Abstract
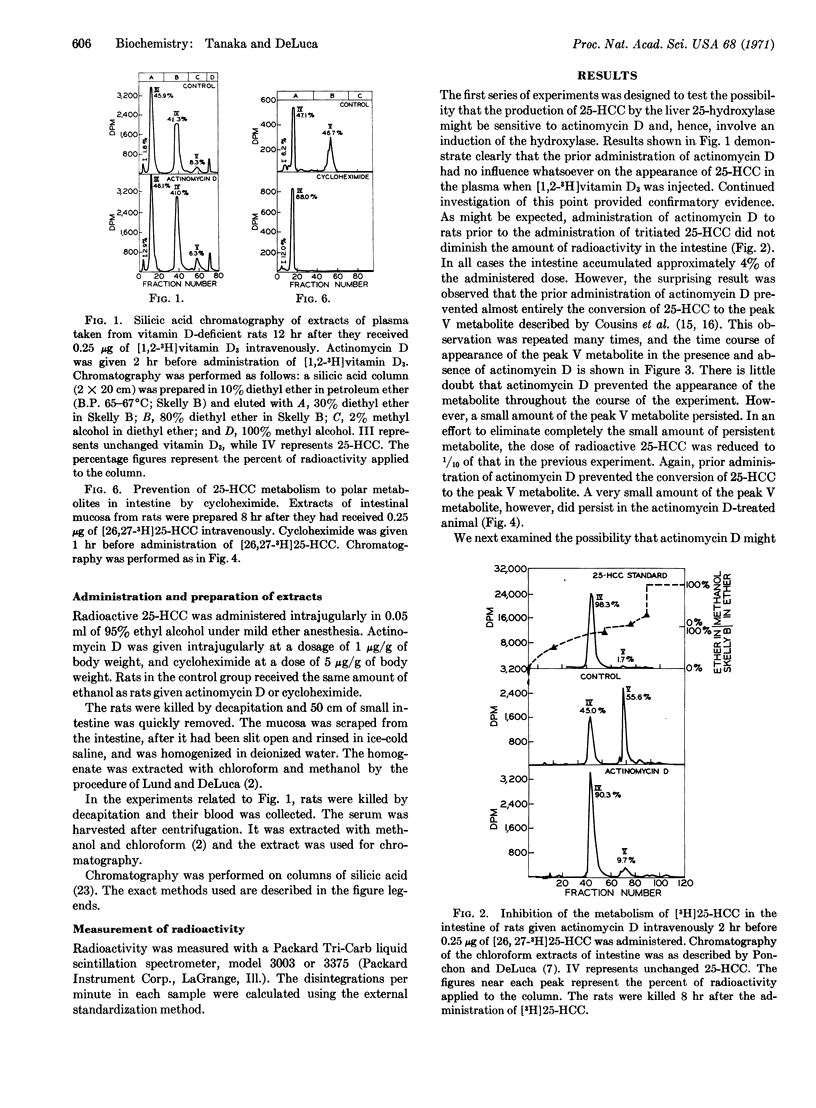
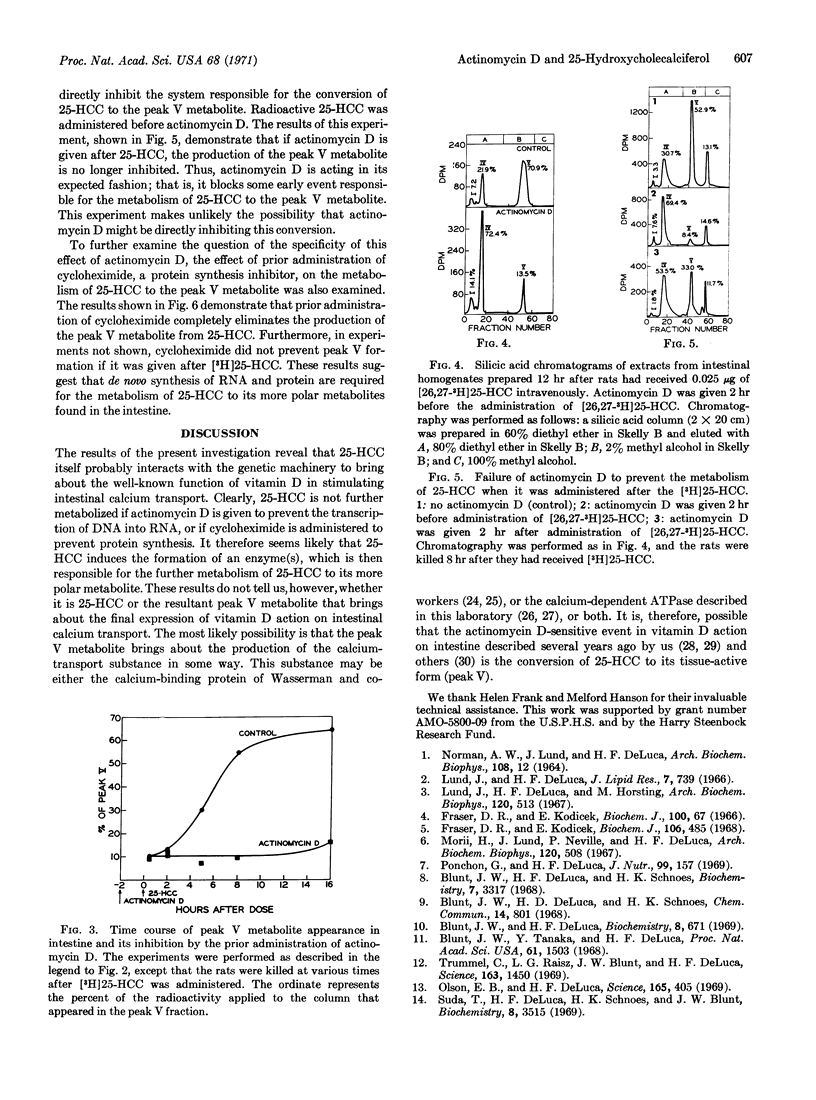
Actinomycin D (or cycloheximide) administered prior to radioactive 25-hydroxycholecalciferol blocks the metabolism of 25-hydroxycholecalciferol to polar metabolites that accumulate in intestinal tissue, while it does not prevent the 25-hydroxylation of vitamin D3 in the liver. Actinomycin D given after radioactive 25-hydroxycholecalciferol does not inhibit 25-hydroxycholecalciferol metabolism. These results indicate that 25-hydroxycholecalciferol must interact with the nuclei of cells to bring about the production of an enzyme(s) that converts it to its polar metabolites.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blunt J. W., DeLuca H. F., Schnoes H. K. 25-hydroxycholecalciferol. A biologically active metabolite of vitamin D3. Biochemistry. 1968 Oct;7(10):3317–3322. doi: 10.1021/bi00850a001. [DOI] [PubMed] [Google Scholar]
- Blunt J. W., DeLuca H. F. The synthesis of 25-hydroxycholecalciferol. A biologically active metabolite of vitamin D3. Biochemistry. 1969 Feb;8(2):671–675. doi: 10.1021/bi00830a031. [DOI] [PubMed] [Google Scholar]
- Blunt J. W., Tanaka Y., DeLuca H. F. Biological activity of 25-hydroxycholecalciferol, a metabolite of vitamin D3. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1503–1506. doi: 10.1073/pnas.61.4.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins R. J., DeLuca H. F., Chen T., Suda T., Tanaka Y. Metabolism and subcellular location of 25-hydroxycholecalciferol in intestinal mucosa. Biochemistry. 1970 Mar 17;9(6):1453–1459. doi: 10.1021/bi00808a021. [DOI] [PubMed] [Google Scholar]
- Cousins R. J., DeLuca H. F., Gray R. W. Metaboism of 25-hydroxycholecalciferol in target and nontarget tissues. Biochemistry. 1970 Sep 15;9(19):3649–3652. doi: 10.1021/bi00821a001. [DOI] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Investigations on vitamin D esters synthesized rats. Detection and identification. Biochem J. 1968 Jan;106(2):485–490. doi: 10.1042/bj1060485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCH J., AHRENS E. H., Jr The separation of complex lipide mixtures by the use of silicic acid chromatography. J Biol Chem. 1958 Aug;233(2):311–20. [PubMed] [Google Scholar]
