Introduction
Carcinogenesis associated with exposure to radiation is widely known, first reported more than 100 years ago in 1902 by Friben and in 1907 by Porter and White [16]. Exposure to therapeutic doses of radiation have also been shown to be associated with an increased risk of a second cancer, although the precise risk remains unknown. Sarcomas are a rare, but recognized, complication of RT for breast carcinoma, and are associated with poor prognosis. The first description of a bone sarcoma after RT of a breast tumor was reported by Beck in 1922, and Warren and Sommer in 1936 [2] reported a soft tissue sarcoma in the breast treatment volume. The long term incidence of sarcoma after radiation therapy for breast cancer is 0.2 % for 10 years [3]. In 1948, Cahan et al. [2] defined the criteria for the diagnosis of radiation-induced sarcoma. EOO is a soft tissue tumor that produces osteoid, bone or chondroid material without attachment to the skeleton as determined by imaging modalities [4]. Wilson reported the first case of EOO in 1941 and so far only around 300 cases have been reported in the English literature [6]. Most reported cases of osteosarcoma of the chest wall following radiation therapy for breast cancer arise from the chest wall skeletal structures [11]. We describe a rare case of EOO involving the chest wall musculature, after radiation therapy for breast cancer.
Case Report
A 60-year-old lady presented with a soft tissue swelling in the right axillary region of 1 month duration. It was progressively increasing in size since she noticed it which was not associated with any complaints. There were no symptoms related to Chest, Lungs, Abdomen or any other generalized complaints. She is a case of Carcinoma of right Breast diagnosed in 2002(cT4bN1M0). As she presented with a locally advanced disease, patient was first treated with neoadjuvant chemotherapy followed by Modified radical mastectomy and chemotherapy (three cycles of CMF regimen pre-operatively and remaining 3 cycles as adjuvant). Following which she received chest wall radiation-45Gy in 20# to chest wall followed by 45 Gy in 20# to the Supraclavicular fossa and a 6gy in single # to PA boost. Patient completed the treatment regimen in April 2003. Since she had a hormone receptor positive tumor she was put on Tamoxifen for 5 years. Patient was on regular outpatient visit till 2008 and then lost for follow-up. Now, she was reporting after a gap of 3 year, to our outpatient department with a fine needle aspiration (FNA) report of the presenting swelling, done at a local centre, as Adenocarcinoma.
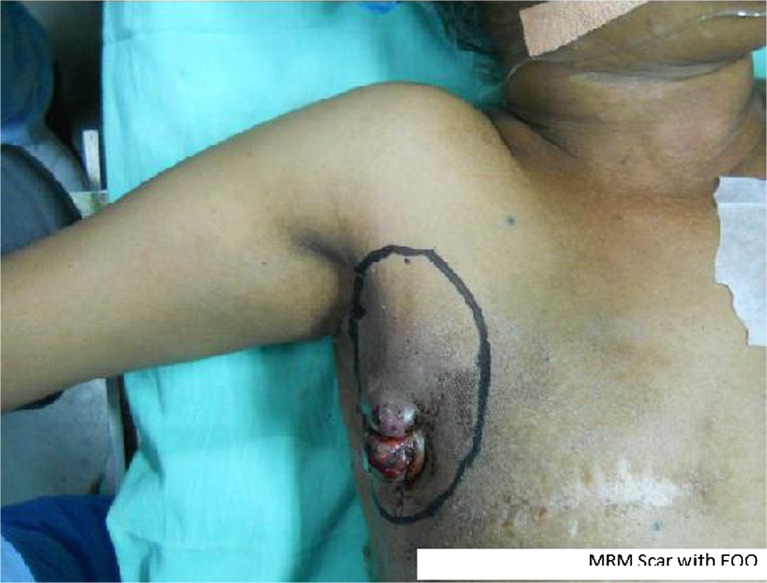
Local examination of the patient revealed a large swelling measuring 6 cm × 5 cm with variable consistency and irregular surface situated in the right axillary region towards the anterior axillary line (Fig. 1). Swelling was fixed to the underlying structures with restricted mobility. Examination of the ipsilateral and contra-lateral axillae, bilateral supraclavicular fossa and contra-lateral breast were non-contributory. Chest and abdominal examination did not reveal any significant finding.
