Abstract
Female space use can have important fitness consequences, which are likely due to differential access to food resources. Many studies have explored spatial competition in solitary species, but little is known about how individuals in social species compete over shared space. In this study, we investigate spatial patterns of aggression among female East African chimpanzees, Pan troglodytes schweinfurthii. This species provides an excellent opportunity to study spatial competition since (1) female chimpanzees occupy overlapping core areas (small areas of the community range in which individuals concentrate their space use) and (2) female core area quality is correlated with reproductive success, suggesting that females compete over long-term access to core areas. Here, we examine how female aggression towards other females varies inside and outside individual female core areas during a 14-year period at Gombe National Park, Tanzania. Overall, females showed higher rates of aggression inside than outside their own core areas. This pattern was driven by spatial variation in aggression in nonfeeding contexts. While food-related aggression did not vary spatially, females were more aggressive in nonfeeding contexts inside their core areas than they were outside their core areas. These results suggest that female chimpanzees follow a mixed strategy in which they compete for long-term access to resources in their core areas as well as for immediate access to food throughout the community range.
Keywords: aggression, chimpanzee, foraging, Pan troglodytes schweinfurthii, resource competition, space use, territoriality
Spatial competition is generally studied in the context of territory defence, in which an individual or group of individuals excludes competitors from a particular range (Maher & Lott, 1995). Excluding competitors can increase reproductive fitness (Stamps, 1994), and the adaptive value of territoriality has been documented in a wide variety of vertebrate taxa (reviewed in Maher & Lott, 2000). Territoriality can occur at an individual level (Eurasian red squirrel, Sciurus vulgaris: Wauters, Lens, & Dhont, 1995; green anoles, Anolis carolinensis: Leuck, 1995; bank voles, Clethrionomys glareolus: Koskela, Mappes, & Ylönen, 1997; Rich Mountain salamanders, Plethodon ouachitae: Anthony, Wicknick, & Jaeger, 1997; brown trout, Salmo trutta: Johnsson, Carlsson, & Sundström, 2000), or at the group level (e.g. cichlids, Neolamprologus pulcher: Taborsky, 1984; gibbons, Hylobates lar: Brockelman & Srikosamatara, 1984; golden lion tamarins, Leontopithecus rosalia: Peres, 1989; fiddler crabs, Uca annulipes: Milner, Booksmythe, Jennions, & Backwell, 2010).
Relatively little is known about spatial competition within social groups that lack individual territories. Nevertheless, home range quality has previously been linked to reproductive outcomes in group-living species that have overlapping individual home ranges (meadow voles, Microtus pennsylvanicus: Ostfeld, Pugh, Seamon, & Tamarin, 1988; red deer, Cervus elaphus: Stopher et al., 2012; chimpanzees, Pan troglodytes: Emery Thompson, Kahlenberg, Gilby, & Wrangham, 2007). Despite a reproductive advantage associated with maintaining a high-quality home range, the mechanisms through which individuals maintain differential access to shared areas within groups are largely unknown.
East African female chimpanzees, Pan troglodytes schweinfurthii, present an excellent opportunity to explore competition for space in a social mammal since they concentrate their space use in small areas of a larger community range. Chimpanzees live in multimale, multifemale communities ranging in size from as few as 12 individuals (Bossou, Guinea: Hockings, Anderson, & Matsuzawa, 2012) to as many as 187 individuals (Ngogo, Kibale National Park, Uganda: Carlson, Rothman, & Mitani, 2013). Males are philopatric and jointly defend the community territory, whereas most females transfer and settle in new communities before breeding (Mitani, Watts, & Muller, 2002). Members of a community form temporary subgroups that vary in size and composition throughout the day. East African chimpanzee females are less gregarious than males and often forage alone or with maternal kin (Wrangham & Smuts, 1980). Females concentrate their space use in small, overlapping core areas of the community range to which they have high fidelity (Gombe, Tanzania: Murray, Mane, & Pusey, 2007; Williams, Pusey, Carlis, Farm, & Goodall, 2002; Wrangham & Smuts, 1980; Budongo Forest, Uganda: Fawcett, 2000; Kanyawara, Kibale National Park, Uganda: Kahlenberg, Emery Thompson, & Wrangham, 2008; Wrangham, Clark, & Isabirye-Basutu, 1992; Mahale National Park, Tanzania: Hasegawa, 1990). This behaviour is thought to minimize feeding competition (Wrangham, 1979) and maximize feeding efficiency through intimate familiarity with the distribution of food resources (Pusey, Williams, & Goodall, 1997; Williams et al., 2002). There is evidence that female chimpanzee core areas vary in the quality of food resources within them (Emery Thompson et al., 2007; Kahlenberg, Emery Thompson, & Wrangham, 2008; Murray, Eberly, & Pusey, 2006) and that these differences in core area quality are correlated with reproductive success (Emery Thompson et al., 2007). The advantages of foraging in a known area have also been demonstrated in other species (red-winged blackbirds, Agelaius phoeniceus: Beletsky & Orians, 1991; western barbastelle bats, Barbastella barbastellus: Hillen, Kiefer, & Veith, 2009).
