Introduction
Testicular tumors are far more common in adults than in children. For this reason, management of pediatric testis tumors has been based on experience in adults. However, there are important differences between testis tumors occurring in children and those occurring in adults. These differences involve tumor histopathology, malignant potential, and pattern of metastatic spread [1]. The majority of primary testis tumors in the Prepubertal Testis Tumor Registry of the Urologic Section of the American Academy of Pediatrics were yolk sac tumors, followed by teratomas and stromal tumors. Because teratomas and most stromal tumors are benign in children, it would follow that fewer than two thirds of prepubertal testis tumors have malignant potential, compared with 90 % of tumors in adults. It is even possible that the majority of prepubertal tumors are benign. Several single-center studies suggest that teratomas are more common than yolk sac tumors in pediatric patients; however, these studies were not limited to prepubertal patients [2–5] .
Paratesticular rhabdomyosarcomas usually present with an enlarging painless scrotal mass. By the time they present it is usually not possible to distinguish from a primary testis tumor on physical examination, though the extra-testicular nature of the tumor is usually apparent on ultrasound. Trans-scrotal biopsies of solid scrotal masses should be avoided due to the risk of seeding of the incision if the mass is indeed a rhabdomyosarcoma. The excision of the tumor should be performed just as for a testicular malignancy. The metastatic evaluation for paratesticular rhabdomyosarcoma includes a CT (Computed tomography) of the chest, abdomen, and pelvis. A majority of patients will have clinical stage 1 disease. The most common sites of metastases are the retroperitoneum and lungs. Patients with retroperitoneal metastases should undergo a modified unilateral nerve-sparing RPLND (Retroperitoneal lymph node dissection). All patients should receive chemotherapy and those with positive retroperitoneal nodes should receive radiation therapy as well [1]. We report on two older children/adolescents who underwent Laparoscopic-RPLND.
Case Report 1
An 11 year old male child with a known history of left paratesticular rhabdomyosarcoma presented to the urology clinic with a large left scrotal mass of 1 month duration. The child had previously undergone a left testis preserving surgery followed by chemotherapy, and was off treatment since 8 years. The child also complained of dragging pain in the left groin region. Physical examination revealed an enlarged, non tender left hemiscrotum. Ultrasonography revealed a 4.7 cm mass in the left hemiscrotum. The CT abdomen also revealed the scrotal mass with no evidence of metastatic disease. The child underwent a left radical orchiectomy with left hemi-scrotectomy and scrotoplasty with biopsy from right testis for germ cell preservation and bone marrow biopsy. The histopathological examination (HPR) of the specimen revealed a paratesticular embryonal rhoabdomyosarcoma. The biopsy from right testis revealed reduced number of germ cells, normal primary spermatocytes with absent spermatids and also reduced leydig cells. The child underwent a left nerve sparing RPLND 3 weeks later.
The child was put in a flank position. Three ports were placed, one 12 mm periumbilical camera port and two 5 mm ports (one midline below the xiphoid and one midline above the pubis). The colon was medialised by incising along the white line of Toldt from the spleen upto the sigmoid colon exposing the aorta and the IVC upto common iliac vessels bifurcation, the testicular vessels and the ureter. First, the left spermatic cord was dissected out and taken down to the point of the previous orchiectomy. The ureter was dissected out from the nearby vessels to avoid ureteral injury. The peri-aortic tissue was then split to begin the dissection of the peri-aortic lymph nodes. Dissection was carried out from the left renal vessels down to the bifurcation of the aorta and IVC. The common iliac, pericaval and interaortocaval lymph nodes were excised out. The total operative time was 410 min and the estimated blood loss was 40 ml. The child tolerated the surgery well and the recovery was uncomplicated and discharged home on postoperative day two. The HPR of the RPLND revealed one positive lymph node for rhabdomyosarcoma (Fig. 1).
