Abstract
Context
Child emotional maltreatment can result in lasting immune dysregulation that may be heightened in the context of more recent life stress. Basal cell carcinoma (BCC) is the most common skin cancer, and the immune system plays a prominent role in tumor appearance and progression.
Objective
To address relationships among recent severe life events, childhood parental emotional maltreatment, depression, and messenger RNA (mRNA) coding for immune markers associated with BCC tumor progression/regression.
Setting
University medical center
Design
We collected information about early parent-child experiences, severe life events in the last year as assessed by the Life Events and Difficulties Schedule (LEDS), depression, and mRNA for immune markers associated with BCC tumor progression/regression from BCC tumor patients.
Participants
91 BCC patients (ages 23–92) who had all had a previous BCC tumor.
Main Outcome Measures
The expression of four BCC tumor mRNA markers (CD25, CD3ε, ICAM-1, and CD68) that have been linked to BCC tumor progression/regression were assessed in BCC tumor biopsies.
Results
Both maternal and paternal emotional maltreatment interacted with the occurrence of severe life events to predict the local immune response to the tumor (adjusted p=0.009, p=0.03, respectively). Among BCC patients who had experienced a severe life event within the past year, those who were emotionally maltreated by their mothers (p=0.007) or fathers (p=0.02) as children had a poorer immune response to the BCC tumor. Emotional maltreatment was unrelated to BCC immune responses among those who did not experience a severe life event. Depressive symptoms were not associated with the local tumor immune response.
Conclusions
Troubled early parent-child relationships, in combination with a severe life event in the past year, predicted immune responses to a BCC tumor. The immunoreactivity observed in BCCs and the surrounding stroma reflects an anti-tumor-specific immune response that can be altered by stress.
Stressful events and the negative emotions they generate can dysregulate immunity sufficient to produce clinically significant alterations.1 Acute and chronic stressors can impair vaccine responses, slow wound healing, promote inflammation, and dampen markers of both innate and adaptive immune function.2–6 Those who experienced adverse childhood events are particularly sensitive to subsequent stressors.7–10
Converging evidence suggests that highly stressful events early in life can have long-term consequences for immune system regulation. Childhood maltreatment has been associated with elevated inflammation and higher antibody titers to herpes simplex virus type 1 (reflecting poorer cellular immune function).11–15 Child maltreatment has also been linked to multiple diseases, including cancer; immune dysregulation likely contributes to these effects.16
Skin cancer, the most common cancer in the United States, is more prevalent than all other malignancies combined.18, 19 The incidence of basal cell carcinoma (BCC), the most common skin cancer, has been doubling every 14 years.20 The risk for subsequent BCCs after an initial tumor is substantial, with 44% developing additional lesions within three years.21 Risk factors for the first or index BCC include age, childhood sun exposure, fair skin, and being male; however, subsequent tumors are not reliably related to these variables.21, 22
The immune system plays a prominent role in BCC tumor appearance and progression.23 A significant increase in the expression of CD3ε (total T cells) and CD25 (IL-2 receptor) has been observed in actively regressing tumors compared with those showing no current or past regression.24 Furthermore, increased expression of ICAM-1 and infiltration of CD68+ cells (macrophages) have been described during BCC tumor regression after treatment with imiquimod (a topical cream that enhances the local immune response against BCCs).25, 26 Histological evaluations of excised BCCs show that 50% provide evidence of at least partial regression. The immunoreactivity observed in BCCs and the surrounding stroma reflects an anti-tumor-specific immune response.23
Immunosuppressive treatments clearly increase BCC incidence. Organ transplant recipients have a ten-fold risk compared to the general population.27 However, even much milder alterations in cell-mediated immunity can be consequential. For example, oral glucocorticoid therapy boosts BCC incidence, likely through decreased immunosurveillance.28, 29
Chronic stressors can be a powerful immunomodulator during critical developmental periods, setting the stage for future alterations in skin cancer tumors. Mice that had been subjected to restraint stress subsequently developed UV-induced squamous cell carcinoma more rapidly than non-stressed control mice.30 Furthermore, stressed mice also had a poorer immune response as assessed by messenger RNA (mRNA) immune markers in their tumors compared to controls. Indeed, even well after the stressor ended, the tumors of stressed mice did not regress like those of controls, suggesting that stressors early in development can continue to influence the immune response long after stress exposure.
Early stressful experiences combined with subsequent stress may be particularly detrimental. Seligman and colleagues exposed young rats to inescapable, escapable, or no shock conditions.17 When these rats reached adulthood, they were injected with cancer cells and exposed to one of the three shock conditions again. Rats exposed to inescapable shock when young (i.e., an early environmental stressor) were more likely to develop tumors if also exposed to either shock condition as adults. However, rats that did not experience inescapable shock when they were young were not more likely to develop a tumor when exposed to shock as adults. These data suggest early life stressors may alter the immune response to subsequent stressors, thus decreasing anti-tumor defenses.
Parental emotional maltreatment is a common stressor in childhood. Maltreatment in childhood has been associated with atypical cortisol production throughout the day.31, 32 In adults, troubled early parent-child relationships have been linked to more pronounced stress-induced glucocorticoid production.33, 34 Compared to those who had healthy parent-child relationships, those who had adverse parent-child relationships are more likely to have emotional difficulties when they encounter subsequent stressors.35
Accordingly, this study addressed how parental emotional maltreatment and subsequent stressors might impact the BCC tumor environment. Assessment of the BCC tumor environment included four mRNA markers (i.e. CD25, CD3ε, ICAM-1, and CD68) that have been linked to BCC tumor progression/regression. BCC tumor biopsies were taken from excised tissue. We hypothesized that childhood parental emotional maltreatment would be associated with a poorer local immune response to a BCC tumor, if accompanied by a recent life stressor.
Methods
Participants
Patients who had a newly diagnosed, histologically verified BCC received a letter from their treating dermatologist that described the study. Disqualifying health problems included immunosuppressive therapies or immunological treatments for other medical conditions, another cancer diagnosed within the last five years (except for a prior BCC), or any history of squamous cell carcinoma (SCC) or melanoma. We assessed childhood experiences and mRNA immune markers associated with BCC tumor progression/regression in 91 participants who all had at least one prior BCC tumor. We chose to study individuals who previously had a BCC tumor because we wanted to know what sets the stage for subsequent BCC tumors that develop within three years in almost half of the people following their BCC.21 The presence of the first tumor indicates that people already had enough sun exposure to get a BCC tumor, and met their personal age threshold to start developing them. Accordingly, by studying individuals who had a prior history, we had a stronger basis for investigating psychosocial influences. This study was approved by the local institutional review board, and participants provided written informed consent.
