Abstract
Gout is a common disease resulting from hyperuricemia which causes acute arthritis. Recently, genome-wide association studies revealed an association between serum uric acid levels and a common variant of leucine-rich repeat-containing 16A (LRRC16A) gene. However, it remains to be clarified whether LRRC16A contributes to the susceptibility to gout. In this study, we investigated the relationship between rs742132 in LRRC16A and gout. A total of 545 Japanese male gout cases and 1,115 male individuals as a control group were genotyped. rs742132 A/A genotype significantly increased the risk of gout, conferring an odds ratio of 1.30 (95 % CI 1.05–1.60; p = 0.015). LRRC16A encodes a protein called capping protein ARP2/3 and myosin-I linker (CARMIL), which serves as an inhibitor of the actin capping protein (CP). CP is an essential element of the actin cytoskeleton, which binds to the barbed end of the actin filament and regulates its polymerization. In the apical membrane of proximal tubular cells in the human kidney, the urate-transporting multimolecular complex (urate transportsome) is proposed to consist of several urate transporters and scaffolding proteins, which interact with the actin cytoskeleton. Thus, if there is a CARMIL dysfunction and regulatory disability in actin polymerization, urate transportsome may be unable to operate appropriately. We have shown for the first time that CARMIL/LRRC16A was associated with gout, which could be due to urate transportsome failure.
Electronic supplementary material
The online version of this article (doi:10.1007/s13577-013-0081-8) contains supplementary material, which is available to authorized users.
Keywords: Gouty arthritis, Single nucleotide polymorphism (SNP), Urate transport, PDZ domain-containing 1 (PDZK1), Sodium–proton exchanger regulatory factor 1 (NHERF1)
Introduction
The leucine-rich repeat-containing 16A (LRRC16A) gene encodes a protein called capping protein ARP2/3 and myosin-I linker (CARMIL), which plays an important role in cell-shape changes and motility [1]. A common variant of LRRC16A gene has been previously reported to be associated with nephrolithiasis [2], platelet count [3], and hemoglobin [4]. In addition, a meta-analysis of genome-wide association studies (GWAS) has revealed an association between serum uric acid (SUA) levels and rs742132, a single nucleotide polymorphism (SNP) in LRRC16A [5]. While elevated SUA levels potentially cause gout [6], it remains to be clarified whether LRRC16A contributes to the susceptibility to gout. In this study, therefore, we investigated the effects of a common variant of LRRC16A on the susceptibility to gout.
Materials and methods
Study participants
All procedures were carried out in accordance with the standards of the institutional ethical committees involved in this project and the Declaration of Helsinki with written informed consent from each subject participating in this study. For cases, 545 male gout patients were assigned from among outpatients of gout clinics in Midorigaoka Hospital (Osaka, Japan). All were clinically diagnosed with primary gout according to the criteria established by the American College of Rheumatology [7]. For the control group, 1,115 males with normal SUA (≤7.0 mg/dl) and without a history of gout were collected from the Japan Multi-Institutional Collaborative Cohort Study (J-MICC Study) [8]. The details and participants in this study are shown in Supplemental Table 1.
Genetic analysis
Genomic DNA was extracted from whole peripheral blood cells [9]. Genotyping of rs742132, a common variant of LRRC16A gene, was performed by TaqMan method (Life Technologies, Carlsbad, CA, USA) with a LightCycler 480 (Roche Diagnostics, Mannheim, Germany) [10, 11]. To confirm their genotypes, DNA sequencing analysis was performed with the following primers: forward 5′-GATCACACTGTGACCACACC-3′, and reverse 5′-GTATCTCTGTGCCTCATATTCCTC-3′. Direct sequencing was performed with a 3130xl Genetic Analyzer (Life Technologies) [11].
For all calculations in the statistical analysis, SPSS v.17.0J (IBM Japan, Tokyo, Japan) were used. The Chi-square test was used for association analysis.
Results
Genotyping results of rs742132 for 545 gout patients and 1,115 controls are shown in Table 1. The call rate for rs742132 was 97.3 %. Its p value for Hardy–Weinberg equilibrium was 0.56 in controls. A p value that suggested mistyping was not obtained. The association analysis (2 × 3 Chi-square test) of the LRRC16A variant, rs742132, showed a significant result (p = 0.027; Table 1). The A-allele of rs742132 was a risk allele for gout in this study, and the risk allele frequency in the gout cases (75.0 %) was higher than in the controls (72.0 %; Table 1). As a result, rs742132 had a borderline significant association for the allele frequency model (p = 0.070; Table 2). In addition, the A/A genotype was observed more frequently in the gout cases (58.5 %) than in the control subjects (52.1 %; Table 1). Although no significant association was observed in the dominant model (p = 0.784), A/A genotype significantly increased gout risk in the recessive model (p = 0.015; odds ratio = 1.30; 95 % CI 1.05–1.60; Table 2).
