Abstract
Objective
The development of a novel surgical tool or technique for mitral valve repair can be hampered by cost, complexity, and time associated with performing animal trials. A dynamically pressurized model was developed to control pressure and flowrate profiles in intact porcine hearts in order to quantify mitral regurgitation and evaluate the quality of mitral valve repair.
Methods
A pulse duplication system was designed to replicate physiological conditions in explanted hearts. To test the capabilities of this system in measuring varying degrees of mitral regurgitation, the output of eight porcine hearts was measured for two different pressure waveforms before and after induced mitral valve failure. Four hearts were further repaired and tested. Measurements were compared with echocardiographic images.
Results
For all trials, cardiac output decreased as left ventricular pressure was increased. After induction of mitral valve insufficiencies, cardiac output decreased, with a peak regurgitant fraction of 71.8%. Echocardiography clearly showed increases in regurgitant severity from post-valve failure and with increased pressure.
Conclusions
The dynamic heart model consistently and reliably quantifies mitral regurgitation across a range of severities. Advantages include low experimental cost and time associated with each trial, while still allowing for surgical evaluations in an intact heart.
Keywords: Dynamic heart model, Mitral regurgitation, Mitral valve repair
INTRODUCTION
The development of new surgical tools and techniques for heart valve repair requires extensive testing and validation before they can be introduced into the clinical setting; however, the preliminary stages of prototype development can be hampered by the time and cost associated with animal trials. While in vivo animal trials are the surgical standard for evaluating new technologies and approaches, and are a necessary step in product validation, they are frequently inefficient choices for the initial exploration of new methods of heart valve repair. The early stages of the design process could be greatly shortened by the utilization of a cost-effective, dynamic model to evaluate emerging heart valve repair techniques, lessening the need for laboratory animal sacrifice.
In this study, we demonstrate the feasibility of an explanted heart model to facilitate the development of surgical tools and techniques for mitral valve repair. This left heart pulse duplication system dynamically pressurizes porcine hearts to replicate a wide range of physiological conditions and mitral valve deficiencies. This system uses a computer-controlled positive displacement pump and the heart’s own valves to precisely direct the flow of saline, subjecting the mitral valve to pressure waveforms and flow rates similar to those found in vivo.
To demonstrate the effectiveness of this system in modeling and quantifying different degrees of mitral regurgitation, experiments were conducted to monitor changes in cardiac output with varying ventricular pressures, before and after induced mitral valve insufficiency, and again after valve repair. Experimental measurements from the system were compared to clinically standard echocardiographic images.
MATERIALS AND METHODS
Construction
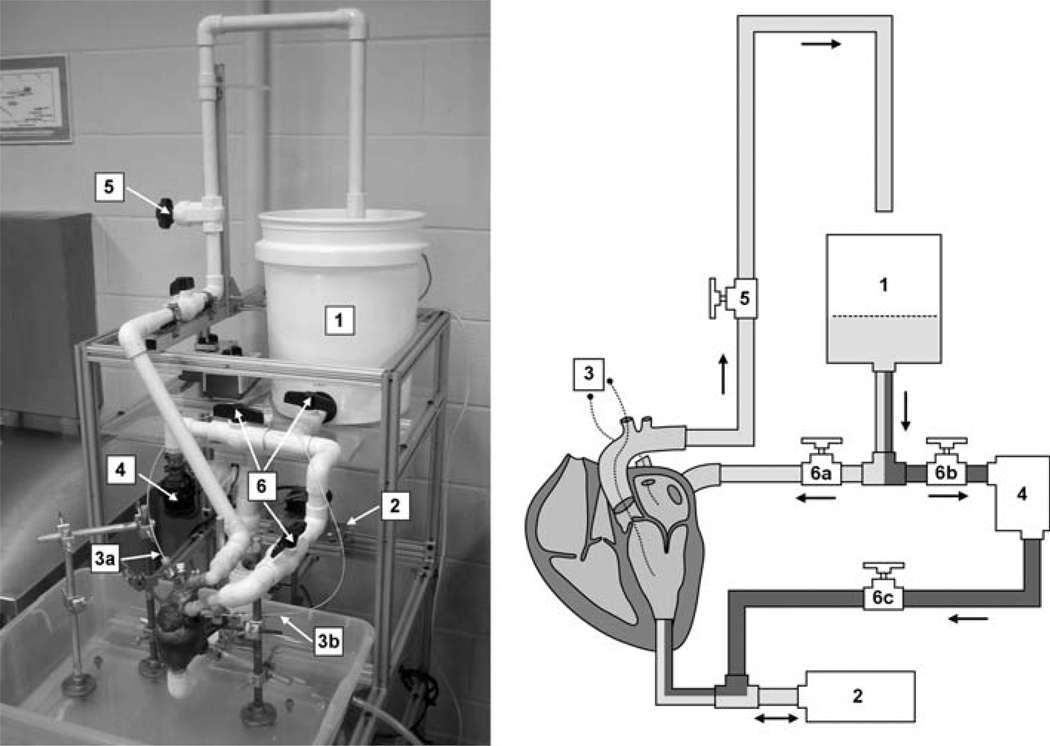
The proposed system consists of three mechanical components connected to a freshly explanted porcine heart: a left atrial reservoir, an aortic outflow pathway, and a computer-controlled positive displacement (PD) pump (Fig. 1). The left atrial reservoir passively fills the left atrium through a pulmonary vein cannula. The height of the fluid in this reservoir can be adjusted to modify left atrial pressure. The aortic outflow pathway, which drains into the atrial reservoir, is connected via an aortic cannula to the heart. The height of this tube can be adjusted to regulate backpressure against the aortic valve. Additionally, a valve on the aortic outflow pathway allows precise modification of flow resistance and can be used to increase peak left ventricular pressure. All primary fluid pathways were constructed using 20.93 mm ID PVC tubing.
FIGURE 1.
The proposed system. Left: photograph of the system in operation. Right: schematic of system components: atrial filling reservoir (1), PD pump (2), pressure catheters (3), centrifugal pump (4), aortic outflow resistance valve (5), and static pressure mode valves (6). Dynamic and static pressure mode pathways are shaded light and dark, respectively.