RNA extraction and cDNA synthesis
We examined the expression of four mRNA markers, CD25 (the alpha chain of the interleukin (IL)-2 receptor expressed on activated T-cells and B-cells), CD3ε (a T-cell receptor marker), ICAM-1 (a cell surface glycoprotein on endothelial cells and immune cells), and CD68 (a marker for monocytes/macrophages) in BCC tumor tissue. The mRNA markers encode for proteins that are expressed on various immune cells; CD25 is the alpha chain of the interleukin (IL)-2 receptor expressed on activated T-cells and B-cells, CD3ε is part of the T-cell receptor-CD3 complex that has an important role in signal transduction after antigen recognition by the T-cell, ICAM-1 is a cell surface glycoprotein on endothelial cells and immune cells that functions in the endothelial transmigration of immune cells, and CD68 is a hematopoietic differentiation marker of the monocyte/macrophage lineage.
Four 10 μm thick sections obtained from each paraffin-embedded diagnostic biopsy were immediately deparaffinized with xylene followed by hydration through graded ethanol washes. The tissue was then centrifuged and digested with 100 μl digestion buffer (0.01M Tris pH7.8, 0.005M EDTA, and 0.5% SDS) plus 100 μl of 20 mg/ml proteinase K for 24 hours at 55°C with agitation. Total RNA was extracted by adding 800 μl Trizol Reagent (Life Technologies) with 200 μg/ml glycogen as a carrier. Total RNA was precipitated with 650 μl of 100% isoproponol at −40°C overnight then centrifuged at maximum speed at room temperature. The pellets were washed twice with 75% ice-cold ethanol. The RNA pellet was resuspended in nuclease-free H2O, and quantified using a Nanodrop spectrophotometer. Total RNA (1 μg) was treated with DNase I (Life Technologies), followed by cDNA synthesis using Superscript III RNase H- reverse transcriptase (Life Technologies). The cDNA was stored at −80°C until used for real-time PCR.
Real-time PCR
TaqMan Gene Expression Assays (Applied Biosystems) were used for both internal positive controls and the genes of interest. Using the Taqman Human Endogenous Control Plates (Applied Biosystems) and the GeNorm software (http://medgen.ugent.be/~jvdesomp/genorm/) we selected the following as best-fit internal positive controls for this study: GAPDH (glyceraldehydes-3-phosphate-dehydrogenase; Assay ID: 4326317E) and RPLP0 (large ribosomal protein; Assay ID 4326314E). The expression of CD3ε (Assay ID: Hs99999153_m1), CD25 (Assay ID: 4328847F), CD68 (Assay ID: Hs00154355_m1), and ICAM-1 (Assay ID: Hs99999152_m1) mRNAs were normalized to the geometric average of the CTs for GAPDH and RPLP0. TaqMan Gene Expression Assay Reagents do not detect genomic DNA sequences; therefore, these are specific to mRNA. The mRNA levels between samples were compared using relative real-time PCR with TaqMan fluorogenic probes, TaqMan PCR Reagent Kit and 7300 Real-Time PCR System (Applied Biosystems). Since the complete study involved running samples in several 96-well plates, one cDNA sample from leukocytes previously characterized to express the genes of interest was also included in all of the PCR runs to serve as the plate control for normalization. All the genes of interest were first normalized to the internal positive control, then normalized to the plate control, and the relative expression of mRNA species was calculated using the comparative CT method as described by the manufacturer (see User bulletin #2 Applied Biosystems, P/N 4303859, 1997).36
Psychological and Health Related Measures
Detailed life event interviews were conducted to assess severe life events in the past year. The Life Events and Difficulty Schedule (LEDS) interview assesses the occurrence of over 200 stressful events, with a goal of understanding the environmental stressors, while avoiding some of the biases that could occur from emotional status or coping resources.37 When events are identified, interviewers gather data about a set of contextual factors that might intensify the meaning and implications of the event. For example, loss of employment is likely more stressful for someone who is already in debt and the sole family provider than one who has alternate sources of income. The LEDS has been widely used in psychiatric research, and severe life events predict the occurrence of psychiatric disorders, particularly depression. There are also documented links between the LEDS and illnesses.38 For example, life stressors assessed by the LEDS were linked to increased cold susceptibility.39
Data gathered during interviews were presented to a set of raters who were blind to the participant’s emotional or subjective reaction to the life stressor, SCID data, child maltreatment scores, BCC mRNA levels, and participant’s health. Raters, who were trained by S. Johnson, judged the severity of each life event on a five-point scale ranging from 1 (marked) to 5 (little or no) negativity using the Bedford College dictionaries, which provide example ratings for over 1,000 life events occurring in different life contexts. They also rated whether events might be related to BCC status (e.g., medical complications, lifestyle changes, or other ways in which the cancer diagnosis may have provoked the stressor). Any discrepancies were resolved through group discussion and consensus ratings. Inter-rater reliability was evaluated each month, and was sustained at a level above intraclass correlations of .80 throughout the study. Inconsistencies across raters were reviewed, and ongoing training was conducted to ensure that raters followed guidelines carefully. Our study focused on the presence of at least one severe life event (as defined by severity ratings of 1 or 2) that was independent of the BCC and other illnesses. Although severe events can differ across individuals, the most common severe life events include the loss of a core confidant relationship, death of a family member, marital separation or divorce, or the loss of employment for the primary wage earner in the family. Because our goal was to document that environmental stressors can predict changes in disease status, we focused on events that were not related to illness to ensure that we were capturing stressors that were unrelated to an underlying biological vulnerability.
Although the LEDS takes approximately 10 hours for interviews, transcribing, and rating, the instrument’s well-documented validity suggest that this care is warranted. It has significantly higher reliability and validity compared with self-report measures.37, 38, 40, 41
The depression module of the Structured Clinical Interview for DSM-IV, nonpatient version (SCID-NP) was administered by clinically-trained interviewers to make relatively rapid and valid DSM-IV diagnoses.42 Inter-rater reliability for SCID-NP diagnoses were calculated using randomly selected audiotapes for 20% of the participants. There was greater than 85% agreement for each of the five diagnoses tested, with Cohen’s kappa ranging from 0.64 to 0.69. This substantial interrater agreement was confirmed with McNemar’s test for marginal proportions (p>0.99 for all diagnoses).
