ABSTRACT
Background
Hot flashes are the most commonly reported vasomotor symptom during the peri- and early post-menopausal period.
Objectives
To systematically review, appraise and summarize the evidence of the impact of different SSRIs on peri-menopausal hot flashes in healthy women in randomized, controlled trials.
Methods
A comprehensive literature search was conducted of MEDLINE™, EMBASE, the Cochrane Central Register of Controlled Trials, Web of Science and Scopus through March 2013. Two independent reviewers selected studies and extracted data. Random effects meta-analysis was used to pool outcomes across studies, and Bayesian mixed treatment methods were used to rank SSRIs in terms of effectiveness.
Results
We included a total of 11 randomized controlled trials with good methodological quality enrolling 2,069 menopausal and post-menopausal women (follow-up 1–9 months, mean age 36–76 years, mean time since menopause 2.3–6.6 years). Compared with placebo, SSRIs were associated with a statistically significant decrease in hot flash frequency (difference in means −0.93; 95 % CI −1.46 to −0.37; I2 = 21 %) and severity assessed by various scales (standardized difference in means −0.34; 95 % CI −0.59 to −0.10; I2 = 47 %). Adverse events did not differ from placebo. Mixed treatment comparison analysis demonstrated the superiority of escitalopram compared to other SSRIs in terms of efficacy.
Conclusion
SSRI use is associated with modest improvement in the severity and frequency of hot flashes but can also be associated with the typical profile of SSRI adverse effects.
KEY WORDS: SSRI, hot flashes, menopause
INTRODUCTION
Hot flashes remain the most commonly reported vasomotor symptom during peri- and early post-menopausal period.1 For some women, they can lead to significant physical distress and functional impairment requiring medical intervention.2 Hot flashes are described as spontaneous sensations of warmth affecting the face, neck and upper chest, and are often associated with palpitation, sweating and anxiety. Although the exact pathophysiology is unknown, estrogen withdrawal, rather than low circulating estrogen levels, has been thought to cause central thermoregulatory center dysfunction, which eventually will lead to hot flashes.3 This process is regulated by multiple neurotransmitters, norepinephrine being the primary neurotransmitter responsible for lowering the thermoregulatory set point and triggering the heat loss mechanisms as described before.4
Hormone replacement therapy (HRT) is the most effective and standard treatment for vasomotor symptoms of menopause.5 However, a randomized controlled trial of 16,608 post-menopausal women receiving estrogen and progesterone HRT versus placebo showed an increased hazard ratio of coronary heart disease and breast cancer, which were present across racial/ethnic and age strata and were not influenced by the antecedent risk status or prior disease. HRT also showed increased risk of stroke and pulmonary embolism. The risk was not counterbalanced by the smaller reduction in the number of hip fractures and colorectal cancer.6 Because of the concerns regarding the safety of HRT, the interest in alternative therapies for improving menopausal symptoms was increased. Such alternatives include stress management, chiropractic interventions, soy supplements and acupuncture; however, evidence of their efficacy is inconclusive.7
Other pharmacological interventions with possible benefit include clonidine, selective serotonin reuptake inhibitors (SSRIs), selective nor-epinephrine reuptake inhibitors and anticonvulsants.8 SSRIs seem to be an attractive alternative in this setting because of their wide use and favorable safety profile demonstrated in various settings. Nevertheless, studies of SSRIs have demonstrated mixed results; some studies demonstrated benefit by reducing hot flashes by 50–60 % while others reported no effect.9–11
Therefore, we conducted a systematic review to synthesize and summarize the best available evidence on the use of SSRIs to treat hot flashes in healthy menopausal women. The aim of this comparative effectiveness review is to evaluate the efficacy and side effect profile to aid in decision making.
METHODS
The study was performed following procedures recommended by the Cochrane collaboration12 and is reported in accordance with the recommendations set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.13
Eligibility Criteria
We included randomized controlled trials that enrolled healthy peri-menopausal women at the beginning of the study who received any SSRI medications (e.g., citalopram, escitalopram, fluoxetine, paroxetine, sertraline, etc.), compared them against placebo or other SSRIs, and evaluated vasomotor symptoms (daily hot flash frequency or improvement in vasomotor scores). We specifically excluded studies that enrolled cancer patients and patients receiving selective estrogen receptor modulators (SERMs) because hot flashes and night sweats are common complaints (up to 80 %) of patients receiving tamoxifen for breast cancer,14–16 women taking hormonal replacement therapy and patients with diagnoses of depression.
Information Sources and Search Methods
A comprehensive literature search was conducted by an expert reference librarian. We searched the electronic databases (MEDLINE™, EMBASE, the Cochrane Central Register of Controlled Trials CENTRAL, Web of Science and Scopus) using various combinations of controlled terms: “menopause,” post-menopause,” “peri-menopause”, “hot flushes,” “hot flashes,” “SSRIs,” “climacteric” and “vasomotor.” No limits were applied for publication date or language, and foreign papers were translated. We searched through March 2013.