Fig. 1.
MRM Scar with EOO
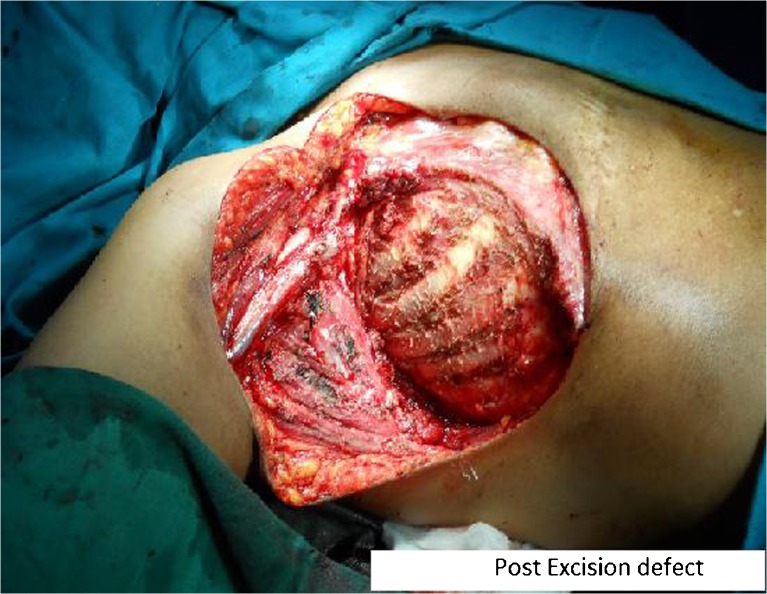
With a working diagnosis of axillary recurrence a decision of radical treatment, if no systemic metastasis, was taken in the Multi Speciality Board. Complete metastatic work-up was done with Contrast Enhanced CT (CECT) of Chest, abdomen and pelvis which did not reveal any visceral metastasis. (CECT of the chest wall was reported as-Right axillary mass with calcification/osteoid formation-swelling arising from the soft tissues of the chest wall. There was no relationship to the bony chest wall). A bone scan was done which ruled out osseous metastasis or primary bone lesions. A repeat FNA was done at our centre which was reported suspicious of a spindle cell neoplasm, suggestive of sarcoma. Later a core needle biopsy was done which was reported as new bone forming atypical spindle cell neoplasm, suggestive of Osteosarcoma. Patient underwent a wide surgical excision of the tumor along with the underlying soft tissue and periosteum of the ribs in contact with the tumor (Fig. 2). The defect was reconstructed with a TRAM flap.
Fig. 2.
Post Excision defect
Pathology of post-resection specimen: Grossly the surgical excision specimen revealed a 6x4cm lobulated grey-white firm to hard tumour with focal calcification. The histopathological examination of the specimen, in our case revealed a malignant neoplasm composed of pleomorphic spindle and polygonal tumour cells with marked nuclear atypia with tumour in the dermal plane and in the overlying epidermis (Fig. 3). Neoplastic cells were arranged as fascicles and diffuse sheets with areas having haemangiopericytomatous pattern. Abundant osteoid was laid down by the tumour cells with foci rich in chondroid lobules and calcified bone also seen (Fig. 4). Numerous osteoclastic giant cells were noted. Mitotic rate was >20/10hpf. The histologic grade was 3(FNCLCC tumour differentiation score). The final diagnosis was Extraosseous Osteosarcoma-pT2 [AJCC]
Fig. 3.
