Abstract
DNA methylation is an epigenetic regulatory mechanism commonly associated with transcriptional silencing. DNA methyltransferases (DNMTs) are a family of related proteins that both catalyze the de novo formation of 5-methylcytosine and maintain these methylation marks in cell-specific patterns in virtually all mitotic cells of the body. In the adult brain, methylation occurs in progenitor cells of the neurogenic zones and in postmitotic neurons. Of the DNMTs, DNMT1 and DNMT3a are most highly expressed in postmitotic neurons. While it has been commonly thought all post-mitotic neurons and glia express DNMTs at comparable levels, the coexpression of selected DNMTs with markers of distinct neurotransmitter phenotypes has not been previously examined in detail in the mouse. To this end, we analyzed the expression of DNMT1 and DNMT3a along with GAD67 in the brains of the glutamic acid decarboxylase67-enhanced green fluorescent protein (GAD67-GFP) knockin mice. After first confirming that GFP-immunopositive neurons were also GAD67-positive, we showed that in the motor cortex, piriform cortex, striatum, CA1 region of the hippocampus, dentate gyrus, and basolateral amygdala (BLA), GFP immunofluorescence coincided with the signal corresponding to DNMT1 and DNMT3a. A detailed examination of cortical neurons, showed that ≈30% of NeuN-immunopositive neurons were also DNMT1-positive. These data do not exclude the expression of DNMT1 or DNMT3a in glutamatergic neurons and glia. However, they suggest that their expression is low compared with the levels present in GABAergic neurons.
INDEXING TERMS: conditional knockout, DNA methylation, epigenetics, GABAergic neuron, gene regulation, schizophrenia
DNA methylation is a major factor involved in the epigenetic regulation of neuronal gene expression underlying changes in synaptic plasticity associated with learning and memory processes (Day and Sweatt, 2010, 2011). Aberrant methylation of candidate gene promoters affects the expression of downstream transcription units. Pathogenic mechanisms associated with psychiatric disorders including mood and cognition disorders are linked to neuronal mRNA changes (Veldic et al., 2004; Abdolmaleky et al., 2005; Grayson et al., 2005, 2006; Ruzicka et al., 2007; Mill et al., 2008; Poulter et al., 2008; Higuchi et al., 2011).
The enzymes that catalyze the transfer of a methyl group from S-adenosyl-methionine to the 5′ position of the C moiety of CpG dinucleotides form a small family of structurally related proteins called the DNA methyltransferases (DNMTs). These include DNMT1, DNMT3a, DNMT3b, and the more recently described DNMT3L (see Goll and Bestor, 2005; Cheng and Blumenthal, 2008; Jurkowska et al., 2011). DNMT1 is the most active of the DNMTs, and while it has a preference for hemimethylated sites, it also catalyzes de novo methylation in CpG islands (Jair et al., 2006; Cheng and Blumenthal, 2008). DNMTs-3a and -3b are typically known as de novo DNMTs and each show distinct developmental patterns of expression in the brain. DNMT3b is expressed in neuroprogenitor cells and is likely important for establishing methylation patterns during neurogenesis, while DNMT3a is expressed in postmitotic, newly differentiated and mature neurons (Feng et al., 2005). DNMT3a may be required for the establishment of tissue-specific methylation patterns of the genome (Watanabe et al., 2006). DNMT3L is catalytically inactive and acts to enhance the activities of DNMT3a and DNMT3b (Van Emburgh and Robertson, 2011).
Interestingly, DNMT1 and DNMT3a are highly expressed in adult mammalian postmitotic neurons, whereas DNMT3b expression is low (Inano et al., 2000; Veldic et al., 2004; Feng et al., 2005; Zhubi et al., 2009). DNMT1 is more highly expressed in GABA neurons of the human cortex and less so in pyramidal neurons or glia (Veldic et al., 2004). DNMT1 is also increased in GABAergic neurons of cortical layers I and II in schizophrenia (SZ) and bipolar disorder patients (BPD) (Veldic et al., 2004, 2005; Ruzicka et al., 2007) and is upregulated in GABAergic medium spiny neurons of the striatum of SZ but not BPD patients (Veldic et al., 2007). More recently, an examination of DNMT3a mRNA in postmortem human cortex showed higher numbers of neurons reaching detection in layers I and II in SZ patients (Zhubi et al., 2009).
Conditional double knockout mice in which DNMT1 and DNMT3a are absent in forebrain excitatory neurons show abnormal long-term plasticity in the CA1 region of the hippocampus and deficits in learning and memory (Feng et al., 2010). In addition, a large number of genes that are deregulated in glutamatergic neurons in response to the loss of DNMT function have been identified. The observed decrease in DNA methylation in the double knockout neurons compared with the single conditional knockout mice suggests the possibility that DNMT1 and DNMT3a have overlapping roles in postmitotic neurons. The studies described in the preceding paragraph with respect to DNMT localization in GABA neurons of the human brain and the loss of function studies carried out in excitatory forebrain neurons suggest that the localization of DNMT1 and 3a with respect to neurons of a defined neurotransmitter phenotype is still not entirely clear.
Only limited studies have been carried out in the mouse brain to show the localization of DNMT1 with markers of GABAergic or pyramidal neurons. For example, Satta et al. (2008) showed that DNMT1 and GAD67 colocalize in layers I and II of the mouse frontal cortex. However, the localization of DNMT3a with respect to neurons of a defined neurotransmitter phenotype is still unknown. The goal of the current study was to provide a comprehensive analysis of the distribution of DNMT1 and DNMT3a in the mouse brain with respect to neurotransmitter phenotype. To this end, we studied the glutamic acid decarboxylase67-enhanced green fluorescent protein (GAD67-GFP) knockin mouse (Tamamaki et al., 2003). This mouse was developed by targeting GFP to the ATG start codon of the GAD67 primary transcript by homologous recombination such that GFP expression is under control of the endogenous GAD67 promoter. These GAD67-GFP mice have previously been examined with respect to the colocalization of GFP and GAD67 and various additional markers of GABAergic neurons such as parvalbumin, calretinin, and somatostatin (Tamamaki et al., 2003). Establishing the distribution of DNMT1 and DNMT3a in these mice will allow us the possibility of using fluorescence-activated cell sorting of neurons to study changes in GABA neuron-specific methylation patterns following different developmental, environmental, and pharmacological manipulations. As a first step, we determined the neurotransmitter phenotype of neurons expressing DNMT1 and DNMT3a by colocalizing the respective immunohistochemistry with markers for GABAergic neurons (GAD67) in the motor and piriform cortices, striata, hippocampi, and BLA of GAD67-GFP knockin mice.
MATERIALS AND METHODS
Animals were housed in a controlled environment and were genotyped prior to use. All experiments were conducted in accordance with the University of Illinois at Chicago Animal Care and Use Committee guidelines.
Animals and tissue preparation
Adult mice, 25–30 g in body weight, were perfused intracardially with ≈25 ml saline (0.9% NaCl) followed by 40–60 ml of 4% paraformaldehyde (Liu et al., 2001). The brains were 1) postfixed for 72 hours in 4% paraformaldehyde and 2) embedded in 30% sucrose in 0.15 M phosphate-buffered saline (PBS), pH 7.4, at 4°C. All experiments were conducted in groups of three to five animals. For each antibody, from four to six sections were processed for each animal.
