Abstract
Favourable clinical results in rheumatoid arthritis (RA) patients with high disease activity (HDA) are difficult to achieve. This study evaluated the clinical efficacy of abatacept according to baseline disease activity compared to adalimumab and tocilizumab. This study included all patients registered in a Japanese multicenter registry treated with abatacept (n = 214), adalimumab (n = 175), or tocilizumab (n = 143) for 24 weeks. Clinical efficacy of abatacept in patients with HDA (DAS28-CRP > 4.1) and low and moderate disease activity was compared. Clinical efficacy of abatacept, adalimumab, and tocilizumab was compared in patients with HDA at baseline. In patients treated with abatacept, multivariate logistic regression identified HDA at baseline as an independent predictor for achieving low disease activity (LDA; DAS28-CRP < 2.7) [OR 0.26, 95 % CI 0.14–0.50] or remission (DAS28-CRP < 2.3) [OR 0.26, 95 % CI 0.12–0.56] at 24 weeks. In patients with HDA at baseline, logistic regression did not identify treatment with adalimumab or tocilizumab as independent predictors of LDA or remission compared to abatacept. Retention rates based on insufficient efficacy were significantly higher in patients treated with abatacept compared to adalimumab and lower than tocilizumab. Retention rates based on adverse events in patients treated with abatacept were significantly lower compared to tocilizumab. Clinical efficacy of abatacept was affected by baseline disease activity. There were no significant differences between the three different classes of biologics regarding clinical efficacy for treating RA patients with HDA, although definitive conclusions regarding long-term efficacy will require further research.
Keywords: Abatacept, Adalimumab, High disease activity, Japanese multicenter registry system, Rheumatoid arthritis, Tocilizumab
Introduction
Biological disease-modifying antirheumatic drugs (DMARDs) are standard treatment for rheumatoid arthritis (RA). Several clinical trials have demonstrated that biological agents significantly reduce disease activity and that suppression of synovitis significantly reduces subsequent joint destruction. However, favourable clinical results are often difficult to achieve in patients with high disease activity (HDA), even in this ‘bio-era’ of drug discovery. Previous reports have demonstrated that lower disease activity at baseline is a predictor of clinical efficacy when using anti-tumour necrosis factor (TNF) agents and tocilizumab, which is a humanized monoclonal antibody against the interleukin-6 receptor. Hydrich et al. reported that a lower Disease Activity Score 28 (DAS28) score at baseline was a significant predictor of clinical remission at 6 months in patients treated with infliximab and etanercept [1]. The TEMPO etanercept study indicated that patients with lower disease activity at baseline were more likely to achieve remission [2]. Similar results were reported in Japanese RA patients treated with infliximab, etanercept [3, 4], and tocilizumab. A higher proportion of patients with moderate disease activity achieved LDA and clinical remission at week 24 [5].
Abatacept is the first member of a new class of biologic agents for RA treatment that inhibits T-cell activation by binding to CD80/86, modulating its interaction with CD28. This strategy is expected to achieve clinical efficacy in patients who are naïve or inadequately respond to other classes of biologics. The efficacy and safety of abatacept has been reported in several clinical trials [6–10]. The effectiveness of abatacept has also been reported in clinical practice in Denmark and Japan [11, 12]. However, there are no available reports describing the effects of baseline disease activity on the clinical efficacy of abatacept. In this study, we evaluated clinical data of patients treated with abatacept and compared the clinical efficacy of adalimumab, tocilizumab, and abatacept in patients with HDA at baseline.
Materials and methods
Participants
All eligible patients were registered in and followed by the Tsurumai Biologics Communication Registry (TBCR), a RA research consortium that includes Nagoya University Hospital and 12 affiliated institutes [13]. TBCR was initiated in October 2008 to study the long-term efficacy and safety of biologics used to treat RA. Data were retrospectively collected from 2003 to 2008 and prospectively after 2008. Patient characteristics and disease activity data are available for all RA patients treated with commercially available biologics at TBCR institutes in Japan. Registered data are updated once per year and include drug continuation, reasons for switching drugs, and adverse events (e.g. surgery, pregnancy) that may have occurred during treatment. The present study included all patients who were treated with abatacept (ABT, n = 214), adalimumab (ADA, n = 175), or tocilizumab (TCZ, n = 143) for 24 weeks at TBCR-affiliated institutes. All patients met the 1987 American College of Rheumatology classification criteria for RA. Patients received abatacept infusions three times every 2 weeks followed by every 4 weeks, adalimumab infusions every 2 weeks, or tocilizumab infusions every 4 weeks according to drug labels and Japan College of Rheumatology guidelines for treatment. Patient anonymity was maintained during data collection, and the security of personal information was strictly controlled. This study was approved by the Nagoya University Graduate School of Medicine ethics committee.
Data collection
Data were retrospectively collected from clinical records. The following demographic data were recorded at the initiation of treatment (baseline, week 0): disease duration, concomitant treatment (methotrexate (MTX) or prednisolone (PSL)), joint damage (Steinbrocker stage), and daily dysfunction (Steinbrocker class). The following disease parameters were recorded at baseline and after 4, 12, and 24 weeks of treatment: tender joint count (TJC) and swollen joint count (SJC) on 28 joints, general health on a visual analogue scale (GH-VAS), and serum c-reactive protein (CRP) levels. Disease activity was evaluated at each time point using DAS28 with CRP (DAS28-CRP).
Disease activity and EULAR response
Disease activity was categorised as follows: DAS28 remission (DAS28-CRP < 2.3), low disease activity (LDA; 2.3 ≤ DAS28-CRP < 2.7), moderate disease activity (MDA; 2.7 ≤ DAS28-CRP ≤ 4.1), and high disease activity (HAD; DAS28-CRP > 4.1) [14]. Disease activity was evaluated at baseline and 24 weeks after treatment. The European League Against Rheumatism (EULAR) response was evaluated at 24 weeks [15].