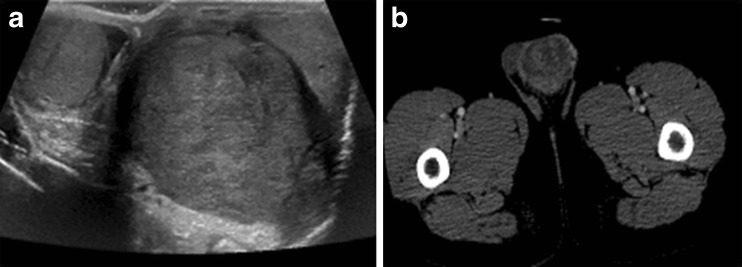
Fig. 1.
a Scrotal ultrasonography depicting the left testicular mass, b CT Scan showing the left testicular mass
Case Report 2
A 16 year old male child/adolescent noted painless mass in left scrotum and approached a primary care physician who advised him antibiotics. Over a period of 3 months the size of the mass increased to double its size. He was referred to a pediatric surgeon, who offered him radical orchiectomy. The pathology read as embryonal rhabdomyosarcoma. He was referred for further management here. PET (Positron emission tomography) scan PET images demonstrated mild uptake at the surgical site in the left groin consistent with post-surgical healing. There is also mild uptake in the right testicle, which is a normal finding and linear uptake in the medial, inferior left hemiscrotum in a region of collapsed scrotal tissue (Fig. 2). The tracer distribution in the remainder of the scanned regions of the body is within normal limits. He underwent left hemiscrotectomy and underwent left sided laparoscopic RPLND. The total operative time was 380 min and the estimated blood loss was 45 ml. The child tolerated the surgery well and the post-operative period was uneventful. HPR of the hemi-scrotectomy specimen and RPLND revealed negative for the lesion (Fig. 3).
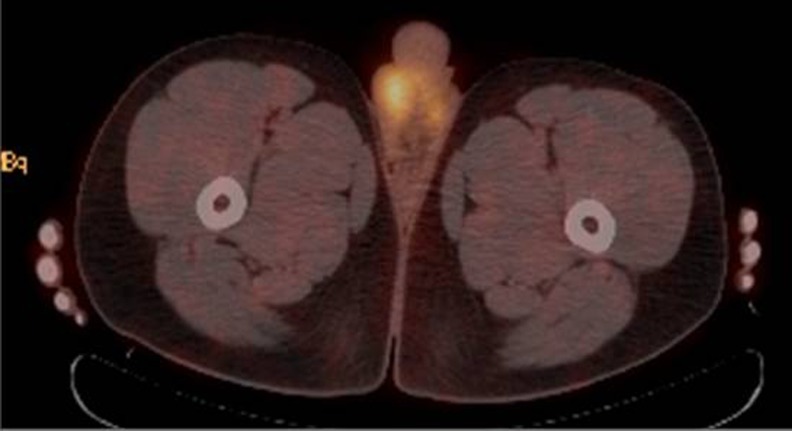
Fig. 2.
FDG PET scan mild uptake in the right testicle, which is a normal finding and linear uptake in the medial, inferior left hemiscrotum in a region of collapsed scrotal tissue
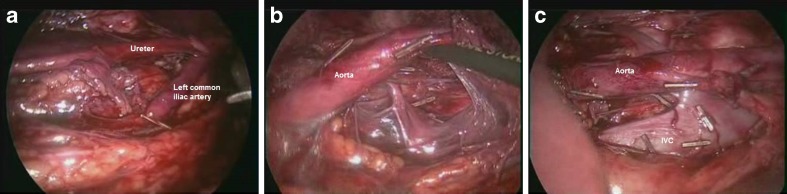
Fig. 3.
a Laparoscopic dissection of left paraaortic group of lymphnodes. b Laparoscopic dissection of retroaortic group of lymphnodes. c Laparoscopically cleared inter aortocaval space
Discussion
Para-testicular rhabdomyosarcomas are rare tumors with aggressive growth patterns. Multimodal therapy including surgery, chemotherapy and radiotherapy provides the child with an excellent long-term prognosis. The role of staging RPLND in patients with clinical stage 1 disease is based on experience in the Intergroup Rhabdomyosarcoma Studies (IRS) [6]. IRS-III (from 1984 to 1991) required a staging unilateral RPLND for all clinical stage 1 patients. In IRS-IV (from 1991 to 1997) patients with a negative CT scan did not undergo staging RPLND (and did not receive radiation to the retroperitoneum). This approach resulted in a worrisome decrease in the percentage of patients diagnosed with metastatic disease in IRS-IV, particularly among adolescents [1].