GAD67-GFP knockin mice
For the experiments described we used heterozygous GAD67-GFP mice in which the cDNA encoding enhanced GFP is inserted into the GAD67 ATG start codon by homologous recombination (Tamamaki et al., 2003). Offspring of the original knockin mice were crossed with CAG-cre transgenic animals (Sakai and Miyazaki, 1997) to remove the loxP-flanked phosphoglycerokinase-neomycin resistance cassette used as a selectable marker (Tamamaki et al., 2003). GAD67-GFP (Δneo) mice were used throughout this study and were backcrossed and maintained in the C57BL/6 background. GAD67 is one of two genes encoding isoforms of the GABA-synthesizing enzyme, glutamic acid decarboxylase (GAD). Heterozygous GAD67-GFP breeder males were mated with wild-type C57BL/6 females and offspring were genotyped at birth. Two primer pairs were used for genotyping to identify both alleles (GAD67-GFP and GAD67/GAD67) of all offspring (for primer sequences, see Tamamaki et al., 2003). The GFP insertion disrupts the GAD67 reading frame so that mice homozygous for the GAD67-GFP knockin allele die just prior to birth (Asada et al., 1997; Condie et al., 1997). For the experiments described, 60–80-day-old animals of either sex were used. GAD67 wild-type littermates were used as controls. Coronal slices (nominally 20–25 μm thick) of fixed mouse brains were cut using a cryostat (Richard Allen Scientific, Kalamazoo, MI). Sections were directly used to view native GFP fluorescence. GFP fluorescence was observed; however, to enhance the signal rabbit anti-GFP antibodies were used in the colocalization studies. All fixed slices were washed in PBS (three washes at 10-minute intervals), then processed for immunohistochemistry and immunofluorescence experimental analysis.
Antibodies
See Table 1 for a complete list of all antibodies, sources, and dilutions used in the present study. Secondary antibodies included: Cy5-labeled goat antimouse, antirabbit IgG (diluted 1:1,000; Amersham Biosciences, Piscat-away, NJ), or Cy2-labeled streptavidin (diluted 1:1,000; Amersham Biosciences) were used as described below.
TABLE 1.
Primary Antibodies
| Antibody | Immunogen | Species | Source, cat. no., clone | Dilution |
|---|---|---|---|---|
| GFP | Recombinant GFP protein | Rabbit | Abcam, Cambridge MA, ab290 | 1:250 |
| GAD67 | Recombinant GAD67 protein | Mouse | Millipore, Billerica MA, MAB5406, clone 1G10.2 | 1:500 |
| Reelin | N-terminus of mouse reelin, amino acids 40—189 | Mouse | Andre Goffinet, Fac Univ Notre-Dames de la Paix School of Medicine, Namur, Belgium (G10) | 1:500 |
| DNMT1 | Synthetic peptide corresponding to amino acids 637–650 (EKDDREDKENAFKR) of human Dnmt1 | Mouse | Imgenex, San Diego CA IMG-261A, clone 60B1220.1 | 1:500 |
| DNMT3a | Recombinant mouse Dnmt3a. The epitope is near the C-terminus (a.a. 705–908) | Mouse | Imgenex, San Diego CA IMG-268A, clone 64B1446 | 1:500 |
| NeuN | Purified cell nuclei from mouse brain | Mouse | Imgenex, San Diego CA, MAB377, clone A60 | 1:500 |
| VGlut2 | Recombinant protein from rat VGLUT2. | Mouse | Millipore, Billerica MA, MAB5504, clone 8G9.2 | 1:500 |
Antibody characterization
Antibody specificity was evaluated by western blot analyses of cortical extracts. Following separation on 4–12% sodium dodecyl sulfate-polyacrylamide gels and blotting to nitrocellulose, major immunoreactive bands of the expected molecular size were detected with all antibodies used.
The GFP polyclonal antibody (directed against purified recombinant GFP protein) detects the enhanced green fluorescent protein in formalin-fixed sections according to the manufacturer’s technical information. Comparison of the confocal images (Fig. 1) from the GAD67-GFP mouse with those of Tamamaki et al. (2003) indicates that these antibodies recognize virtually the same neurons in both studies. When slices from wildtype C57BL/6 mice are incubated with both primary antibody and secondary antibodies, no signal is observed.
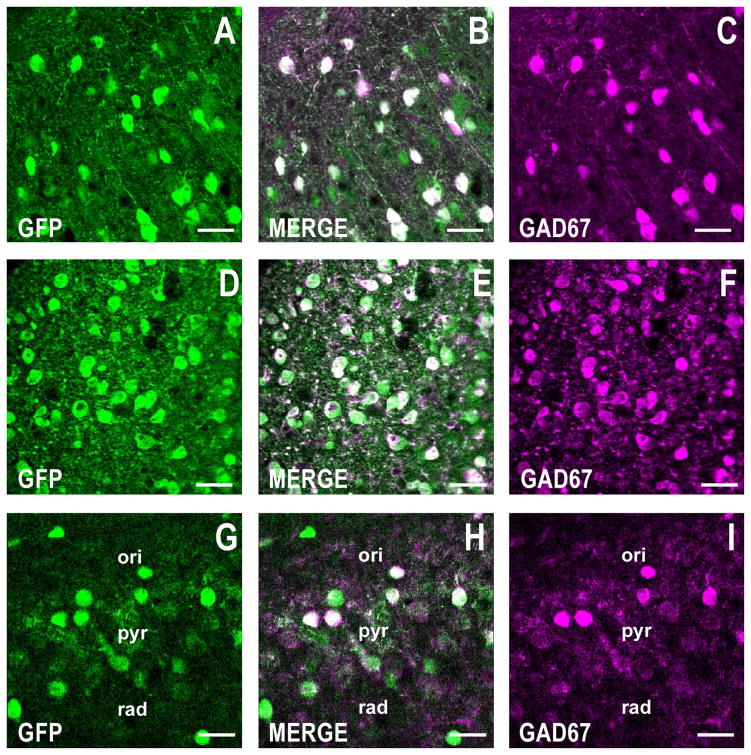
Figure 1.
GFP is coexpressed with GAD67 (the prototypic GABAergic marker) in telencephalic neurons from a GAD67-GFP knockin mouse. Confocal double fluorescence images corresponding to GFP (green), GAD67 (magenta), and merged images (white). A–C: Motor cortex, layers I/II. Coronal section corresponding roughly to bregma +1.4 mm is shown. D–F: Striatum. Coronal plane shown as described for A–C. G–I: CA1 region of the hippocampus showing the stratum oriens (ori), stratum pyramidale (pyr), and stratum radiatum (rad). Coronal section corresponds roughly to bregma −2 mm. Note that in the three brain areas studied, more than 90% of GFP-positive cells are also positive for GAD67. Scale bars = 40 μm.
The GAD67 (clone 1G10.2) monoclonal antibody, directed against recombinant GAD67 protein), reacts with GAD67 from rat, mouse, and human. There is no detectable crossreactivity with GAD65 based on western blot analysis of brain lysates when compared with blots probed with antibodies that react with both GAD65 and GAD67 (manufacturer’s data sheets). Immunostaining of mouse cortical slices showed selective neuronal staining similar to the pattern previously published (Liu et al., 2001).
The reelin (clone G-10) monoclonal antibody was directed against the hinge region of the protein (amino acids 40–189) and recognizes multiple reelin related products based on western analysis (de Bergeyck et al., 1998). The antibody stained Cajal-Retzius cells in the embryonic forebrain of mice (de Bergeyck et al., 1997). Immunostaining of adult wildtype cortical slices is consistent with previous findings (Liu et al., 2001) and no staining was visible in slices processed from the reeler mouse.