- Haussler M. R., Myrtle J. F., Norman A. W. The association of a metabolite of vitamin D3 with intestinal mucosa chromatin in vivo. J Biol Chem. 1968 Aug 10;243(15):4055–4064. [PubMed] [Google Scholar]
- Lawson D. E., Wilson P. W., Kodicek E. Metabolism of vitamin D. A new cholecalciferol metabolite, involving loss of hydrogen at C-1, in chick intestinal nuclei. Biochem J. 1969 Nov;115(2):269–277. doi: 10.1042/bj1150269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J., DeLuca H. F. Biologically active metabolite of vitamin D3 from bone, liver, and blood serum. J Lipid Res. 1966 Nov;7(6):739–744. [PubMed] [Google Scholar]
- Martin D. L., Melancon M. J., Jr, DeLuca H. F. Vitamin D stimulated, calcium-dependent adenosine triphosphatase from brush borders of rat small intestine. Biochem Biophys Res Commun. 1969 Jun 27;35(6):819–823. doi: 10.1016/0006-291x(69)90697-4. [DOI] [PubMed] [Google Scholar]
- Melancon M. J., Jr, DeLuca H. F. Vitamin D stimulation of calcium-dependent adenosine triphosphatase in chick intestinal brush borders. Biochemistry. 1970 Apr 14;9(8):1658–1664. doi: 10.1021/bi00810a002. [DOI] [PubMed] [Google Scholar]
- Myrtle J. F., Haussler M. R., Norman A. W. Evidence for the biologically active form of cholecalciferol in the intestine. J Biol Chem. 1970 Mar 10;245(5):1190–1196. [PubMed] [Google Scholar]
- NORMAN A. W., LUND J., DELUCA H. F. BIOLOGICALLY ACTIVE FORMS OF VITAMIN D3 IN KIDNEY AND INTESTINE. Arch Biochem Biophys. 1964 Oct;108:12–21. doi: 10.1016/0003-9861(64)90349-2. [DOI] [PubMed] [Google Scholar]
- Olson E. B., DeLuca H. F. 25-hydroxycholecalciferol: direct effect on calcium transport. Science. 1969 Jul 25;165(3891):405–407. doi: 10.1126/science.165.3891.405. [DOI] [PubMed] [Google Scholar]