How does a female chimpanzee maintain access to her core area? Several lines of evidence support the hypothesis that female chimpanzees engage in aggressive competition for long-term access to space. First, resident females have been observed killing the newborn infants of within-community females at Gombe (Goodall, 1986; Pusey et al., 2008), Budongo (Townsend, Slocombe, Emery Thompson, & Zuberbühler, 2007), and have been suspected to do so at Taï National Park (Boesch & Boesch-Achermann, 2000). Such infanticide accounted for 7.2% to an estimated maximum of 31.3% of the mortality of infants in their first 2 months at Gombe over a 19-year period (Pusey et al., 2008) and has been suggested to be an extreme manifestation of resource competition (Muller, 2007; Pusey et al., 2008). Second, resident females are often aggressive to newly immigrated females (Goodall, 1986; Kahlenberg, Emery Thompson, Muller, & Wrangham, 2008; Kahlenberg, Emery Thompson, & Wrangham, 2008; Nishida, 1989; Pusey, 1980) and can even prevent immigrant females from settling in a new community (Pusey et al., 2008).
Finally, evidence suggests that females actively compete over long-term access to core areas within the community range. Social dominance rank is correlated with core area quality at Gombe and Kanyawara (Kahlenberg, Emery Thompson, & Wrangham, 2008; Murray et al., 2006). At Gombe, higher-ranking females occupy smaller ranges to which they have higher fidelity (Murray et al., 2007). Also, newly immigrated females tend to settle away from high-ranking females (Murray et al., 2007; Williams et al., 2002). Nevertheless, the extent to which females aggressively defend and maintain access to their core areas is not known. In general, aggressive interactions among females are infrequent but occur most commonly over food resources as would be expected based on nutritional constraints on female reproduction (Goodall, 1986; Wittig & Boesch, 2003; reviewed in Murray et al., 2007). It is unknown whether females are more likely to fight for food in their core areas, but it seems probable since those areas contain known resources that may be contestable.
In this study, we use 14 years of long-term data from Gombe National Park to test the hypothesis that female chimpanzees defend access to their core areas by showing higher rates and severity of aggression towards females within their core areas than they do outside their core areas. Because females may be more protective of food resources in their own core areas, we examined interactions in feeding and nonfeeding contexts separately.
METHODS
Study Population
We investigated how aggression among female chimpanzees varied in relation to location in the Kasekela community in Gombe National Park, Tanzania. Gombe is a small park (ca. 35 km2) with a diverse habitat ranging from evergreen forest in the valleys to grasslands on the ridges (Clutton-Brock & Gillett, 1979). Researchers have studied the Kasekela community since 1960, with data on space use standardized in 1973. Since then, pairs of Tanzanian researchers have conducted almost daily dawn-to-dusk ‘follows’ of focal individuals, during which they record subgroup composition, feeding behaviour and location on a map at 15 min point samples (Gilby, Eberly, Pintea, & Pusey, 2006; Goodall, 1986). They also record detailed narrative notes on conspicuous social interactions, including aggression, among all group members. The researchers rotate through adult community members each month to ensure adequate sampling of all individuals.
We examined patterns of female aggression during a 14-year period (1995–2008), for which social interaction notes had been completely translated from Swahili into English. During this period, the community ranged in size from 41 to 62 members and contained 10–13 adult males and 13–26 adult females.
Female Core Areas
We defined core areas as areas in which females spent their time when alone or with adult female kin. ‘Alone’ locations were determined as the locations at which a female was encountered alone, with dependent offspring, or with an adult daughter (and dependent offspring) by the target individual for the follow (as described above); this allowed us to include females that were not regularly followed by field staff but were nevertheless frequently observed. While previous studies at Gombe employed kernels to delineate core areas (Murray et al., 2007; Murray, Gilby, Mane, & Pusey, 2008; Williams et al., 2002), we defined core areas as the 50% minimum convex polygon of alone locations of nonoestrous females to create a contiguous area that could be easily delineated. We excluded oestrous alone locations, as ranging patterns during this time are more likely to reflect sexual and social needs rather than personal space use. The resulting polygon indicated where a female concentrated her space use during a given period. Areas in which a female ranges alone are highly consistent, and presumably extremely important to foraging; competitors in this area may represent a particular risk to foraging efficiency. While female chimpanzees exhibit high site fidelity and often use the same general area for their entire lives, their core areas sometimes shift slightly over time (Murray et al., 2007; Williams et al., 2002). For that reason, we divided our study into three periods (1995–1999, 2000–2004, 2005–2008) and determined core areas for each period. These 5-year segments allowed us to capture site fidelity while accounting for slight shifts over time in response to events such as immigration or death of an individual in the community. We classified every aggressive event directed at another female for each adult female according to its location (inside versus outside) relative to her core area during the period in which it occurred. Delineation of core areas and all spatial analyses were conducting using Biotas™ 2.0 Alpha (Ecological Software Solutions LLC, Hegymagas, Hungary).