The DNMT1 (clone 60B1220.1) monoclonal antibody (directed against amino acids 637–650) has been purified by protein G chromatography and recognizes a single band of ≈190 kd based on western blot analysis (Kundakovic et al., 2007; manufacturer’s data sheet). The antibody was generated against amino acids 637–650 of the human protein but also crossreacts with mouse DNMT1. Preincubation of the antibody with the antigen eliminates the signal. This antibody has been used to detect DNMT immunohistochemically in cortical neurons (Satta et al., 2008) and in olfactory receptor neurons following formalin fixation (MacDonald et al., 2005).
The DNMT3a (clone 64B1446) monoclonal antibody was generated against recombinant mouse DNMT3a and has been purified by G protein chromatography. The epitope was subsequently mapped to the carboxyl terminus (Chen et al., 2002). Western blot analysis shows a single band that is eliminated upon preabsorption with recombinant DNMT3a (manufacturer’s data sheets). Immunohistochemistry data have been reported using this antibody with paraformaldehyde fixed mouse cortical slices (Feng et al., 2005) and olfactory receptor neurons (MacDonald et al., 2005) during development and in the adult.
The NeuN (clone A60) monoclonal antibody was generated against purified brain cell nuclei and recognizes the neuron-specific nuclear protein NeuN (Mullen et al., 1992; Wolf et al., 1996). The antibody recognizes two to three similar-sized bands in the range of 46–48 kd on western blots (manufacturer’s data sheets). The antibody has been used extensively to identify neurons in brain slices from a wide variety of species. Immunohistochemical staining is primarily localized in the nucleus of neurons with lighter staining in the cytoplasm. Of those neurons not recognized by the antibody (Purkinje, mitral, and photoreceptor neurons), none are known to be located in coronal slices of the motor cortex (as in Fig. 8).
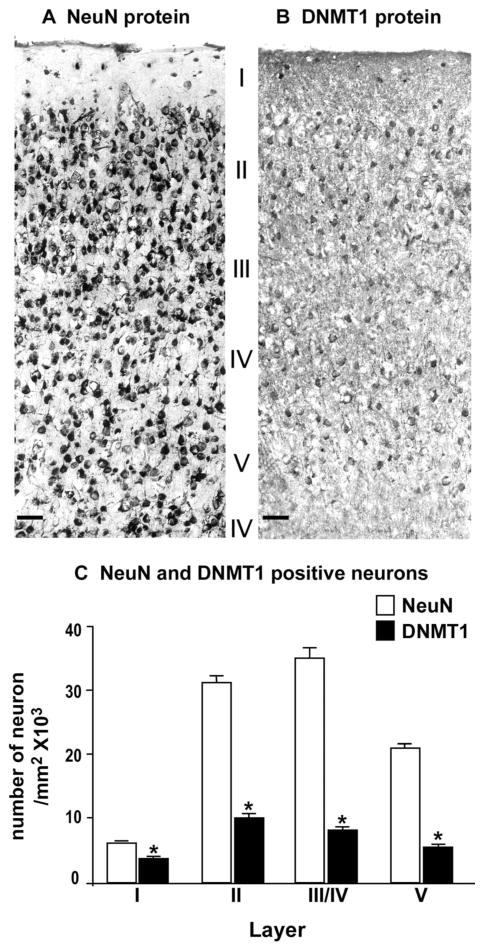
Figure 8.
Photomicrographs showing examples of DNMT1 and NeuN immunolabeling in a coronal section of motor cortex (bregma +1.4 mm) from the GAD67-GFP knockin mouse. A: NeuN immunostaining. B: DNMT1 immunostaining. C: Average numbers of NeuN- and DNMT1-positive neurons in different cortical layers of a coronal block of motor cortex included between 1.4 mm and 1.0 mm anterior to bregma. Each bar is derived from the mean ± SE of 5 mice per group. *P < 0.01 for NeuN vs. DNMT1. ANOVA followed by Bonferroni multiple comparison. Scale bars = 40 μm.
The VGlut2 (clone 8G9.2) monoclonal antibody was generated using recombinant rat vesicular glutamate transporter 2. The protein A purified antibody recognizes a single band of ≈65 kd on SDS gels following western blotting and antibody preabsorbed with the VGlut2 immunizing antigen eliminated the signal (Agis-Balboa et al., 2006). Immunohistochemistry in the absence of the primary antibody showed no detectable product (Agis-Balboa et al., 2006; Griffin et al., 2010). We have previously used this antibody to stain glutamatergic neurons in multiple regions of the mouse brain (Agis-Balboa et al., 2007).
Confocal double fluorescence microscopy
Immunofluorescence labeling was performed by following a modification of the procedure described by Pesold et al. (1998), Veldic et al. (2007), and Agis-Balboa et al. (2007). After labeling with the primary antibodies, slices were incubated with Cy5-labeled goat antimouse or anti-rabbit IgG (diluted 1:1,000; Amersham Biosciences) to produce red fluorescent staining or Cy2-labeled streptavidin (diluted 1:1,000; Amersham Biosciences) to produce green fluorescent staining. The reactions were carried out in 3% normal goat serum, and 1% bovine serum albumin (BSA) in PBS for 1 hour.
The number of cells in which green and red fluorescence colocalize compared with the number of cells that express only green or only red fluorescence was quantified using confocal microscopy (Leica, Bannockburn, IL) at a magnification of 40× in a counting box of 100 × 100 × 20 μm. The red images were subsequently converted to magenta as indicated below.
Immunohistochemistry
To obtain neuron-specific nuclear protein (NeuN) and DNMT1 immunolabeling, 20-μm floating sections were incubated in parallel with 1) monoclonal anti-DNMT1 antiserum (1:500; Imgenex, San Diego, CA) or 2) monoclonal anti-NeuN antiserum (diluted 1:500; Chemicon, Billerica, MA). The sections were incubated with the primary antibody for 48 hours at 4°C and 2 hours at room temperature. After addition of the secondary antibody, sections were stained following a previously described protocol and sections were reacted with 3, 3′-diaminobenzidine tetrahydrochloride (DAB) with nickel-ammonium sulfate to obtain a dark-brown reaction product (Satta et al., 2008). To estimate the relative density of NeuN- and DNMT1-positive neurons in motor cortex, coronal cortical blocks (from 1.4 to +1 mm anterior to bregma) were studied. For each brain area, five to six sections were taken (one every fourth slice) and cells were counted using an “assumption-based” bidimensional cell counting method (Benes and Lange, 2001). The NeuN and DNMT1 counts were performed blindly in three randomly selected squares in each of five to six sections; thus, a total of 15–18 squares per sample were counted. All analyses were carried out in comparable areas (100 × 100 μm) under the same optical and illumination conditions (for example, light intensity was set to 8 on a scale of 1–10, and exposure time was set to 1.5 ms) in a Zeiss MicroImager. Black and white images were digitized and viewed on a computer using AxioVision software.
Statistical analysis
Results are expressed as the mean ± SE. Analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison test was used. The criterion for significance was *P < 0.05.
Digital photomicrography and production
DAB stained images were captured by AxioVision 3.1 (Zeiss), while confocal immunofluorescent images were captured using a Leica Confocal Microscope (Leica Microsystems). Photoshop CS4 (Extended v. 11.0, Adobe Systems, San Jose, CAQ) was used to adjust contrast, brightness, and sharpness of the captured images. Red-green fluorescent images were converted to magenta-green images also using Adobe Photoshop CS4. Finally, images were imported into CanvasX System v. 10.5.8 for final figure production and subsequent conversion to tif-format.