Dynamic pressurization of the left ventricle is achieved using a computer-controlled PD pump, connected via an apical cannula. During diastole (ventricular depressurization), retrograde motion of the piston draws fluid from the left atrium through the mitral valve and into the left ventricle and piston cylinder. During systole (ventricular pressurization), forward piston motion expels fluid from the left ventricle through the aortic valve. The PD pump consists of a LinMot model PS01–37×120 actuator (LinMot, Inc., Elkhorn, WI) and a custom-built piston and cylinder. The actuator’s linear encoder has a resolution of 100 µm, enabling feedback control of pump volume with a resolution of 0.0216 mL.
In addition to its dynamic pressure mode, the proposed system can be operated in static pressure mode for mitral valve tests requiring constant ventricular pressure. In static pressure mode, valves on the aortic and atrial lines (valves 5 and 6a, Fig. 1) are closed, preventing circulative flow from the atrial reservoir. A small centrifugal pump (Model 59510–0012, Jabsco, Glocester, MA) draws fluid from the atrial reservoir and pressurizes the left ventricle via apical cannulation to the desired level (up to 155 mmHg). This mode is used once the mitral valve has been exposed, so any regurgitant flow is collected in a basin beneath the heart stand.
Computer Control
Data acquisition and control of the PD pump are coordinated using a dedicated computer system programmed in LabVIEW Real-Time 8.5 (National Instruments, Austin, TX). This controller allows a wide range of customizable settings, including heart rate (HR, 30–180 bpm), pump stroke volume (PV, 0–150 mL), cardiac output rate (0–27 L/min), and systolic fraction (SF, 0–100%). In addition, customized pressure waveforms and flow rates can be achieved by controlling the pump trajectory. The control system also acquires, displays, and stores measurements from two pressure catheters at a sampling frequency of 1 kHz. For convenience in gating echocardiographic image capture, the system outputs a simulated ECG signal synchronized to the PD pump motion.
Heart Preparation
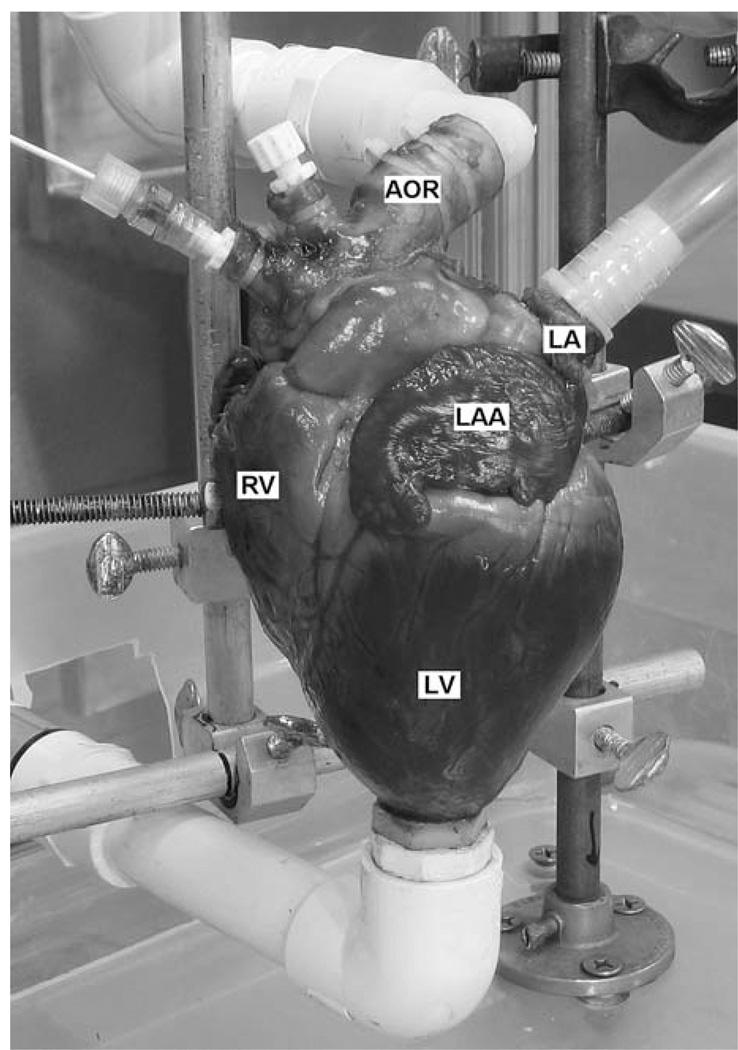
Fresh hearts from 70 to 100 kg swine are obtained from a local abattoir and trimmed of excess tissue. A 1.27 cm OD apical cannula is inserted through an undersized circular incision in the left ventricular dimple at the apex of the heart and secured with cyanoacrylate adhesive. A 2 cm OD aortic cannula is inserted and secured 2–3 cm distal to the aortic branch to preserve aortic compliance. The brachiocephalic and left common carotid/subclavian arteries are fitted at the base with Tuohy-Borst adapters for introduction of pressure catheters and endoscopic devices. The largest pulmonary vein is cannulated and secured with a 1.5 cm OD feed tube, while the smaller pulmonary vein is fitted with Tuohy-Borst adapters. All remaining veins and branches are closed using hemostat clamps. The heart is then secured to the test stand via right ventricle clamps and connected to the appropriate components (Fig. 2). After ensuring correct operation and no system leakage, the left and right coronary arteries are tied closed at the base of the aorta using suture to prevent any loss of output due to coronary perfusion.
FIGURE 2.
Heart connected to system, showing left ventricle (LV), right ventricle (RV), left atrium (LA), left atrial appendage (LAA), and aorta (AOR).
Determining Mitral Regurgitation
In a rigid pulsatile system using only explanted valves, the volumetric output of each pump stroke (pump stroke volume, PV) would exactly match the output of the aorta (cardiac output volume, CO). Because the proposed system uses intact hearts, which are compliant, some of the PV is used to expand the left ventricular walls during the systolic (pressurization) phase, causing the CO to be less than the PV. The difference between PV and CO is directly related to the pressure inside the left ventricle and will be constant for all cardiac cycles at the same pressure.