Patterns of Aggression
We only considered aggressive behaviour by resident adult females in this study. To be considered a resident adult, a female must have given birth before the start of a period as motherhood implies that a female is an established member of the community and is unlikely to emigrate (Goodall, 1986). Aggression by residents included directed threats (cough threat, arm raise), directed displays, chases and attacks (Goodall, 1986) directed at other females. We excluded females from our analysis when they had sexual swellings, to maintain consistency with our space methods and because their social context is very different (Muller, Kahlenberg, & Wrangham, 2009).
Recipients included all females aged 10 years and older in the community. At age 10, females experience their first adult sexual swellings (Goodall, 1986; Nishida, Takasaki, & Takahata, 1990; Pusey, 1990). As a result, adolescents become attractive to males and experience their first mating (Pusey, 1990), marking the rapid reduction in time spent in close proximity to their mothers. This indicates the onset of adult behaviour and the ranging patterns most reflective of personal space use. Therefore, these adolescent females represent true competitors to adult females, particularly because approximately 50% of the females in our study community do not emigrate (Pusey et al., 1997). We also included adult daughters as recipients of aggression in this study, because they represent viable competitors as well.
The observers recorded aggressive events between any group members on an ad libitum or all-occurrence basis (Altmann, 1974) from full-day focal follows of adult individuals that lasted from morning to evening. While it is possible that subtle aggression involving nonfocal individuals might be missed, most aggressive interactions are loud and obvious events, and are reliably recorded on all group members (Gilby et al., 2013). We categorized each aggressive event based on three criteria: context (food-related or not), contact (whether physical contact was made or not) and location (inside or outside the aggressor's core area). We classified an event as food-related if the focal chimpanzee was feeding at the time of the interaction, thus indicating that there was an accessible food resource at the time and place of the aggressive event. That is, the aggressor (or victim) was not necessarily feeding when the aggression occurred, but because others were eating, the fact that food was available indicated the potential for feeding competition. To support our assumption that nonfocal individuals were likely to be feeding if the focal was feeding, we identified periods in which two individuals were the subject of focal follows in the same party. During 1926 h in which the behaviour of two focal individuals was recorded simultaneously for 60 min or longer, we found that, when one focal was feeding, the other focal was feeding, on average (±SE), 78.1 ± 0.01% of the time. Our validation matches results found in other populations that have compared focal and group-level data collection (Gilby, Pokempner, & Wrangham, 2010). We considered all other aggressive events as nonfood related. We calculated rates of aggression by each resident female as follows: total number of aggressive events by location and context given by female X/total number hours that female X spent with at least one other female in that location and context × 100.
For example, to determine the rate of food-related aggression within the core area of female X, we divided the number of aggressive events in which female X was the aggressor in food-related contexts inside her core area by the total number of hours she was observed with any other adult female in a feeding context inside her core area. We calculated rates for each female across the entire period she was included in the study. We then made the following comparisons: rates of food-related aggression versus nonfood-related aggression (regardless of location), rates of aggression inside versus outside a core area (regardless of context), and food-related versus nonfood-related events by location.
Finally, we calculated the proportion of events in which female X directed physical contact aggression as follows: number of events in which female X exhibited contact aggression/total number of aggressive events for female X. We compared this value according to location and context as above.
Analyses
To be included as aggressors in our analyses, adult females had to have (1) at least 20 alone locations (within a period) for core area generation, a number that was previously shown to be sufficient for quantifying space use (Murray et al., 2007), (2) been observed directing aggression at other females and (3) been alive for over 65% of the period in which events were categorized. As a result, the number and identity of the individuals we analysed varied from period to period; 11 different females were included (see Results, Table 1).
Table 1.