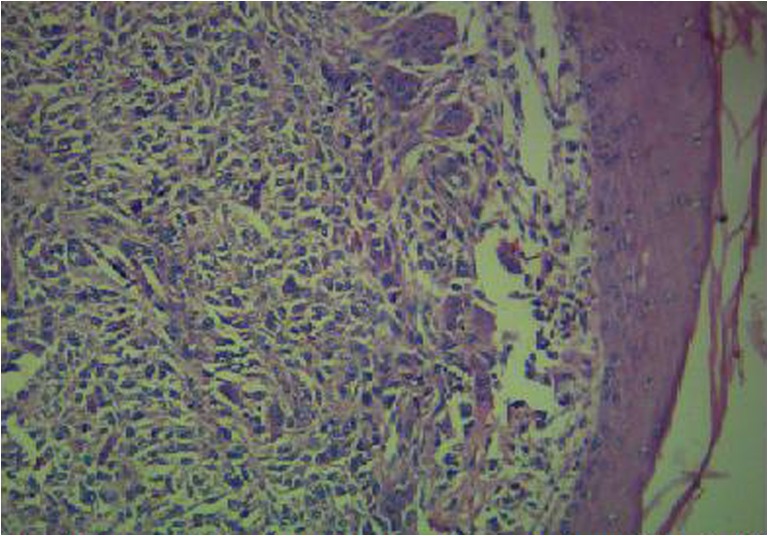
Tumour in the dermal plane with overlying epidermis [H & E,200X]
Fig. 4.
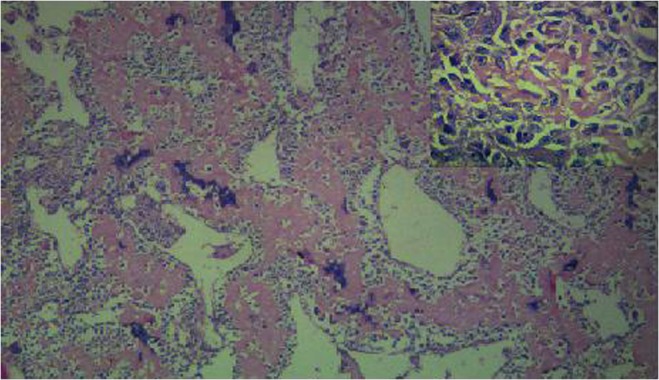
Broad interlacing trabeculae showing abundant osteoid surrounded by tumour cells [H & E, 200X] Pleomorphic tumour cells with newly laid osteoid [H & E,400X](inset)
Discussion
The occurrence of sarcomas in irradiated tissues is an uncommon but well-documented long-term complication of radiotherapy. The incidence of radiation-induced tumors is increasing in the oncology population as a result of increased survival, with improvement in cancer treatment [18]. Although the pathogenesis is unknown, various predisposing factors have been suggested. In addition to radiation dose, development of post irradiation sarcoma is probably influenced by other factors: age of the patient at radiation exposure, association of chemotherapy, and genetic predisposition [17]. It is suggested that the patients who harbour mutations in tumor suppressor genes like p53 and RB1 are more prone to develop radiation-induced osteosarcoma. Many authors have suggested that the lack of RB expression in osteosarcoma and soft tissue sarcoma may be common since RB gene transcripts were not detected. Moreover, they consider that lack of RB gene expression occurred only after the tumor develops. Nevertheless, radiation-induced DNA alteration is well demonstrated, so we could consider that deletion or inactivation of this tumor suppressor gene may be an important factor for post irradiation sarcoma [19] with a relative risk of RIS 400-fold greater than the general population [19]. The Li-Fraumeni syndrome, von Recklinghausen’s disease, and other hereditary syndromes have also been identified as risk factors for post irradiation sarcoma [17].