Table 1.
Distributions of genotypes of rs742132 in LRRC16A gene
| G/G | G/A | A/A | RAFa | p valueb | |
|---|---|---|---|---|---|
| Gout cases | 47 | 179 | 319 | 0.750 | 0.027 |
| Controls | 88 | 424 | 558 | 0.720 | – |
RAF Risk allele frequency
aA is risk allele
b2 × 3 Chi square test of rs742132 genotype
Table 2.
The risk of gout due to a common variant of LRRC16A gene, rs742132
| p value | OR | 95 % CI | |
|---|---|---|---|
| Allele frequency model | 0.070 | 1.17 | 0.99–1.38 |
| Recessive model (G/G or G/A versus A/A) | 0.015 | 1.30 | 1.05–1.60 |
| Dominant model (G/G versus G/A or A/A) | 0.784 | 0.95 | 0.66–1.37 |
OR odds ratio, CI confidence interval
Discussion
Gout is a common disease as a consequence of hyperuricemia which increases the risks of hypertension [6, 12], cardiovascular diseases [13], cerebrovascular diseases [14], and renal failure [15]. Previous studies identified several transporter genes associated with gout, such as ATP-binding cassette transporter, subfamily G, member 2 (ABCG2/BCRP) [11, 16–18], GLUT9/SLC2A9 [19–21], monocarboxylate transporter 9 (MCT9/SLC16A9) [22], and organic anion transporter 4 (OAT4/SLC22A11) [23]. In the present study, we have shown for the first time that a common variant of LRRC16A has a significant association with gout. Although rs742132 is reported to associate with SUA [5], another study revealed no significant association between LRRC16A and gout [19]. This is partly because the participants in that study were medical history reading or self-reported patients, whereas we performed this study using only clinically diagnosed cases for a better understanding of the genetic basis of gout. While the functional role of rs742132 remains unknown and further studies are necessary, it may well be possible that this intronic SNP would regulate LRRC16A gene expression or be a surrogate marker for other functional SNPs.
LRRC16A encodes CARMIL, a large protein which is the most abundant in kidney and other epithelial tissues [1]. It serves as an inhibitor of the heterodimeric actin capping protein (CP), an essential element of the actin cytoskeleton which binds to the barbed end of the actin filament and regulates its polymerization [1, 24] (Supplemental Fig. 1). Therefore, LRRC16A mutation may cause the dysfunction of CARMIL to dislodge the capping protein from the actin filament which results in uncontrolled elongation at the barbed end of the filament.
Recently, in the apical membrane of proximal tubular cells in the human kidney, a urate-transporting multimolecular complex (urate transportsome) [25] is proposed to be composed of the following transporters: urate transporter 1 (URAT1/SLC22A12), ABCG2/BCRP, OAT4/SLC22A11, type 1 sodium-dependent phosphate transporter (NPT1/SLC17A1), and multidrug resistance protein 4 (MRP4/ABCC4) [26] (Fig. 1). These transporters are scaffolded by a PDZ domain-containing 1 (PDZK1) and sodium–proton exchanger regulatory factor 1 (NHERF1) [26]. NHERF1 interacts with the actin cytoskeleton through the ezrin protein. Hence, if there is CARMIL dysfunction and regulatory disability in actin polymerization, urate transportsome may be unable to operate appropriately, which results in urate transport failure (Fig. 1). In addition to these transporters, shown in Fig. 1, a type 4 sodium-dependent phosphate transporter (NPT4/SLC17A3) is also reported to be a urate transporter expressed in kidney [27]. CARMIL may also have effects on NPT4 by regulating urate transportsome, because NPT4 is supposed to bind PDZK1 and/or NHERF1.
Fig. 1.