Aggression rates in female chimpanzees by context and location
| Female | Demography, aggressor rates and number of events |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Inclusion criteria |
Demographic information |
Overall |
Inside core area |
Outside core area |
|||||
| Periods included | Date of birth | Date of death | Feeding | Nonfeeding | Feeding | Nonfeeding | Feeding | Nonfeeding | |
| CD | 1,2,3 | 02 Jul 1969 | 02 Oct 2007 | 0.046 (1) | 0.133 (2) | 0.262 (1) | 0.000 (0) | 0.000 (0) | 0.147 (2) |
| DL | 3 | 17 Jun 1986 | — | 0.084 (1) | 0.119 (1) | 0.000 (0) | 0.000 (0) | 0.096 (1) | 0.143 (1) |
| FF | 1,2 | 02 Jul 1958 | 01 Sep 2004 | 0.210 (6) | 0.697 (23) | 0.418 (2) | 0.706 (6) | 0.168 (4) | 0.693 (17) |
| FN | 1,2,3 | 19 Mar 1981 | — | 0.402 (18) | 0.574 (28) | 0.230 (2) | 0.815 (9) | 0.444 (16) | 0.504 (19) |
| GM | 1,2,3 | 19 Nov 1970 | — | 0.208 (8) | 0.398 (16) | 0.424 (3) | 0.504 (5) | 0.159 (5) | 0.363 (11) |
| JF | 1,2,3 | 02 Jul 1973 | — | 0.224 (3) | 0.361 (5) | 0.380 (1) | 0.416 (1) | 0.186 (2) | 0.350 (4) |
| PI | 1,2 | 02 Jul 1961 | 03 Oct 2005 | 0.224 (4) | 0.244 (4) | 0.299 (1) | 0.448 (2) | 0.230 (3) | 0.168 (2) |
| SA | 1,2,3 | 26 Oct 1973 | — | 0.132 (5) | 0.194 (7) | 0.097 (1) | 0.419 (4) | 0.144 (4) | 0.113 (3) |
| SW | 1,2,3 | 02 Jul 1958 | — | 0.143 (5) | 0.455 (16) | 0.000 (0) | 0.495 (3) | 0.172 (5) | 0.447 (13) |
| TG | 3 | 22 Apr 1989 | — | 0.188 (2) | 0.087 (1) | 0.731 (2) | 0.340 (1) | 0.000 (0) | 0.000 (0) |
| TZ | 1,2,3 | 02 Jul 1978 | — | 0.155 (5) | 0.341 (11) | 0.000 (0) | 0.514 (3) | 0.194 (5) | 0.302 (8) |
Includes demographic data for female chimpanzees that met inclusion criteria in terms of observation time, core area generation and aggressive events, as well as aggression rates (aggressive events/100 h with >1 other female) and number of aggressive events (in parentheses) for each female. Period 1:1995-1999; Period 2: 2000-2004; Period 3: 2005-2008.
For all analyses, we used nonparametric Wilcoxon signed-ranks tests, using the PROC UNIVARIATE function of SAS version 9.3 (SAS Institute, Cary, NC, U.S.A.).
Ethical Note
This project and its protocols were conducted in compliance with Tanzanian regulations and were approved by Tanzanian National Parks, Tanzania Wildlife Research Institute, and Tanzanian Commission for Science and Technology, and adhered to the ASAB/ABS Guidelines for the Use of Animals in Research.
RESULTS
Rates and Context of Aggression
During the study period, female subjects directed a mean ± SE of 0.26 ± 0.04 aggressive acts/100 h towards at least one other adult female (individual rates of aggression are summarized in Table 1).
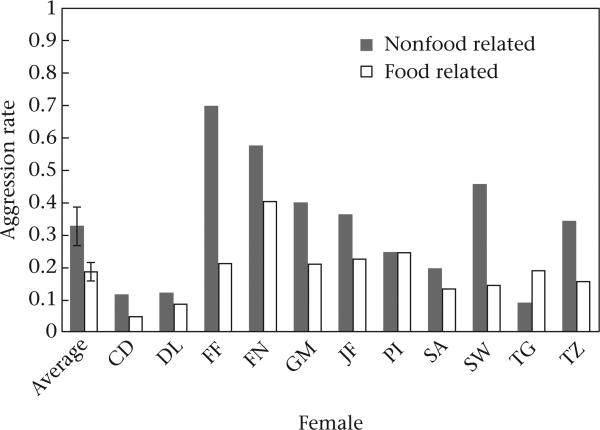
Overall, females were significantly more likely to engage in aggression during nonfeeding contexts than during feeding contexts (mean ± SE aggressive events/100 h with at least one other female: nonfeeding contexts: 0.33 ± 0.06; feeding contexts: 0.19 ± 0.03; Wilcoxon signed-ranks test: T = –0.26, N = 11 females, P = 0.02; Fig. 1, Table 1).
Figure 1.
Mean ± SE rate of aggression by female chimpanzees (aggressive events/100 h with ≥1 other female) in nonfood-related and food-related contexts. Includes averages for all females included in analyses in addition to individual values.
Spatial Patterns of Aggression
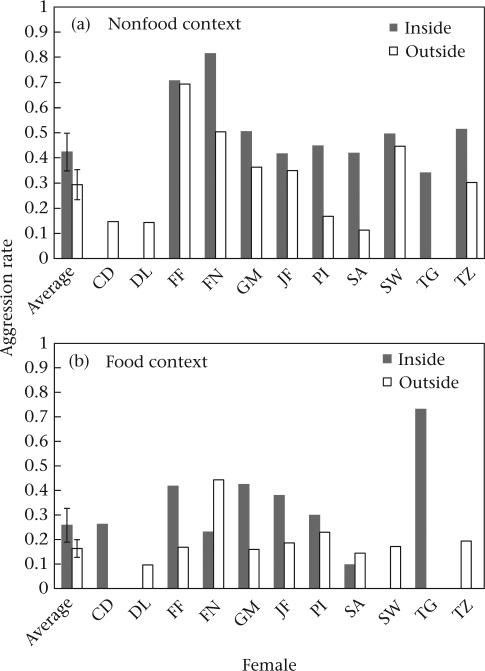
Females were significantly more likely to direct aggression at other adult females inside their core area than outside their core area (mean ± SE aggressive events/100 h with at least one other female: inside core area: 0.35 ± 0.06; outside core area: 0.23 ± 0.04; T = 2.32, N = 11 females, P = 0.03; Fig. 2, Table 1). This pattern was driven by spatial variation in nonfeeding aggression. Females were also significantly more likely to show aggression in nonfeeding contexts inside their core area than outside their core area (mean ± SE aggressive events/100 h with at least one other female: inside core area: 0.42 ± 0.08; outside core area: 0.29 ± 0.06; T = 2.44, N = 11 females, P = 0.05; Fig. 2a, Table 1). By contrast, there was no significant difference in aggression in feeding contexts inside and outside a female's core area (T = 1.12, N = 11 females, P = 0.29; Fig. 2b, Table 1).