A history of parental emotional maltreatment was assessed with the antipathy and neglect subscales of the Childhood Experience of Care and Abuse questionnaire (CECA.Q),43 which examines emotional maltreatment by parents from birth to age 17. All items from the questionnaire were derived from the well-validated CECA interview.44 On a 5-point Likert type scale ranging from (Yes, Definitely) to (No, Not at All), the antipathy (mother and father) scale assessed hostile and rejecting parenting (e.g., “She was very critical of me,” “He made me feel unwanted”), and the neglect subscale assessed the extent to which parents provided material or emotional support for their children (e.g., “he was concerned about my whereabouts,” and “she tried to make me feel better when I was upset”). A one unit increase/decrease in maltreatment reflects a unit increase on the 1 to 5 scale. The neglect scale was highly correlated with the antipathy subscale for both parents (Mothers: r=0.72, p<0.0001; Fathers: r=0.77, p<0.0001). In accord with prior work we combined both scales;45 maternal antipathy and neglect and paternal antipathy and neglect scores were averaged to create an overall mother emotional maltreatment composite, and father emotional maltreatment composite.
The CECA.Q possesses good test-retest reliability and alternate forms of reliability when compared to the CECA interview in both community and clinical populations.46 Furthermore, the antipathy and neglect scales of the CECA.Q converges with other popular measures of childhood adversity, including the Parental Bonding Instrument,47 suggesting this measure possesses good convergent validity. The questionnaire instructs respondents to fill out the CECA.Q in reference to the mother and father figure who they were “with the longest,” or the one they found “most difficult to live with.” Ninety-four percent of participants filled out the questionnaire in reference to their biological mother, and 93% responded in reference to their biological father.
The Center for Epidemiological Studies Depressive Symptoms Scale (CES-D) provided data on current depressive symptoms.48, 49 Studies have shown acceptable test-retest reliability and excellent construct validity.49 It has been widely used in cancer studies.50
The Pittsburgh Sleep Quality Index provided data on sleep quality and sleep disturbances.51
The Older Adults Resources Survey (OARS) Multidimensional Functional Assessment Questionnaire assessed underlying diseases, as well as associated medications.67 Several studies have found excellent agreement between self-reports and hospital or physician records for specific conditions including myocardial infarction, stroke, and diabetes.52, 53
Statistical Methods
The mRNA markers (levels of CD25, CD3ε, ICAM-1, and CD68) were highly correlated, with pairwise Spearman’s correlation coefficients ranging from 0.78 to 0.91 and a Cronbach’s alpha of 0.95 (using log-transformed values). Thus, a single composite mRNA index was created for each subject using z-scores. For each subject, the z-scores for each (base 10) log-transformed mRNA marker were calculated, and these four z-scores were averaged to produce the summary construct. These cell surface markers operate together in vivo; this combined index reflects this coordinated immune response to the BCC tumor. We used this average mRNA z-score as the primary outcome of interest. Secondary analyses used depressive symptoms (CES-D) scores as the outcome.
Linear regression analyses were conducted to evaluate relationships between parental emotional maltreatment, the experience of severe life events, and each outcome. The LEDS life events variable was dichotomized at none versus one or more in the past year. Adjusted models controlled for age and gender. Models for the mRNA z-score index additionally controlled for smoking status, alcohol consumption, comorbid conditions, sleep quality (Pittsburgh Sleep Quality index), and BCC tumor type. Residual plots were examined for all models, and when the normality assumption was found to have failed, outcomes were log-transformed. Alpha was set to 0.05, and two-sided tests were conducted. All analyses were performed in SAS version 9.1.
Results
Participant sample characteristics are summarized in Table 1. There were 48 males and 43 females in the sample, the majority of whom had at least some college education (n=72, 79%). This is a cancer that occurs among those with fair skin, and all participants self described themselves as white.54 The average age at interview was 58.2 years (SD=13.5). The 91 participants were generally healthy with few comorbid conditions: n=4 (4%), asthma, n=5 (5%), emphysema, n=2 (2%), heart disease, n=8 (9%), hypertension, n=27 (30%), kidney disease, n=2 (2%), liver disease, n=1 (1%), stroke, n=2 (2%) and thyroid disease, n=4 (4%). Comorbid conditions did not include psychiatric conditions. Neither father nor mother’s emotional maltreatment was associated with age (Mothers: r=.14, p=0.20, Fathers: r=0.17, p=0.11). Parental maltreatment was not associated with the experience of any severe life events (Mothers: r=0.10, p=0.35, Fathers: r=0.17, p=0.10).
Table 1.
Characteristics of the Study Sample (n=91)
| Demographics | - | - |