Study Identification
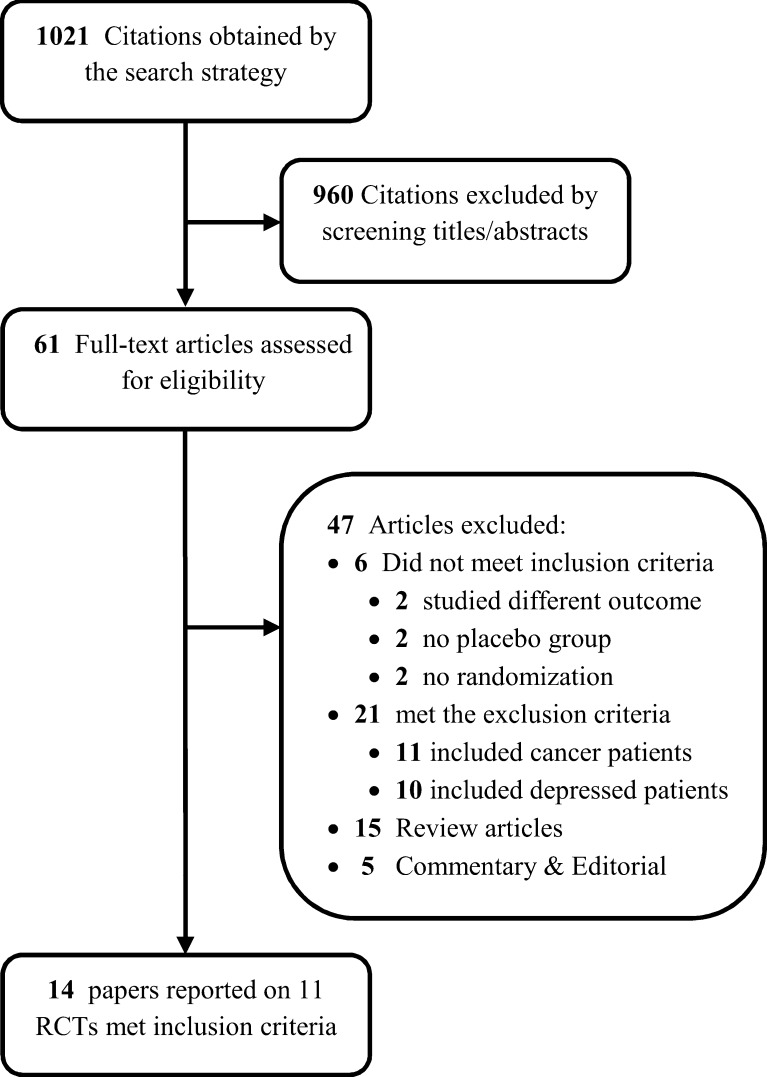
Previously described data sources were searched by two independent reviewers (TS & BF); they reviewed the abstracts, agreed on a-priori eligibility criteria including the inclusion and exclusion criteria of each study and decided which of the eligible studies to include. Disagreements between reviewers were resolved by consensus. The kappa statistic for agreement on study selection was 0.87. If a study was deemed relevant, the manuscript was obtained and reviewed in full text versions for further assessment. The final search identified 61 RCTs; of these, 11 fulfilled the inclusion criteria (Fig. 1).
Figure 1.
Flow chart of the study.
Data Collection and Extraction
Data from included studies were extracted by two independent reviewers (TS and FH) using a standardized, piloted data extraction sheet. We abstracted data on patient demographics and baseline characteristics (including age, menopause status, race, smoking status and body mass index); study design; sample size; intervention type [including type and dose of the SSRI (selective serotonin reuptake inhibitor) versus placebo or versus the type and dose of another SSRI]; type of outcome measure (including the frequency of hot flashes, improvement in the vasomotor score of a validated scale).
One review author extracted the data from included studies, and a second author verified the extracted data. Disagreements were resolved by discussion between the two review authors. The number of events in each trial was extracted, when available, on the basis of the intention-to-treat approach. For collected and abstracted data, please see Table 1.
Table 1.
Characteristics of Included Studies
| Study ID | Origin | Sample size | Study arms | Primary outcomes | Age (years; range or mean ± SD) | Frequency of hot flashes at baseline | Time since menopause (years; mean) | Race (%) | Smoking (%) | BMI (mean ± SD) | Treatment max duration |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Paroxetine (3 trials) | |||||||||||
| Simon 2012 | USA | 568 | Arm 1: low-dose mesylate salt of paroxetine (LDMP) 7.5 mg/day Arm 2: Placebo | Frequency and severity of HF | 54.4 ± 5.6 | 10.86 ± 3.91 | - | 75.5 % white; 21.5 % AA; 3 % others | - | 28.14 ± 5 | 24 weeks |
| Kaunitz 2012 | USA | 606 | Arm 1: LDMP 7.5 mg/day Arm 2: Placebo | Frequency and severity of HF | 54.7 ± 6.1 | 11.72 ± 4.63 | - | 64.7 % white; 32.8 % AA; 2.5 % others | - | 29.5 ± 6.1 | 12 weeks |
| Stearns 2003 | USA | 165 | Arm 1: paroxetine 12.5 mg /day Arm 2: paroxetine 25 mg /day Arm 3: placebo | Frequency of HF, disability score and adverse events | 36-76 | - | - | 78 % ≥ 12 months | - | - | 6 weeks |
| Escitalopram (2 trials) | |||||||||||
| Freeman 2011 | USA | 205 | Arm 1: escitalopram 10–20 mg/day Arm 2: placebo | Frequency of HF/day and HF severity score | 40-62 | 9.77 ± 5.62 | Postmenopause 81 %; late transition 15 %; early transition 3 % | 50 % white; 46 % AA; 6 % others | 23 | 29.1 ± 6 | 8 weeks |
| Freedman 2011 | USA | 26 | Arm 1: escitalopram 10–20 mg/day Arm 2: placebo | Frequency of HF/day | 49-59 | 20.28 ± 5.31 | 5.8 ± 4 | 26 % white; 71 % AA; 0.2 % Hispanic | - | 26.8 ± 3 | 8 weeks |
| Citalopram (3 trials); fluoxetine (1 trial) | |||||||||||
| Akhavan 2011 | Iran | 80 | Arm 1: cipram Arm 2: cipram plus HT Arm 3: placebo Arm 4: placebo plus HT | Frequency of HF/day | 51.4 ± 3.5 | 15.0 ± 3.0 | 3.3 ± 1 | - | - | - | 8 weeks |
| Kalay 2007 | Turkey | 50 | Arm 1: cipram Arm 2: cipram plus HT Arm 3: placebo Arm 4: placebo plus HT | Frequency of HF/day and MENQOL HF score | 53 ± 4.5 | 7.39 ± 0.8 | 6.6 ± 4 | - | 15 | 27.8 ± 4 | 8 weeks |
| Suvanto-Luukkonen 2005 | Finland | 150 | Arm 1: citalopram Arm 2: fluoxetine Arm 3: placebo | Frequency of HF/day and Modified Kupperman-index | 45-66 | 5.51 ± 2.25 | 4 ± 4 | - | 25 | - | 36 weeks |
| Sertraline (3 trials) | |||||||||||
| Aedo 2011 | Chile | 33 | Arm 1: sertraline 50 mg/day Arm 2: placebo | Somatic menopause score | 45-60 | - | 2.3 ± 4 | - | 6 | 28.3 ± 3 | 13 weeks |
| Grady 2007 | USA | 89 | Arm 1: sertraline 100 mg/day Arm 2: placebo | Frequency of HF/day and hot flash score | 40-60 | 8.95 ± 5.78 | 3.5 ± 4 | 57 % white; 26 % AA; 17 % others | 23 | - | 6 weeks |
| Gordon 2006 | USA | 97 | Arm 1: sertraline 50 mg/day Arm 2: placebo | Frequency of HF/day and hot flash score | 40-65 | 6.7 ± 4.26 | - | 80 % white; 13 % Hispanic; 6 % others | 16 | - | 8 weeks (4 weeks each crossover period) |
Risk of Bias Assessment
