diabetes may have a low BMI at the time of data collection due to the advanced disease state and an increased risk of mortality, which may give rise to the likely erroneous appearance that a higher BMI among those with diabetes is protective.
A recent study sought to address this concern by using BMI measurements taken within a year of diabetes diagnosis to investigate mortality risk associated with higher BMIs, and found a U-shaped relationship between BMI and mortality, where overweight individuals had the lowest risk.42 This study, however, should also be interpreted with caution due to both low statistical power and insufficient accounting for potential bias related to reverse causation. Our results also suggest weight in adults with diabetes may be associated with different impacts on mortality than are observed in the general population. However, our ability to determine optimal weight among adults with diabetes is precluded by both 1) the cross-sectional nature of our data that produced BMI measurements among participants with prevalent and not the more informative incident diabetes (or BMI at diabetes diagnosis), and 2) our inability to address the potential for reverse causation, where the presence of preexisting illnesses are associated with weight loss or wasting.
We have additional limitations. Our data are based on self-report, including heights and weights used to calculate BMIs. Studies have, however, shown that BMI values based on self-report largely produce the same risk estimates as those based on measurements.37,40,43,44 We also relied on self-reported diabetes, and as many as 27 % of individuals with diabetes are undiagnosed.45 Due to data collection procedure, we were unable to separate participants with type 1 and type 2 diabetes to exclude participants with type 1 diabetes from the group reporting diabetes status. We were, however, able to exclude those who reported a diabetes diagnosis associated with insulin use prior to 25 years of age. Nonetheless, type 1 diabetes represents 5 % of those with diabetes, making it reasonable to assume that most cases were type 2. Lastly, the higher risk among those in the lowest BMI quintile may be due to under-treatment, poor diabetes control, type 1 diabetes due to longer duration of diabetes and higher rates of complications, and/or poor health conditions that lead to wasting; however, we did not have the information necessary to assess these conditions.
Despite the limitations, our study had important strengths, as our data were based on: 1) a nationally representative cohort with results that are generalizable to the US population; 2) a large sample size; 3) a large number of deaths overall and from diabetes; 4) comparable data for those with and without diabetes that allowed a direct comparison of risk patterns; and 5) recently collected data.
As the BMI–mortality relationship appears inverse among adults with diagnosed prevalent diabetes, our study suggests diabetes status should be taken into account, much like heart disease and cancer, in future BMI–mortality studies, to obtain valid estimates. In terms of public health and clinical implications, data derived from the previously described types of study designs—due to potential design and methodological limitations—should be interpreted with caution when developing or employing treatment recommendations/strategies for diabetes. As it is established that BMI is positively associated with increased risk of morbidity and poorer health-related quality of life,46 achieving and maintaining a healthy weight should continue to be recommended for both individuals with and without diabetes.
Electronic Supplementary Material
(DOC 162 kb)
Acknowledgements
Drs. Brancati, Yeh and Wang were supported by a Diabetes Research and Training Center grant from NIDDK (P60 DK079637). Dr. Brancati was supported by a grant from NIDDK (K24 DK62222), Dr. Yeh was supported by a grant from NHLBI (P50HL105187) and Dr. Wang was supported by a grant from NCATS (UL1 RR025005). Drs. Hu and Jackson were supported by TREC (1U54CA155626-01). The funding sources were not involved in the data collection, data analysis, manuscript writing or publication. Our beloved colleague, Dr. Brancati, died before the final acceptance of this study.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA: j Am Med Assoc. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Lamb MM, Carroll MD, Flegal KM. Obesity and socioeconomic status in adults: United States, 2005–2008. NCHS Data Brief 2010:1–8. [PubMed]
- 3.Overweight, obesity, and health risk. National Task Force on the Prevention and Treatment of Obesity. Archives of internal medicine. 2000;160:898–904. [DOI] [PubMed]
- 4.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 5.Calle EE, Teras LR, Thun MJ. Obesity and mortality. N Engl J Med. 2005;353:2197–2199. doi: 10.1056/NEJM200511173532020. [DOI] [PubMed] [Google Scholar]
- 6.Schneider HJ, Wittchen HU, Wallaschofski H. Obesity and risk of death. N Engl J Med. 2009;360:1043. [PubMed] [Google Scholar]
- 7.Finkelstein EA, Fiebelkorn IC, Wang G. State-level estimates of annual medical expenditures attributable to obesity. Obes Res. 2004;12:18–24. doi: 10.1038/oby.2004.4. [DOI] [PubMed] [Google Scholar]
- 8.Flegal KM, Graubard BI, Williamson DF, Cooper RS. Reverse causation and illness-related weight loss in observational studies of body weight and mortality. Am J Epidemiol. 2011;173:1–9. doi: 10.1093/aje/kwq341. [DOI] [PubMed] [Google Scholar]
- 9.National Center for Health Statistics, Centers for Disease Control and Prevention. National Health Interview Survey. Hyattsville, MD. Available at: http://www.cdc.gov/nchs/nhis.htm. Accessed June, 2013.