| Female sex, Number (%) | 43 | (47) |
| White race, Number (%) | 91 | (100) |
| Education, Number (%) | ||
| High school or less | 19 | (21) |
| Some college | 26 | (29) |
| College degree | 46 | (51) |
| Marital status, Number (%) | ||
| Single | 7 | (8) |
| Married | 69 | (76) |
| Common law | 2 | (2) |
| Divorced | 9 | (10) |
| Widowed | 4 | (4) |
| Age (years) | ||
| Mean (SD) | 58.2 | (13.5) |
| Range | 23–92 | |
| Self-Report Measures | ||
| Number of LEDS events, Number (%) | ||
| 0 | 70 | (77) |
| 1 | 17 | (19) |
| 2+ | 4 | (4) |
| Months from most recent LEDS event to biopsy (n=21) | ||
| Mean (SD) | 5.5 | (3.2) |
| Range | 0.1–11 | |
| History of major depression, Number (%) | 30 | (33) |
| Center for Epidemiological Studies Depression Scale (CES-D) | ||
| Mean (SD) | 7.8 | (9.0) |
| Median (IQR) | 6 | (2–9) |
| Range | 0–49 | |
| Pittsburgh Sleep Quality Index (PSQ) | ||
| Mean (SD) | 4.9 | (2.7) |
| Range | 0–15 | |
| Any comorbid conditions, Number (%) | 65 | (71) |
| Current smoker, Number (%) | 10 | (11) |
| Consume alcohol, Number (%) | 46 | (51) |
| Emotional Maltreatment (CECA.Q) | ||
| Mother | ||
| Mean (SD) | 12.6 | (5.5) |
| Range | 8–31.5 | |
| Father | ||
| Mean (SD) | 14.4 | (6.5) |
| Range | 8–31 | |
| mRNA Levels | ||
| CD25 | ||
| Median (IQR) | 38 | (12–103) |
| Range | 2.5–1284 | |
| CD3ε | ||
| Median (IQR) | 0.23 | (0.10–0.56) |
| Range | 0.020–16 | |
| ICAM-1 | ||
| Median (IQR) | 0.24 | (0.13–0.50) |
| Range | 0.015–3.5 | |
| CD68 | ||
| Median (IQR) | 1.5 | (0.56–3.6) |
| Range | 0.11–38 | |
| Clinical Characteristics | ||
| BCC tumor type, Number (%) | ||
| Nodular | 27 | (30) |
| Superficial | 15 | (16) |
| Mixed type | 49 | (54) |
| BCC site, Number (%) | ||
| Head and neck | 50 | (55) |
| Trunk | 17 | (19) |
| Upper limbs | 15 | (16) |
| Lower limbs | 3 | (3) |
| Multi-site | 6 | (7) |
| Skin type - Burning, Number (%) | ||
| Always burn | 11 | (12) |
| Usually burn | 17 | (19) |
| Burn moderately | 31 | (34) |
| Burn minimally | 21 | (23) |
| Rarely/never burn | 11 | (12) |
| Number of sunburns before age 19, Number (%) | ||
| 0 | 4 | (4) |
| 1–9 | 44 | (48) |
| 10–19 | 23 | (25) |
| 20+ | 19 | (21) |
| Unknown | 1 | (1) |
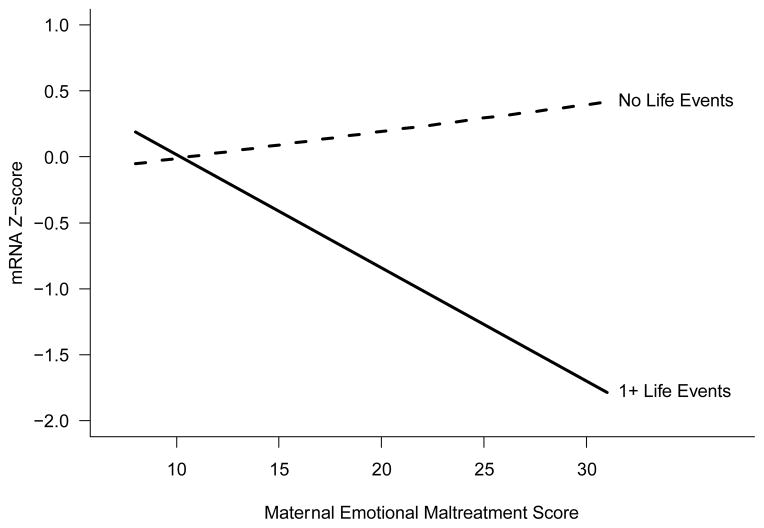
Table 2 summarizes the association of mother’s emotional maltreatment and life events with the average mRNA z-score. In the unadjusted model there was a significant interaction between mother’s emotional maltreatment and the experience of any severe life events, and this interaction persisted in the adjusted model (unadjusted p=0.02, adjusted p=0.009). The experience of severe life events led to a negative association between mother’s emotional maltreatment and mRNA z-score. In the adjusted model, a one unit increase in mother’s emotional maltreatment was not significantly associated with mRNA z-score for participants with no life events (B=0.020, 95% CI: −0.024 to 0.065, p=0.37). However, for participants with one or more severe life events, a one unit increase in maternal emotional maltreatment score led to a 0.086 point decrease in mRNA z-score (95% CI: −0.15 to −0.017, p=0.02). This interaction is illustrated in Figure 1.
Table 2.
Association of mother’s emotional maltreatment and LEDS events with average mRNA z-score. Results from linear regression models (n=91).
| Variable | Unadjusted Model | Adjusted Model | ||||
|---|---|---|---|---|---|---|
| R2 = 0.08 | R2 = 0.28 | |||||
| Estimate | (95% CI) | p-value | Estimate | (95% CI) | p-value | |
| Mom’s emotional maltreatment | 0.0077 | (−0.033, 0.048) | 0.71 | 0.020 | (−0.024, 0.065) | 0.37 |
| Any LEDS Events | 0.98 | (−0.099, 2.1) | 0.07 | 1.1 | (0.044, 2.1) | 0.04 |
| Mom’s emotional maltreatment x Any LEDS Events | −0.10 | (−0.18, −0.019) | 0.02 | −0.11 | (−0.18, −0.028) | 0.009 |
| Dad’s emotional maltreatment | −0.013 | (−0.046, 0.021) | 0.45 | |||
| Age (years) | −0.0019 | (−0.017, 0.013) | 0.81 | |||
| Female (ref: male) | −0.45 | (−0.86, −0.038) | 0.03 | |||
| Comorbid conditions | −0.060 | (−0.28, 0.16) | 0.59 | |||
| Smoker (ref: non-smoker) | −0.037 | (−0.63, 0.56) | 0.90 | |||
| Drinks alcohol (ref: non-drinker) | 0.037 | (−0.35, 0.42) | 0.85 | |||
| Pittsburgh Sleep Quality Index | 0.034 | (−0.036, 0.11) | 0.34 | |||
| Tumor type (ref: Mixed) | ||||||
| Nodular | −0.81 | (−1.2, −0.40) | 0.0002 | |||
| Superficial | −0.38 | (−0.90, 0.14) | 0.15 | |||
Figure 1.
Interaction plot illustrating the relationship between mother’s emotional maltreatment, severe life events, and composite mRNA z-score, from adjusted model in Table 2. Values of all other variables in the model are set to their sample means.
Table 3 summarizes a similar interaction between father’s emotional maltreatment and life events in predicting mRNA z-score (unadjusted p=0.02, adjusted p=0.03). In the adjusted model, a one unit increase in father’s emotional maltreatment was significantly associated with a 0.063 point decrease in mRNA z-score (95% CI: −0.12 to −0.010, p=0.02) for participants who had experienced any severe life events. Participants who had not experienced any life events did not have a significant relationship between father’s emotional maltreatment and mRNA z-score (B=0.009, 95% CI: −0.031 to 0.050, p=0.65).
Table 3.