Methodological quality was defined as the control of bias assessed through the reported methods in each individual trial. Two reviewers independently assessed trial quality by examining several components: generation of allocation sequence (classified as adequate if based on computer-generated random numbers, tables of random numbers or similar), concealment of allocation (classified as adequate if based on central randomization, sealed envelopes or similar), blinding (patients, care givers or outcome assessors), baseline imbalance and lost to follow-up. Disagreements between the reviewers were resolved by discussion or arbitrated with a third coauthor. We used the Cochrane Collaboration’s risk-of-bias tool to assess the quality of included randomized trials.17 The two reviewers extracting risk of bias data were blinded to the study authors, institution and journal name.
Summary of Measures
Our primary outcome measure was the daily frequency of hot flashes. We also extracted hot flashes/vasomotor symptoms assessed by scores (e.g., hot flash score,10 vasomotor score,11 modified Kupperman index,20 Rand mood score,21 hot flash-related daily interference scale22 and menopausal rating score23) whenever available and from those studies that did not report daily hot flash frequency.
Statistical Analysis
From each trial, we calculated the mean difference (MD) and 95 % confidence intervals (CI) as the measure of effect. When the mean response is not measured on the same scale, the standardized mean difference (SMD) was calculated allowing for pooling across trials on the same scale.18 The average effects for the outcomes across trials was estimated using a random effects model, as described by DerSimonian.19 We chose the random effects method as primary analysis because of its conservative summary estimate and incorporation of between- and within-study variance. We also tested the fixed-effect method to ascertain robustness of findings, and this model is mentioned only if it changed the conclusions. To assess the heterogeneity of treatment effect among trials, we used the I2 statistic. The I2 statistic represents the proportion of heterogeneity of treatment effect across trials that were not attributable to chance or random error. Hence, a value of 50 % reflects significant heterogeneity due to real differences in study populations, protocols, interventions and outcomes.20 The p-value threshold for statistical significance was set at 0.05 for the effect size. The level of agreement between the reviewers was estimated using Cohen’s kappa statistic, a measure of inter-rater agreement. Publication bias was evaluated using the Begg-Mazumdar rank correlation21 and Egger’s linear regression22,23 method. Analyses were conducted using RevMan v5.1 (The Nordic Cochrane Center, Copenhagen, Denmark).
Mixed Treatment Comparison
We anticipated that the majority of the trials would include comparisons with placebo, with only few head-to-head trials. Therefore, we conducted a mixed treatment comparison (MTC) analysis. This analysis pools evidence from direct and indirect comparisons to facilitate simultaneous inference regarding all treatments.24 MTC analysis was conducted using Bayesian methods. The goodness of fit was checked using the incoherent value. When incoherent value of the fixed-effect model or random-effect model was obtained, the model with the lower value was used. When residual deviance was similar between the two models, a random-effect model was used. The mean effect and standard error in the treatment and placebo groups of each eligible trial were conducted simultaneously using the fixed- and the random-effect Bayesian method.
For all outcomes, a burn-in of 30,000 simulations was discarded and the results presented based on a further 270,000 simulations. For each comparison, we estimated the 95 % credible intervals (CrI, the Bayesian version of the confidence intervals) of the estimates.25 Analyses were performed using WinBUGS 1.4 statistical software (MRC Biostatistics Unit, Cambridge, UK).
RESULTS
Search Results and Study Description
A total of 1,021 potentially relevant references were identified by the electronic search strategy, of which 61 full-text articles met the eligibility for assessment. A total of 11 trials (reported in 14 papers) met the inclusion criteria and were pooled in the meta-analysis;10,11,26–37 47 articles were excluded. Figure 1 depicts the results of the search strategy and study selection. All included randomized trials had a parallel design and only one had a crossover design,27 for which we only included the first arm of the crossover.
The 11 trials included 2,069 menopausal and post-menopausal women. In these trials, women were followed for a period of 1 to 9 months. Their mean age ranged from 36 to 76 years old. The mean time since menopause prior to enrollment in those trials ranged from 2.3 to 6.6 years. The most prevalent races among trials’ populations are Caucasian and African American. Smoking rate ranged from 6 % to 25 %. Table 1 describes and summarizes the characteristics of the included studies.