Statistical analysis
Demographic and disease characteristics are reported using descriptive statistics. All results are expressed as mean ± standard deviation or percentage. Student’s t test was used for two-group comparisons, and the chi-square test was used for categorical variables. The last observation carried forward (LOCF) method was used in each analysis. To determine predictors of LDA, clinical remission, and a moderate EULAR response at 24 weeks, multivariate logistic regression analysis was performed. All statistical tests were two-sided, and significance was defined as p < 0.05. All analyses were performed with SPSS version 20.0.0 software (IBM Corp., Armonk, NY, USA).
Results
Demographic data
We compared the clinical parameters of patients with low and moderate disease activity (≤MDA, DAS28-CRP ≤ 4.1) and HDA at baseline. Characteristics of patients treated with abatacept in the ≤MDA and HDA groups are shown in Table 1. There were no significant differences in age, RA disease duration, gender, stage, and class. There were no differences in the proportion of patients treated concomitantly with MTX and PSL and mean MTX and PSL doses. Mean DAS28-CRP and related components (SJC, TJC, CRP, and GH) were higher in the HDA group, while mean matrix metalloproteinase-3 (MMP-3) values did not differ between groups.
Table 1.
Baseline characteristics of rheumatoid arthritis patients who received abatacept and of the patients with high disease activity who received adalimumab or tocilizumab
| Abatacept | Adalimumab | Tocilizumab | ||||
|---|---|---|---|---|---|---|
| Disease activity at baseline | (in patients with HDA) | |||||
| ≤MDA | HDA | |||||
| (n = 86) | (n = 128) | p value | (n = 120) | (n = 97) | p valueb | |
| Age (years) | 64.1 ± 11.4 | 64.9 ± 10.9 | 0.64 | 57.3 ± 14.4 | 55.8 ± 13.8 | <0.001 |
| Gender (% female) | 82.6 | 80.5 | 0.725 | 82.5 | 78.4 | 0.761 |
| Disease duration (years) | 10.9 ± 10.1 | 13.0 ± 10.8 | 0.177 | 13.8 ± 10.6 | 10.7 ± 8.9 | 0.074 |
| Stage (I/II/III/IV %) | 11.6/26.7/34.9/26.7 | 10.2/12.5/41.4/35.9 | 0.052 | 12.5/15.0/30.0/42.5 | 13.4/23.7/24.7/38.1 | 0.097 |
| Class (I/II/III/IV %) | 11.6/45.3/41.9/1.2 | 3.2/45.3/48.4/3.1 | 0.071 | 8.3/50.8/37.5/3.3 | 12.4/44.3/43.3/0.0 | 0.069 |
| Prior use of biologics (%) | 51.2 | 52.3 | 0.89 | 32.5 | 62.9 | <0.001 |
| MTX use (%) | 51.2 | 48.4 | 0.781 | 76.7 | 36.1 | <0.001 |
| MTX dose (mg/week)a | 7.3 ± 2.5 | 7.2 ± 2.3 | 0.79 | 7.0 ± 1.9 | 7.9 ± 1.6 | 0.106 |
| Oral steroid use (%) | 52.3 | 54.7 | 0.779 | 60.8 | 71.9 | 0.041 |
| Oral steroid dose (mg/day)a | 4.2 ± 2.0 | 4.4 ± 2.3 | 0.643 | 5.2 ± 2.7 | 4.8 ± 2.1 | 0.181 |
| MMP-3 (ng/mL) | 226.9 ± 746.5 | 276.7 ± 271.6 | 0.568 | 335.1 ± 365.1 | 378.1 ± 307.8 | 0.066 |
| SJC, 0-28 | 2.7 ± 3.0 | 7.2 ± 5.9 | <0.001 | 7.9 ± 5.5 | 8.2 ± 5.9 | 0.357 |
| TJC, 0-28 | 2.7 ± 2.4 | 10.4 ± 7.2 | <0.001 | 9.2 ± 6.3 | 10.5 ± 7.6 | 0.345 |
| CRP (mg/dL) | 0.9 ± 1.3 | 3.1 ± 3.2 | <0.001 | 3.7 ± 3.3 | 3.7 ± 2.9 | 0.257 |
| GH, VAS 0–100 mm | 32.8 ± 20.8 | 70.4 ± 20.1 | <0.001 | 64.6 ± 20.6 | 61.5 ± 23.6 | 0.007 |
| DAS28-CRP | 3.2 ± 0.6 | 5.4 ± 0.9 | <0.001 | 5.3 ± 0.9 | 5.5 ± 1.0 | 0.656 |
Data are presented as mean ± standard deviation except when otherwise indicated
Stage Steinbrocker’s stages, Class Steinbrocker’s classes, MTX methotrexate, MMP-3 matrix metalloproteinase-3, SJC swollen joint count, TJC tender joint count, CRP c-reactive protein, GH general health, VAS visual analog scale, DAS28 Disease Activity Score in 28 joints
aMean among patients receiving the drug
bObtained from analysis of variance (ANOVA) between Abatacept (HDA), Adalimumab, and Tocilizumab groups
Clinical efficacy and retention in patients treated with abatacept in the ≤ MDA and HDA groups
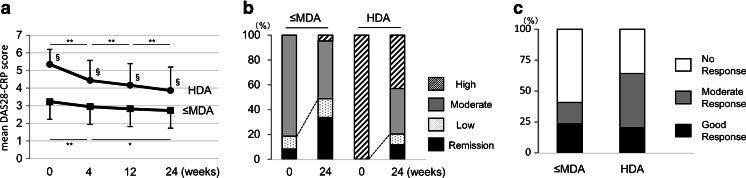
As shown in Fig. 1a, the mean DAS28-CRP score significantly decreased from baseline to 4 weeks at 3.23 ± 0.64 to 2.95 ± 0.81 in the ≤ MDA group (p < 0.01) and 5.35 ± 0.86 to 4.44 ± 1.14 in the HDA group (p < 0.01). Significant differences in DAS28-CRP scores were observed between 4 and 12 weeks (4.16 ± 1.24, p < 0.01) and 12 and 24 weeks (3.87 ± 1.33, p < 0.01) in the HDA group, while only 4 and 24 weeks (2.74 ± 0.85, p < 0.028) in the ≤MDA group. The difference between the ≤MDA and HDA groups remained significant at 24 weeks.