Figure 2.
Mean ± SE rate of aggression by female chimpanzees (aggressive events/100 h with ≥1 other female) inside and outside their core area in (a) nonfeeding and (b) feeding contexts. Includes averages for all females included in analyses in addition to individual values.
Spatial Patterns of Contact Aggression
Aggression by a female was equally likely to involve physical contact whether it occurred inside or outside her core area (mean ± SE proportion of aggressive events involving physical contact: inside core area: 0.43 ± 0.10; outside core area: 0.34 ± 0.09; T = 0.71, N = 11 females, P = 0.80). The average proportion of aggressive events involving physical contact also did not vary by feeding context (mean ± SE proportion of events involving physical contact: feeding contexts: 0.40 ± 0.12; nonfeeding contexts: 0.48 ± 0.10; T = 0.62, N = 11 females, P = 0.26).
DISCUSSION
Understanding how individuals maintain and defend ranges is a primary goal in the field of animal behaviour. This is especially true for species in which space use correlates with reproductive success (meadow voles, Microtus pennsylvanicus: Ostfeld et al., 1988; buffleheads, Bucephala albeola: Gauthier, 1990; bushy-tailed woodrats, Neotoma cinerea: Moses & Millar, 1994; wild turkeys, Meleagris gallopavo: Badyaev & Faust, 1996). The majority of studies examining how aggression varies over space have focused on species that maintain exclusive, individual foraging or breeding territories (Eurasian red squirrels: Wauters et al., 1995; bank voles: Jonsson, Hartikainen, Koskela, & Mappes, 2002; de Kort, Eldermire, Cramer, & Vehrencamp, 2009). By comparison, the mechanisms for negotiating shared space within social groups are not well understood. Here, we investigate spatial competition among female chimpanzees, a social species that lacks individual territories. We found that a given female was more likely to be aggressive towards other females within her (the aggressor's) preferred area of the community range. The intriguing combination of low aggression rates, spatially explicit aggression (this study) and the association between space use and reproductive success (Emery Thompson et al., 2007) suggests that female chimpanzees compete for long-term access to core areas.
Female reproductive success is affected by nutrition and accordingly limited by access to current and future food resources (Gadgil & Bossert, 1970). The relationship between diet quality and reproductive success has been well documented in a variety of female mammals (black bears, Ursus americanus: Elowe & Dodge, 1989; Antarctic fur seals, Arctocephalus gazella: Lunn, Boyd, & Croxall, 1994; Eurasian red squirrels: Lurz, Garson, & Wauters, 1997). In chimpanzees, previous studies suggest that aggression may give females immediate access to resources, as aggression over food is one of the most common contexts of female aggression (Wittig & Boesch, 2003). In this study, we observed the opposite pattern: aggression was significantly less likely to occur in food-related contexts. It is possible that rates of food-related aggression were lower in our study because of socioecological components of female feeding patterns at Gombe. Based on the distribution of resources, females may be more likely to feed in large groups at Gombe than at other sites. If resources are clumped yet abundant enough to support multiple individuals, exclusion of competitors may not be feasible. In addition, males have been observed to ‘police’ female interactions, reducing rates of aggression in large groups (Kahlenberg, Emery Thompson, & Wrangham, 2008). Future studies should aim to explore the nuances of group size and composition on patterns of both feeding and aggression in chimpanzees.
We predicted that female aggression would be highest inside a female's core area since she would be most familiar with the food resources therein. However, we found no difference in the location of food-related aggression relative to a female's core area. This suggests that aggression in the context of feeding is equally bene-ficial regardless of location. The immediate gain can have important consequences for reproductive success. More intriguing, however, are our results on nonfeeding aggression, which supports a longer-term strategy.
Compared to aggression in the context of feeding, we found that females were significantly more likely to engage in nonfood-related aggression inside their core areas than outside their core areas. It seems likely that maintaining a familiar space would give individuals a foraging advantage when resources are less abundant and/or predictable. Chimpanzees are predominantly frugivorous with a diet that changes within and between seasons as different fruits become ripe (Goodall, 1986). Ideally, females should concentrate their space use in areas that contain resources throughout the year. This is possible when core areas are of high value throughout the year or when females are able to exploit smaller patches during ‘lean’ times. In either scenario, a female will gain reproductive benefits by excluding competitors. We suggest that aggression over food will have benefits regardless of location; nonfeeding aggression may serve to exclude competitors from an area that has long-term benefits to the female that occupies it.