There were four papers reported on one trial;34–37 we referred to them as Freeman et al. Included trials reported outcome measures at different periods: two trials reported at 6 weeks, five trials reported at 8 weeks and four trials reported at >12 weeks. We used data reported at 6- to 8-week periods. The trial by Gordon et al., reported in two papers,27,38 is a crossover trial; outcomes were both prior to crossover (at 4 weeks) and at the end of the study (8 weeks). For frequency of hot flashes, we used data before crossover had occurred, at end of a 4-week period, which is optimal. For hot flash severity scores, data before crossover were not available, and we used the available data at the end of the study, an 8-week period. Simon et al. and Kaunitz et al. are both conference abstracts presented at the 23rd Annual Meeting of The North American Menopause Society in October 2012 at Orlando, FL. The authors were contacted and provided us with the presentation and further information about those studies; we included data reported at 8 weeks. Aedo et al. only reported on hot flash severity scores, although they was not included in the analysis as the reported data are dichotomous. Our inclusion criteria initially excluded all studies containing >10 % of breast cancer patients or >10 % of patients on hormonal therapy. We included Stearns et al. as breast cancer patients comprise only 7 % of the patient population, and 7 % were also on hormonal therapy. Only 9 of the 11 included trials reported the frequency of hot flashes per day; only six trials reported the severity scores of hot flashes (see Fig. 2).
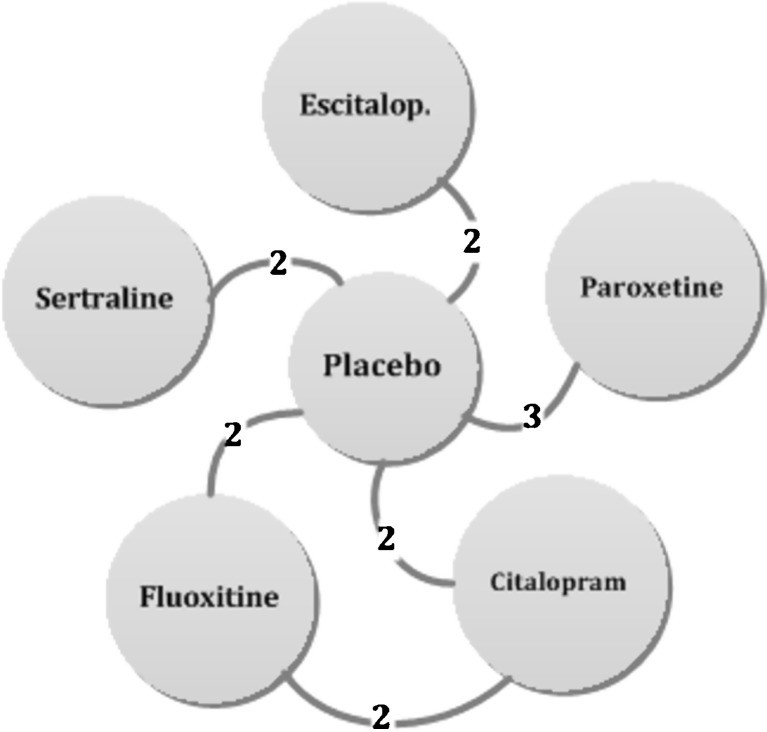
Figure 3.
SSRI network: Each edge (circle) represents a treatment; connecting lines indicate pairs of treatments that have been directly compared in randomized trials. The numbers on the lines indicate the numbers of trials making that comparison.
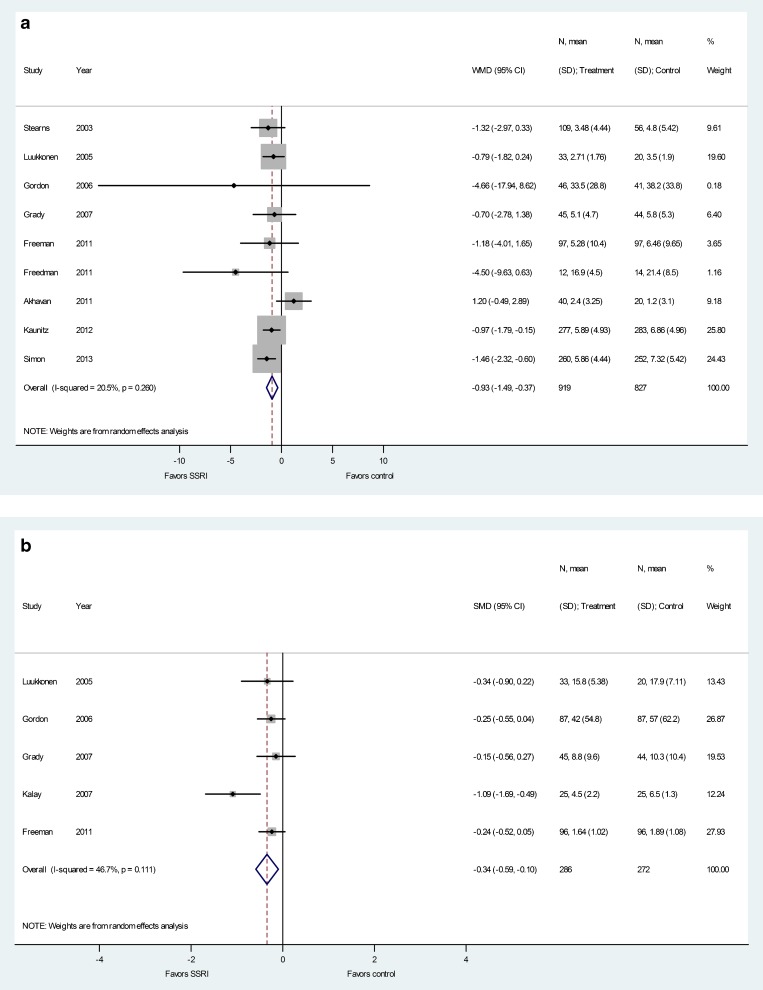
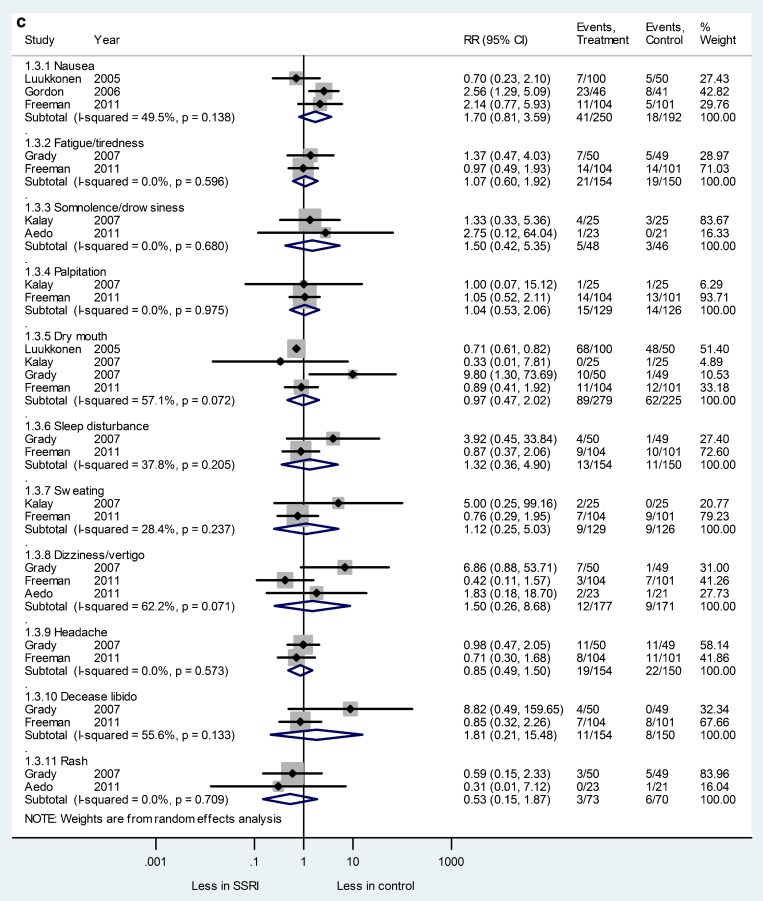
Figure 2.
Meta-analyses. Panel a: Improvement in hot flash frequency per day. Panel b: Improvement in standardized hot flash scale scores. Panel c: Adverse events.
In general, the overall quality of the 11 trials was appropriate with likely low risk of bias. Table 2 describes the methodological quality of the 11 RCTs included in this systematic review.
Table 2.
Quality Assessment of Included Studies*
| Study ID | Adequate sequence generation | Allocation concealment | Blinding | Baseline characteristics imbalance | Lost to follow-up (%) | Intention to treat analysis applied | Source of study funding | Other source of bias |
|---|---|---|---|---|---|---|---|---|
| Simon 2012 | Yes; method not mentioned | Unclear | Double-blind at first, then single-blind after 12 days | None | 12 at week 8; 102 at week 24 | Mentioned | For-profit organization | Both Simon et al. and Kauntiz et al. has same co-authors from Yale Medical Group and Noven Pharmaceuticals |
| Kaunitz 2012 | Yes; method not mentioned | Unclear | Double-blind at first, then single-blind after 12 days | None | 44 at week 8; 62 at week 12 | Mentioned | For-profit organization | |