A valvular insufficiency, such as mitral regurgitation, allows some of the PV to be leaked, or regurgitated, into the left atrium, further reducing the total CO. The amount of regurgitation, like compliance loss, is also related to pressure in the left ventricle: as the pressure increases, so does the amount of regurgitant flow.3
If the cardiac output of a heart is measured before and after a failure is induced in the mitral valve, the decrease in CO will equal the regurgitant volume. As long as the pump volume and pressure waveforms remain constant, the compliance loss will be the same for both cases and will be removed from the equation. That is, for identical left ventricular pressure waveforms:
where CON and COD represent the measured cardiac output volumes associated with a normal and damaged mitral valve, respectively, PV represents the pump stroke volume, CL represents the compliance loss, and MR represents mitral regurgitation.
Using the same approach, the increase in cardiac function following a mitral valve repair operation can be determined by comparing the cardiac output of the repaired valve with that of the failed and normal valve states.
Normal Valve Data Collection
To demonstrate how the proposed system can be used to quantify mitral regurgitation in explanted porcine hearts, experiments were conducted to monitor changes in cardiac output of hearts before and after induction of mitral insufficiency. A repair operation was performed on a subset of these hearts to show how the system can be useful for evaluating surgical techniques.
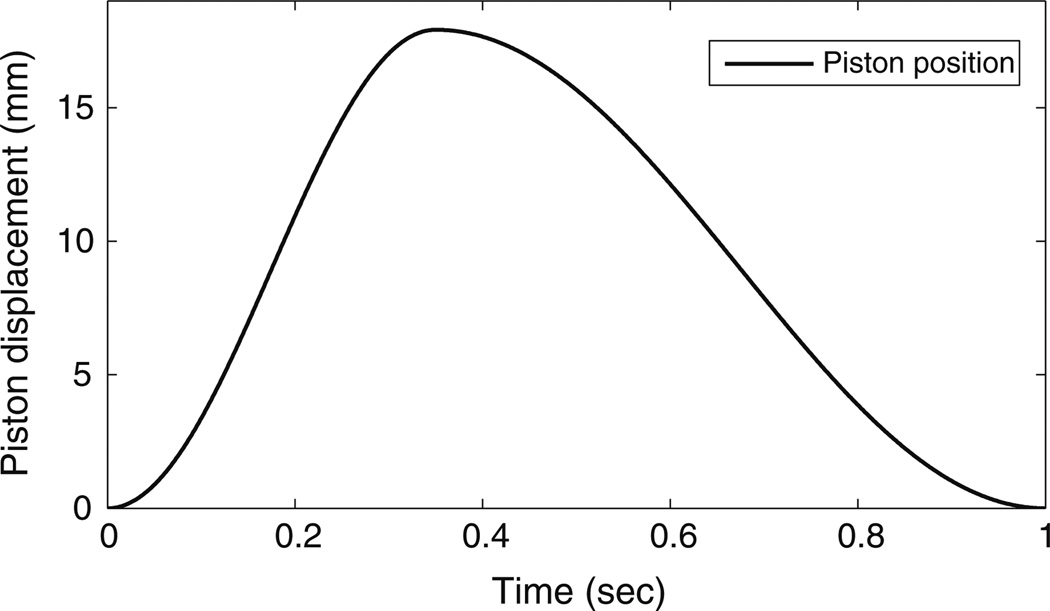
For each of eight trials, a prepared heart was connected to the system and flooded with 0.9% saline to ensure an atrial preload of 15 mmHg. Aortic backpressure (afterload) was set to 75 mmHg. A 5F microtip pressure catheter (MPC-500, Millar, Houston, TX) was inserted through the aortic valve into the left ventricle. An identical pressure catheter was inserted through a pulmonary vein into the left atrium. The left ventricle was dynamically pressurized at a heart rate (HR) of 60 bpm, with a pump stroke volume (PV) of 35 mL, a systolic fraction (SF) of 35% (systolic duration of 350 ms), and a pump trajectory as shown in Fig. 3. These values were chosen based on in vivo porcine data collected from a survey of published works.5,16 The system was operated at room temperature (roughly 25 °C). Throughout the experiment, saline was applied to the surface of the heart to prevent it from drying out. Additionally, data from the pump encoder and pressure catheters were acquired and stored at a 1 kHz sampling frequency for post experimental analysis.
FIGURE 3.
Pump trajectory used to obtain 60 bpm HR, 35 mL PV, and 35% SF.
To obtain baseline cardiac output measurements for a normal, undamaged mitral valve, the aortic outflow resistance was increased until the peak left ventricular pressure (pLVP) reached 120 mmHg. The heart was dynamically pressurized for 60 cycles (1 min) and the cardiac output collected and measured using a graduated cylinder. Next, the outflow resistance was increased until the pLVP reached 150 mmHg and the measurement cycle repeated. To confirm the precision of volumetric measurements, several trial runs were conducted where the cardiac outputs for 60 cycles were collected and measured. In each case, the variation in cardiac output was less than 5 mL (the resolution of the graduated cylinder), or less than 0.083 mL/cycle.
Induction of Valve Insufficiency
After collecting baseline data, an atriotomy was performed to expose the mitral valve. A 3 cm incision was made in the left atrium and the mitral valve and annulus were manipulated to induce two common symptoms of mitral valve failure—annular dilation and chordal rupture. Previous studies have shown that in cases of annular dilation, the primary enlargement occurs in the anterior-posterior direction.13 For this reason, annular dilation was replicated by manually stretching the mitral annulus in the anterior–posterior and transverse directions until a 25% increase in the anterior–posterior distance occurred without decreasing the transverse length. Anterior–posterior distance was defined as the straight-line distance from anterior annulus to posterior annulus, while transverse distance was defined as the straight-line distance from the left to right commissures.
Next, to induce a flail posterior leaflet, the P2 segment (using Carpentier nomenclature4) of the posterior leaflet was lifted and chordae tendineae connecting the P2 leaflet to the papillary muscles were severed until a satisfactory level of failure was achieved. Satisfactory failure was defined as valve leakage under constant ventricular pressure, without total valve incompetence. Valve leakage was visually assessed by switching the system to static pressure mode and applying 35 mmHg backpressure to the mitral valve. The atriotomy was then closed using sutures and cyanoacrylate adhesive.
Damaged Valve Data Collection
To obtain cardiac output measurements for the damaged mitral valve, the heart was dynamically pressurized using the same system settings as previously (HR 60 bpm, PV 35 mL, SF 35%). Aortic outflow resistance was increased until pLVP reached 120 mmHg and cardiac output was measured for 60 cycles (1 min). pLVP was then increased to 150 mmHg and the measurement routine repeated.