Interestingly, females showed no tendency to engage in physical contact (i.e. ‘escalate’) more often over food or within their core areas. This is in marked contrast to numerous studies from solitary species that demonstrate that the territory owner is more likely to escalate and win encounters over the ‘intruder’ (green anoles: Leuck, 1995; Rich Mountain salamanders: Anthony et al., 1997; brown trout: Johnsson et al., 2000). However, escalation may be constrained by other factors in chimpanzees. Unlike many territorial mammals that leave offspring in a den or nest, female chimpanzees travel and forage with dependent offspring (Goodall, 1986). The presence of offspring makes escalated aggression more costly (Muller, 2002), and may therefore decrease the likelihood of direct physical contests.
Unlike individuals in solitary, territorial species, female chimpanzees must negotiate space use with other members of their community. We demonstrate that female chimpanzees appear to have clear mechanisms for diminishing competition within their social groups. Our results and those from other studies suggest that female chimpanzees use aggression to exclude long-term competitors in three contexts: (1) location-dependent aggression (this study); (2) aggression towards immigrants and (3) infanticide. These three forms of rare, yet strategic, aggression likely play an integral role in long-term competition between females and lay the foundation for future research on the nuances of female competition. Our results demonstrate that female aggression is an important component to negotiating access to food resources and shared space, and are an important step in understanding the subtleties of female chimpanzee competition. This study lays the foundation for further research in gregarious species with overlapping home ranges within a community territory.
Acknowledgments
We thank Tanzania National Parks, the Tanzania Wildlife Research Institute and the Tanzanian Council for Science and Technology for granting us permission to work in Gombe National Park. We also thank the Jane Goodall Institute for funding long-term research at Gombe, the Gombe Stream Research Center staff for maintaining data collection and Dr Jane Goodall for granting us permission to work with the long-term data set. Digitization and analysis of behavioural data were supported by grants from the National Science Foundation (DBS-9021946, SBR-9319909, BCS-0452315, LTREB-1052693), the National Institutes of Health (5R00HD057992 and R01 A1058715), the University of Minnesota, the Harris Steel Group, the Windibrow Foundation, the Jane Goodall Institute, the Carnegie Corporation, Minnesota Base Camp and Duke University. Funding for this specific project was provided by the Duke Undergraduate Research Support office. We are extremely grateful to Joseph Feldblum, Dr Amy Schreier, Dr Leslie Digby and Aaron Sandel for their support throughout the duration of this project.
References
- Altmann J. Observational study of behavior: sampling methods. Behaviour. 1974;49:227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Anthony CD, Wicknick JA, Jaeger RG. Social interactions in two sympatric salamanders: effectiveness of a highly aggressive strategy. Behaviour. 1997;134:71–88. [Google Scholar]
- Badyaev AV, Faust JD. Nest site fidelity in female wild turkey: potential causes and reproductive consequences. Condor. 1996;98:589–594. [Google Scholar]
- Beletsky LD, Orians GH. Effects of breeding experience and familiarity on site fidelity in female red-winged blackbirds. Ecology. 1991;72:787–796. [Google Scholar]
- Boesch C, Boesch-Achermann H. The chimpanzees of the Taï Forest: Behavioural ecology and evolution. Oxford University; Oxford, UK: 2000. [Google Scholar]
- Brockelman W, Srikosamatara S. The maintenance and evolution of social structure in gibbons. In: Preuschoft H, Chivers D, Brockelman W, Creel N, editors. The lesser apes: Evolutionary and behavioural biology. Edinburgh University Press; Edinburgh, UK: 1984. pp. 298–323. [Google Scholar]
- Carlson BA, Rothman JM, Mitani JC. Diurnal variation in nutrients and chimpanzee foraging behavior. American Journal of Primatology. 2013;75:342–349. doi: 10.1002/ajp.22112. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock TH, Gillett JB. A survey of forest composition in the Gombe National Park, Tanzania. African Journal of Ecology. 1979;17:131–158. [Google Scholar]
- Elowe KD, Dodge WE. Factors affecting black bear reproductive success and cub survival. Journal of Wildlife Management. 1989;53:962–968. [Google Scholar]
- Emery Thompson M, Kahlenberg SM, Gilby IC, Wrangham RW. Core area quality is associated with variance in reproductive success among female chimpanzees at Kibale National Park. Animal Behaviour. 2007;73:501–512. [Google Scholar]