| Freedman 2011 | Yes; generated through a computer software | Yes; a distant institute kept the blinding code | Yes; the placebo and active drug capsules were identical | The active drug group had a significant higher BMI | 14 | Not mentioned | Non-for-profit organization | • Adverse effect of medication was not mentioned• Participant were paid for their participation |
| Freeman 2011 | Yes; through a dynamic randomization algorithm | Yes; they used a secure web-based database | Yes; participant ants and study site personnel | None | 5.4 | Not mentioned | Non-for-profit organization | The dose increase if HF was not reduced ≥50 % |
| Akhavan 2011 | Yes, they used blocked randomization | Unclear | Yes; methods unclear | There is significant difference in mean age of study groups | Unclear | Mentioned | Unclear | - |
| Suvanto-Luukkonen 2005 | Yes; the randomization done at distant institute | Yes; opaque boxes were used | Yes; randomization codes opened only after all women had completed the study | None | 36 | Not mentioned | Non-for-profit organization | Some adverse effect of medication was not mentioned (only nausea and dry mouth were mentioned) |
| Kalay 2007 | Yes; generated through a computer software | No | Yes; only participants were blinded | Kupperman index for climacteric symptoms score was higher in the Citalopram group than in placebo group | 0 | Not mentioned | Unclear; not reported | • The dose was increased to 40 mg/day in cases where insufficient improvement was observed. • Investigators know the treatment and the placebo groups |
| Grady 2007 | Yes; through randomly permuted blocks | Yes; see next cell | Yes; all investigators, study staff and participants were blinded to study medication status until the trial was completed | Treatment group participants were younger, had higher percentage of African American race, and were less educated than Placebo group | 10.1 | Not mentioned | For-profit organization | - |
| Gordon 2006 | Yes; method not mentioned | Yes; randomization code was not unmasked until the collection of data was completed | Yes; The randomization code was not unmasked until the collection of data was completed | None | 25 | Mentioned | Unclear; not reported | - |
| Gordon 2006 | Yes; generated through a computer software | Yes; a pharmacist who had no contact with study participants determined the sequence numbers | Yes; all study participants and study personnel were blinded | None | 14.7 | Not mentioned | For-profit organization | Participant were paid for their participation |
| Stearns 2003 | Yes; patients randomized in 1:1:1 ratio; method not mentioned | Unclear | Yes; methods unclear | None | 0 | Not mentioned | For-profit organization | - |
*We used the Cochrane risk-of-bias tool 17 for assessing the quality of included randomized clinical trials
Improvement in Hot Flash Frequencies and Scores
Compared with placebo, SSRIs were associated with a statistically significant decrease in hot flash frequency at end of 4 to 8 weeks (MD −0.93; 95 % CI −1.49 to −0.37; I2 = 21 %). Also, scores reflecting hot flashes assessed on different standardized scales showed improvement in SSRI groups (SMD −0.34; 95 % CI −0.59 to −0.10; I2 = 47 %). Results are depicted in Fig. 2, panels a and b, and Fig. 3. There was no evidence of publication bias (P > 1.0 using the methods of both Begg-Mazumdar and Egger). This improvement in hot flashes, although statistically significant, is modest and likely has questionable clinical significance.