- Ponchon G., Deluca H. F. Metabolites of vitamin D3 and their biologic activity. J Nutr. 1969 Oct;99(2):157–167. doi: 10.1093/jn/99.2.157. [DOI] [PubMed] [Google Scholar]
- Suda T., DeLuca H. F., Schnoes H. K., Blunt J. W. The isolation and identification of 25-hydroxyergocalciferol. Biochemistry. 1969 Sep;8(9):3515–3520. doi: 10.1021/bi00837a005. [DOI] [PubMed] [Google Scholar]
- Suda T., DeLuca H. F., Schnoes H. K., Ponchon G., Tanaka Y., Holick M. F. 21,25-dihydroxycholecalciferol. A metabolite of vitamin D3 preferentially active on bone. Biochemistry. 1970 Jul 7;9(14):2917–2922. doi: 10.1021/bi00816a025. [DOI] [PubMed] [Google Scholar]
- Suda T., DeLuca H. F., Tanaka Y. Biological activity of 25-hydroxyergocalciferol in rats. J Nutr. 1970 Sep;100(9):1049–1052. doi: 10.1093/jn/100.9.1049. [DOI] [PubMed] [Google Scholar]
- Trummel C. L., Raisz L. G., Blunt J. W., Deluca H. F. 25-Hydroxycholecalciferol: stimulation of bone resorption in tissue culture. Science. 1969 Mar 28;163(3874):1450–1451. doi: 10.1126/science.163.3874.1450. [DOI] [PubMed] [Google Scholar]
- Wasserman R. H., Corradino R. A., Taylor A. N. Vitamin D-dependent calcium-binding protein. Purification and some properties. J Biol Chem. 1968 Jul 25;243(14):3978–3986. [PubMed] [Google Scholar]
- Wasserman R. H., Taylor A. N. Vitamin D-dependent calcium-binding protein. Response to some physiological and nutritional variables. J Biol Chem. 1968 Jul 25;243(14):3987–3993. [PubMed] [Google Scholar]
- Zull J. E., Czarnowska-Misztal E., DeLuca H. F. On the relationship between vitamin D action and actinomycin-sensitive processes. Proc Natl Acad Sci U S A. 1966 Jan;55(1):177–184. doi: 10.1073/pnas.55.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zull J. E., Czarnowska-Misztal E., Deluca H. F. Actinomycin D Inhibition of Vitamin D Action. Science. 1965 Jul 9;149(3680):182–184. doi: 10.1126/science.149.3680.182. [DOI] [PubMed] [Google Scholar]