Osteosarcoma may arise de novo or in association with well-recognized precursors such as Paget’s disease, radiation injury, bone infarcts, osteomyelitis, and certain clinical syndromes. Osteosarcoma after radiation typically develops after a latency period of 5–10 years after doses in excess of 30 Gy and these tumors characteristically occur at the edge of the radiation field because the administered radiation is unable to cause cell death but is sufficient to induce malignant transformation [9]. The shortest latency period has been reported to be only 3 months and the median latency period ranges from 10 to 14.37 years [11]. Laskin et al. reported that the latency period of the radiation induced EOO had latency period less than the mean compared with all post irradiation sarcomas [11]. Histologically, osteosarcoma contains two basic components: proliferating tumor cells arising from undifferentiated bone-forming mesenchyma and an extracellular osteoid matrix. On microscopy, depends up on the morphologic variability it is been divided in to osteoblastic, chondroblastic, or fibroblastic subtypes [9]. The single most important criteria for diagnosis of this tumor is the presence of neoplastic osteoid and bone; sometimes with neoplastic cartilage [9]. The histological variants of EOO include osteoblastic, chondroblastic, fibroblastic, osteoclastic or giant cell type, telangiectatic, small cell or well differentiated forms [8]. The immunohistochemical profile includes the expression of osteocalcin and osteonectin which are specific for osteosarcoma [9].
By definition, EOO is defined as malignant mesenchymal neoplasms that are located in the soft tissues without attachment to bone or periosteum, having a uniform sarcomatous pattern, produces osteoid with or without a cartilaginous matrix, and has no epithelial component [12]. In 1948, Cahan et al. [2] described 11 cases of sarcomas arising from irradiated bones. They established four criteria for the diagnosis of radiation-induced osteosarcoma that are still valid today: (a) the origin of the neoplasm in the radiation field, (b) the non malignant nature of the initial bone condition (this criteria was modified by Arlen et al. [17]: “the tumors developed in bone not known to have a primary malignant osteoblastic lesion when the radiotherapy was given”), (c) the histological diagnosis of the neoplasm, and (d) a relatively long latency period. Our patient fulfils all the above criteria because she was treated for carcinoma breast and now she has a histologically proven osteosarcoma arising 9 years after the irradiation of the chest wall. The length of time required between radiation exposure and sarcoma formation is the one criterion that has been modified by most investigators from the several year requirement originally proposed. This latency period is necessary to differentiate a RT-induced sarcoma from a second primary that may predate the radiation; because no accurate molecular or pathologic markers exist, any spontaneous sarcomas that may appear in a radiation field cannot reliably be excluded from analysis [15]. The minimum latency period used to call a tumor radiation-associated, in the literature, is 2 to 5 years and the median latency period is 10 to 14.3 years [10]. EOO is a rare type of tumor making up only 1 % of all soft tissue sarcomas [1] and 4 % of all osteosarcoma [5]. Younger population is affected by the bone sarcomas, in contrast to it EOO tends to present at an older age (median age 50.7 years) [7]. The most common site of EOO is the thigh and only a few cases of EOO have been reported in the soft tissues of the chest wall [11]. The major differential diagnosis includes metaplastic carcinoma, sarcoma arising in a phylloides tumor and metastatic lesions from bone primary elsewhere[11]. In our case, the tumor was involving only the muscles-Pectoralis major, Latissimus dorsi and Serratus anterior, but not the skeletal framework.
The incidence of radiation-associated EOO is reported in the literature in the range of 3.8 %–10 % of all EOO [7] and 13 % of all radiation induced sarcomas [13]. Pendlebury et al. [14]reviewed the literature on radiation-associated sarcoma following radiotherapy for breast cancer. Osteosarcoma was found to be the most frequent tumor (50 %), usually localised to the skeletal chest wall or bones of the upper extremity. Only one case of EOO was reported, and it was localized to the supraclavicular fossa. When only soft tissue sarcomas were analyzed angiosarcoma was the most frequent histologic type (46 %), most commonly arising in the conserved breast. Largest retrospective study of radiation induced sarcomas in breast cancer patients (13,472 patients) by Youlina M et al. [2] from Curie institute; Paris gives the result as follows. The latency period ranged from 3 years to 20.3 years. There were 27 cases of post irradiation sarcoma, out of which only 4 cases were Osteosarcomas.