- Fawcett K. Doctoral thesis. University of Edinburgh; UK: 2000. Female relationships and food availability in a forest community of chimpanzees. [Google Scholar]
- Gadgil M, Bossert WH. Life historical consequences of natural selection. American Naturalist. 1970;104:1–24. [Google Scholar]
- Gauthier G. Philopatry, nest-site fidelity and reproductive performance in buffleheads. Auk. 1990;107:126–132. [Google Scholar]
- Gilby IC, Brent L, Wroblewski E, Rudicell RS, Hahn BH, Goodall J, et al. Fitness benefits of coalitionary aggression in male chimpanzees. Behavioral Ecology and Sociobiology. 2013;67:373–381. doi: 10.1007/s00265-012-1457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilby IC, Eberly LE, Pintea L, Pusey AE. Ecological and social in-fluences on the hunting behaviour of wild chimpanzees (Pan troglodytes schweinfurthii). Animal Behaviour. 2006;72:169–180. [Google Scholar]
- Gilby IC, Pokempner AA, Wrangham RW. A direct comparison of scan and focal sampling methods for measuring wild chimpanzee feeding behavior. Folia Primatologica. 2010;81:254–264. doi: 10.1159/000322354. [DOI] [PubMed] [Google Scholar]
- Goodall J. The chimpanzees of Gombe. Harvard University Press; Cambridge, MA, USA: 1986. [Google Scholar]
- Hasegawa T. Sex differences in ranging patterns. In: Nishida T, editor. The chimpanzees of the Mahale Mountains. University of Tokyo Press; Tokyo, Japan: 1990. pp. 100–114. [Google Scholar]
- Hillen J, Kiefer A, Veith M. Foraging site fidelity shapes the spatial organization of a population of female western barbastelle bats. Biological Conservation. 2009;142:817–823. [Google Scholar]
- Hockings KJ, Anderson JR, Matsuzawa T. Socioecological adaptations by chimpanzees, Pan troglodytes verus, inhabiting an anthropogenically impacted habitat. Animal Behaviour. 2012;83:801–810. [Google Scholar]
- Johnsson JI, Carlsson M, Sundström LF. Habitat preference increases territorial defense in brown trout (Salmo trutta). Behavioral Ecology and Socio-biology. 2000;48:373–377. [Google Scholar]
- Jonsson P, Hartikainen T, Koskela E, Mappes T. Determinants of reproductive success in voles: space use in relation to food and litter size manipulation. Evolutionary Ecology. 2002;16:455–467. [Google Scholar]
- Kahlenberg SM, Emery Thompson M, Muller MN, Wrangham RW. Immigration costs for female chimpanzees and male protection as an immigrant counterstrategy to intrasexual aggression. Animal Behaviour. 2008;76:1497–1509. [Google Scholar]
- Kahlenberg SM, Emery Thompson M, Wrangham RW. Female competition over core areas in Pan troglodytes schweinfurthii, Kibale National Park, Uganda. International Journal of Primatology. 2008;29:931–947. [Google Scholar]
- de Kort SR, Eldermire ERB, Cramer ERA, Vehrencamp SL. The deterrent effect of bird song in territory defense. Behavioral Ecology. 2009;20:200–206. doi: 10.1093/beheco/arn135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskela E, Mappes T, Ylönen H. Territorial behaviour and reproductive success of bank vole Clethrionomys glareolus females. Journal of Animal Ecology. 1997;66:341–349. [Google Scholar]
- Leuck BE. Territorial defense by male green anoles: an experimental test of the roles of residency and resource quality. Herpetological Monographs. 1995;9:63–74. [Google Scholar]
- Lunn NJ, Boyd IL, Croxall JP. Reproductive performance of female Antarctic fur seals: the influence of age, breeding experience, environmental variation and individual quality. Journal of Animal Ecology. 1994;63:827–840. [Google Scholar]
- Lurz PWW, Garson PJ, Wauters LA. Effects of temporal and spatial variation in habitat quality on red squirrel dispersal behaviour. Animal Behaviour. 1997;54:427–435. doi: 10.1006/anbe.1996.0486. [DOI] [PubMed] [Google Scholar]
- Maher CR, Lott DF. Definitions of territoriality used in the study of variation in vertebrate spacing systems. Animal Behaviour. 1995;49:1581–1597. [Google Scholar]
- Maher CR, Lott DF. A review of ecological determinants of territoriality within vertebrate species. American Midland Naturalist. 2000;143:1–29. [Google Scholar]
- Milner RNC, Booksmythe I, Jennions MD, Backwell PRY. The battle of the sexes? Territory acquisition and defence in male and female fiddler crabs. Animal Behaviour. 2010;79:735–738. [Google Scholar]
- Mitani JC, Watts DP, Muller MN. Recent developments in the study of wild chimpanzee behavior. Evolutionary Anthropology. 2002;11:9–25. [Google Scholar]
- Moses RA, Millar JS. Philopatry and motheredaughter associations in bushy-tailed woodrats: space use and reproductive success. Behavioral Ecology and Sociobiology. 1994;35:131–140. [Google Scholar]