RESULTS
Colocalization of GFP and GAD67
To confirm the original findings with respect to the colocalization of GFP and GAD67 in the knockin mice, we performed double immunofluorescence histochemistry using antibodies directed against GFP and GAD67. In this first group of experiments we examined the colocalization of these antigens in the frontal cortex, striatum, and hippocampus of heterozygous GAD67-GFP knockin mice with confocal microscopy. As shown in Figure 1, the extent of overlap between GAD67 and GFP fluorescence was greater than 90%. The colocalization of GFP and GAD67 was extended to include additional brain regions including the piriform cortex and BLA. Cerebellar Purkinje cells showed inconsistent staining, as previously reported (Tamamaki et al., 2003; data not shown).
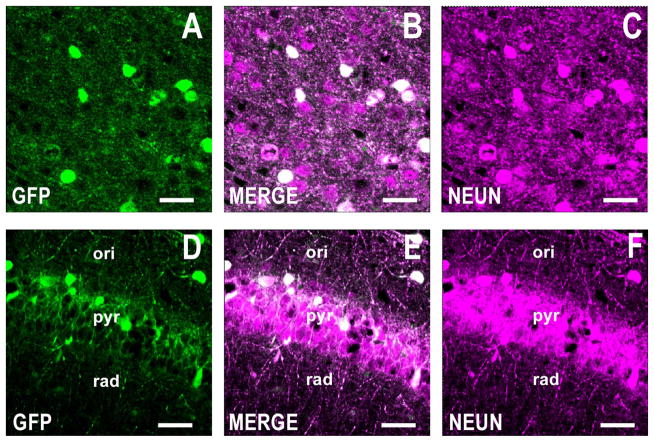
To confirm previous findings regarding neuronal expression of GFP in the GAD67-GFP knockin mouse (Tamamaki et al., 2003), we examined the colocalization of GFP and NeuN by immunofluorescence histochemistry. In the cortex and hippocampus, virtually all of the GFP-positive cells coexpress NeuN, indicating that GFP is expressed in neurons and not in glial cells (Fig. 2). Collectively, these results demonstrate that GFP expression is regulated by the endogenous GAD67 promoter and that GFP-positive cells are indeed GABAergic neurons.
Figure 2.
GFP is expressed in NeuN-positive cells of the GAD67-GFP knockin mouse. Roughly 100% of GFP-positive cells are positive for NeuN. Confocal double immunofluorescence labeling of GFP coded in green (left panels), NeuN is color-coded in magenta (right panels), and merged images in white (center panels). A–C: Motor cortex, layers I/II. Coronal section corresponds roughly to bregma +1.4 mm. D–F: CA1 field of the hippocampus. Coronal section corresponds roughly to bregma −2 mm. Scale bars = 40 μm.
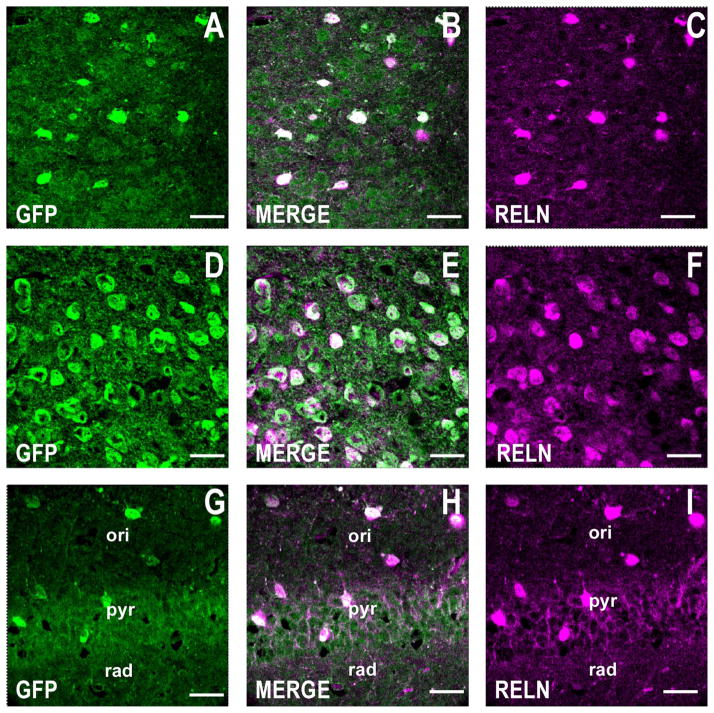
In the following experiments we used GAD67-GFP mice to investigate whether GFP colocalizes with RELN and the vesicular glutamate transporter 2 (VGLUT2). While RELN is primarily expressed in telencephalic GABAergic neurons in the adult brain (Pesold et al., 1999), VGLUT2 is mostly expressed in glutamatergic neurons in cortical layers IV and VI (Fremeau et al., 2001), in layer V pyramidal neurons (Agis-Balboa et al., 2007), and in principal excitatory neurons in other telencephalic areas. Similar to GAD67, RELN immunofluorescence is only expressed in GFP-positive neurons (Fig. 3). As anticipated, VGLUT2-positive neurons fail to express GFP immunofluorescence (Fig. 4) or RELN (data not shown).
Figure 3.
RELN is coexpressed with GFP in telencephalic GABAergic neurons in the GAD67-GFP knockin mouse. A large proportion of the GFP-positive GABAergic neurons are also positive for RELN. Confocal double immunofluorescence labeling of GFP coded in green (left panels), RELN color coded in magenta (right panels), and merged images in white (center panels). A–C: Motor cortex, layers I/II. Coronal sections correspond to bregma +1.4 mm. D–F: Striatum. Coronal sections are as described in A–C. G–I: CA1 field of the hippocampus. Coronal section corresponds roughly to bregma −2 mm. Scale bars = 40 μm.
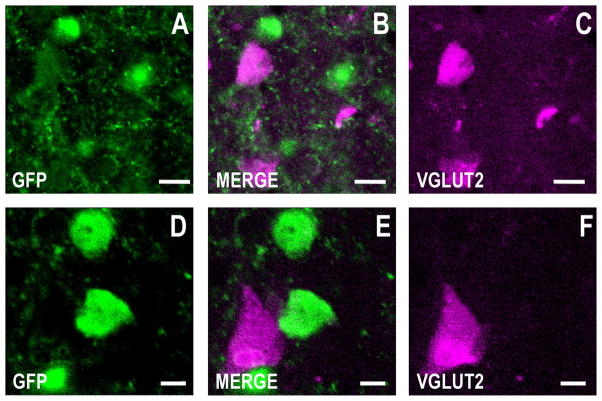
Figure 4.
GFP is not expressed in cortical glutamatergic neurons in the GAD67-GFP knockin mouse. Note the absence of colocalization between GFP and VGLUT2 immunofluorescence. Confocal double immunofluorescence labeling of GFP coded in green (left panels), RELN color-coded in magenta (right panels), and merged images in white (center panels). A–C: Motor cortex, layer II. D–F: Motor cortex, layer III. Coronal sections correspond roughly to bregma +1.4 mm. Scale bars = 20 μm in A–C; 5 μm in D–F.
Colocalization of GFP and DNMTs
We next examined the colocalization of DNMT1 with GFP in the GAD67-GFP mouse (Fig. 5). In the motor cortex, piriform cortex, striatum, CA1 region of the hippocampus, dentate gyrus, and BLA, GFP immunofluorescence coincides with the signal corresponding to DNMT1. It is noteworthy that at larger magnifications GFP immunofluorescence is present throughout the neuronal somata and also includes the neuropil (axon terminals and dendrites), whereas DNMT1 fluorescence is mostly confined to the somata of the GFP-positive cells (Fig. 6).