- 10.Wilcosky T, Hyde J, Anderson JJ, Bangdiwala S, Duncan B. Obesity and mortality in the Lipid Research Clinics Program Follow-up Study. J clin epidemiol. 1990;43:743–752. doi: 10.1016/0895-4356(90)90232-E. [DOI] [PubMed] [Google Scholar]
- 11.Wolters KM. Introduction to Variance Estimation. New York, NY: Springer; 1990. [Google Scholar]
- 12.Rao JN, Scott AJ. A simple method for the analysis of clustered binary data. Biometrics. 1992;48:577–585. doi: 10.2307/2532311. [DOI] [PubMed] [Google Scholar]
- 13.Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carnethon MR, De Chavez PJ, Biggs ML, et al. Association of weight status with mortality in adults with incident diabetes. JAMA. 2012;308:581–590. doi: 10.1001/jama.2012.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleischmann E, Teal N, Dudley J, May W, Bower JD, Salahudeen AK. Influence of excess weight on mortality and hospital stay in 1,346 hemodialysis patients. Kidney Int. 1999;55:1560–1567. doi: 10.1046/j.1523-1755.1999.00389.x. [DOI] [PubMed] [Google Scholar]
- 17.Badheka AO, Rathod A, Kizilbash MA, et al. Influence of obesity on outcomes in atrial fibrillation: yet another obesity paradox. Am J Med. 2010;123:646–651. doi: 10.1016/j.amjmed.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 18.Chazot C, Gassia JP, Di BA, Cesare S, Ponce P, Marcelli D. Is there any survival advantage of obesity in Southern European haemodialysis patients? Nephrol Dial Transplant. 2009;24:2871–2876. doi: 10.1093/ndt/gfp168. [DOI] [PubMed] [Google Scholar]
- 19.Curtis JP, Selter JG, Wang Y, et al. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165:55–61. doi: 10.1001/archinte.165.1.55. [DOI] [PubMed] [Google Scholar]
- 20.Fonarow GC, Srikanthan P, Costanzo MR, Cintron GB, Lopatin M. An obesity paradox in acute heart failure: analysis of body mass index and inhospital mortality for 108,927 patients in the Acute Decompensated Heart Failure National Registry. Am Heart J. 2007;153:74–81. doi: 10.1016/j.ahj.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Galal W, van Gestel YR, Hoeks SE, et al. The obesity paradox in patients with peripheral arterial disease. Chest. 2008;134:925–930. doi: 10.1378/chest.08-0418. [DOI] [PubMed] [Google Scholar]
- 22.Kumakura H, Kanai H, Aizaki M, et al. The influence of the obesity paradox and chronic kidney disease on long-term survival in a Japanese cohort with peripheral arterial disease. J Vasc Surg. 2010;52:110–117. doi: 10.1016/j.jvs.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Mullen JT, Moorman DW, Davenport DL. The obesity paradox: body mass index and outcomes in patients undergoing nonbariatric general surgery. Ann Surg. 2009;250:166–172. doi: 10.1097/SLA.0b013e3181ad8935. [DOI] [PubMed] [Google Scholar]
- 24.Bertoni AG, Saydah S, Brancati FL. Diabetes and the risk of infection-related mortality in the U.S. Diabetes Care. 2001;24:1044–1049. doi: 10.2337/diacare.24.6.1044. [DOI] [PubMed] [Google Scholar]
- 25.Habbu A, Lakkis NM, Dokainish H. The obesity paradox: fact or fiction? Am J Cardiol. 2006;98:944–948. doi: 10.1016/j.amjcard.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 26.McAuley PA, Kokkinos PF, Oliveira RB, Emerson BT, Myers JN. Obesity paradox and cardiorespiratory fitness in 12,417 male veterans aged 40 to 70 years. Mayo Clin Proc. 2010;85:115–121. doi: 10.4065/mcp.2009.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt DS, Salahudeen AK. Obesity-survival paradox-still a controversy? Semin Dial. 2007;20:486–492. doi: 10.1111/j.1525-139X.2007.00349.x. [DOI] [PubMed] [Google Scholar]