Fig. 1.
a Clinical efficacy of abatacept in rheumatoid arthritis patients. Mean and standard deviation for the Disease Activity Score based on 28 joints (DAS28-CRP). §p < 0.01 between the HDA and ≤MDA groups. b Changes in DAS28-CRP defined disease activity over 24 weeks of abatacept treatment. High DAS28-CRP > 4.1, Moderate 4.1 ≥ DAS28-CRP ≥ 2.7, Low 2.7 > DAS28-CRP ≥ 2.3, Remission DAS28-CRP < 2.3. c Comparison of European League Against Rheumatism (EULAR) responses at 24 weeks between patients with high disease activity at baseline (HDA) and patients with lower disease activity (≤MDA) at baseline. *p < 0.05; **p < 0.01
Disease activities as assessed by DAS28-CRP score at baseline and after 24 weeks of abatacept therapy in the ≤MDA and HDA groups are shown in Fig. 1b. The proportion of patients who achieved LDA gradually increased over time after initiation of abatacept treatment in the ≤MDA and HDA groups. The proportion of patients who achieved LDA was significantly higher in the ≤MDA group (48.8 %) compared to the HDA group (20.3 %, p < 0.001). The proportion of patients who achieved a moderate or good EULAR response was significantly higher in the HDA group (64.1 %) compared to the ≤MDA group (40.7 %, p < 0.001; Fig. 1c).
Given that multiple confounding factors may contribute to the clinical efficacy of abatacept, multivariate logistic regression was performed to confirm the influence of disease activity at baseline on disease activity at 24 weeks (Table 2). Odds ratios (ORs) were adjusted for the following parameters: age, gender, disease duration, class, DAS28-CRP at baseline, prior use of biologics, and concomitant MTX and PSL use. Multivariate analysis confirmed that HDA (DAS28-CRP > 4.1) at baseline, class 3 or 4, and prior use of biologics were independent negative factors for achieving LDA or a moderate EULAR response at 24 weeks.
Table 2.
Multivariate logistic regression analysis for the achievement of low disease activity (LDA), clinical remission, and moderate or good EULAR response at 24 weeks in the overall patients using abatacept (upper column) or in the patients with high disease activity at baseline using abatacept, adalimumab, or tocilizumab (lower column)
| LDA at 24 weeks | Remission at 24 weeks | Moderate or good EULAR response at 24 weeks | ||||
|---|---|---|---|---|---|---|
| Adjusted OR (95 % CI) | p value | Adjusted OR (95% CI) | p value | Adjusted OR (95% CI) | p value | |
| Overall patients taking abatacept | ||||||
| DAS28-CRP > 4.1 | 0.261 (0.135–0.503) | <0.001 | 0.264 (0.124–0.564) | 0.001 | 3.010 (1.626–5.574) | <0.001 |
| Steinbrocker class 1–2 (vears 3–4) | 2.427 (1.145–5.147) | 0.021 | 2.003 (0.853–4.707) | 0.111 | 1.670 (0.841–3.318) | 0.143 |
| No previous use of biologics | 2.346 (1.185–4.642) | 0.014 | 2.056 (0.938–4.508) | 0.072 | 2.824 (1.520–5.250) | 0.001 |
| Concomitant MTX use | 0.698 (0.353–1.381) | 0.302 | 0.652 (0.299–1.425) | 0.284 | 0.798 (0.433–1.472) | 0.47 |
| Patients with HDA at baseline | ||||||
| Adalimumab use (vs abatacept) | 1.361 (0.683–2.711) | 0.381 | 1.611 (0.712–3.648) | 0.252 | 0.740 (0.402–1.363) | 0.334 |
| Tocilizumab use (vs abatacept) | 1.479 (0.733–2.983) | 0.275 | 1.057 (0.430–2.598) | 0.904 | 2.594 (1.316–5.114) | 0.006 |
| DAS28-CRP score at baseline | 0.768 ( 0.551–1.070) | 0.119 | 0.600 (0.386–0.933) | 0.023 | 0.929 (0.700–1.232) | 0.608 |
| Steinbrocker class 1–2 (vears 3–4) | 2.809 (1.570–5.029) | 0.001 | 3.254 (1.509–7.019) | 0.003 | 1.830 (1.119–2.994) | 0.016 |
| No previous use of biologics | 2.030 (1.187–3.473) | 0.01 | 3.070 (1.558–6.049) | 0.001 | 2.152 (1.299–3.567) | 0.003 |
| Concomitant MTX use | 0.934 (0.522–1.672) | 0.818 | 1.102 (0.543–2.237) | 0.788 | 1.157 (0.676–1.982) | 0.594 |
OR odds ratio, CI confidence interval, EULAR European League Against Rheumatism, DAS28 Disease Activity Score in 28 joints, MTX methotrexate
The retention rate of patients treated with abatacept was compared between the ≤MDA and HDA groups and analysed based on reasons for discontinuation. Kaplan–Meier curves for time to discontinuation due to insufficient efficacy (insufficiency) and adverse events (AEs) were generated. Over 24 weeks, 3 of the 86 patients in the ≤MDA group and 16 of the 126 patients in the HDA group withdrew from abatacept treatment due to insufficiency. The retention rate based on insufficiency was significantly higher in the ≤MDA group compared to the HDA group (96.4 vs. 88.4 %, p = 0.023). There were no significant differences in retention rates due to AEs (95.2 vs. 97.5 %, p = 0.226).