Association of father’s emotional maltreatment and LEDS events with average mRNA z-score. Results from linear regression models (n=91).
| Variable | Unadjusted Model | Adjusted Model | ||||
|---|---|---|---|---|---|---|
| R2 = 0.08 | R2 = 0.26 | |||||
| Estimate | (95% CI) | p-value | Estimate | (95% CI) | p-value | |
| Dad’s emotional maltreatment | 0.010 | (−0.027, 0.048) | 0.59 | 0.0094 | (−0.031, 0.050) | 0.65 |
| Any LEDS Events | 1.1 | (−0.073, 2.2) | 0.07 | 1.0 | (−0.14, 2.2) | 0.08 |
| Dad’s emotional maltreatment x Any LEDS Events | −0.076 | (−0.14, −0.011) | 0.02 | −0.073 | (−0.14, −0.0086) | 0.03 |
| Mom’s emotional maltreatment | −0.010 | (−0.049, 0.030) | 0.63 | |||
| Age (years) | −0.0025 | (−0.018, 0.013) | 0.75 | |||
| Female (ref: male) | −0.39 | (−0.80, 0.023) | 0.06 | |||
| Comorbid conditions | −0.0090 | (−0.23, 0.21) | 0.94 | |||
| Smoker (ref: non-smoker) | −0.090 | (−0.70, 0.52) | 0.77 | |||
| Drinks alcohol (ref: non-drinker) | −0.076 | (−0.46, 0.31) | 0.69 | |||
| Pittsburgh Sleep Quality Index | 0.023 | (−0.049, 0.10) | 0.52 | |||
| Tumor type (ref: Mixed) | ||||||
| Nodular | −0.80 | (−1.2, −0.38) | 0.0003 | |||
| Superficial | −0.42 | (−0.94, 0.11) | 0.12 | |||
Additional analyses evaluated the effect of removing father’s emotional maltreatment from the adjusted models in Table 2 and mother’s emotional maltreatment from the adjusted model in Table 3 because father’s and mother’s emotional maltreatment were moderately correlated (r=0.42, p<0.0001). Removing paternal emotional maltreatment had negligible effects on estimates for maternal emotional maltreatment and vice versa in both mRNA z-score models. We also considered an interaction between mother’s and father’s emotional maltreatment to assess any added effect when both parents emotionally maltreated their children; results were non-significant.
Post-hoc analyses were performed by repeating the models in Tables 2 and 3 using each of the individual mRNA markers (log-transformed) as the outcome (8 total separate adjusted regression models). Results matched the results of the composite models and were consistent across all mRNA variables. The emotional maltreatment by LEDS interaction significantly predicted each individual mRNA variable in all but one model; the only non-significant interaction was in the model using paternal emotional maltreatment predicting CD3ε, and the effect was in the expected direction (p=0.13).
A separate linear regression model to assess the relationship between parental emotional maltreatment, severe life events, and CES-D is presented in Table 4. The interaction between parental emotional maltreatment and life events predicting depressive symptoms was not significant, so we dropped the interaction term from Table 4 as is the standard convention.55 Father’s emotional maltreatment was positively associated with depressive symptoms. A one unit increase in father’s emotional maltreatment was associated with a 4.0% increase in the CES-D score (p=0.03). Neither mother’s emotional maltreatment nor severe life events were significantly associated with CES-D, though slope estimates were in the expected direction. A history of major depression (as assessed by the SCID) was not associated with mother’s emotional maltreatment (Wilcoxon rank sum p=0.80), father’s emotional maltreatment (Wilcoxon rank sum p=0.08), or severe life events (chi-square p=0.27).
Table 4.
Association of maternal and paternal emotional maltreatment and LEDS events with depressive symptoms (CES-D*). Results from a linear regression model (n=91).
| Variable | Adjusted Model | ||
|---|---|---|---|
| R2 = 0.15 | |||
| Estimate | (95% CI) | p-value | |
| Age (years) | −0.005 | (−0.020, 0.010) | 0.51 |
| Female (ref: male) | 0.25 | (−0.16, 0.66) | 0.24 |
| Any LEDS Events | 0.32 | (−0.16, 0.79) | 0.19 |
| Mom’s emotional maltreatment | 0.004 | (−0.037, 0.044) | 0.85 |
| Dad’s emotional maltreatment | 0.039 | (0.0043, 0.074) | 0.03 |
Outcome (CES-D) is natural log transformed
The CES-D scores were not associated with mRNA z-score in unadjusted or adjusted models and did not mediate the effects of parental emotional maltreatment on mRNA z-score (results not shown). The same conclusions held when we looked at the effect of a history of major depression (as assessed by the SCID) in place of CES-D. Adding CES-D or history of major depression as a predictor did not change the point estimates or significance of father’s or mother’s emotional maltreatment or life events or their interaction in the models presented in Tables 2 and 3. In the CES-D model (Table 4), removing maternal emotional maltreatment resulted in a small (4%) increase in the point estimate for paternal emotional maltreatment, with a subsequent decrease in the p-value to p=0.01. Adding sunburn history, skin type, and/or time since life events as predictors did not change the point estimates or significance levels of our results presented in Tables 2 and 3. If we included illness related severe events, only two additional people fell into the severe life event group; all of the analyses remained the same (i.e. point estimates did not change substantially, and significance levels were identical). The number of previous BCCs did not predict differences in mRNA levels or alter our findings. Finally, the pattern of results remained the same when the antipathy and neglect subscales of the CECA.Q were assessed independently.
Discussion
Our results show that among BCC patients who experienced a severe stressor in the past year, those who were emotionally maltreated by their mothers or fathers as children were more likely to have poorer immune responses as reflected in lower levels of mRNA for CD25, CD3ε, ICAM-1, and CD68 to their BCC tumors. Being emotionally maltreated by one’s father was also linked to higher depressive symptoms. However, depressive symptoms and a history of depression, per se, were not directly linked to the BCC immune responses related to the BCC tumor. Females had a poorer immune response to the BCC tumor than males; most of the prior literature on BCC immune responses has not analyzed responses by sex, so it is unclear if this is typical or not.
The immune system plays a prominent role in response to BCC tumors because they are immunogenic, unlike many other common cancers that do not show the same responsiveness to the immune system.23 Studies addressing control of BCC tumor progression show that inflammatory cells both in the peritumoral milieu and those that infiltrate BCC tumors have important roles.24, 56, 57 Although key risk factors for a person’s first BCC include childhood sun exposure, fair skin, and being male, subsequent tumors are not reliably related to these variables.21, 22 Psychological stress may play an important role in the tumor environment for this immunogenic tumor, and have important implications for subsequent BCC tumors. Future studies should further investigate the clinical implication of the current findings.
Mechanistically, troubled parent-child relationships can alter the set point for the stress response system. Individuals who had adverse childhood relationships are more physiologically reactive to stress as adults compared to those that did not.58 As described previously, early life adversity has been linked to subsequent dysregulated immune function in adults, and physiological responsiveness to subsequent stressors.11–14, 59
We examined mRNAs encoding for proteins that are expressed on various immune cells and have been implicated in their function; mRNAs carry the information that specify the properties of the protein end product. The expression of mRNAs for CD3ε, CD68, CD25, and ICAM-1 indicates a coordinated immune response to the BCC tumor. In general, BCC tumor tissue has higher levels of mRNA immune markers than normal tissue because the immune system is responding to the tumor. The presence of these markers in BCC tumors is suggestive of infiltration of immune cells as part of the anti-tumor immune response.24, 56, 60–63
This study extends animal work demonstrating that stress, especially early in life, can impact tumor growth and progression.17 Our findings complement work demonstrating that early life stress increases vulnerability to tumor development when exposed to an additional stressor in adulthood.17
Our findings also complement studies from Lutgendorf and colleagues that have addressed the relationship between psychosocial factors and immune markers within the tumor environment.63, 64 Ovarian cancer patients who were more distressed had poorer natural killer (NK) cell activity in tumor-infiltrating lymphocytes than those who were less distressed.64, 65 Furthermore, those who had more social support had greater NK cell activity in tumor-infiltrating lymphocytes than those who had less support. The current study is the first to show that early life stressors can also influence the tumor environment in humans.