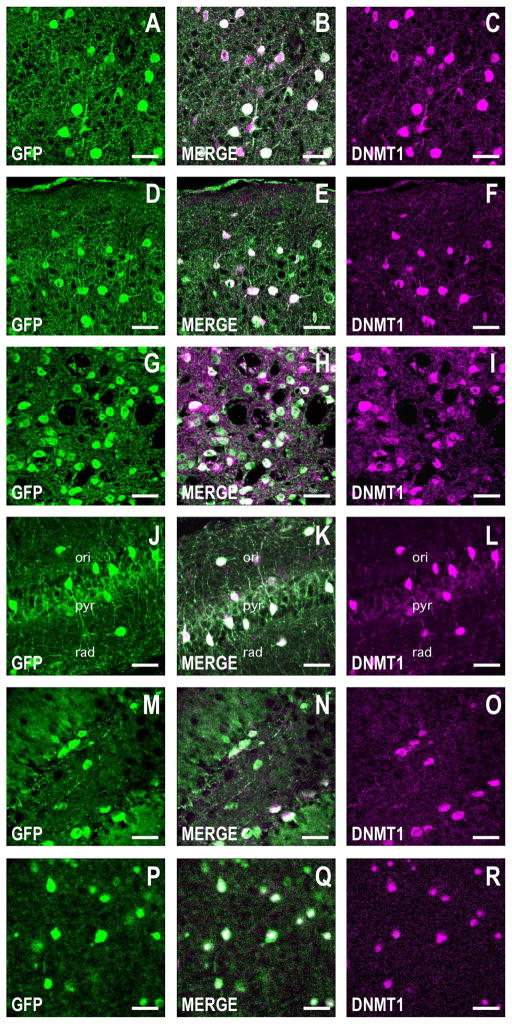
Figure 5.
DNMT1 is coexpressed with GFP in corticolimbic GABAergic neurons in the GAD67-GFP knockin mouse. Confocal double immunofluorescence labeling of GFP coded in green (left panels), DNMT1 color coded in magenta (right panels), and merged images in white (center panels). A–C: Layers I and II of the motor cortex. Coronal sections correspond roughly to bregma +1.4 mm. D–F: Layers I and II of the piriform cortex. Coronal section as described in A–C. G–I: Striatum. Coronal section as described in A–C. J–L: CA1 field of the hippocampus. Coronal section corresponding roughly to bregma −2 mm. M–O: Dentate gyrus. Coronal section as in J–L. P–R: Basolateral amygdala (BLA). Coronal section as described in M–O. Note that in all corticolimbic areas studied, greater than 90% of GFP-positive cells are also positive for DNMT1. Scale bars = 40 μm.
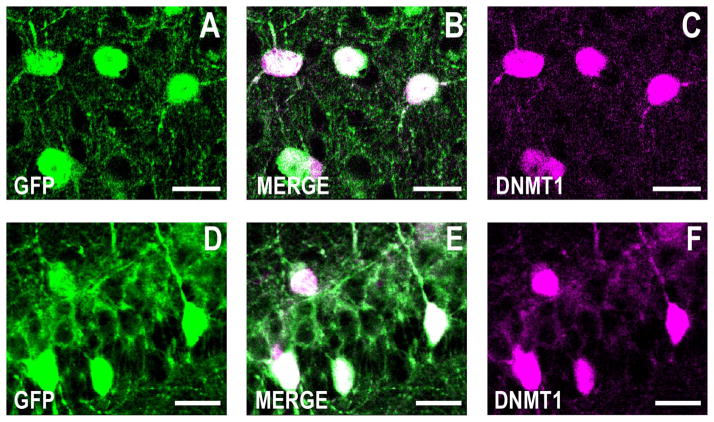
Figure 6.
Larger magnification of GFP and DNMT1 immunofluorescence showing that both colocalize to the neuronal somata. Confocal double immunofluorescence labeling of GFP in green (left panels), DNMT1 in magenta (right panels), and merged images in white (middle panels). A–C: Larger magnification of Figure 2D–F showing double labeling of GFP and DNMT1 immunofluorescence in the piriform cortex. Coronal section corresponding roughly to bregma +1.4 mm. D–F: Larger magnification of Figure 2J–L showing double labeling of GFP and DNMT1 immunofluorescence in the CA1 hippocampal formation. Coronal sections corresponding to bregma −2 mm. Note that while DNMT1 immunofluorescence is primarily limited to the somata, GFP immunofluorescence is also abundant in the neuropil. Scale bars = 20 μm.
We also report that similar to DNMT1, in the motor cortex, striatum, hippocampus, and amygdala, GFP immunofluorescence colocalizes with the fluorescent immunohistochemical signal detected with the DNMT3a antibody (Fig. 7).
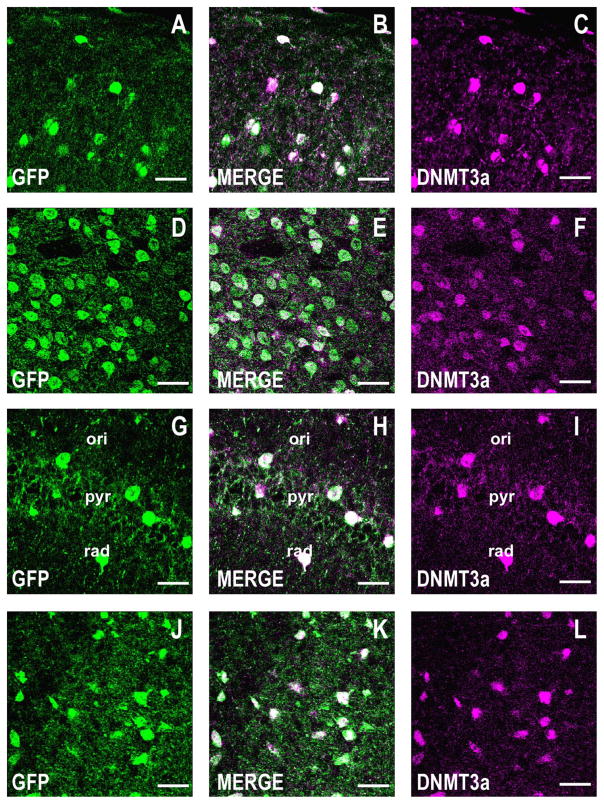
Figure 7.
DNMT3a is coexpressed with GFP in corticolimbic GABAergic neurons of the GAD67-GFP knockin mouse. Confocal double immunofluorescence labeling of GFP (green), DNMT3a (magenta), and merged images in white (center panels). A–C: Layers I and II of the motor cortex. Coronal sections correspond roughly to bregma +1.4 mm. D–F: Striatum. Coronal sections as described in A–C. G–I: CA1 field of the hippocampus. Coronal sections correspond roughly to bregma −2 mm. J–L: Basolateral amygdala. Coronal section corresponding roughly to bregma −2 mm. Scale bars = 40 μm.
NeuN- and DNMT1-positive neurons in the frontal cortex
In Figure 8 we show that NeuN-immunopositive cells are significantly more abundant than DNMT1-positive cells, particularly in cortical layers III to V. The data reflect a ratio of ≈30% between DNMT1 (GABAergic interneurons) and the total number of neurons in this brain area.
DISCUSSION
The expression and activity of DNMTs is abundant in dividing cells and is very high, particularly during development. Previous studies have reported that in adult mammalian brains, which primarily contain postmitotic terminally differentiated neurons and glial cells, the expression levels of DNMT1 and DNMT3a mRNA and proteins are evident but, interestingly, their neuronal/cellular distribution is uneven (Inano et al., 2000; Veldic et al., 2004, 2007; Feng et al., 2005). For example, immunohistochemical studies and biochemical measurements of DNMT1 in laser microdissected neurons from prefrontal cortex (PFC) postmortem human brain indicate that DNMT1 is highly expressed in GABAergic interneurons but, in contrast, is expressed at very low levels in pyramidal neurons and glial cells (Veldic et al., 2005, 2007; Ruzicka et al., 2007; Zhubi et al., 2009).