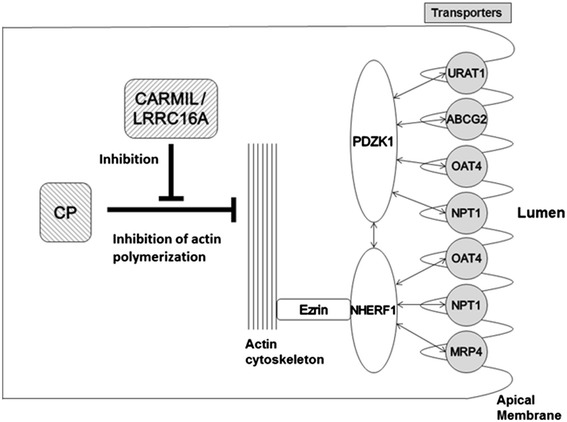
A proposed model of CARMIL/LRRC16A-mediated urate transportsome regulation. In the urate transportsome of renal proximal tubular cells, urate transporters are scaffolded by PDZK1 and NHERF1, which interacted with the actin cytoskeleton through ezrin [ref. 25, 26]. In this study, we propose a new model of urate transportsome regulation by CARMIL. In this model, CARMIL dysfunction, which causes uncontrolled elongation of actin filament, could relate to the pathophysiology of gout
Until now, the multiple biochemical mechanisms associated with CARMIL raise many possibilities for its intracellular function [1, 24]. We suggest that CARMIL/LRRC16A has a novel mechanism associated with gout due to urate transportsome failure.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors would like to thank all the patients and healthy volunteers involved in this study. We also are indebted to J. Abe, K. Gotanda, Y. Morimoto, N. Katsuta, Y. Utsumi, S. Terashige, Y. Kato, H. Ogata, Y. Shichijo, A. Akashi, Y. Tanahashi, Y. Yasuda and H. Nakashima, National Defense Medical College, Tokorozawa, Japan, for genetic analysis and helpful discussion. This study was supported by grants from the Ministry of Education, Science, and Culture of Japan, the Ministry of Health, Labor and Welfare of Japan, the Ministry of Defense of Japan, the Japan Society for the Promotion of Science, the Kawano Masanori Memorial Foundation for Promotion of Pediatrics, the AstraZeneca VRI Research Grant, the Takeda Science Foundation, and the Gout Research Foundation of Japan.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
M. Sakiyama and H. Matsuo contributed equally to this work.
References
- 1.Yang C, Pring M, Wear MA, Huang M, Cooper JA, Svitkina TM, et al. Mammalian CARMIL inhibits actin filament capping by capping protein. Dev Cell. 2005;9(2):209–221. doi: 10.1016/j.devcel.2005.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tore S, Casula S, Casu G, Concas MP, Pistidda P, Persico I, et al. Application of a new method for GWAS in a related case/control sample with known pedigree structure: identification of new loci for nephrolithiasis. PLoS Genet. 2011;7(1):e1001281. doi: 10.1371/journal.pgen.1001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qayyum R, Snively BM, Ziv E, Nalls MA, Liu Y, Tang W, et al. A meta-analysis and genome-wide association study of platelet count and mean platelet volume in African Americans. PLoS Genet. 2012;8(3):e1002491. doi: 10.1371/journal.pgen.1002491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Z, Tang H, Qayyum R, Schick UM, Nalls MA, Handsaker R, et al. Genome-wide association analysis of red blood cell traits in African Americans: the COGENT Network. Hum Mol Genet. 2013;22(12):2529–2538. doi: 10.1093/hmg/ddt087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolz M, Johnson T, Sanna S, Teumer A, Vitart V, Perola M, et al. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. 2009;5(6):e1000504. doi: 10.1371/journal.pgen.1000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359(17):1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yu TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20(3):895–900. doi: 10.1002/art.1780200320. [DOI] [PubMed] [Google Scholar]
- 8.Hamajima N. J-MICC Study Group. The Japan Multi-Institutional Collaborative Cohort Study (J-MICC Study) to detect gene–environment interactions for cancer. Asian Pac J Cancer Prev. 2007;8(2):317–323. [PubMed] [Google Scholar]
- 9.Matsuo H, Kamakura K, Saito M, Okano M, Nagase T, Tadano Y, et al. Familial paroxysmal dystonic choreoathetosis: clinical findings in a large Japanese family and genetic linkage to 2q. Arch Neurol. 1999;56(6):721–726. doi: 10.1001/archneur.56.6.721. [DOI] [PubMed] [Google Scholar]
- 10.Margraf RL, Mao R, Wittwer CT. Rapid diagnosis of MEN2B using unlabeled probe melting analysis and the LightCycler 480 instrument. J Mol Diagn. 2008;10(2):123–128. doi: 10.2353/jmoldx.2008.070111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuo H, Takada T, Ichida K, Nakamura T, Nakayama A, Ikebuchi Y, et al. Common defects of ABCG2, a high-capacity urate exporter, cause gout: a function-based genetic analysis in a Japanese population. Sci Transl Med. 2009;1(5):5ra11. doi: 10.1126/scitranslmed.3000237. [DOI] [PubMed] [Google Scholar]