Aedo et al. reported a successful response, defined as reduction of 50 % or more in the sum of somatic and psychological domains of the Menopause Rating Scale (MRS), in 81.3 % of women in the sertraline group compared to 35.3 % in the placebo group at 90 days of follow-up.
Adverse Events
SSRIs had no significantly higher or significantly lower adverse events compared to placebo, but there was a trend toward more adverse effects in the SSRI group. Pooled effects for adverse events are: nausea (RR 1.7; CI 0.81 to 3.59), fatigue/tiredness (RR 1.07; CI 0.60 to 1.92), somnolence/drowsiness (RR 1.50; CI 0.42 to 5.35), palpitation (RR 1.04; CI 0.53 to 2.06), dry mouth (RR 1.29; CI 0.69 to 2.40), sleep disturbance (RR 1.32; CI 0.36 to 4.90), sweating (RR 1.12; CI 0.25 to 5.03), dizziness/vertigo (RR 1.5; CI 0.26 to 8.68), headache (RR 0.85; CI 0.49 to 1.5), decreased libido (RR 1.81; CI 0.21 to 15.48) and rash (RR0.53; CI 0.15 to 1.87). Simon et al. and Kaunitz et al. did not report adverse events at 8-week intervals. Results are provided in Fig. 2, panel c.
Mixed Treatment Comparison Analysis
In Table 3, the corresponding mean effects using Bayesian methods are presented. As expected, and when using the fixed- or random-effect Bayesian MTC, each treatment from the SSRI family performs better than placebo. Table 3 also demonstrates the relationship of SSRIs compared to each other. Escitalopram has the highest probability to be ranked first among other SSRIs in terms of efficacy. In sensitivity analysis, the fixed effect results were very similar to the random effect results, suggesting the robustness the of analysis to the choice of model
Table 3.
Results of Mixed Treatment Comparison Comparing the Efficacy of Escitalopram, Paroxetine, Sertraline, Citalopram and Fluoxetine, Conducted Using the Random-Effect Bayesian Method (Total Residual Deviance = 18.87)
| Comparison | Mean effect | 95 % CrI | Probability treatment is best | Rank |
|---|---|---|---|---|
| Placebo | Reference | - | 0 | - |
| Escitalopram | −2.05 | −4.82 to 0.62 | 61 % | 1 |
| Paroxetine | −1.23 | −2.39 to −0.12 | 18 % | 2 |
| Sertraline | −0.83 | −3.44 to 1.64 | 16 % | 3 |
| Citalopram | −0.54 | −2.00 to 0.83 | 3.4 % | 4 |
| Fluoxetine | −0.14 | −1.55 to 1.30 | 0.9 % | 5 |
| Escitalopram vs. Citalopram | 1.511 | −1.55 to 4.58 | - | - |
| Escitalopram vs. Fluoxetine | 1.914 | −1.11 to5.06 | - | - |
| Escitalopram vs. Sertraline | 1.225 | −2.44 to 4.89 | - | - |
| Escitalopram vs. Paroxetine | 0.82 | −2.06 to 3.82 | - | - |
| Citalopram vs. Fluoxetine | 0.40 | −1.02 to 1.92 | - | - |
| Citalopram vs. Sertraline | −0.29 | −3.32 to 2.57 | - | - |
| Citalopram vs. Paroxetine | −0.69 | −2.47 to 1.14 | - | - |
| Fluoxetine vs. Sertraline | −0.69 | −3.64 to 2.12 | - | - |
| Fluoxetine vs. Paroxetine | −1.09 | −2.94 to 0.70 | - | - |
| Sertraline vs. Paroxetine | −0.40 | −3.11 to 2.46 | - | - |
DISCUSSION
Main Findings
We conducted a systematic review and meta-analysis comparing the effect of SSRIs on menopausal hot flashes. The use of SSRIs is associated with a statistically significant decrease in the number of hot flashes per day after 8 weeks of use. This analysis was associated with minimal heterogeneity suggesting high confidence in this estimate. SSRI use was also associated with a significant, although heterogeneous, improvement in the scores of standardized scales for hot flashes.
The patients enrolled in the included trials had moderate-to-severe hot flashes, with an average frequency of ten per day. In terms of hot flash frequency, our analysis showed a decrease of one hot flash per day. In comparison, this 10 % reduction is less than that noticed with estrogen. The mean reduction observed with estrogen was (−2.7; 95 % CI – 4.7 to – 0.7) for conjugated equine estrogen, (−2.4; 95 % CI –3.3 to −1.45) for oral 17 β-estradiol and (−3.2; 95 % CI –5.1 to −1.48) for trans-dermal 17 β-estradiol.5Therefore, the effect of SSRIs on the frequency of hot flashes compared to estrogen, the most effective treatment, is smaller. In terms of hot flash severity, we noticed an improvement of 0.70 in standard deviation units across multiple scales. This improvement is considered a moderate effect size. Therefore, SSRIs appear to be a reasonable alternative to estrogen in women who cannot take hormonal therapy or are concerned about the long-term effects of estrogen.