Furthermore, adolescents with clinical stage 1 disease in IRS-IV had a 3-year event-free survival (EFS) of only 68 % compared to a 100 % survival for patients with recognized lymph node involvement (who received radiation and more intense chemotherapy) — presumably due to under-staging and under-treatment of those stage 1 patients with occult retroperitoneal disease. Such discrepancies were not seen in the prepubertal patients. Based on these data, patients over 10 years of age with or without radiographic evidence of retroperitoneal disease should undergo a staging RPLND and receive radiation in addition to chemotherapy if the lymph nodes are positive. Younger children with a normal abdominal CT may be treated with chemotherapy alone without a staging RPLND. Overall survival rates for patients with para-testicular rhabdomyosarcoma approach 90 % [6] .
The increased use of minimally invasive surgery has spread to RPLND, and the initial report of laparoscopic RPLND (L-RPLND) was in 1992 [7] and robot-assisted (R-RPLND) in 2006 [8]. These techniques are feasible and safe with equivalent intermediate-term oncologic outcomes in appropriately selected adult patients [9, 10]. Subsequently, L-RPLND was reported in adolescents for both para-testicular rhabdomyosarcoma (PTRMS) and Testicular germ cell tumor (T-GCT) [11, 12] .
Tomaszewski et al. [11] reported on their experience with laparoscopic RPLND in high-risk pediatric patients with para-testicular rhabdomyosarcoma (PTRMS). Three patients, mean age 13.6 years (range 10–16 years), underwent modified template LRPLND after radical orchiectomy for preoperative rhabdomyosarcoma stage T(1a)N(0)M(0), T(1b)N(0)M(0), and T(2b)N(0)M(0), respectively. Average operative time was 382 min (range 245–656 min). Mean estimated blood loss was 53 mL (range 10–75 mL), and mean postoperative hospital stay was 2.5 days (range 2–3 days). There were no postoperative complications. They concluded that LRPLND for high-risk pediatric patients with PTRMS was a safe diagnostic and therapeutic procedure with the benefit of rapid convalescence, enabling early commencement of adjuvant chemotherapy. In addition, multiple series on L-RPLND for T-GCT include adolescent patients and demonstrate equivalent intermediate-term oncologic out-comes with lower morbidity than open RPLND [9, 12] .
Cost et al. [13] reported on two cases of adolescents who were treated using robot-assisted laparoscopic RPLND (R-RPLND)—one with PT-RMS and one with T-GCT with good outcomes and low morbidity. Based on the robot-assisted laparoscopic prostatectomy experience, robot assistance enables physicians to more easily and safely in-corporate advanced laparoscopic skills into clinical practice [14]. Thus, robot assistance could allow for wider dissemination of minimally invasive RPLND [15]. Also, robot assistance allows for enhanced three-dimensional visualization and more precise surgical instrumentation. This would hopefully enable the surgeon to achieve a complete resection and potentially better nerve sparing.