Clinical efficacy of abatacept, adalimumab, and tocilizumab in patients with HDA at baseline
Clinical efficacy of abatacept in patients with HDA was insufficient compared to efficacy in patients with lower activity. Therefore, we compared the clinical efficacy of abatacept with agents in different biologic classes in patients with active RA. RA patients with HDA at baseline were treated with the TNF inhibitor adalimumab (n = 120) and the IL-6R inhibitor tocilizumab (n = 97).
Table 1 shows baseline characteristics of patients with HDA treated with abatacept, adalimumab, and tocilizumab. Post hoc analysis demonstrated that patients treated with abatacept were significantly older (p < 0.001, vs. ADA and TCZ). Fewer patients treated with adalimumab had a history of being treated with biologics (p = 0.002, vs. ABT; p < 0.001, vs. TCZ) and a higher proportion concomitantly used MTX (p < 0.001, vs. ABT and TCZ). A lower proportion of patients treated with abatacept concomitantly used oral steroids (p = 0.013, vs. TCZ). Disease activity parameters (DAS28, TJC, SJC, CRP, and MMP-3) did not show significant differences, except for increased GH-VAS in patients treated with abatacept (p = 0.007, vs. TCZ).
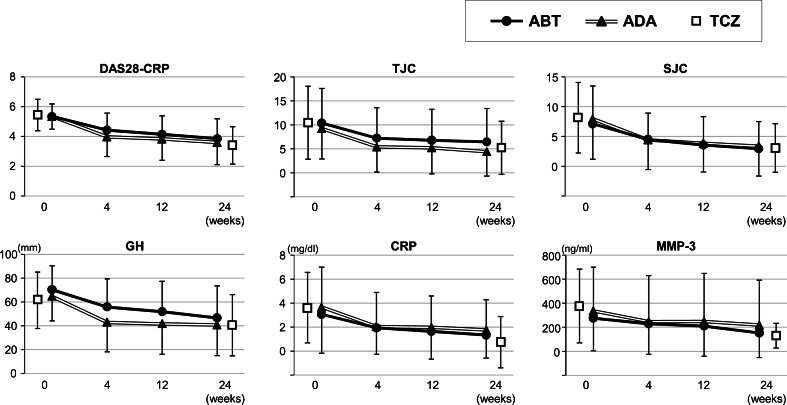
As shown in Fig. 2, changes in disease activity parameters were similar between patients treated with abatacept and adalimumab throughout the study period. Because clinical data at 4 and 12 weeks were unavailable for patients treated with tocilizumab, we only show data at baseline and 24 weeks. ANOVA demonstrated significant differences in TJC (p = 0.029), CRP (p = 0.004), and MMP-3 (p = 0.036) at 24 weeks between the three treatments. Post hoc analysis (Bonferroni method) showed a significant difference in TJC between abatacept and adalimumab (6.5 ± 7.0 vs. 4.4 ± 5.1, p = 0.029) and CRP and MMP-3 between adalimumab and tocilizumab (1.8 ± 2.5 vs. 0.76 ± 2.1, p = 0.003; 219.1 ± 373.3 vs. 130.8 ± 102.8, p = 0.048, respectively).
Fig. 2.
Overall clinical efficacy of abatacept (ABT), adalimumab (ADA), and tocilizumab (TCZ) in rheumatoid arthritis patients with high disease activity at baseline. Mean and standard deviations for the Disease Activity Score based on 28 joints (DAS28-CRP) and tender joint count (TJC), swollen joint count (SJC), general health on a visual analogue scale (GH-VAS), c-reactive protein (CRP), and matrix metalloproteinase-3 (MMP-3) are shown
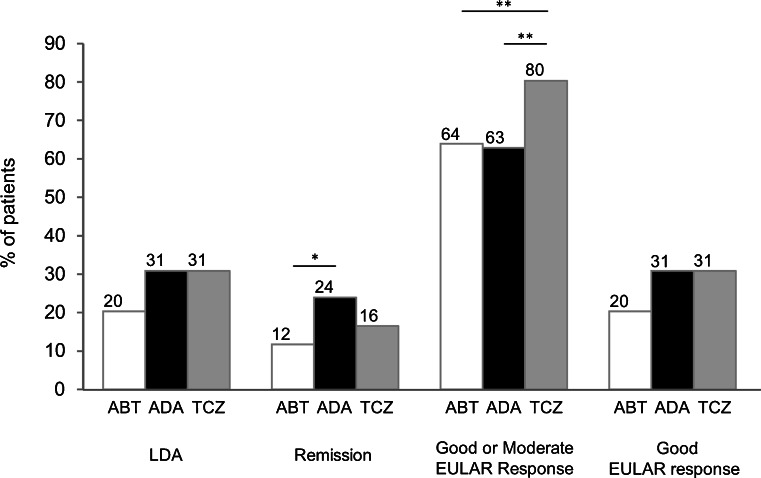
The proportion of patients who achieved LDA, clinical remission, a moderate or good EULAR response, and a good EULAR response at 24 weeks was compared following treatment with abatacept, adalimumab, and tocilizumab (Fig. 3). There was no significant difference in the proportion of patients who achieved LDA at 24 weeks. Although a lower proportion of patients treated with abatacept achieved clinical remission compared to adalimumab, the difference was not significant compared to tocilizumab. A higher proportion of patients treated with tocilizumab achieved a moderate or good EULAR response compared to patients treated with abatacept and adalimumab. There was no significant difference in the proportion of patients who achieved a good EULAR response between the three groups.