- 12.Forman JP, Choi H, Curhan GC. Uric acid and insulin sensitivity and risk of incident hypertension. Arch Intern Med. 2009;169(2):155–162. doi: 10.1001/archinternmed.2008.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuttle KR, Short RA, Johnson RJ. Sex differences in uric acid and risk factors for coronary artery disease. Am J Cardiol. 2001;87(12):1411–1414. doi: 10.1016/S0002-9149(01)01566-1. [DOI] [PubMed] [Google Scholar]
- 14.Lin YH, Hsu HL, Huang YC, Lee M, Huang WY, Huang YC, et al. Gouty arthritis in acute cerebrovascular disease. Cerebrovasc Dis. 2009;28(4):391–396. doi: 10.1159/000235626. [DOI] [PubMed] [Google Scholar]
- 15.Talaat KM, el-Sheikh AR. The effect of mild hyperuricemia on urinary transforming growth factor beta and the progression of chronic kidney disease. Am J Nephrol. 2007;27(5):435–440. doi: 10.1159/000105142. [DOI] [PubMed] [Google Scholar]
- 16.Woodward OM, Köttgen A, Coresh J, Boerwinkle E, Guggino WB, Köttgen M. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci USA. 2009;106(25):10338–10342. doi: 10.1073/pnas.0901249106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ichida K, Matsuo H, Takada T, Nakayama A, Murakami K, Shimizu T, et al. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat Commun. 2012;3:764. doi: 10.1038/ncomms1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuo H, Ichida K, Takada T, Nakayama A, Nakashima H, Nakamura T, et al. Common dysfunctional variants in ABCG2 are a major cause of early-onset gout. Sci Rep. 2013;3:2014. doi: 10.1038/srep02014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stark K, Reinhard W, Grassl M, Erdmann J, Schunkert H, Illig T, et al. Common polymorphisms influencing serum uric acid levels contribute to susceptibility to gout, but not to coronary artery disease. PLoS ONE. 2009;4(11):e7729. doi: 10.1371/journal.pone.0007729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuo H, Chiba T, Nagamori S, Nakayama A, Domoto H, Phetdee K, et al. Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am J Hum Genet. 2008;83(6):744–751. doi: 10.1016/j.ajhg.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dinour D, Gray NK, Campbell S, Shu X, Sawyer L, Richardson W, et al. Homozygous SLC2A9 mutations cause severe renal hypouricemia. J Am Soc Nephrol. 2010;21(1):64–72. doi: 10.1681/ASN.2009040406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakayama A, Matsuo H, Shimizu T, Ogata H, Takada Y, Nakashima H, et al. A common missense variant of monocarboxylate transporter 9 (MCT9/SLC16A9) gene is associated with renal overload gout, but not with all gout susceptibility. Hum Cell. 2013 (Epub ahead of print). [DOI] [PMC free article] [PubMed]
- 23. Sakiyama M, Matsuo H, Shimizu S, Nakashima H, Nakayama A, Chiba T, et al. A common variant of organic anion transporter 4 (OAT4/SLC22A11) gene is associated with renal underexcretion type gout. Drug Metab Pharmacokinet. 2013 (Epub ahead of print). [DOI] [PubMed]
- 24.Takeda S, Minakata S, Koike R, Kawahata I, Narita A, Kitazawa M, et al. Two distinct mechanisms for actin capping protein regulation—steric and allosteric inhibition. PLoS Biol. 2010;8(7):e1000416. doi: 10.1371/journal.pbio.1000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anzai N, Kanai Y, Endou H. New insights into renal transport of urate. Curr Opin Rheumatol. 2007;19(2):151–157. doi: 10.1097/BOR.0b013e328032781a. [DOI] [PubMed] [Google Scholar]
- 26.Ichida K. What lies behind serum urate concentration? Insights from genetic and genomic studies. Genome Med. 2009;1(12):118. doi: 10.1186/gm118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jutabha P, Anzai N, Kitamura K, Taniguchi A, Kaneko S, Yan K, et al. Human sodium phosphate transporter 4 (hNPT4/SLC17A3) as a common renal secretory pathway for drugs and urate. J Biol Chem. 2010;285(45):35123–35132. doi: 10.1074/jbc.M110.121301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