Our work may have broader implications for other cancers. In a large prospective study with over one million participants, those with nonmalignant skin cancers were 20–30% more likely to die from other noncutaneous cancers; the relative risk for mortality from other cancers was 1.30 in men and 1.26 in women.66 A recent meta-analysis reported that individuals who were more stress reactive were at greater risk for cancer mortality those who were not.67 Accordingly, BCCs may have some prognostic value for broader cancer risks.
These findings could also be relevant to recent work linking child maltreatment with cancer incidence. One study demonstrated a dose-response relationship between the number of exposures to abuse or household dysfunction during childhood and cancer incidence.16 In other work, those who were physically abused as children had 49% higher odds of having a cancer diagnosis than those who were not abused.68 The findings remained after adjusting for health behaviors such as smoking and exercise; immune dysregulation may have contributed to this link.68
One major strength of this study was the use of the LEDS to assess life events. The LEDS allowed us to exclude life events that were related to underlying medical issues. Furthermore, because the LEDS uses objective ratings of stress severity, biases related to depressive symptoms do not influence people’s ratings of their life stress. Accordingly, the LEDS allowed us to assess links between severe stressors and immune function with control over biased reporting of stress related to depressive symptoms and poor health.
Despite the potential importance of understanding how early adverse experiences influence cancer risk, there are limitations that should be acknowledged. Our participants could have been biased when reporting the degree to which their parents emotionally maltreated them as children. However, adults generally underreport rather than over report childhood abuse and neglect.69 In addition, there are many other forms of child adversity not assessed in the current study that could also impact immune function such as low socioeconomic status.70 Future work should take these factors into account as well. Another limitation is our exclusive focus on BCC tumors. We chose this disease because of the known immunogenic properties of BCC tumors, but future work assessing other types of tumors will be important in order to generalize our findings to cancer more broadly. Finally, our sample was exclusively white, which is not surprising given the nature of the disease.
BCC is a substantial public health concern; it is highly prevalent and carries risks for scarring and disfigurement.21 Furthermore, it may be prognostic for other cancers. A better understanding of the factors that contribute to BCC incidence and reoccurrence is clinically relevant. Troubled early parental experiences are linked to greater stress reactivity in adulthood, and poorer immune regulation. This is the first study to show troubled early parental experiences, in combination with a severe life event in the past year, predict local immune responses to a BCC tumor. These data complement and expand growing evidence that the consequences of early parental experiences extend well beyond childhood.
Acknowledgments
This study was supported in part by a grant from the National Cancer Institute CA100243 (RG), The Gilbert and Kathryn Mitchell Endowment (RG), the Ohio State University Comprehensive Cancer Center, CA16058, and an American Cancer Society Postdoctoral Fellowship Grant PF-11-007-01-CPPB awarded to the first author. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We would also like to thank Dr. Gailen Marshall for his very helpful suggestions.
Footnotes
The corresponding author had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: Implications for health. Nature Reviews Immunology. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 2.Kiecolt-Glaser JK, Marucha PT, Malarkey WB, Mercado AM, Glaser R. Slowing of wound healing by psychological stress. Lancet. 1995;346:1194–1196. doi: 10.1016/s0140-6736(95)92899-5. [DOI] [PubMed] [Google Scholar]
- 3.Kiecolt-Glaser JK, Glaser R, Gravenstein S, Malarkey WB, Sheridan J. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proceedings of the National Academy of Sciences. 1996;93(7):3043. doi: 10.1073/pnas.93.7.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiecolt-Glaser JK, Preacher K, MacCallum R, Atkinson C, Malarkey W, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(15):9090. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ironson G, Wynings C, Schneiderman N, Baum A, Rodriguez M, Greenwood D, Benight C, Antoni M, LaPerriere A, Huang HS. Posttraumatic stress symptoms, intrusive thoughts, loss, and immune function after Hurricane Andrew. Psychosomatic Medicine. 1997;59(2):128. doi: 10.1097/00006842-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Glaser R, Kennedy S, Lafuse WP, Bonneau RH, Speicher C, Kiecolt-Glaser JK. Psychological stress-induced modulation of IL-2 receptor gene expression and IL-2 production in peripheral blood leukocytes. Archives of General Psychiatry. 1990;47:707–712. doi: 10.1001/archpsyc.1990.01810200015002. [DOI] [PubMed] [Google Scholar]
- 7.Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. American Journal of Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- 8.Luecken LJ. Childhood attachment and loss experiences affect adult cardiovascular and cortisol function. Psychosomatic Medicine. 1998;60(6):765. doi: 10.1097/00006842-199811000-00021. [DOI] [PubMed] [Google Scholar]