Fig. 3.
Proportion of patients who achieved DAS28-CRP defined as low disease activity (LDA), clinical remission, good or moderate EULAR response, and good EULAR response. *p < 0.05; **p < 0.01. ABT abatacept, ADA adalimumab, TCZ tocilizumab, EULAR European League Against Rheumatism
Multivariate analysis confirmed that none of the three biologics had significant advantages in achieving LDA or clinical remission at 24 weeks (Table 2). Adalimumab was not an independent factor for achieving LDA, remission, or a moderate EULAR response at 24 weeks. Tocilizumab was an independent factor for achieving a moderate EULAR response at 24 weeks compared to abatacept. Class 1 or 2 and no prior history of biologic use were independent factors for LDA, remission, and a moderate EULAR response. ORs were adjusted for the following parameters: age, gender, disease duration, class, DAS-CRP at baseline, prior biologic use, and concomitant MTX and PSL treatment.
Retention rates in patients with HDA at baseline treated with abatacept, adalimumab, and tocilizumab
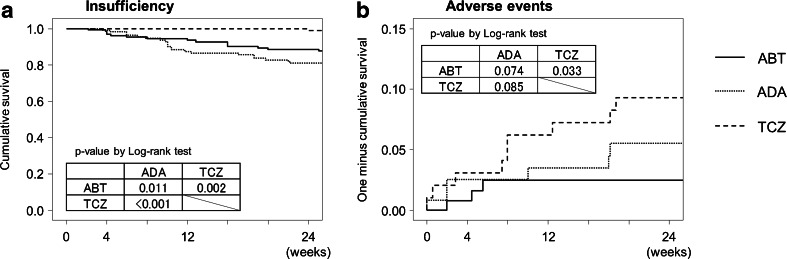
Retention rates were evaluated based on reasons for discontinuation. Kaplan–Meier curves for time to discontinuation for each agent due to insufficient efficacy and AEs are shown in Fig. 4a, b, respectively. Retention rates due to insufficient efficacy in patients treated with abatacept were significantly higher than in patients treated with adalimumab and lower than in patients treated with tocilizumab. Retention rates due to AEs in patients treated with abatacept were significantly lower than in patients treated with tocilizumab.
Fig. 4.
Kaplan–Meier curves for time to discontinuation for each biologic. Withdrawal was due to a insufficient clinical efficacy (insufficiency) and b adverse events. Retention rates were compared using the log-rank test among groups. ABT abatacept, ADA adalimumab, TCZ tocilizumab
Discussion
Baseline disease activity had a significant influence on the clinical efficacy of abatacept. In patients with HDA, the clinical efficacy of abatacept appeared to be insufficient compared with efficacy in patients with a lower disease activity. The clinical efficacy of abatacept in HDA patients was similar to the efficacy of adalimumab and tocilizumab. Some physicians perceive abatacept as being difficult to use in RA patients with HDA due to insufficient efficacy. However, adequate clinical responses were not obtained in any of the patients evaluated, regardless of the class of biologic used. Based on the present data, abatacept can be selected to treat RA patients with low, moderate, and high disease activity.
A recent head-to-head clinical trial (AMPLE trial) demonstrated that subcutaneous abatacept was not inferior to adalimumab [16]. The ADACTA head-to-head trial reported that tocilizumab monotherapy was superior to adalimumab monotherapy in reducing RA activity in patients for whom MTX was ineffective or inappropriate [17]. Although the data suggest equivalent clinical efficacies between different classes of biologics, patients in these trials were generally uniform and are different from real-world patients with diverse characteristics seen in clinical practice. The Danish DANBIO registry reported similar abatacept and tocilizumab efficacies in RA patients in clinical practice [11]. Multicenter registries can provide real-world long-term data relevant to safety, efficacy, or future outcomes in patients with comorbidities. The value of such registries in accumulating and evaluating relevant data cannot be underestimated.
In this study, differences in the DAS28-CRP score between patients treated with abatacept in the ≤MDA and HDA groups were consistent throughout the study period and remained significant at 24 weeks. The proportion of patients who achieved LDA or remission at 24 weeks was significantly lower in the HDA group. Multivariate regression analysis demonstrated that HDA at baseline was an independent negative predictor for achieving LDA and remission. Abatacept treatment in patients with HDA appeared to yield poor clinical results. However, similar results have been reported in previous studies related to TNF inhibitors and tocilizumab [1–5, 18]. Thus, inferior clinical efficacy in HDA patients is not abatacept specific, but is likely due to common features of DMARDs. The proportion of patients who achieved a moderate or good EULAR response was higher in the HDA group at 24 weeks. There was a sharp downward trend in the HDA group between 12 and 24 weeks, whereas the trend was gradual in the ≤MDA group after 4 weeks. A longer period is necessary to evaluate whether disease activity in the HDA group can eventually correspond to activity in the ≤MDA group.