Repaired Valve Data Collection
To demonstrate the ability of the proposed system to evaluate repair, hearts five through eight underwent valve repair surgery. First, the atriotomy was reopened to expose the mitral valve. The flail leaflet was repaired with a triangular resection using 5–0 Prolene suture (Ethicon, Inc., Somerville, NJ). To correct the dilated annulus, an annuloplasty ring, either a Carpentier-Edwards Physio ring (Edwards Lifesciences, Irvine, CA), or a proprietary ring of our own design, was inserted and attached using 12 to 14 knots of 2–0 TI·CRON braided polyester suture (USS, Norwalk, CT). After closing the atriotomy, the heart was dynamically pressurized using the same system settings as previously and the measurement routine repeated at a pLVP of 120 and 150 mmHg.
Echocardiographic Analysis
During one of the heart trials, echocardiographic images and video were taken by a trained veterinary echocardiologist using an Acuson Sequoia C256 ultrasound system (Siemens, Malvern, PA) with both 7 and 10 MHz cardiac probes (models 7V3 and 10V4, respectively). For optimal imaging and velocity measurements, the echo probes were placed directly on the surface of the heart. At each experimental state, levels of mitral and aortic regurgitation were graded based on echocardiographic images.
Endoscopic Analysis
To illustrate another feature of the proposed system, several hearts were examined during dynamic pressurization using an endoscope (DyoCam 750, Smith & Nephew, London, UK) to verify valve condition and quantify the quality and durability of the repair. To avoid interference with data collection, endoscopic imaging was not performed concomitantly with the pressure and volumetric measurements described previously.
RESULTS
For each heart, the experimental data was divided into four cases: (1) a normal, functional, mitral valve with pLVP of 120 mmHg, (2) a normal, functional valve with pLVP of 150 mmHg, (3) a damaged, semifunctional, mitral valve with pLVP of 120 mmHg, and (4) a damaged, semi-functional valve with pLVP of 150 mmHg. Hearts undergoing repair provided two additional cases: (5) a repaired mitral valve with pLVP of 120 mmHg, and (6) a repaired valve with pLVP of 150 mmHg. The experimental measurements for all cases are presented in Table 1.
TABLE 1.
Cardiac output per cycle of each heart at both operating pressures before and after induction of mitral valve defects.
| Heart | pLVP (mmHg) | CON (mL) | COD (mL) | MR (mL) | RF % | COR (mL) | RP % |
|---|---|---|---|---|---|---|---|
| 1 | 120 | 18.9 | 14.4 | 4.4 | 23.6 | – | – |
| – | 150 | 15.4 | 10.2 | 5.2 | 34.1 | – | – |
| 2 | 120 | 22.7 | 17.3 | 5.5 | 24.1 | – | – |
| – | 150 | 19.7 | 12.0 | 7.7 | 39.1 | – | – |
| 3 | 120 | 17.2 | 10.5 | 6.8 | 39.2 | – | – |
| – | 150 | 14.3 | 5.3 | 9.0 | 62.8 | – | – |
| 4 | 120 | 22.3 | 16.3 | 6.0 | 26.8 | – | – |
| – | 150 | 20.5 | 13.1 | 7.4 | 36.0 | – | – |
| 5 | 120 | 15.2 | 8.1 | 7.1 | 46.9 | 12.5 | 82.3 |
| – | 150 | 13.3 | 3.8 | 9.5 | 71.8 | 7.9 | 59.6 |
| 6 | 120 | 25.2 | 20.0 | 5.2 | 20.5 | 20.7 | 82.1 |
| – | 150 | 21.3 | 16.7 | 4.7 | 21.9 | 17.2 | 80.5 |
| 7 | 120 | 21.3 | 15.8 | 5.5 | 25.8 | 18.3 | 85.9 |
| – | 150 | 17.3 | 12.7 | 4.7 | 26.9 | 16.2 | 93.3 |
| 8 | 120 | 19.2 | 11.4 | 7.8 | 40.7 | 16.7 | 87.0 |
| – | 150 | 17.0 | 9.1 | 7.9 | 46.7 | 14.0 | 82.4 |
COD = damaged valve cardiac output; CON = normal valve cardiac output; pLVP = peak left ventricular pressure; MR = mitral regurgitation; RF = regurgitant fraction; COR = repaired valve cardiac output; RP = repair percentage (percent return to normal valve output).
Changes in Cardiac Output
As shown in Table 1, cardiac output at a given pLVP was greatly reduced following induction of mitral valve defects. This regurgitant fraction, defined as the percent decrease in cardiac output resulting from mitral regurgitation, was as high as 46.9% at 120 mmHg pLVP and 71.8% at 150 mmHg pLVP in the fifth heart, which had the most severe failure. The regurgitant fraction was also shown to increase with increasing pLVP, with an average rise of 35.1% from 120 to 150 mmHg. Additionally, cardiac output for normal valve states reduced as pLVP increased from 120 to 150 mmHg due to ventricular wall compliance, by as much as 18.8% in the seventh heart.
After valve repair, the average cardiac output was improved to 84.3% of the normal valve output at 120 mmHg pLVP and 78.9% at 150 mmHg pLVP.