The results of comparative effectiveness mixed treatment analysis suggest that escitalopram may be more effective than other SSRIs. All other SSRIs (escitalopram, citalopram and fluoxetine) were more effective than placebo. The adverse effects of SSRIs when used to alleviate hot flashes were minor and did not differ significantly from placebo, but there was a trend toward more adverse effects in the SSRI group. The adverse effects reported were nausea, dry mouth, fatigue, tiredness, decreased libido, sweating, dizziness and rash. In general, inferences about adverse effects should be derived from trials and observational studies of SSRIs used in the general population and with various indications. Such studies would provide a larger body of evidence with longer follow-up than studies of a limited indications, such as the case here.
Implications for Practice and Research
In this review, we excluded studies of women who are being treated with selective estrogen receptor modulators (SERM) or other hormonal treatments, such as tamoxifen and raloxifene, as they may suffer from flashing and hot flashes caused by different mechanisms than those related to natural menopause.16 SSRIs in these women may also speed the metabolism of tamoxifen to inactive metabolites, possibly reducing the severity of side effects, including hot flashes.39 Therefore, our findings relate to women suffering from natural peri- and post-menopausal symptoms.
Our results are consistent with previous guidelines. The Royal College of Obstetricians and Gynecologists Scientific Advisory Committee acknowledged SSRIs as the most commonly used drugs in clinical practice for the alleviation of menopause symptoms as an alternative to HRT.40,41 HRT was considered the most effective treatment for vasomotor symptoms including hot flashes, but its use is associated with several complications and adverse effects, particularly venous thromboembolism and stroke. The committee described side effects of SSRIs, such as nausea and reduced libido, as a possible drawback. In our analysis, there was no statistical significance in adverse event rates between SSRI and placebo, although rates of nausea and libido appeared to be higher in the SSRI arm. It is likely that analysis of a small number of RCTs is underpowered to detect these adverse effects, and data from larger observational studies in menopausal women or trials in conditions other than menopausal treatment are needed for more precise harm outcomes.
The limitations of this review include the short follow-up period in most of the included studies; only one study followed patients up to 9 months, whereas most of the studies had less than 3 months of follow-up. Other limitations are the small sample size in each individual study, which ranged from 26 to 606 patients. The quality of the overall evidence (confidence in the estimates) was moderate to high for efficacy vs. placebo, low to moderate for side effects (limited by imprecision) and likely low for the ranking suggesting superiority of escitalopram (limited by indirectness of comparative data).42,43
In conclusion, given the promising positive effects of SSRIs on hot flashes and the likely favorable side effect profile, their use seems to be an acceptable option for treating menopausal women with hot flashes and is a good alternative to HRT. Future trials should investigate different SSRIs over a long time period (e.g., 6–12 months) and should not use placebo for comparison in order to provide high-quality comparative effectiveness evidence.
CONCLUSIONS
SSRI use is associated with modest improvement in the severity and frequency of hot flashes and can also be associated with the typical profile of SSRI adverse effects.
Acknowledgement
Source of Funding
None
Disclosures
This study was made possible by support from the Center for the Science of Healthcare Delivery, Mayo Clinic, and CTSA grant UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Taghreed Shams and Belal Firwana contributed equally to this work.
REFERENCES
- 1.Freeman EW, Sherif K. Prevalence of hot flushes and night sweats around the world: a systematic review. Climacteric. 2007;10(3):197–214. doi: 10.1080/13697130601181486. [DOI] [PubMed] [Google Scholar]
- 2.National Institutes of Health State-of-the-Science Conference statement: management of menopause-related symptoms. Ann Intern Med. 2005;142(12 Pt 1):1003–13. [PubMed]
- 3.Nelson HD, Vesco KK, Haney E, Fu R, Nedrow A, Miller J, et al. Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA. 2006;295(17):2057–71. doi: 10.1001/jama.295.17.2057. [DOI] [PubMed] [Google Scholar]
- 4.Shanafelt TD, Barton DL, Adjei AA, Loprinzi CL. Pathophysiology and treatment of hot flashes. Mayo Clin Proc. 2002;77(11):1207–18. doi: 10.4065/77.11.1207. [DOI] [PubMed] [Google Scholar]
- 5.Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA. 2004;291(13):1610–20. doi: 10.1001/jama.291.13.1610. [DOI] [PubMed] [Google Scholar]
- 6.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 7.Newton KM, Buist DS, Keenan NL, Anderson LA, LaCroix AZ. Use of alternative therapies for menopause symptoms: results of a population-based survey. Obstet Gynecol. 2002;100(1):18–25. doi: 10.1016/S0029-7844(02)02005-7. [DOI] [PubMed] [Google Scholar]
- 8.Hall E, Frey BN, Soares CN. Non-hormonal treatment strategies for vasomotor symptoms: a critical review. Drugs. 2011;71(3):287–304. doi: 10.2165/11585360-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Loprinzi CL, Sloan JA, Perez EA, Quella SK, Stella PJ, Mailliard JA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol. 2002;20(6):1578–83. doi: 10.1200/JCO.20.6.1578. [DOI] [PubMed] [Google Scholar]
- 10.Grady D, Cohen B, Tice J, Kristof M, Olyaie A, Sawaya GF. Ineffectiveness of sertraline for treatment of menopausal hot flushes: a randomized controlled trial. Obstet Gynecol. 2007;109(4):823–30. doi: 10.1097/01.AOG.0000258278.73505.fa. [DOI] [PubMed] [Google Scholar]
- 11.Kalay AE, Demir B, Haberal A, Kalay M, Kandemir O. Efficacy of citalopram on climacteric symptoms. Menopause. 2007;14(2):223–9. doi: 10.1097/01.gme.0000243571.55699.4a. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration; updated March 2011.