Radiation-induced sarcomas have traditionally been viewed as aggressive, high-grade tumors with poor prognosis. Majority of authors recommends a complete excision with a negative margin as the treatment of choice for radiation induced EOO. EOO has a local recurrence rate of 26 % to 69 % [7] with a 5 year survival of 25 % to 37 % [21]. Survival of the patient was not affected by the factors like latency period, location, amount of radiation or histological type (bone v/s soft tissue sarcoma) [10]. Tumor size and grade are the two most important prognostic factors for soft tissue sarcomas, including those associated with radiation therapy.
Some authors have recommended neoadjuvant chemotherapy before definitive surgery is undertaken [20]. But still the role of adjuvant therapy in radiation-induced sarcomas is unknown. In majority of the published cases and series the chemotherapeutic agents used in the treatment of radiation induced EOO are ifosfamide, methorexate, and doxorubicin. But there are no randomised trials to compare the results with that of the treatment of primary osteosarcoma. Surgery with a three dimensional negative margin appears to offer the best chance of cure. Previous irradiation may have played a role in obscuring anatomic and tumor planes, preventing surgeons from appreciating true tumor margins. This underscores the necessity for aggressive and wide resections, especially considering that a positive surgical margin (gross or microscopic) will reduce survival by nearly half [22]. Radical resection of the adjacent structures has no advantage in the local control of the disease when compare to a three dimensional wide local excision [7, 22]. However, as osteosarcoma metastasizes by the haematogenous route, there is rationale for the addition of adjuvant chemotherapy [18]. There are no large studies to determine the effectiveness of various treatments for post irradiation osteosarcoma. But there is another school of thought that believes that there is no survival benefit for those patients receiving adjuvant chemotherapy or radiotherapy compared with those treated only with surgery that acheives negative margin [22]. The prognosis of radiation-induced sarcoma is generally thought to be worse than primary sarcomas, regardless of site. The cumulative disease-free survival at 5 years for patients with a post irradiation osteosarcoma was 17 %, with a median survival estimate of 1 year [17]. Long-term follow-up is mandatory for patients who have been treated with RT, and any abnormality found in the irradiated field should be aggressively biopsied. If a sarcoma is detected, the treatment of choice for radiation-induced sarcomas as of today is surgical resection with negative margins.
References
- 1.Rodney E (2010) Wegner, Extraosseous Osteosarcoma of the Esophagus: A Case Report. Sarcoma, Volume [DOI] [PMC free article] [PubMed]
- 2.Kirova YM, Vilcoq JR, et al. Radiation-induced sarcomas after radiotherapy for breast carcinoma. A large-scale single-institution review. Cancer. 2005;104:856–63. doi: 10.1002/cncr.21223. [DOI] [PubMed] [Google Scholar]
- 3.Pendlebury SC, Bilous M, et al. Sarcoma following radiation therapy for breast cancer: A report of 3 cases and review of literature. Int J Radiat Oncol Biol Phys. 1995;31:505–410. doi: 10.1016/0360-3016(95)93157-3. [DOI] [PubMed] [Google Scholar]
- 4.Alonge TO, et al. Extraosseous osteosarcoma in Ibadan: Case series over a 20-year period. Rare Tumors. 2009;1:e3. doi: 10.4081/rt.2009.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCarter MD, Lewis JJ, Antonescu CR, et al. Extraskeletal osteosarcoma: analysis of outcome of a rare neoplasm. Sarcoma. 2000;4:119–23. doi: 10.1080/13577140020008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmad SA, Patel SR, Ballo MT, et al. Extraosseous osteosarcoma. Response to treatment and long term outcome. J Clin Oncol. 2002;20:521–7. doi: 10.1200/JCO.20.2.521. [DOI] [PubMed] [Google Scholar]