Pressure Waveforms
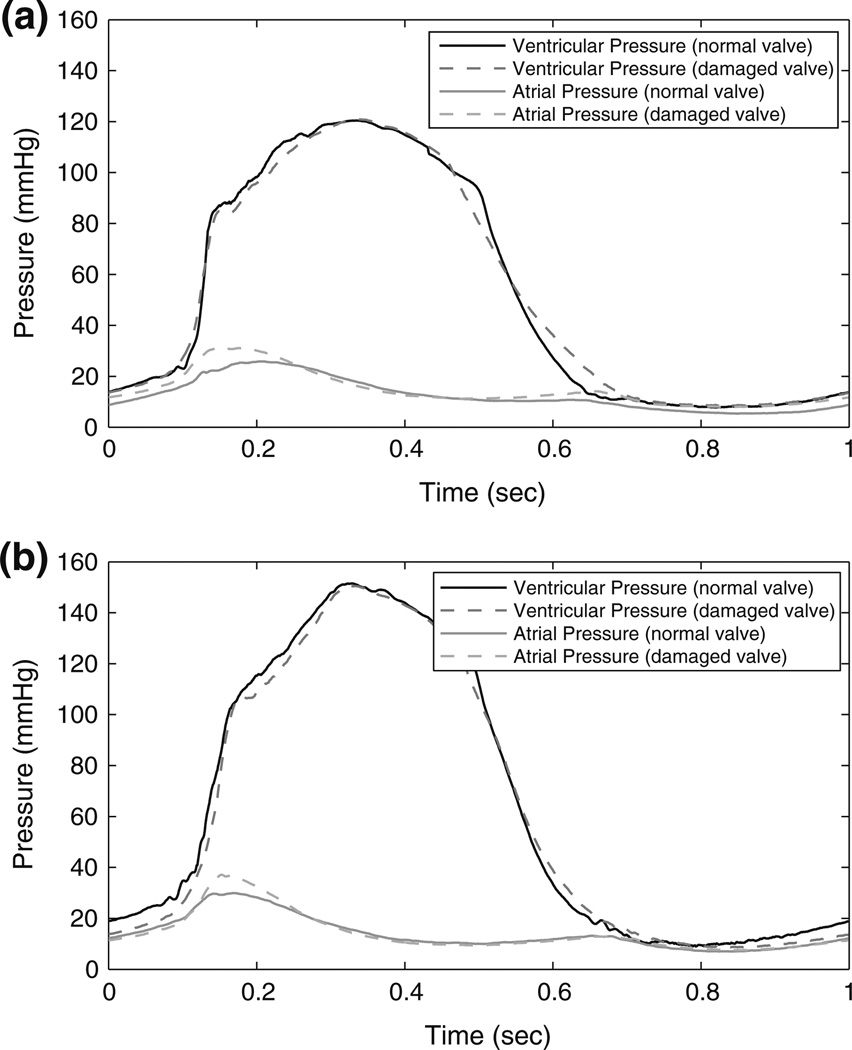
For each case, 10 cycles of measured pressure were averaged to compute mean left ventricular and left atrial pressure waveforms (Fig. 4). Statistical tests were performed on each group of pLVP and peak left atrial pressure (pLAP) values from each case. The Student’s t-test, with α = 0.05, showed a difference (p < 0.0001) in pLAP between all cases for each heart. At 120 mmHg pLVP, the pLAP increased by an average of 9.0% after the induction of valvular defects, while at 150 mmHg pLVP, the pLAP increased by an average of 12.6%. Increasing the pLVP within the same valve condition also increased pLAP: an average 6.0% increase with a normal valve, and an average 9.6% increase with a damaged valve. Because it was a controlled parameter, no significant differences were found between pLVP when comparing pressure data from any heart trial.
FIGURE 4.
Pressure waveform comparisons: (a) normal and damaged valve with 120 mmHg pLVP; and (b) normal and damaged valve with 150 mmHg pLVP.
For the four hearts that underwent valve repair, the pLAP pressure decreased to within an average 6% of the pLAP for a normal valve at both 120 and 150 mmHg pLVP.
Echocardiographic Analysis
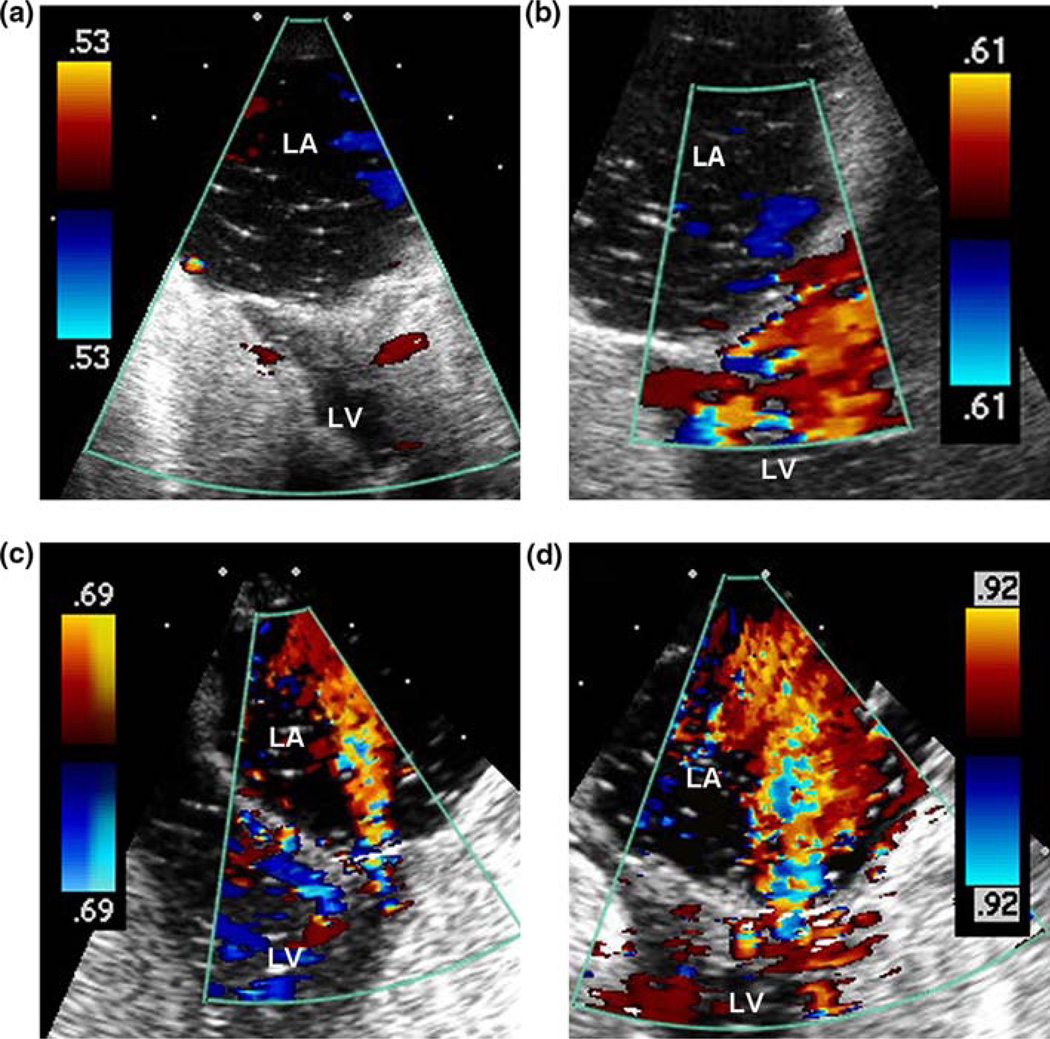
Echocardiographic examination of Heart 1, acquired using a 10 MHz probe during peak systole, revealed no visible mitral regurgitation in either of the normal valve cases (Figs. 5a and 5b). Following the induction of valve defects, MR jets were visible in both low and high pressure cases (Figs. 5c and 5d). Due to the intensity of these regurgitant jets, a 7 MHz probe was used for more accurate velocity visualization. MR jet severity was graded using three primary levels (Mild, Moderate, Severe) and two intermediary levels (Mild-to-Moderate and Moderate-to-Severe). During Case 3 (damaged valve with 120 mmHg pLVP), the visible MR jet was graded Mild-to-Moderate, with an estimated jet area equal to 25% of the left atrial area. During Case 4 (damaged valve with 150 mmHg pLVP), the MR jet was graded Moderate-to-Severe, with an estimated jet area equal to 50% of the left atrial area. The Case 4 jet was described as being more visible and organized than in the previous low pressure case. Thus, with a 25% increase in pLVP, the MR echo severity increased by two grades.