Our data show that the neuronal localization of DNMT1 in adult GAD67-GFP mice is consistent with previous studies of DNMT1 and DNMT3a in mouse neurons (Satta et al., 2008). DNMT1 and DNMT3a are highly expressed in the GAD67-GFP mouse brain and, more important, similar to reports of studies of the human brain, the expression of these proteins is almost exclusively localized to GABAergic interneurons (Veldic et al., 2004, 2005, 2007; Zhubi et al., 2009). For example, virtually all GABAergic GFP-positive neurons in cortex, striatum, hippocampus, and BLA also express high levels of DNMT1 and 3a immunoreactivity. DNMT immunostaining is virtually absent in pyramidal and other glutamatergic principal neurons in the telencephalon of these mice.
We have previously shown in the adult telencephalon of mice (Liu et al., 2001), Patas monkeys (Rodriguez et al., 2002), and humans (Veldic et al., 2005; Ruzicka et al., 2007) that RELN and GAD67 colocalize. This was further explored in the current study in which we demonstrate a high percentage of GFP-positive neurons are also RELN-positive. In contrast, there is no overlap between GFP and VGLUT2, one of two excitatory amino acid transporters and a marker for glutamatergic neurons (Fremeau et al., 2001; Agis-Balboa et al., 2007). Collectively, these data suggest that DNMT1 and DNMT3a may play important roles in the epigenetic regulation of GABAergic gene expression.
In future experiments, we will take advantage of GAD67/GFP mice to isolate populations of cells enriched in GABAergic neurons to study the role of DNMT in the epigenetic control of GABAergic function. GABAergic inter-neurons are involved in complex tasks such as shaping receptive fields of pyramidal neurons, synchronizing the activity of cortical neurons, controlling information processing within the PFC, and more generally, controlling principal neuron excitability in the cortex, hippocampus, and amygdala (Mountcastle, 1998). To accomplish these functions, GABAergic interneurons express unique patterns of electrical activity and are easily recognized compared with other types of neurons because of their fast-spiking discharge rate in response to sudden changes in synaptic activity (Gonzalez-Burgos and Lewis, 2008). Hence, a possible role for the high levels of DNMTs in telencephalic GABAergic interneurons may be that of controlling specific GABAergic genes via epigenetic mechanisms regulating transcription. This would include promoters corresponding to genes such as GAD67, RELN, members of the NMDA receptor subunits, various Ca2+ binding proteins, and GABA-transporter-1, all of which contribute to the control of neuronal excitability and maintenance of synaptic plasticity (Guidotti et al., 2011).
The absence of detectable DNMT immunoreactivity in glutamatergic principal neurons or glia does not exclude the presence of lower levels of DNMT in these cells. Rather, it indicates that in pyramidal neurons and glia, the levels of DNMT are below the threshold for immunohistochemical detection and are low compared to the level of DNMTs in GABAergic interneurons. In fact, recent studies suggest that DNMTs are present in cortical pyramidal neurons and their presence may play an important role in dendritic arborization and synaptic plasticity (Feng et al., 2010).
To study DNMT1 and DNMT3a loss of function in neurons, conditional mutant mice have been generated and analyzed (Fan et al., 2001; Nguyen et al., 2007). Various cre-recombinase-expressing mouse lines have been used to target DNMT deletions to distinct neurons at specific times during development and in the adult. The promoters that have been used to drive cre expression include nestin (Fan et al., 2001; Nguyen et al., 2007), empty spiracles homeobox-1 (Emx1, Golshani et al., 2005; Hutnick et al., 2009), and CamK (Fan et al., 2001; Feng et al., 2010). Nestin is expressed in many types of cells during early development and in neuronal precursor cells (Dubois et al., 2006). Emx1 is expressed in differentiating and mature glutamatergic cortical neurons (Bishop et al., 2003) and CamK is selectively expressed in a subpopulation of excitatory neurons that show no overlap with GAD67-expressing neurons (Jones et al., 1994). The conditional mutant mice generated with each of these cre-driver lines show clear phenotypes that have been associated with alterations in DNA methylation and the downregulation of affected mRNAs. In Emx1-cre/DNMT1 mutant mice, loss of DNMT1 in Emx1-positive neurons disrupts development of the somatosensory barrel cortex (Golshani et al., 2005) and impairs postnatal neuronal maturation (Hutnick et al., 2009). The double DNMT1/3a conditional knockout in CamK-expressing neurons shows deficits in synaptic plasticity, learning, and memory (Feng et al., 2010). While our results show that GABAergic neurons express much higher amounts of both DNMT1 and 3a, the above studies indicate a function for DNMT1 and DNMT3a in glutamatergic neurons during development and following maturation.
As both DNMT1 and DNMT3a mRNAs are more abundantly expressed in GABAergic neurons in all brain regions examined, the availability of GAD67 cre (Higo et al., 2009)- and parvalbumin cre (Tanahira et al., 2009)-mice provides a unique opportunity to examine the function of DNMTs in GABAergic neurons. Our previous studies demonstrating the increased expression of DNMT1 and DNMT3a in GABAergic neurons of schizophrenia patients (see Guidotti et al., 2005, 2011, for recent reviews) provide a compelling rationale for examining DNMT function in these neurons to gain novel insight into the pathogenesis of this devastating psychiatric disorder.
Acknowledgments
Grant sponsor: National Institutes of Health; Grant number: 1R01MH093348-01A1 (to A.G.).
The authors thank Dr. Yuchio Yanagawa, Department of Genetic and Behavioral Neuroscience, Gumma University Graduate School of Medicine, for permission to use the GAD67-GFP mice for this study. These mice were obtained locally from Dr. Ken Mackie, Department of Psychological and Brain Sciences, Indiana University, Bloomington, Indiana.