- Muller MN. Agonistic relations among Kanyawara chimpanzees. In: Boesch C, Hohmann G, Marchant LF, editors. Behavioural diversity in chimpanzees and bonobos. Cambridge University Press; Cambridge, UK: 2002. pp. 112–124. [Google Scholar]
- Muller MN. Chimpanzee violence: femmes fatales. Current Biology. 2007;10:365–366. doi: 10.1016/j.cub.2007.03.037. [DOI] [PubMed] [Google Scholar]
- Muller MN, Kahlenberg SM, Wrangham RW. Male aggression against females and sexual coercion in chimpanzees. In: Muller MN, Wrangham RW, editors. Sexual coercion in primates and humans: An evolutionary perspective on male aggression against females. Harvard University Press; Cambridge, MA, USA: 2009. pp. 184–217. [Google Scholar]
- Murray CM, Eberly LE, Pusey AE. Foraging strategies as a function of season and dominance rank among wild female chimpanzees (Pan troglodytes schweinfurthii). Behavioral Ecology. 2006;17:1020–1028. [Google Scholar]
- Murray CM, Gilby IC, Mane SV, Pusey AE. Male chimpanzees inherit maternal ranging patterns. Current Biology. 2008;18:20–24. doi: 10.1016/j.cub.2007.11.044. [DOI] [PubMed] [Google Scholar]
- Murray CM, Mane SV, Pusey AE. Dominance rank influences female space use in wild chimpanzees, Pan troglodytes: towards an ideal despotic distribution. Animal Behaviour. 2007;74:1795–1804. [Google Scholar]
- Nishida T. Social interactions between resident and immigrant female chimpanzees. In: Heltne PG, Marquardt LA, editors. Understanding chimpanzees. Harvard University Press; Cambridge, MA, USA: 1989. pp. 68–89. [Google Scholar]
- Nishida T, Takasaki H, Takahata Y. Demography and reproductive profiles. In: Nishida T, editor. The chimpanzees of the Mahale Mountains. University of Tokyo Press; Tokyo, Japan: 1990. pp. 63–97. [Google Scholar]
- Ostfeld RS, Pugh SR, Seamon JO, Tamarin RH. Space use and reproductive success in a population of meadow voles. Journal of Animal Ecology. 1988;57:385–394. [Google Scholar]
- Peres CA. Costs and benefits of territorial defense in wild golden lion tamarins, Leontopithecus rosalia. Behavioral Ecology and Sociobiology. 1989;25:227–233. [Google Scholar]
- Pusey AE. Inbreeding avoidance in chimpanzees. Animal Behaviour. 1980;28:543–552. [Google Scholar]
- Pusey AE. Behavioural changes at adolescence in chimpanzees. Behaviour. 1990;115:203–246. [Google Scholar]
- Pusey AE, Murray C, Wallauer W, Wilson M, Wroblewski E, Goodall J. Severe aggression among female Pan troglodytes schweinfurthii at Gombe National Park, Tanzania. International Journal of Primatology. 2008;29:949–973. [Google Scholar]
- Pusey AE, Williams JM, Goodall J. The influence of dominance rank on the reproductive success of female chimpanzees. Science. 1997;277:828–831. doi: 10.1126/science.277.5327.828. [DOI] [PubMed] [Google Scholar]
- Stamps J. Territorial behavior: testing the assumptions. Advances in the Study of Behavior. 1994;23:173–232. [Google Scholar]
- Stopher KV, Walling CA, Morris A, Guinness FE, Clutton-Brock TH, Pemberton JM, et al. Shared spatial effects on quantitative genetic parameters: accounting for spatial autocorrelation and home range overlap reduces estimates of heritability in wild red deer. Evolution. 2012;66:2411–2426. doi: 10.1111/j.1558-5646.2012.01620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taborsky M. Broodcare helpers in the cichlid fish Lamprologus brichardi: their costs and benefits. Animal Behaviour. 1984;32:1236–1252. [Google Scholar]
- Townsend SW, Slocombe KE, Emery Thompson M, Zuberbühler K. Female-led infanticide in wild chimpanzees. Current Biology. 2007;17:355–356. doi: 10.1016/j.cub.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Wauters LA, Lens L, Dhont AA. Variation in territory fidelity and territory shifts among red squirrel, Sciurus vulgaris. Animal Behaviour. 1995;49:187–193. [Google Scholar]
- Williams JM, Pusey AE, Carlis JV, Farm BP, Goodall J. Female competition and male territorial behaviour influence female chimpanzees’ ranging patterns. Animal Behaviour. 2002;63:347–360. [Google Scholar]
- Wittig RM, Boesch C. Food competition and linear dominance hierarchy among female chimpanzees of the Taï National Park. International Journal of Primatology. 2003;24:847–867. [Google Scholar]
- Wrangham RW. On the evolution of ape social systems. Social Science Information. 1979;18:336–368. [Google Scholar]
- Wrangham RW, Clark AP, Isabirye-Basutu G. Female social relationships and social organization of Kibale Forest chimpanzees. In: Nishida T, McGrew WC, Marler P, Pickford M, de Waal F, editors. Topics in primatology: Human origins. University of Tokyo Press; Tokyo, Japan: 1992. pp. 81–98. [Google Scholar]
- Wrangham RW, Smuts BB. Sex differences in the behavioral ecology of chimpanzees in the Gombe National Park, Tanzania. Journal of Reproduction and Fertility. 1980;1980(Suppl. 28):13–31. [PubMed] [Google Scholar]