FIGURE 5.
Color doppler images of left atrium (LA) and left ventricle (LV) during peak systole: (a) normal valve, 120 mmHg pLVP; (b) normal valve, 150 mmHg pLVP; (c) failed valve, 120 mmHg pLVP; and (d) failed valve, 150 mmHg pLVP.
Endoscopic Analysis
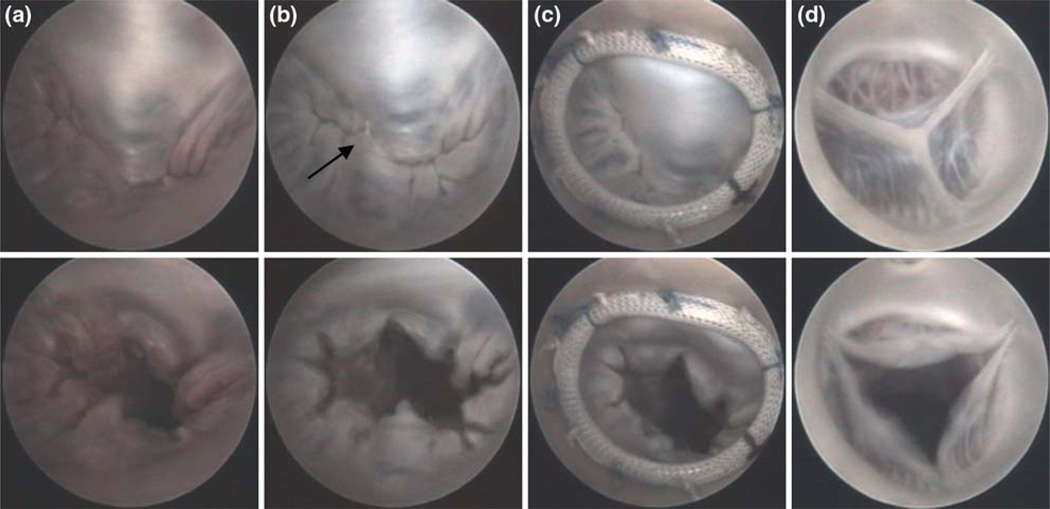
Endoscopic access provided excellent visualization of the mitral and aortic valves, and enabled assessment of mitral valve repair. Figure 6 shows images of a normal, damaged, and repaired mitral valve from the same heart as well as a functional aortic valve. In Fig. 7, examples of repair faults are presented.
FIGURE 6.
Endoscopic images showing states of open and closed mitral valve. (a) normal; (b) damaged (arrow depicts flail leaflet); and (c) repaired. Functional aortic valve is also shown (d).
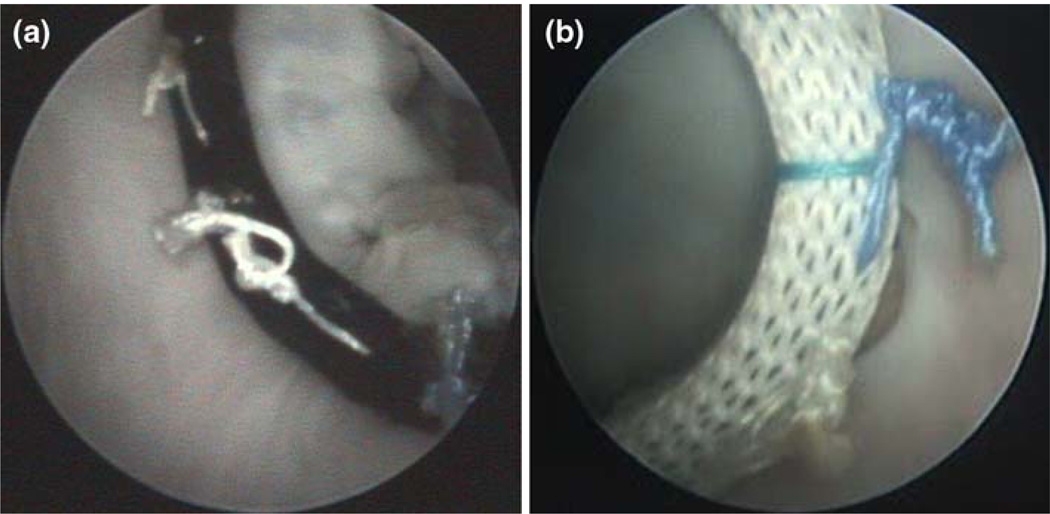
FIGURE 7.
Endoscopic images of repair problems discovered during testing. (a) Loose suture knot revealed during dynamic testing; (b) location where annuloplasty ring has pulled away from the annulus, indicating either incorrect suture placement or a knot that was not tightened properly.