Abbreviations
- BLA
Basolateral amygdala
- BPD
Bipolar disorder
- CA
Cornu ammonis
- CamK
Calmodulin-kinase IIa
- CNS
Central nervous system
- DAB 3
3′-Diaminobenzidine tetrahydrochloride
- DNMT
DNA methyltransferase
- Emx1
Empty spiracles homeobox
- GAD67
Glutamic acid decarboxylase 67
- GFP
Enhanced green fluorescent protein
- NeuN
Neuronal nuclei
- ori
Stratum oriens
- pyr
Stratum pyramidale
- rad
Stratum radiatum
- RELN
Reelin
- PFC
Prefrontal cortex
- SZ
Schizophrenia
- VGLUT2
Vesicular glutamate transporter
LITERATURE CITED
- Abdolmaleky HM, Cheng KH, Russo A, Smith CL, Faraone SV, Wilcox M, Shafa R, Glatt SJ, Nguyen G, Ponte JF, Thiagalingam S, Tsuang MT. Hypermethylation of the RELN (RELN) promoter in the brain of schizophrenic patients: a preliminary report. Am J Med Genet B Neuropsychiatr Genet. 2005;134B:60–66. doi: 10.1002/ajmg.b.30140. [DOI] [PubMed] [Google Scholar]
- Agis-Balboa RC, Pinna G, Pibiri F, Kadriu B, Costa E, Guidotti A. Down-regulation of neurosteroid biosynthesis in corticolimbic circuits mediates social isolation-induced behavior in mice. Proc Natl Acad Sci U S A. 2007;104:18736–18741. doi: 10.1073/pnas.0709419104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada H, Kawamura Y, Maruyama K, Kume H, Ding RG, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Cleft palate and decreased brain gamma-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 1997;94:6496–6499. doi: 10.1073/pnas.94.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Lange N. Two-dimensional versus three-dimensional cell counting: a practical perspective. Trends Neurosci. 2001;24:11–17. doi: 10.1016/s0166-2236(00)01660-x. [DOI] [PubMed] [Google Scholar]
- Bishop KM, Garel S, Nakagawa Y, Rubenstein JL, O’Leary DD. Emx1 and Emx2 cooperate to regulate cortical size, lamination, neuronal differentiation, development of cortical efferents, and thalamocortical pathfinding. J Comp Neurol. 2003;457:345–360. doi: 10.1002/cne.10549. [DOI] [PubMed] [Google Scholar]
- Chen T, Ueda Y, Xie S, Li E. A novel Dnmt3a isoform produced from an alternative promoter localizes to euchromatin and its expression correlates with active de novo methylation. J Biol Chem. 2002;277:38746–38754. doi: 10.1074/jbc.M205312200. [DOI] [PubMed] [Google Scholar]
- Cheng X, Blumenthal RM. Mammalian DNA methyltransferases: a structural perspective. Structure. 2008;16:341–350. doi: 10.1016/j.str.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condie BG, Bain G, Gottlieb DI, Capecchi MR. Cleft palate in mice with a targeted mutation in the gamma-amino-butyric acid-producing enzyme glutamic acid decarboxylase 67. Proc Natl Acad Sci U S A. 1997;94:11451–11455. doi: 10.1073/pnas.94.21.11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Sweatt JD. DNA methylation and memory formation. Nat Neurosci. 2010;13:1319–1323. doi: 10.1038/nn.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Sweatt JD. Cognitive neuroepigenetics: a role for epigenetic mechanisms in learning and memory. Neurobiol Learn Mem. 2011;96:2–12. doi: 10.1016/j.nlm.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bergeyck V, Nakajima K, Lambert de Rouvroit C, Naerhuyzen B, Goffinet AM, Miyata T, Ogawa M, Mikoshiba K. A truncated Reelin protein is produced but not secreted in the ‘Orleans’ reeler mutation (Reln[rl-Orl]) Brain Res Mol Brain Res. 1997;50:85–90. doi: 10.1016/s0169-328x(97)00166-6. [DOI] [PubMed] [Google Scholar]
- de Bergeyck V, Naerhuyzen B, Goffinet AM, Lambert de Rouvroit C. A panel of monoclonal antibodies against reelin, the extracellular matrix protein defective in reeler mutant mice. J Neurosci Methods. 1998;82:17–24. doi: 10.1016/s0165-0270(98)00024-7. [DOI] [PubMed] [Google Scholar]
- Dubois NC, Hofmann D, Kaloulis K, Bishop JM, Trumpp A. Nestin-Cre transgenic mouse line Nes-Cre1 mediates highly efficient Cre/loxP mediated recombination in the nervous system, kidney, and somite-derived tissues. Genesis. 2006;44:355–360. doi: 10.1002/dvg.20226. [DOI] [PubMed] [Google Scholar]
- Fan G, Beard C, Chen RZ, Csankovszki G, Sun Y, Siniaia M, Biniszkiewicz D, Bates B, Lee PP, Kuhn R, Trumpp A, Poon C, Wilson CB, Jaenisch R. DNA hypomethylation perturbs the function and survival of CNS neurons in post-natal animals. J Neurosci. 2001;21:788–797. doi: 10.1523/JNEUROSCI.21-03-00788.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Chang H, Li E, Fan G. Dynamic expression of de novo DNA methyltransferases Dnmt3a and Dnmt3b in the central nervous system. J Neurosci Res. 2005;79:734–746. doi: 10.1002/jnr.20404. [DOI] [PubMed] [Google Scholar]
- Feng J, Zhou Y, Campbell SL, Le T, Li E, Sweatt JD, Silva AJ, Fan G. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat Neurosci. 2010;13:423–430. doi: 10.1038/nn.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Goll MG, Bestor TH. Eukaryotic cytosine methyltransferases. Annu Rev Biochem. 2005;74:481–514. doi: 10.1146/annurev.biochem.74.010904.153721. [DOI] [PubMed] [Google Scholar]
- Golshani P, Hutnick L, Schweizer F, Fan G. Conditional Dnmt1 deletion in dorsal forebrain disrupts development of somatosensory barrel cortex and thalamocortical long-term potentiation. Thalamus Relat Syst. 2005;3:227–233. doi: 10.1017/S1472928807000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson DR, Jia X, Chen Y, Sharma RP, Mitchell CP, Guidotti A, Costa E. Reelin promoter hypermethylation in schizophrenia. Proc Natl Acad Sci U S A. 2005;102:9341–9346. doi: 10.1073/pnas.0503736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson DR, Chen Y, Costa E, Dong E, Guidotti A, Kundakovic M, Sharma RP. The human reelin gene: transcription factors (+), repressors (−) and the methylation switch (+/−) in schizophrenia. Pharmacol Ther. 2006;111:272–286. doi: 10.1016/j.pharmthera.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Griffin GD, Ferri-Kolwicz SL, Reyes BA, Van Bockstaele EJ, Flanagan-Cato LM. Ovarian hormone-induced reorganization of oxytocin-labeled dendrites and synapses lateral to the hypothalamic ventromedial nucleus in female rats. J Comp Neurol. 2010;518:4531–4545. doi: 10.1002/cne.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Dong E, Grayson DR, Veldic M, Zhang X, Costa E. GABAergic dysfunction in schizophrenia: new treatment strategies on the horizon. Psychopharmacology (Berl) 2005;180:191–205. doi: 10.1007/s00213-005-2212-8. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Chen Y, Davis JM, Dong E, Gavin DP, Grayson DR, Matrisciano F, Pinna G, Satta R, Sharma RP, Tremolizzo L, Tueting P. Epigenetic GABAergic targets in schizophrenia and bipolar disorder. Neuropharmacology. 2011;60:1007–1016. doi: 10.1016/j.neuropharm.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higo S, Akashi K, Sakimura K, Tamamaki N. Subtypes of GABAergic neurons project axons in the neocortex. Front Neuroanat. 2009;3:25. doi: 10.3389/neuro.05.025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi F, Uchida S, Yamagata H, Otsuki K, Hobara T, Abe N, Shibata T, Watanabe Y. State-dependent changes in the expression of DNA methyltransferases in mood disorder patients. J Psychiatr Res. 2011;45:1295–300. doi: 10.1016/j.jpsychires.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Hutnick LK, Golshani P, Namihira M, Xue Z, Matynia A, Yang XW, Silva AJ, Schweizer FE, Fan G. DNA hypomethylation restricted to the murine forebrain induces cortical degeneration and impairs postnatal neuronal maturation. Hum Mol Genet. 2009;18:2875–2888. doi: 10.1093/hmg/ddp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inano K, Suetake I, Ueda T, Miyake Y, Nakamura M, Okada M, Tajima S. Maintenance-type DNA methyltransferase is highly expressed in post-mitotic neurons and localized in the cytoplasmic compartment. J Biochem. 2000;128:315–321. doi: 10.1093/oxfordjournals.jbchem.a022755. [DOI] [PubMed] [Google Scholar]