References
- 1.Ross JH, Kay R. Prepubertal testis tumor. Rev Urol. 2004;6:11–18. [PMC free article] [PubMed] [Google Scholar]
- 2.Rushton HG, Belman AB. Testis-sparing surgery for benign lesions of the prepubertal testis. Urol Clin N Am. 1993;20:27–37. [PubMed] [Google Scholar]
- 3.Farivar-Mohseni H, Bagli DJ, McLorie G, et al. Prepubertal testicular and paratesticular tumours. J Urol. 1996;155(suppl):392A. [Google Scholar]
- 4.Shukla AR, Woodard C, Carr MC, et al. Testicular teratoma at the Children’s Hospital of Philadelphia: the role of testis preserving surgery. J Urol. 2002;167(suppl):109. [Google Scholar]
- 5.Sugita Y, Clarnette TD, Cooke-Yarborough CC, et al. Testicular and paratesticular tumours in children: 30 years’ experience. Aust N Z J Surg. 1999;69:505–508. doi: 10.1046/j.1440-1622.1999.01612.x. [DOI] [PubMed] [Google Scholar]
- 6.Wiener ES, Anderson JR, Ojimba JI, et al. Controversies in the management of paratesticular rhabdomyosarcoma: is staging retroperitoneal lymph node dissection necessary for adolescents with resected paratesticular rhabdomyosarcoma? Semin Pediatr Surg. 2001;10:146. doi: 10.1053/spsu.2001.24695. [DOI] [PubMed] [Google Scholar]
- 7.Rukstalis DB, Chodak GW. Laparoscopic retroperitoneal lymph node dissection in a patient with stage 1 testicular carcinoma. J Urol. 1992;148:1907–1910. doi: 10.1016/s0022-5347(17)37068-4. [DOI] [PubMed] [Google Scholar]
- 8.Davol P, Sumfest J, Rukstalis D. Robotic-assisted laparoscopic retroperitoneal lymph node dissection. Urology. 2006;67:199. doi: 10.1016/j.urology.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 9.Abdel-Aziz KF, Anderson JK, Svatek R, et al. Laparoscopic and open retroperitoneal lymph-node dissection for clinical stage I non-seminomatous germ-cell testis tumors. J Endourol. 2006;20:627–631. doi: 10.1089/end.2006.20.627. [DOI] [PubMed] [Google Scholar]
- 10.Weizer AZ, Montgomery JS. The role of lymphadenectomy in minimally invasive urologic oncology. J Endourol. 2010;24:1229–1240. doi: 10.1089/end.2009.0562. [DOI] [PubMed] [Google Scholar]
- 11.Tomaszewski JJ, Sweeney DD, Kavoussi LR, Ost MC. Laparoscopic retroperitoneal lymph node dissection for high-risk pediatric patients with paratesticular rhabdomyo- sarcoma. J Endourol. 2010;24:31–34. doi: 10.1089/end.2009.0161. [DOI] [PubMed] [Google Scholar]
- 12.Skolarus TA, Bhayani SB, Chiang HC, et al. Laparoscopic retroperitoneal lymph node dissection for low-stage testicular cancer. J Endourol. 2008;22:1485–1489. doi: 10.1089/end.2007.0442. [DOI] [PubMed] [Google Scholar]
- 13.Cost NG, DaJusta DG, Granberg CF, Cooksey RM, Laborde CE, Wickiser JE, Gargollo PC. Robot-assisted laparoscopic retroperitoneal lymph node dissection in an adolescent population. J Endourol. 2012;26:635–640. doi: 10.1089/end.2011.0214. [DOI] [PubMed] [Google Scholar]
- 14.Leroy TJ, Thiel DD, Duchene DA, et al. Safety and peri-operative outcomes during learning curve of robot-assisted laparoscopic prostatectomy: a multi-institutional study of fellowship-trained robotic surgeons versus experienced open radical prostatectomy surgeons incorporating robot- assisted laparoscopic prostatectomy. J Endourol. 2010;24:1665–1669. doi: 10.1089/end.2009.0657. [DOI] [PubMed] [Google Scholar]
- 15.Ahlering TE, Skarecky D, Lee D, Clayman RV. Successful transfer of open surgical skills to a laparoscopic environment using a robotic interface: Initial experience with laparoscopic radical prostatectomy. J Urol. 2003;170:1738–1741. doi: 10.1097/01.ju.0000092881.24608.5e. [DOI] [PubMed] [Google Scholar]