DISCUSSION
A positive relationship between left ventricular pressure and MR has been well established in the literature3; its demonstration in this study confirms the capabilities of the proposed system to detect changes in MR. Echocardiographic imaging, the clinical standard for MR diagnosis, was used to verify the existence and severity of MR quantified using the proposed system. Echocardiography is an extremely user-dependent imaging modality and its limitations in precisely measuring hemodynamic variables are widely documented.10 For this reason, quantifying MR using echocardiography was not attempted; instead, a standard grading scale was adopted. While the Proximal Isovelocity Surface Area method (PISA) is currently seen as the best method for semi-quantitative analysis of MR,6,7,13 the required convergence flow field was not consistently visible in these experiments, thus PISA was not performed.
The measured pressure waveforms were consistent with in vivo measurements. Increases in left atrial pressure, an additional indicator of MR,15 were easily detected using the proposed system. Pressure waves associated with atrial contracture were absent, as expected, since pressure profiles in the system are generated by the motion of the PD pump, and not by external activation of cardiac muscle. Additionally, the viscosity differences between saline and blood affect the pressure output. A more viscous fluid would provide some damping effects on the measured waveforms. The choice of saline in these trails was a pragmatic one; the transparent properties of saline allowed for excellent endoscopic visualization of the mitral valve. Regardless, the proposed system is capable of circulating a wide range of fluids.
Following valve repair, the system was able to quantify the increase in cardiac output, enabling assessment of repair effectiveness. Endoscopic access allowed for visualization of the mitral valve and revealed certain repair shortcomings such as knot loosening and incorrect suture placement. Such evaluations could greatly improve the abilities of a practicing surgeon.
The proposed system was designed to be an effective and affordable precursor to animal and clinical trials. By keeping experimental costs low, numerous preliminary studies can be performed to evaluate newly emerging heart repair techniques and devices. Animal trials, which require lengthy approval by the Institutional Animal Care and Use Committee (IACUC), are less efficient for initial testing of surgical prototypes. Based on customary charges at NC State College of Veterinary Medicine, the experimental costs (neglecting labor) associated with an equivalent animal trial on a live 70 to 100 kg swine are approximately 2500 USD per specimen, which includes a standard 14 day acclimation time. Alternatively, porcine hearts are easily obtained from local sources, enabling emerging valve repair techniques to be tested within hours of the idea’s conception, thus maximizing use of available resources and minimizing the time required to verify a new concept. The cost of materials needed to run these experiments on the proposed system was under 25 USD per trial—1% the cost of the corresponding animal trials.
In recent years, similar systems have been developed to examine cardiac or valvular function in isolated models. Several designs rely on static pressure to evaluate explanted porcine mitral valve competency,9,14 making them unable to evaluate the valve under dynamic pressurization. The benefits of using a dynamic system lie in the repeated number of cycles to which the valve is subjected. In pulsatile systems, like the proposed system, output from all cycles is averaged to provide a better estimation of valvular function, minimizing any variability between valve closures. A dynamic system is better able to test the robustness of repairs, such as suture knot durability, and other aspects the single closure from a static test would be unable to evaluate. As shown by Espino et al.8,9 the pressure chosen for a static test plays a critical role in determining mitral regurgitation severity, with some failures not evident until higher pressures. Furthermore, the results gathered from constant pressure tests are not easily comparable to clinical data, as they are in terms of flow rates at a certain pressure, and not regurgitant fraction. With a dynamic system, physiological pressure waveforms and flow rates can be used, better correlating the results to what is seen in vivo.
Other investigators have constructed similar closed-loop pulse duplication systems to test various aspects of explanted porcine mitral valves in a dynamically pressurize setting.2,11 As with the static pressure models, these systems require the mitral valve and accompanying papillary muscles to be removed from the heart and attached to a predetermined template prior to testing. While they offer the benefit of complete control over all hemodynamic parameters, they bypass many of the features of individual heart specimens and further remove the valve from its natural setting.
Other advancements have increased the viability of using isolated working heart models. Both Chinchoy et al.5 and Araki et al.1 have had success in maintaining isolated swine hearts for several hours. Recently, Hill et al.12 have managed to adapt this technique for use on isolated human hearts. These systems are ideal for many ex vivo studies, but they require complex preparation and rigorous adherence to proper transplantation techniques. After the transition to working heart mode, there is a limited and variable window of time for consistent physiological operation of each heart. To date, these systems have been used for intracardiac anatomical imaging; attempts to stop and restart the heart after initial reanimation (required steps in evaluating changes in heart output) have not been reported. Further, these systems still require laboratory animals for heart transplantation, which negates the benefit of using beating heart models as efficient precursors to animal trials.
We sought to develop a dynamic pressurization system that allowed the use of intact hearts, minimizing changes to the natural valve structure and preserving the variation between heart samples for a better understanding of how repair methods will be affected by differing morphologies. Additionally, by utilizing intact hearts, the proposed system allows for surgical evaluations within a natural, anatomically correct setting. The proposed system serves as an effective tool for evaluating mitral valve function in explanted porcine hearts because it provides repeatable control of all variables for the duration of experimental testing. Since mitral regurgitation can be easily and accurately quantified using the system, the proposed system can be used to demonstrate and test emerging heart repair techniques, both surgical and device based.
LIMITATIONS
Proper operation of the proposed system relies on the use of heart specimens that are defect free, since comparisons must be made between normal functioning valves and induced defections. The open design of the proposed system allows easy detection of problems such as septal defects, both ventricular and atrial in nature, and other defects which result in system leakage. Proper valve function, both mitral and aortic, can be visually assessed using an endoscopic camera, or with the use of echocardiography. Throughout these experiments, endoscopic imaging showed full competency of the aortic valve.
All tests were performed at room temperature (roughly 25 °C). While the heart tissue and circulating fluid all have some dependence on temperature, their effects in these trials was found to be negligible. Nonetheless, since completion of this experiment, a temperature controller has been integrated into the proposed system for other studies requiring precise control of operating temperature.
The explanted hearts used in the proposed system are dead and lack contractile function; therefore, exact physiological motion during the cardiac cycle cannot be replicated. To minimize the effects of using a dead heart, the porcine hearts were collected from an abattoir, prepared and tested within 24 h of mortality. Regardless, ventricular and papillary muscle activation, two important factors in recreating an exact left heart model, are absent, which results in the ventricular volume increasing in systole and decreasing during diastole. The basic functionality of the mitral valve remains the same, but exact changes in annular shape during in vivo cardiac cycles13 cannot be duplicated; therefore, mitral valve defects and repair techniques which primarily involve ventricular wall function cannot be reliably tested using this system.