- 28.Kalantar-Zadeh K, Kopple JD. Relative contributions of nutrition and inflammation to clinical outcome in dialysis patients. Am J Kidney Dis. 2001;38:1343–1350. doi: 10.1053/ajkd.2001.29250. [DOI] [PubMed] [Google Scholar]
- 29.Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2001;38:1251–1263. doi: 10.1053/ajkd.2001.29222. [DOI] [PubMed] [Google Scholar]
- 30.Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 31.Banack HR, Kaufman JS. The “obesity paradox” explained. Epidemiology. 2013;24:461–462. doi: 10.1097/EDE.0b013e31828c776c. [DOI] [PubMed] [Google Scholar]
- 32.Ferreira I, Stehouwer CD. Obesity paradox or inappropriate study designs? Time for life-course epidemiology. J Hypertens. 2012;30:2271–2275. doi: 10.1097/HJH.0b013e32835b4fe0. [DOI] [PubMed] [Google Scholar]
- 33.McEwen LN, Karter AJ, Waitzfelder BE, et al. Predictors of mortality over 8 years in type 2 diabetic patients: Translating Research Into Action for Diabetes (TRIAD) Diabetes Care. 2012;35:1301–1309. doi: 10.2337/dc11-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zoppini G, Verlato G, Targher G, Bonora E, Trombetta M, Muggeo M. Variability of body weight, pulse pressure and glycaemia strongly predict total mortality in elderly type 2 diabetic patients. The Verona Diabetes Study. Diabetes Metab Res Rev. 2008;24:624–628. doi: 10.1002/dmrr.897. [DOI] [PubMed] [Google Scholar]
- 35.Ross C, Langer RD, Barrett-Connor E. Given diabetes, is fat better than thin? Diabetes Care. 1997;20:650–652. doi: 10.2337/diacare.20.4.650. [DOI] [PubMed] [Google Scholar]
- 36.Chaturvedi N, Fuller JH. Mortality risk by body weight and weight change in people with NIDDM. The WHO Multinational Study of Vascular Disease in Diabetes. Diabetes Care. 1995;18:766–774. doi: 10.2337/diacare.18.6.766. [DOI] [PubMed] [Google Scholar]
- 37.Berrington de GA, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. [DOI] [PMC free article] [PubMed]
- 38.Zheng W, McLerran DF, Rolland B, et al. Association between body-mass index and risk of death in more than 1 million Asians. N Engl J Med. 2011;364:719–729. doi: 10.1056/NEJMoa1010679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Byers T. Body-mass index and mortality. N Engl J Med. 2000;342:286–287. doi: 10.1056/NEJM200004203421609. [DOI] [PubMed] [Google Scholar]
- 40.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 41.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 42.Logue J, Walker JJ, Leese G, et al. The association between bmi measured within a year after diagnosis of type 2 diabetes and mortality. Diabetes care. 2012. [DOI] [PMC free article] [PubMed]
- 43.Stommel M, Schoenborn CA. Accuracy and usefulness of BMI measures based on self-reported weight and height: findings from the NHANES & NHIS 2001–2006. BMC Publ Health. 2009;9:421. doi: 10.1186/1471-2458-9-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States A, GA: Centers for Disease Control and Prevention; 2011. Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs%5F2011.pdf. Accessed June 2013.
- 46.Sturm R, Wells KB. Does obesity contribute as much to morbidity as poverty or smoking? Public health. 2001;115:229–235. doi: 10.1038/sj/ph/1900764. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 162 kb)