- 9.Luecken LJ, Lemery KS. Early caregiving and physiological stress responses. Clinical Psychology Review. 2004;24(2):171–191. doi: 10.1016/j.cpr.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Pace TWW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. American Journal of Psychiatry. 2006;163(9):1630. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 11.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences. 2007;104(4):1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiecolt-Glaser J, Gouin J, Weng N, Malarkey W, Beversdorf D, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosomatic Medicine. 2011;73(1):16. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danese A, Moffitt TE, Harrington HL, Milne BJ, Polanczyk G, Pariante CM, Poulton R, Caspi A. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Archives of Pediatrics and Adolescent Medicine. 2009;163(12):1135. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biological psychiatry. 2006;60(8):819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Shirtcliff EA, Coe CL, Pollak SD. Early childhood stress is associated with elevated antibody levels to herpes simplex virus type 1. Proceedings of the National Academy of Sciences. 2009 Feb 24;106(8):2963–2967. doi: 10.1073/pnas.0806660106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felitti M, Vincent J, Anda M, Robert F, Nordenberg M. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The adverse childhood experiences (ACE) study. American Journal of Preventive Medicine. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 17.Seligman MEP, Visintainer MA. Tumor rejection and early experience of uncontrollable shock in the rat. In: Brush FR, Overmier JB, Solomon RL, editors. Affect, conditioning, and cognition: essays on the determinants of behavior. Hillsdale, N.J: Lawrence Erlbaum Associates; 1985. pp. 203–210. [Google Scholar]
- 18.Otley CC. Immunosuppression and skin cancer. Archives of Dermatology. 2002;138:827–828. doi: 10.1001/archderm.138.6.827. [DOI] [PubMed] [Google Scholar]
- 19.Ricotti C, Bouzari N, Agadi A, Cockerell CJ. Malignant skin neoplasms. Medical Clinics of North America. 2009;93(6):1241–1264. doi: 10.1016/j.mcna.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Leman JA, McHenry PM. Basal cell carcinoma: Still an enigma. Archives of Dermatology. 2001;137:1239–1240. doi: 10.1001/archderm.137.9.1239. [DOI] [PubMed] [Google Scholar]
- 21.Marcil I, Stern RS. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer. Archives of Dermatology. 2000;136:1524–1530. doi: 10.1001/archderm.136.12.1524. [DOI] [PubMed] [Google Scholar]
- 22.Ramachandran S, Fryer AA, Smith AG, Lear JT, Bowers B, Griffiths CEM, Jones PW, Strange RC. Basal cell carcinoma. Cancer. 2000;89(5):1012–1018. doi: 10.1002/1097-0142(20000901)89:5<1012::aid-cncr10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 23.Urosevic M, Dummer R. Immunotherapy for nonmelanoma skin cancer. Cancer. 2002;94:477–485. doi: 10.1002/cncr.10178. [DOI] [PubMed] [Google Scholar]
- 24.Hunt MJ, Halliday GM, Weedon D, Cooke BE, Barnetson RS. Regression in basal cell carcinoma: an immunohistochemical analysis. British Journal of Dermatology. 1994;130(Jan):1–8. doi: 10.1111/j.1365-2133.1994.tb06873.x. [DOI] [PubMed] [Google Scholar]
- 25.Urosevic M, Maier T, Benninghoff B, Slade H, Burg G, Dummer R. Mechanisms underlying imiquimod-induced regression of basal cell carcinoma in vivo. Archives of Dermatology. 2003;139(10):1325–1332. doi: 10.1001/archderm.139.10.1325. [DOI] [PubMed] [Google Scholar]
- 26.De Giorgi V, Salvini C, Chiarugi A, Paglierani M, Maio V, Nicoletti P, Santucci M, Carli P, Massi D. In vivo characterization of the inflammatory infiltrate and apoptotic status in imiquimod-treated basal cell carcinoma. International Journal of Dermatology. 2009;48(3):312–321. doi: 10.1111/j.1365-4632.2009.03916.x. [DOI] [PubMed] [Google Scholar]
- 27.Wong C, Strange R, Lear J. Basal cell carcinoma. British Medical Journal. 2003;327(7418):794. doi: 10.1136/bmj.327.7418.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karagas MR, Cushing GLJ, Greenberg ER, Mott LA, Spencer SK, Nierenberg DW. Non-melanoma skin cancers and glucocorticoid therapy. British Journal of Cancer. 2001;85(5):686–686. doi: 10.1054/bjoc.2001.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen AØ, Thomsen HF, Engebjerg M, Olesen AB, Friis S, Karagas M, Sørensen HT. Use of oral glucocorticoids and risk of skin cancer and non-Hodgkin’s lymphoma: a population-based case–control study. British Journal of Cancer. 2008;100(1):200–205. doi: 10.1038/sj.bjc.6604796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saul AN, Oberyszyn TM, Daugherty C, Kusewitt D, Jones S, Jewell S, Malarkey WB, Lehman A, Lemeshow S, Dhabhar FS. Chronic stress and susceptibility to skin cancer. Journal of the National Cancer Institute. 2005;97:1760–1767. doi: 10.1093/jnci/dji401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dozier M, Manni M, Gordon MK, Peloso E, Gunnar MR, Stovall-McClough KC, Eldreth D, Levine S. Foster children’s diurnal production of cortisol: An exploratory study. Child Maltreatment. 2006;11(2):189. doi: 10.1177/1077559505285779. [DOI] [PubMed] [Google Scholar]
- 32.Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Development and Psychopathology. 2001;13(03):677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- 33.Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 34.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33(6):693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Dougherty LR, Klein DN, Davila J. A growth curve analysis of the course of dysthymic disorder: The effects of chronic stress and moderation by adverse parent-child relationships and family history. Journal of consulting and clinical psychology. 2004;72(6):1012. doi: 10.1037/0022-006X.72.6.1012. [DOI] [PubMed] [Google Scholar]
- 36.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 37.Brown GW, Harris TO. The Bedford College Life–Events and Difficulty Schedule: Directory of severity for long–term difficulties. Bedford College, University of London; London: 1979. [Google Scholar]
- 38.Brown GW, Harris TO. Life events and illness. The Guilford Press; 1989. [Google Scholar]
- 39.Cohen S, Frank E, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM., Jr Types of stressors that increase susceptibility to the common cold in healthy adults. Health Psychology. 1998;17(3):214. doi: 10.1037//0278-6133.17.3.214. [DOI] [PubMed] [Google Scholar]
- 40.McQuaid JR, Monroe SM, Roberts JR, Johnson SL, Garamoni GL, Kupfer DJ, Frank E. Toward the standardization of life stress assessment: Definitional discrepancies and inconsistencies in methods. Stress Medicine. 1992;8(1):47–56. [Google Scholar]
- 41.Gorman D. A review of studies comparing checklist and interview methods of data collection in life event research. Behavioral Medicine. 1993;19(2):66–73. doi: 10.1080/08964289.1993.9937567. [DOI] [PubMed] [Google Scholar]
- 42.First MB, Spitzer R, Williams J. Structured clinical interview for DSM-IV research version. New York: Biometrics Research Department, New York Psychiatric Institute; 1995. [Google Scholar]
- 43.Bifulco A, Bernazzani O, Moran PM, Jacobs C. The childhood experience of care and abuse questionnaire (CECA.Q): Validation in a community series. British Journal of Clinical Psychology. 2005;44:1–20. doi: 10.1348/014466505X35344. [DOI] [PubMed] [Google Scholar]