- Jair KW, Bachman KE, Suzuki H, Ting AH, Rhee I, Yen RW, Baylin SB, Schuebel KE. De novo CpG island methylation in human cancer cells. Cancer Res. 2006;66:682–692. doi: 10.1158/0008-5472.CAN-05-1980. [DOI] [PubMed] [Google Scholar]
- Jones EG, Huntley GW, Benson DL. Alpha calcium/cal-modulin-dependent protein kinase II selectively expressed in a subpopulation of excitatory neurons in monkey sensory-motor cortex: comparison with GAD-67 expression. J Neurosci. 1994;14:611–629. doi: 10.1523/JNEUROSCI.14-02-00611.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkowska RZ, Jurkowski TP, Jeltsch A. Structure and function of mammalian DNA methyltransferases. Chem Biochem. 2011;12:206–222. doi: 10.1002/cbic.201000195. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Chen Y, Costa E, Grayson DR. DNA methyltransferase inhibitors coordinately induce expression of the human reelin and glutamic acid decarboxylase 67 genes. Mol Pharmacol. 2007;71:644–653. doi: 10.1124/mol.106.030635. [DOI] [PubMed] [Google Scholar]
- Liu WS, Pesold C, Rodriguez MA, Carboni G, Auta J, Lacor P, Larson J, Condie BG, Guidotti A, Costa E. Down-regulation of dendritic spine and glutamic acid decarboxylase 67 expressions in the reelin haploinsufficient heterozygous reeler mouse. Proc Natl Acad Sci U S A. 2001;97:3550–3555. doi: 10.1073/pnas.051614698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald JL, Gin CS, Roskams AJ. Stage-specific induction of DNA methyltransferases in olfactory receptor neuron development. Dev Biol. 2005;288:461–473. doi: 10.1016/j.ydbio.2005.09.048. [DOI] [PubMed] [Google Scholar]
- Mill J, Tang T, Kaminsky Z, Khare T, Yazdanpanah S, Bouchard L, Jia P, Assadzadeh A, Flanagan J, Schumacher A, Wang SC, Petronis A. Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. Am J Hum Genet. 2008;82:696–711. doi: 10.1016/j.ajhg.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle VB. Perceptual neuroscience: the cerebral cortex. Cambridge, MA: Harvard University Press; 1998. [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Nguyen S, Meletis K, Fu D, Jhaveri S, Jaenisch R. Ablation of de novo DNA methyltransferase Dnmt3a in the nervous system leads to neuromuscular defects and shortened lifespan. Dev Dyn. 2007;236:1663–1676. doi: 10.1002/dvdy.21176. [DOI] [PubMed] [Google Scholar]
- Pesold C, Impagnatiello F, Pisu MG, Uzunov DP, Costa E, Guidotti A, Caruncho HJ. Reelin is preferentially expressed in neurons synthesizing gamma-aminobutyric acid in cortex and hippocampus of adult rats. Proc Natl Acad Sci U S A. 1998;95:3221–3226. doi: 10.1073/pnas.95.6.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesold C, Liu WS, Guidotti A, Costa E, Caruncho HJ. Cortical bitufted, horizontal, and Martinotti cells preferentially express and secrete reelin into perineuronal nets, nonsynaptically modulating gene expression. Proc Natl Acad Sci U S A. 1999;96:3217–3222. doi: 10.1073/pnas.96.6.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter MO, Du L, Weaver IC, Palkovits M, Faludi G, Merali Z, Szyf M, Anisman H. GABAA receptor promoter hyper-methylation in suicide brain: implications for the involvement of epigenetic processes. Biol Psychiatry. 2008;64:645–652. doi: 10.1016/j.biopsych.2008.05.028. [DOI] [PubMed] [Google Scholar]
- Rodriguez MA, Caruncho HJ, Costa E, Pesold C, Liu WS, Guidotti A. In Patas monkey, glutamic acid decarboxylase-67 and reelin mRNA coexpression varies in a manner dependent on layers and cortical areas. J Comp Neurol. 2002;451:279–288. doi: 10.1002/cne.10341. [DOI] [PubMed] [Google Scholar]
- Ruzicka WB, Zhubi A, Veldic M, Grayson DR, Costa E, Guidotti A. Selective epigenetic alteration of layer I GABAergic neurons isolated from prefrontal cortex of schizophrenia patients using laser-assisted microdissection. Mol Psychiatry. 2007;12:385–397. doi: 10.1038/sj.mp.4001954. [DOI] [PubMed] [Google Scholar]
- Sakai K, Miyazaki J. A transgenic mouse line that retains Cre recombinase activity in mature oocytes irrespective of the cre transgene transmission. Biochem Biophys Res Commun. 1997;237:318–324. doi: 10.1006/bbrc.1997.7111. [DOI] [PubMed] [Google Scholar]
- Satta R, Maloku E, Zhubi A, Pibiri F, Hajos M, Costa E, Guidotti A. Nicotine decreases DNA methyltransferase 1 expression and glutamic acid decarboxylase 67 promoter methylation in GABAergic interneurons. Proc Natl Acad Sci U S A. 2008;105:16356–16361. doi: 10.1073/pnas.0808699105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Tanahira C, Higo S, Watanabe K, Tomioka R, Ebihara S, Kaneko T, Tamamaki N. Parvalbumin neurons in the forebrain as revealed by parvalbumin-Cre transgenic mice. Neurosci Res. 2009;63:213–323. doi: 10.1016/j.neures.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Van Emburgh BO, Robertson KD. Modulation of Dnmt3b function in vitro by interactions with Dnmt3L, Dnmt3a and Dnmt3b splice variants. Nucleic Acids Res. 2011;39:4984–5002. doi: 10.1093/nar/gkr116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldic M, Caruncho HJ, Liu WS, Davis J, Satta R, Grayson DR, Guidotti A, Costa E. DNA-methyltransferase 1 mRNA is selectively overexpressed in telencephalic GABAergic interneurons of schizophrenia brains. Proc Natl Acad Sci U S A. 2004;101:348–353. doi: 10.1073/pnas.2637013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldic M, Guidotti A, Maloku E, Davis JM, Costa E. In psychosis, cortical interneurons overexpress DNA-methyltransferase 1. Proc Natl Acad Sci U S A. 2005;102:2152–2157. doi: 10.1073/pnas.0409665102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldic M, Kadriu B, Maloku E, Agis-Balboa RC, Guidotti A, Davis JM, Costa E. Epigenetic mechanisms expressed in basal ganglia GABAergic neurons differentiate schizophrenia from bipolar disorder. Schizo Res. 2007;91:51–61. doi: 10.1016/j.schres.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe D, Uchiyama K, Hanaoka K. Transition of mouse de novo methyltransferases expression from Dnmt3b to Dnmt3a during neural progenitor cell development. Neuroscience. 2006;142:727–737. doi: 10.1016/j.neuroscience.2006.07.053. [DOI] [PubMed] [Google Scholar]
- Wolf HK, Buslei R, Schmidt-Kastner R, Schmidt-Kastner PK, Pietsch T, Wiestler OD, Blümcke I. NeuN: a useful neuronal marker for diagnostic histopathology. J Histochem Cytochem. 1996;44:1167–1171. doi: 10.1177/44.10.8813082. [DOI] [PubMed] [Google Scholar]
- Zhubi A, Veldic M, Puri NV, Kadriu B, Caruncho H, Loza I, Sershen H, Lajtha A, Smith RC, Guidotti A, Davis JM, Costa E. An upregulation of DNA-methyltransferase 1 and 3a expressed in telencephalic GABAergic neurons of schizophrenia patients is also detected in peripheral blood lymphocytes. Schizophr Res. 2009;111:115–122. doi: 10.1016/j.schres.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]