Clinical efficacy in patients with HDA was similar between abatacept, adalimumab, and tocilizumab. This is the first study to demonstrate the clinical efficacy of three different classes of biologics in RA patients with HDA in clinical practice from the TBCR. Given that neither adalimumab nor tocilizumab were independent factors for achieving LDA or remission compared with abatacept, we suggest that there are no significant differences between the three biologics regarding clinical efficacy for HDA RA. However, several differences were observed. Tocilizumab treatment resulted in a higher proportion of patients who achieved a moderate EULAR response at 24 weeks and was an independent factor for a moderate EULAR response at 24 weeks compared to abatacept. Cytokine-blocking biologics, specifically tocilizumab, led to normal acute phase reactants (e.g. CRP or ESR) in almost all patients. While this constitutes a positive treatment effect, improvement in CRP might lead to an overestimation of the EULAR response rate based on DAS28-CRP [19]. Abatacept had an average discontinuation rate due to insufficient clinical efficacy. Adalimumab had the highest discontinuation rate due to insufficiency despite a higher proportion of biologic-naïve patients and concomitant MTX use compared to abatacept and tocilizumab, which could be due to relatively low MTX doses in Japan compared to doses used in European countries and the USA. It will be necessary to study patients treated with higher doses of MTX in the future. Tocilizumab had the lowest discontinuation rate due to insufficiency despite having the lowest proportion of biologic-naïve patients and concomitant MTX use. This could be partially explained by a higher rate of achieving a EULAR response, which generally encourages physicians to continue treatment even if clinical efficacy appears to be inadequate. It will be necessary to study whether low discontinuation rates can be maintained for longer periods of time. In contrast, tocilizumab demonstrated the highest discontinuation rate due to adverse events compared to abatacept. Abatacept satisfactorily balances clinical efficacy and safety in RA patients with HDA.
‘Biologic-naïve’ was an independent factor for achieving LDA or EULAR responses at 24 weeks in patients treated with abatacept. Similar to other classes of biologics, abatacept likely demonstrates optimal efficacy in biologic-naïve patients. Several reports have indicated that adalimumab or tocilizumab demonstrate higher efficacy in biologic-naïve patients compared to patients with a history of prior biologic use [5, 20–26]. Advanced functional impairment has also been reported as a negative factor regarding clinical efficacy of adalimumab and tocilizumab [1, 18, 27, 28]. ‘Better physical function’ in all patients treated with abatacept was an independent factor for achieving LDA. In patients with HDA at baseline using abatacept, adalimumab, or tocilizumab, ‘biologic-naïve’ or ‘better physical function’ were independent factors for achieving LDA or remission rather than the specific agent used. Thus, baseline characteristics are more critical than treatment agents for predicting favourable clinical efficacy in RA patients with HDA.
This study has several limitations. It was observational and not randomised, and treatments were likely influenced by patient characteristics and other factors such as preference of administration routes. Although concomitant MTX use was not an independent factor for achieving LDA, remission, or a moderate EULAR response in all patients treated with abatacept and patients with HDA treated with abatacept, adalimumab, or tocilizumab, the mean MTX dose was relatively low in this study. Future studies with higher MTX doses are necessary to conclude whether MTX has a synergistic effect. In addition, radiographic data were not available. Due to the importance of joint protective effects in demonstrating clinical efficacy, evaluating radiographic changes in patients treated with abatacept will be necessary in the future.
In conclusion, this study demonstrated the clinical efficacy of abatacept, adalimumab, and tocilizumab in clinical practice. The clinical efficacy in RA patients with HDA was similar between the three classes of biologics. We suggest that abatacept can be used to treat patients with low, moderate, and high disease activity in clinical practice.
Acknowledgments
We thank Dr. Toshihisa Kanamono (Department of Orthopedic Surgery, Nagano Red Cross Hospital, Nagano, Japan), Dr. Yukiyoshi Oh-ishi (Department of Rheumatology, Toyohashi Municipal Hospital, Toyohashi, Japan), Dr. Yoshito Etoh (Department of Orthopedic Surgery, Higashi Nagoya National Hospital, Nagoya, Japan), and Dr. Masahiro Kobayakawa (Department of Orthopedic Surgery, Fukuroi Municipal Hospital, Fukuroi, Japan) for their kind suggestions.
Conflicts of interest
N. Ishiguro, T. Kojima, Y. Hirano, and A. Kaneko have received speaking fees (less than $5,000) from Abbott Japan Co. Ltd., Eisai Co. Ltd., Mitsubishi Tanabe Pharma Corporation, Pfizer Co. Ltd, Chugai Pharmaceutical Co. Ltd., and Bristol-Myers Squibb Co. Ltd. The other authors declare no conflicts of interest.
References
- 1.Hyrich KL, Watson KD, Silman AJ, Symmons DP, British Society for Rheumatology Biologics R Predictors of response to anti-TNF-alpha therapy among patients with rheumatoid arthritis: results from the British society for rheumatology biologics register. Rheumatology. 2006;45(12):1558–1565. doi: 10.1093/rheumatology/kel149. [DOI] [PubMed] [Google Scholar]