- 44.Bifulco A, Brown GW, Harris TO. Childhood experience of care and abuse (CECA): A retrospective interview measure. Journal of Child Psychology and Psychiatry. 1994;35:1419–1435. doi: 10.1111/j.1469-7610.1994.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 45.Lumley MN, Harkness KL. Childhood maltreatment and depressotypic cognitive organization. Cognitive Therapy and Research. 2009;33(5):511–522. [Google Scholar]
- 46.Smith N, Lam D, Bifulco A, Checkley S. Childhood experience of care and abuse questionnaire (CECA. Q) Social Psychiatry and Psychiatric Epidemiology. 2002;37(12):572–579. doi: 10.1007/s00127-002-0589-9. [DOI] [PubMed] [Google Scholar]
- 47.Parker G. The parental bonding instrument. Social Psychiatry and Psychiatric Epidemiology. 1990;25(6):281–282. doi: 10.1007/BF00782881. [DOI] [PubMed] [Google Scholar]
- 48.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 49.Basco MR, Krebaum SR, Rush AJ. Outcome measures of depression. In: Strupp HH, Horowitz LM, Lambert MJ, editors. Measuring patient changes in mood, anxiety, and personality disorders. Washington D. C: American Psychological Association; 1997. pp. 207–245. [Google Scholar]
- 50.Demark-Wahnefried W, Morey MC, Clipp EC, Pieper CF, Snyder DC, Sloane R, Cohen HJ. Leading the way in exercise and diet (Project LEAD): Intervening to improve function among older breast and prostate cancer survivors. Controlled Clinical Trials. 2003;24(2):206–223. doi: 10.1016/s0197-2456(02)00266-0. [DOI] [PubMed] [Google Scholar]
- 51.Buysse DJ, Reynolds CFI, Monk TH, Berman SB, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 52.Bush TL, Miller SR, Golden AL, Hale WE. Self-report and medical record report agreement of selected medical conditions in the elderly. American Journal of Public Health. 1989;79:1554–1556. doi: 10.2105/ajph.79.11.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kehoe R, Wu SY, Leske MC, Chylack LT. Comparing self-reported and physician reported medical history. American Journal of Epidemiology. 1994;139:813–818. doi: 10.1093/oxfordjournals.aje.a117078. [DOI] [PubMed] [Google Scholar]
- 54.Rubin AI, Chen EH, Ratner D. Current concepts - Basal-cell carcinoma. New England Journal of Medicine. 2005 Nov 24;353(21):2262–2269. doi: 10.1056/NEJMra044151. [DOI] [PubMed] [Google Scholar]
- 55.Kleinbaum D, Kupper L, Muller K, Nizam A. Chapter 11 Confounding and interaction in regression. In: Kleinbaum DG, Kupper LL, Muller KE, Nizam A, editors. Applied Regression Analysis and Other Multivariable Methods. 3. Pacific Grove, CA, USA: Brooks/Cole Publishing Company; 1998. pp. 186–211. [Google Scholar]
- 56.Kaur P, Mulvaney M, Carlson JA. Basal cell carcinoma progression correlates with host immune response and stromal alterations: A histologic analysis. American Journal of Dermatopathology Aug. 2006;28(4):293–307. doi: 10.1097/00000372-200608000-00002. [DOI] [PubMed] [Google Scholar]
- 57.Schlecker C, Pierer G, Haug M. Spontaneous regression of two giant basal cell carcinomas in a single patient after incomplete excision. Tumori. 2009;95(2):258–263. doi: 10.1177/030089160909500223. [DOI] [PubMed] [Google Scholar]
- 58.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 59.Tyrka AR, Price LH, Kao HT, Porton B, Marsella SA, Carpenter LL. Childhood maltreatment and telomere shortening: preliminary support for an effect of early stress on cellular aging. Biological Psychiatry. 2010 Mar 15;67(6):531–534. doi: 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Urosevic M, Maier T, Benninghoff B, Slade H, Burg G, Dummer R. Mechanisms underlying imiquimod-induced regression of basal cell carcinoma in vivo. Archives of Dermatology. 2003;139:1325–1332. doi: 10.1001/archderm.139.10.1325. [DOI] [PubMed] [Google Scholar]
- 61.De Giorgi V, Salvini C, Chiarugi A, Paglierani M, Maio V, Nicoletti P, Santucci M, Carli P, Massi D. In vivo characterization of the inflammatory infiltrate and apoptotic status in imiquimod treated basal cell carcinoma. International Journal of Dermatology. 2009;48(3):312–321. doi: 10.1111/j.1365-4632.2009.03916.x. [DOI] [PubMed] [Google Scholar]
- 62.Pollack SV, Goslen JB, Sherertz EF, Jegasothy BV. The biology of basal cell carcinoma: A review. Journal of the American Academy of Dermatology. 1982;7(5):569–577. doi: 10.1016/s0190-9622(82)70136-7. [DOI] [PubMed] [Google Scholar]
- 63.Howell BG, Solish N, Lu C, Watanabe H, Mamelak AJ, Freed I, Wang B, Sauder DN. Microarray profiles of human basal cell carcinoma: Insights into tumor growth and behavior. Journal of Dermatological Science. 2005;39(1):39–51. doi: 10.1016/j.jdermsci.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 64.Lutgendorf SK, Sood AK, Anderson B, McGinn S, Maiseri H, Dao M, Sorosky JI, De Geest K, Ritchie J, Lubaroff DM. Social support, psychological distress, and natural killer cell activity in ovarian cancer. Journal of Clinical Oncology. 2005 Oct 1;23(28):7105–7113. doi: 10.1200/JCO.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 65.Lutgendorf SK, Lamkin DM, DeGeest K, Anderson B, Dao M, McGinn S, Zimmerman B, Maiseri H, Sood AK, Lubaroff DM. Depressed and anxious mood and T-cell cytokine expressing populations in ovarian cancer patients. Brain Behav Immun Aug. 2008;22(6):890–900. doi: 10.1016/j.bbi.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kahn HS, Tatham LM, Patel AV, Thun MJ, Heath CW., Jr Increased cancer mortality following a history of nonmelanoma skin cancer. Journal of the American Medical Association. 1998;280:910–912. doi: 10.1001/jama.280.10.910. [DOI] [PubMed] [Google Scholar]
- 67.Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nature Clinical Practice Oncology. 2008;5(8):466–475. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- 68.Fuller Thomson E, Brennenstuhl S. Making a link between childhood physical abuse and cancer. Cancer. 2009;115(14):3341–3350. doi: 10.1002/cncr.24372. [DOI] [PubMed] [Google Scholar]
- 69.Dill DL, Chu JA, Grob MC, Eisen SV. The reliability of abuse history reports: A comparison of two inquiry formats. Comprehensive Psychiatry. 1991;32(2):166–169. doi: 10.1016/0010-440x(91)90009-2. [DOI] [PubMed] [Google Scholar]
- 70.Chen E, Miller G, Kobor M, Cole S. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Molecular Psychiatry. 2011;16:729–737. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]