- 7.Lee JSY, Fetsch JF, Wasdhal DA, et al. A review of 40 patients with extraskeletal osteosarcoma. Cancer. 1995;76(11):2253–2259. doi: 10.1002/1097-0142(19951201)76:11<2253::AID-CNCR2820761112>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Weiss SW, Goldblum J (2001) Osseous soft tissue tumours in Enzingers and Weiss: Soft tissue tumours 4th ed. Pg 1389–1417, Mosby St Loius
- 9.Ling-Ling Chan, Bogdan A et al. (2000) Radiation-induced osteosarcoma after bilateral childhood retinoblastoma. AJR: 174 [DOI] [PubMed]
- 10.Brady MS, Gaynor JJ, Merman MF. Radiation associated sarcoma of the bone and soft tissues. Arch Surg. 1992;127:1379–1385. doi: 10.1001/archsurg.1992.01420120013002. [DOI] [PubMed] [Google Scholar]
- 11.Lumang O, Ulana S, Alisan G, Ira B, Shabnam J. Radiation-assoaicated extraskeletal osteosarcoma of the chest wall. Arch pathol Lab Med. 2006;130:198–200. doi: 10.5858/2006-130-198-REOOTC. [DOI] [PubMed] [Google Scholar]
- 12.Allan CJ, Soule EH. Osteogenic sarcoma of the somatic soft tissues: Clinicopathological study of 26 cases and review of literature. Cancer. 1971;27:1121–1133. doi: 10.1002/1097-0142(197105)27:5<1121::AID-CNCR2820270519>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 13.Laskin WB, Silverman TA, Enzinger FM. Postradiation soft tissue sarcomas: An anlaysis of 53 cases. Cancer. 1988;62:2230–2340. doi: 10.1002/1097-0142(19881201)62:11<2330::AID-CNCR2820621113>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Pendlebury SC, Bilous M, Langlands AO. Sarcomas following radiation therapy for breast cancer: A report of three cases and review of the literature. Int J Radiat Oncol Biol Phys. 1995;31:405–410. doi: 10.1016/0360-3016(95)93157-3. [DOI] [PubMed] [Google Scholar]
- 15.Cha C, Antonescu CR, Quan ML, Maru S, Brennan MF. Long-term results with resection of radiation-induced soft tissue sarcomas. Annals of Surgery. 2004;6:903–910. doi: 10.1097/01.sla.0000128686.51815.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rustemeyer P, Micke O, Blasius S, Peters PE. Radiation induced malignant mesenchymoma of the chest wall following treatment for breast cancer. The British Journal of Radiology. 1997;70:424–426. doi: 10.1259/bjr.70.832.9166083. [DOI] [PubMed] [Google Scholar]
- 17.Chabchoub I, Gharbi O, Remadi S, Limem S, Trabelsi A, Hochlef M, Fatma LB, Landolsi A, Mokni M, Kraiem C, Ahmed SB. Postirradiation osteosarcoma of the maxilla: A case report and current review of literature. J Oncol. 2009;876138:4. doi: 10.1155/2009/876138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prakash O, Varghese BT, Mathews A, Nayak N, Ramchandran K, Pandey M. Radiation induced osteogenic sarcoma of the maxilla. World Journal of Surgical Oncology. 2005;3, article 49:1–4. doi: 10.1186/1477-7819-3-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong FL, Boice JD, Jr, Abramson DH, et al. Cancer incidence after retinoblastoma: Radiation dose and sarcoma risk. The Journal of the American Medical Association. 1997;278(15):1262–1267. doi: 10.1001/jama.1997.03550150066037. [DOI] [PubMed] [Google Scholar]
- 20.Patel SR. Radiation-induced sarcoma. Current Treatment Options in Oncology. 2000;1(3):258–261. doi: 10.1007/s11864-000-0037-6. [DOI] [PubMed] [Google Scholar]
- 21.Jensen ML, Schumacher B, Jensen OM, Keller J, Nielsen OS. Extra skeletal osteosarcoma: A clinicopathological study of 25 cases. Am J Surg Pathol. 1998;22:588–594. doi: 10.1097/00000478-199805000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Cha C, Antonescu CR, Quan ML, Maru S, Brennan MF. Long-term results with resection of radiationinduced soft tissue sarcomas. Annals of Surgery. 2004;239(6):903–910. doi: 10.1097/01.sla.0000128686.51815.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]