The functional limitations must be taken into consideration when analyzing results from the proposed system and any system utilizing explanted hearts or valves. While it’s undeniable that this model is not a true physiological representation of in vivo heart function, and has serious deficiencies in the area of cardiac and papillary muscle contracture, the ability to maintain natural valve variability, the open design for surgical evaluations, the ease of imaging (both endoscopic and ultrasonic), and the consistency of operation all present an improvement upon current “explanted valve” techniques. The ability to measure changes in mitral valve function in a pulsatile environment makes the proposed system a useful tool for basic evaluations of mitral valve repair and can greatly decrease development time of new techniques, allow for surgical practice of those techniques in a natural setting, and better identify good candidates for in vivo testing.
ACKNOWLEDGMENTS
This work was funded by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH), Grant Number 1 R01 HL075489–03A1. The authors would like to thank Dr. Teresa DeFrancesco for her expertise in acquiring echocardiographic images, William Griffin for his assistance with the design of the system, and Molly Purser for her aid in experimental trials.
REFERENCES
- 1.Araki Y, Usui A, Kawaguchi O, Saito S, Song MH, Akita T, Ueda Y. Pressure-volume relationship in isolated working heart with crystalloid perfusate in swine and imaging the valve motion. Eur. J. Cardiothorac. Surg. 2005;28(3):435–442. doi: 10.1016/j.ejcts.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 2.Arita M, Tono S, Kasegawa H, Umezu M. Multiple purpose simulator using a natural porcine mitral valve. Asian Cardiovasc. Thorac. Ann. 2004;12(4):350–356. doi: 10.1177/021849230401200415. [DOI] [PubMed] [Google Scholar]
- 3.Braunwald E, Welch GH, Jr, Sarnoff SJ. Hemodynamic effects of quantitatively varied experimental mitral regurgitation. Circ. Res. 1957;5(5):539–545. doi: 10.1161/01.res.5.5.539. [DOI] [PubMed] [Google Scholar]
- 4.Carpentier AF, Lessana A, Relland JY, Belli E, Mihaileanu S, Berrebi AJ, Palsky E, Loulmet DF. The “physio-ring”: An advanced concept in mitral valve annuloplasty. Ann. Thorac. Surg. 1995;60(5):1177–1185. doi: 10.1016/0003-4975(95)00753-8. discussion 1185–1186. [DOI] [PubMed] [Google Scholar]
- 5.Chinchoy E, Soule CL, Houlton AJ, Gallagher WJ, Hjelle MA, Laske TG, Morissette J, Iaizzo PA. Isolated four-chamber working swine heart model. Ann. Thorac. Surg. 2000;70(5):1607–1614. doi: 10.1016/s0003-4975(00)01977-9. [DOI] [PubMed] [Google Scholar]
- 6.Enriquez-Sarano M, Miller FA, Jr, Hayes SN, Bailey KR, Tajik AJ, Seward JB. Effective mitral regurgitant orifice area: Clinical use and pitfalls of the proximal isovelocity surface area method. J. Am. Coll. Cardiol. 1995;25(3):703–709. doi: 10.1016/0735-1097(94)00434-R. [DOI] [PubMed] [Google Scholar]
- 7.Enriquez-Sarano M, Sinak LJ, Tajik AJ, Bailey KR, Seward JB. Changes in effective regurgitant orifice throughout systole in patients with mitral valve prolapse. A clinical study using the proximal isovelocity surface area method. Circulation. 1995;92(10):2951–2958. doi: 10.1161/01.cir.92.10.2951. [DOI] [PubMed] [Google Scholar]
- 8.Espino DM, Hukins DW, Shepherd DE, Buchan KG. Mitral valve repair: an in vitro comparison of the effect of surgical repair on the pressure required to cause mitral valve regurgitation. J. Heart Valve Dis. 2006;15(3):375–381. [PubMed] [Google Scholar]
- 9.Espino DM, Hukins DW, Shepherd DE, Watson MA, Buchan K. Determination of the pressure required to cause mitral valve failure. Med. Eng. Phys. 2006;28(1):36–41. doi: 10.1016/j.medengphy.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Feigenbaum HB, Armstrong WG, Ryan TP. Feigenbaum’s Echocardiography. Philadelphia: Lippincott Williams & Wilkins; 2005. p. 790. [Google Scholar]
- 11.He S, Fontaine AA, Schwammenthal E, Yoganathan AP, Levine RA. Integrated mechanism for functional mitral regurgitation: leaflet restriction versus coapting force: in vitro studies. Circulation. 1997;96(16):1826–1834. doi: 10.1161/01.cir.96.6.1826. [DOI] [PubMed] [Google Scholar]
- 12.Hill AJ, Laske TG, Coles JA, Jr, Sigg DC, Skadsberg ND, Vincent SA, Soule CL, Gallagher WJ, Iaizzo PA. In vitro studies of human hearts. Ann. Thorac. Surg. 2005;79(1):168–177. doi: 10.1016/j.athoracsur.2004.06.080. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan SR, Bashein G, Sheehan FH, Legget ME, Munt B, Li XN, Sivarajan M, Bolson EL, Zeppa M, Arch MZ, Martin RW. Three-dimensional echocardiographic assessment of annular shape changes in the normal and regurgitant mitral valve. Am. Heart J. 2000;139(3):378–387. doi: 10.1016/s0002-8703(00)90077-2. [DOI] [PubMed] [Google Scholar]
- 14.Katoh T, Ikeda N, Nishi K, Gohra H, Hamano K, Noda H, Fujimura Y, Esato K. A newly designed adapter for testing an ex vivo mitral valve apparatus. Artif. Organs. 1999;23(10):920–923. doi: 10.1046/j.1525-1594.1999.06293.x. [DOI] [PubMed] [Google Scholar]
- 15.Moulopoulos SD. Cardiomechanics. Springfield, IL: Charles C. Thomas; 1963. p. 193. [Google Scholar]
- 16.Rosenstrauch D, Akay HM, Bolukoglu H, Behrens L, Bryant L, Herrera P, Eya K, Tuzun E, Clubb FJ, Jr, Radovancevic B, Frazier OH, Kadipasaoglu KA. Ex vivo resuscitation of adult pig hearts. Tex. Heart Inst. J. 2003;30(2):121–127. [PMC free article] [PubMed] [Google Scholar]