- 13.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Bonneterre J, Thurlimann B, Robertson JF, Krzakowski M, Mauriac L, Koralewski P, et al. Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability study. J Clin Oncol. 2000;18(22):3748–57. doi: 10.1200/JCO.2000.18.22.3748. [DOI] [PubMed] [Google Scholar]
- 15.Hunter MS, Grunfeld EA, Mittal S, Sikka P, Ramirez AJ, Fentiman I, et al. Menopausal symptoms in women with breast cancer: prevalence and treatment preferences. Psychooncology. 2004;13(11):769–78. doi: 10.1002/pon.793. [DOI] [PubMed] [Google Scholar]
- 16.Gupta P, Sturdee DW, Palin SL, Majumder K, Fear R, Marshall T, et al. Menopausal symptoms in women treated for breast cancer: the prevalence and severity of symptoms and their perceived effects on quality of life. Climacteric. 2006;9(1):49–58. doi: 10.1080/13697130500487224. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol. 2008;61(8):763–9. doi: 10.1016/j.jclinepi.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 19.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105–14. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 22.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53(11):1119–29. doi: 10.1016/S0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
- 24.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23(20):3105–24. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 25.Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. 2012. [PubMed]
- 26.Suvanto-Luukkonen E, Koivunen R, Sundstrom H, Bloigu R, Karjalainen E, Haiva-Mallinen L, et al. Citalopram and fluoxetine in the treatment of postmenopausal symptoms: a prospective, randomized, 9-month, placebo-controlled, double-blind study. Menopause. 2005;12(1):18–26. doi: 10.1097/00042192-200512010-00006. [DOI] [PubMed] [Google Scholar]
- 27.Gordon PR, Kerwin JP, Boesen KG, Senf J. Sertraline to treat hot flashes: a randomized controlled, double-blind, crossover trial in a general population. Menopause. 2006;13(4):568–75. doi: 10.1097/01.gme.0000196595.82452.ca. [DOI] [PubMed] [Google Scholar]
- 28.Aedo S, Cavada G, Campodonico I, Porcile A, Irribarra C. Sertraline improves the somatic and psychological symptoms of the climacteric syndrome. Climacteric. 2011;14(5):590–5. doi: 10.3109/13697137.2011.568645. [DOI] [PubMed] [Google Scholar]
- 29.Freedman RR, Kruger ML, Tancer ME. Escitalopram treatment of menopausal hot flashes. Menopause. 2011;18(8):893–6. doi: 10.1097/gme.0b013e31820ccae9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akhavan S, Zandvakili F, Arab M, Karimi H, Gharibi F. Comparison of the therapeutic effects of fluoxetine, citalopram, estrogen and progesterone and placebo in the treatment of hot flushes in perimenopausal women. Scientific Journal of Kurdistan University of Medical Sciences. 2011;16(3):31–8. [Google Scholar]
- 31.Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA. 2003;289(21):2827–34. doi: 10.1001/jama.289.21.2827. [DOI] [PubMed] [Google Scholar]
- 32.Simon JA, Sanacora G, Bhaskar S, Lippman J. Safety and efficacy of low-dose mesylate salt of paroxetine (LDMP) for the treatment of vasomotor symptoms (VMS) associated with menopause: A 24-week, randomized, placebo-controlled phase 3 study. Menopause. 2012;19(12):1371. [Google Scholar]
- 33.Kaunitz A, Sanacora G, Bhaskar S, Lippman J. Safety and efficacy of low-dose mesylate salt of paroxetine (LDMP) for the treatment of vasomotor symptoms (VMS) associated with menopause: A 12-week, randomized, placebo-controlled phase 3 study. Menopause. 2012;19(12):1389. [Google Scholar]
- 34.Freeman EW, Guthrie KA, Caan B, Sternfeld B, Cohen LS, Joffe H, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305(3):267–74. doi: 10.1001/jama.2010.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reed SD, Guthrie KA, Joffe H, Shifren JL, Seguin RA, Freeman EW. Sexual function in nondepressed women using escitalopram for vasomotor symptoms: A randomized controlled trial. Obstetrics and Gynecology. 2012;119(3):527–38. doi: 10.1097/AOG.0b013e3182475fa4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ensrud KE, Joffe H, Guthrie KA, Larson JC, Reed SD, Newton KM, et al. Effect of escitalopram on insomnia symptoms and subjective sleep quality in healthy perimenopausal and postmenopausal women with hot flashes: a randomized controlled trial. Menopause. 2012;19(8):848–55. doi: 10.1097/gme.0b013e3182476099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carpenter JS, Guthrie KA, Larson JC, Freeman EW, Joffe H, Reed SD, et al. Effect of escitalopram on hot flash interference: a randomized, controlled trial. Fertility and sterility. 2012;97(6):1399–404. doi: 10.1016/j.fertnstert.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kerwin JP, Gordon PR, Senf JH. The variable response of women with menopausal hot flashes when treated with sertraline. Menopause. 2007;14(5):841–5. doi: 10.1097/gme.0b013e31802e7f22. [DOI] [PubMed] [Google Scholar]
- 39.Desmarais JE, Looper KJ. Interactions between tamoxifen and antidepressants via cytochrome P450 2D6. J Clin Psychiatry. 2009;70(12):1688–97. doi: 10.4088/JCP.08r04856blu. [DOI] [PubMed] [Google Scholar]
- 40.Reid R, Blake J, Abramson B, Khan A, Senikas V, Fortier M. Menopause and Osteoporosis Update 2009. SOGC Clinical Practice Guidelines. 2009;31(1):Supplement 1. [DOI] [PubMed]
- 41.Rees MCP, Panay N. Alternatives to HRT for the Management of Symptoms of the Menopause (SAC Opinion Paper 6). 2010.
- 42.Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence–imprecision. J Clin Epidemiol. 2011;64(12):1283–93. doi: 10.1016/j.jclinepi.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 43.Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence–indirectness. J Clin Epidemiol. 2011;64(12):1303–10. doi: 10.1016/j.jclinepi.2011.04.014. [DOI] [PubMed] [Google Scholar]