- 2.van der Heijde D, Klareskog L, Landewe R, Bruyn GA, Cantagrel A, Durez P, Herrero-Beaumont G, Molad Y, Codreanu C, Valentini G, Zahora R, Pedersen R, MacPeek D, Wajdula J, Fatenejad S. Disease remission and sustained halting of radiographic progression with combination etanercept and methotrexate in patients with rheumatoid arthritis. Arthritis Rheum. 2007;56(12):3928–3939. doi: 10.1002/art.23141. [DOI] [PubMed] [Google Scholar]
- 3.Iwamoto N, Kawakami A, Fujikawa K, Aramaki T, Kawashiri SY, Tamai M, Arima K, Ichinose K, Kamachi M, Yamasaki S, Nakamura H, Nakashima M, Mizokami A, Goto A, Fukuda T, Matsuoka N, Ueki Y, Tsukada T, Migita K, Shoumura F, Kawabe Y, Shibatomi K, Mine M, Ida H, Origuchi T, Aoyagi K, Eguchi K. Prediction of DAS28-ESR remission at 6 months by baseline variables in patients with rheumatoid arthritis treated with etanercept in Japanese population. Mod Rheumatol Jpn Rheum Assoc. 2009;19(5):488–492. doi: 10.1007/s10165-009-0187-8. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka Y, Takeuchi T, Inoue E, Saito K, Sekiguchi N, Sato E, Nawata M, Kameda H, Iwata S, Amano K, Yamanaka H. Retrospective clinical study on the notable efficacy and related factors of infliximab therapy in a rheumatoid arthritis management group in Japan: one-year clinical outcomes (RECONFIRM-2) Mod Rheumatol Jpn Rheum Assoc. 2008;18(2):146–152. doi: 10.1007/s10165-008-0026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burmester GR, Feist E, Kellner H, Braun J, Iking-Konert C, Rubbert-Roth A. Effectiveness and safety of the interleukin 6-receptor antagonist tocilizumab after 4 and 24 weeks in patients with active rheumatoid arthritis: the first phase IIIb real-life study (TAMARA) Ann Rheum Dis. 2011;70(5):755–759. doi: 10.1136/ard.2010.139725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud-Mendoza C, Szechinski J, Li T, Ge Z, Becker JC, Westhovens R. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2006;144(12):865–876. doi: 10.7326/0003-4819-144-12-200606200-00003. [DOI] [PubMed] [Google Scholar]
- 7.Kremer JM, Russell AS, Emery P, Abud-Mendoza C, Szechinski J, Westhovens R, Li T, Zhou X, Becker JC, Aranda R, Peterfy C, Genant HK. Long-term safety, efficacy and inhibition of radiographic progression with abatacept treatment in patients with rheumatoid arthritis and an inadequate response to methotrexate: 3-year results from the AIM trial. Ann Rheum Dis. 2011;70(10):1826–1830. doi: 10.1136/ard.2010.139345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiff M, Keiserman M, Codding C, Songcharoen S, Berman A, Nayiager S, Saldate C, Li T, Aranda R, Becker JC, Lin C, Cornet PL, Dougados M. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis. 2008;67(8):1096–1103. doi: 10.1136/ard.2007.080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiff M, Pritchard C, Huffstutter JE, Rodriguez-Valverde V, Durez P, Zhou X, Li T, Bahrt K, Kelly S, Le Bars M, Genovese MC. The 6-month safety and efficacy of abatacept in patients with rheumatoid arthritis who underwent a washout after anti-tumour necrosis factor therapy or were directly switched to abatacept: the ARRIVE trial. Ann Rheum Dis. 2009;68(11):1708–1714. doi: 10.1136/ard.2008.099218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westhovens R, Robles M, Ximenes AC, Nayiager S, Wollenhaupt J, Durez P, Gomez-Reino J, Grassi W, Haraoui B, Shergy W, Park SH, Genant H, Peterfy C, Becker JC, Covucci A, Helfrick R, Bathon J. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis. 2009;68(12):1870–1877. doi: 10.1136/ard.2008.101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leffers HC, Ostergaard M, Glintborg B, Krogh NS, Foged H, Tarp U, Lorenzen T, Hansen A, Hansen MS, Jacobsen MS, Dreyer L, Hetland ML. All departments of rheumatology in D (2011) efficacy of abatacept and tocilizumab in patients with rheumatoid arthritis treated in clinical practice: results from the nationwide Danish DANBIO registry. Ann Rheum Dis. 2011;70(7):1216–1222. doi: 10.1136/ard.2010.140129. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi N, Kojima T, Terabe K, Kaneko A, Kida D, Hirano Y, Fujibayashi T, Yabe Y, Takagi H, Oguchi T, Miyake H, Kato T, Fukaya N, Ishikawa H, Hayashi M, Tsuboi S, Kato D, Funahashi K, Matsubara H, Hattori Y, Hanabayashi M, Hirabara S, Yoshioka Y, Ishiguro N. Clinical efficacy of abatacept in Japanese rheumatoid arthritis patients. Mod Rheumatol Jpn Rheum Assoc. 2012 doi: 10.1007/s10165-012-0760-4. [DOI] [PubMed] [Google Scholar]
- 13.Kojima T, Kaneko A, Hirano Y, Ishikawa H, Miyake H, Oguchi T, Takagi H, Yabe Y, Kato T, Ito T, Terabe K, Fukaya N, Kanayama Y, Shioura T, Funahashi K, Hayashi M, Kato D, Matsubara H, Fujibayashi T, Kojima M, Ishiguro N, TBC Study protocol of a multicenter registry of patients with rheumatoid arthritis starting biologic therapy in Japan: tsurumai biologics communication registry (TBCR) study. Mod Rheumatol Jpn Rheum Assoc. 2011 doi: 10.1007/s10165-011-0518-4. [DOI] [PubMed] [Google Scholar]
- 14.Matsui T, Kuga Y, Kaneko A, Nishino J, Eto Y, Chiba N, Yasuda M, Saisho K, Shimada K, Tohma S. Disease activity score 28 (DAS28) using C-reactive protein underestimates disease activity and overestimates EULAR response criteria compared with DAS28 using erythrocyte sedimentation rate in a large observational cohort of rheumatoid arthritis patients in Japan. Ann Rheum Dis. 2007;66(9):1221–1226. doi: 10.1136/ard.2006.063834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Gestel A, van Riel P. American college of rheumatology preliminary definition of improvement in rheumatoid arthritis: comment on the article by Felson et al. Arthritis Rheum. 1996;39(3):535–537. doi: 10.1002/art.1780390325. [DOI] [PubMed] [Google Scholar]
- 16.Weinblatt ME, Schiff M, Valente R, van der Heijde D, Citera G, Zhao C, Maldonado M, Fleischmann R. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis. Arthritis Rheum. 2012 doi: 10.1002/art.37711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gabay C, Emery P, van Vollenhoven R, Dikranian A, Alten R, Klearman M, Musselman D, Agarwal S, Green J, Kavanaugh A. Tocilizumab (TCZ) Monotherapy is superior to adalimumab (ADA) Monotherapy in reducing disease activity in patients with rheumatoid arthritis (RA): 24-week data from the phase 4 ADACTA trial. Ann Rheum Dis. 2012;71(Suppl 3):152. [Google Scholar]
- 18.Kristensen LE, Kapetanovic MC, Gulfe A, Soderlin M, Saxne T, Geborek P. Predictors of response to anti-TNF therapy according to ACR and EULAR criteria in patients with established RA: results from the south Swedish arthritis treatment group register. Rheumatology. 2008;47(4):495–499. doi: 10.1093/rheumatology/ken002. [DOI] [PubMed] [Google Scholar]
- 19.Smolen JS, Aletaha D. Interleukin-6 receptor inhibition with tocilizumab and attainment of disease remission in rheumatoid arthritis: the role of acute-phase reactants. Arthritis Rheum. 2011;63(1):43–52. doi: 10.1002/art.27740. [DOI] [PubMed] [Google Scholar]
- 20.Takeuchi T, Tanaka Y, Kaneko Y, Tanaka E, Hirata S, Kurasawa T, Kubo S, Saito K, Shidara K, Kimura N, Nagasawa H, Kameda H, Amano K, Yamanaka H. Effectiveness and safety of adalimumab in Japanese patients with rheumatoid arthritis: retrospective analyses of data collected during the first year of adalimumab treatment in routine clinical practice (HARMONY study) Mod Rheumatol Jpn Rheum Association. 2012;22(3):327–338. doi: 10.1007/s10165-011-0516-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bombardieri S, Ruiz AA, Fardellone P, Geusens P, McKenna F, Unnebrink K, Oezer U, Kary S, Kupper H, Burmester GR, Research in Active Rheumatoid Arthritis Study G Effectiveness of adalimumab for rheumatoid arthritis in patients with a history of TNF-antagonist therapy in clinical practice. Rheumatology. 2007;46(7):1191–1199. doi: 10.1093/rheumatology/kem091. [DOI] [PubMed] [Google Scholar]
- 22.Kaneko A, Hirano Y, Fujibayashi T, Hattori Y, Terabe K, Kojima T, Ishiguro N. Twenty-four-week clinical results of adalimumab therapy in Japanese patients with rheumatoid arthritis: retrospective analysis for the best use of adalimumab in daily practice. Mod Rheumatol Jpn Rheum Assoc. 2012 doi: 10.1007/s10165-012-0705-y. [DOI] [PubMed] [Google Scholar]
- 23.Yamanaka H, Tanaka Y, Inoue E, Hoshi D, Momohara S, Hanami K, Yunoue N, Saito K, Amano K, Kameda H, Takeuchi T. Efficacy and tolerability of tocilizumab in rheumatoid arthritis patients seen in daily clinical practice in Japan: results from a retrospective study (REACTION study) Mod Rheumatol Jpn Rheum Assoc. 2011;21(2):122–133. doi: 10.1007/s10165-010-0366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yabe Y, Kojima T, Kaneko A, Asai N, Kobayakawa T, Ishiguro N. A review of tocilizumab treatment in 122 rheumatoid arthritis patients included in the tsurumai biologics communication registry (TBCR) study. Mod Rheumatol Jpn Rheum Assoc. 2012 doi: 10.1007/s10165-012-0648-3. [DOI] [PubMed] [Google Scholar]
- 25.Nakashima Y, Kondo M, Harada H, Horiuchi T, Ishinishi T, Jojima H, Kuroda K, Miyahara H, Nagamine R, Nakashima H, Otsuka T, Saikawa I, Shono E, Suematsu E, Tsuru T, Wada K, Iwamoto Y. Clinical evaluation of tocilizumab for patients with active rheumatoid arthritis refractory to anti-TNF biologics: tocilizumab in combination with methotrexate. Mod Rheumatol Jpn Rheum Assoc. 2010;20(4):343–352. doi: 10.1007/s10165-010-0290-x. [DOI] [PubMed] [Google Scholar]
- 26.Bykerk VP, Ostor AJ, Alvaro-Gracia J, Pavelka K, Ivorra JA, Graninger W, Bensen W, Nurmohamed MT, Krause A, Bernasconi C, Stancati A, Sibilia J. Tocilizumab in patients with active rheumatoid arthritis and inadequate responses to DMARDs and/or TNF inhibitors: a large, open-label study close to clinical practice. Ann Rheum Dis. 2012;71(12):1950–1954. doi: 10.1136/annrheumdis-2011-201087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mancarella L, Bobbio-Pallavicini F, Ceccarelli F, Falappone PC, Ferrante A, Malesci D, Massara A, Nacci F, Secchi ME, Manganelli S, Salaffi F, Bambara ML, Bombardieri S, Cutolo M, Ferri C, Galeazzi M, Gerli R, Giacomelli R, Grassi W, Lapadula G, Cerinic MM, Montecucco C, Trotta F, Triolo G, Valentini G, Valesini G, Ferraccioli GF, group G Good clinical response, remission, and predictors of remission in rheumatoid arthritis patients treated with tumor necrosis factor-alpha blockers: the GISEA study. J Rheumatol. 2007;34(8):1670–1673. [PubMed] [Google Scholar]
- 28.Takeuchi T, Tanaka Y, Amano K, Hoshi D, Nawata M, Nagasawa H, Sato E, Saito K, Kaneko Y, Fukuyo S, Kurasawa T, Hanami K, Kameda H, Yamanaka H. Clinical, radiographic and functional effectiveness of tocilizumab for rheumatoid arthritis patients–REACTION 52-week study. Rheumatology. 2011;50(10):1908–1915. doi: 10.1093/rheumatology/ker221. [DOI] [PMC free article] [PubMed] [Google Scholar]