Abstract
Mesenchymal stem cells (MCSs) have been shown to have therapeutic potential in multiple disease states associated with vascular instability including traumatic brain injury (TBI). In the present study, Tissue Inhibitor of Matrix Metalloproteinase-3 (TIMP3) is identified as the soluble factor produced by MSCs that can recapitulate the beneficial effects of MSCs on endothelial function and blood brain barrier (BBB) compromise in TBI. Attenuation of TIMP3 expression in MSCs completely abrogates the effect of MSCs on BBB permeability and stability, while intravenous administration of rTIMP3 alone can inhibit BBB permeability in TBI. Our results demonstrate that MSCs increase circulating levels of soluble TIMP3, which inhibits VEGF-A induced breakdown of endothelial AJs in vitro and in vivo. These findings elucidate a clear molecular mechanism for the effects of MSCs on the BBB in TBI, and directly demonstrate a role for TIMP3 in regulation of BBB integrity.
INTRODUCTION
Mesenchymal Stem Cells (MSCs) have been shown to have therapeutic potential in multiple disease states characterized by vascular instability(1–4). Our past work has shown that MSCs modulate endothelial cell (EC) permeability both in vitro and in vivo in a rodent model of traumatic brain injury (TBI). MSCs were found to inhibit blood brain barrier (BBB) permeability in TBI through preservation of cerebral endothelial adherens junctions (AJs) and tight junctions (TJs)(3). Interestingly, the beneficial effects of MSCs were recapitulated by conditioned culture media (CM) collected from MSCs cultured in contact with ECs, suggesting soluble factors produced by MSC-EC contact mediate the observed effects. Further support of this concept is found in vivo where MSCs exert potent effects on the brain, despite the lack of large numbers of MSCs in the brain. Most of the intravenously (IV) administered cells are found in the lungs, liver and spleen(5, 6). Since ECs are the first cell type intravenous MSCs encounter, we hypothesized that the interaction between MSCs and ECs (i.e. in the lungs) may trigger the synthesis and release of a vascular stabilizing factor (s).
In the present study, we sought to identify the active factor(s) and employed a gene expression based approach to screen for soluble factors that are regulated by contact between pulmonary endothelial cells (PECs) and MSCs. In this screen, the factor Tissue Inhibitor of Matrix Mettaloproteinases-3 (TIMP3), a soluble and matrix bound protein that can recapitulate the effects of MSCs alone, was identified. TIMP3, a member of the tissue inhibitor of Matrix Mettaloproteinases(TIMPs), has modulatory effects on extracellular matrix remodeling, inflammation and angiogenesis(7–10). TIMP3 has specifically been shown to inhibit VEGF-A signaling by directly binding to VEGFR2, a critical regulator of vascular endothelial permeability(11). Our studies in vitro demonstrate 1) TIMP3 is induced in both MSCs and PECs when co-cultured; 2) siRNA-mediated knockdown of TIMP3 expression in MSCs reduces the protective effects of MSC conditioned media (CM) on VEGF-A induced PEC permeability, matrigel vascular network formation and AJ breakdown, and 3) recombinant human TIMP3 (rTIMP3) recapitulates the effects of MSC CM in vitro. In a mouse model of TBI, we show that 1) IV administration of MSCs increases lung and serum levels of TIMP3, but does not change TIMP3 levels in brain, spleen, or liver; 2) knockdown of TIMP3 by siRNA in MSCs abrogates the effect of MSCs on BBB permeability and AJ stability and 3) IV administration of rTIMP3 recapitulates the effects of MSCs by decreasing BBB permeability and preserving AJs in the injured brain. These results suggest that MSCs promote vascular stability after TBI through increased production of TIMP3, a protein that inhibits VEGF-A induced compromise of the endothelial barrier. TBI is the leading cause of death in children and young adults between the ages of 18–44(12) with few therapeutic options for treating cerebral edema and increased intracranial pressure (ICP). These findings elucidate a clear molecular mechanism for the effects of MSCs on the BBB in TBI and potentially demonstrate a therapeutic role for TIMP3 in regulation of BBB integrity.
RESULTS
Gene Expression Studies Reveal Significant Changes in Multiple Genes Induced by MSC-PEC Contact
Since the main goal of this work was to identify soluble factors produced by IV MSC administration, a gene expression based approach that has been used successfully by others for similar purposes was employed(13). The objective was to identify the soluble factors produced by either ECs or MSCs or both after co-culture with contact in vitro. In these experiments human pulmonary endothelial cells (PECs) were chosen in light of abundant data by our group and others showing that the majority of MSCs administered IV are lodged in the pulmonary microvasculature due to a first pass effect(6) thereby making PECs one of the main cells that MSCs interact with. To study these effects and re-create the MSC therapeutic niche in vitro, ECs and MSCs were co-cultured in contact for 24 hours at a 1:5 ratio of MSCs to PECs (Figure 1A). Cells were harvested and separated using CD31 antibody coated beads by MACS separation. After separation four groups of cells were generated. MCO and PCO were MSC and PEC cell groups that were co-cultured and separated, whereas PEC and MSC groups were cultured alone. All groups were subjected to the separation process and RNA was prepared for Affymetrix total genome array analysis. To ensure that our MACS separation was complete without contamination from the other cell type, surface antigen expression by flow cytometry was analyzed in the MACS sorted cell. Cell separation yielded a pure population of CD31+/CD44− PECs and CD31−/CD44+ MSCs (Figure S1A) CD44 message, found predominantly in MSCs, was not detected in the PCO group (Figure S1B) indicating a clean complete separation. Affymetrix Human Genome GeneChip® was used for gene profiling. Each array contains 54,675 probe sets.
Figure 1. Direct MSC-PEC interaction dramatically alters gene expression in both cell types.
(A) Schematic of MSC-PEC co-culture, MACS separation, and RNA generation to generate four groups: MSCs cultured alone (MSC), MSCs co-cultured with PECs then separated (MCO), PECs cultured alone (PEC), and PECs co-cultured with MSCs then separated (PCO). (B) Two-way clustering heat maps for PECs vs PCOs and (C) MSCs vs MCOs shows large changes with co-culture in both cell types. (D) Gene ontogeny analysis reveals that secreted, cell adhesion, and extracellular matrix proteins are classes of genes altered by MSC-PEC contact. (E) Confirmatory qPCR and (F) western blot data shows significant increases in TIMP3 expression in both MSCs and PECs following co-culture (*p<0.05). Data expressed as mean ± SEM; n =2–4.
The first observation made upon completion of this analysis was the pronounced differences in gene expression between the groups PEC vs PCO and MSC vs MCO as seen by the two-way clustering heat maps (Figure 1B and Figure 1C). The groups cultured alone and with MSCs separated out from each other by the Pearson correlations, indicating large differences between the groups. (Figure S1C). We made two comparisons, between PCO and PEC and between MCO and MSC based on gene expression data. We identified a number of genes whose expression was significantly changed by co-culture (Figure S1D). Gene ontogeny analysis indicates that genes altered by contact between the two cell types fall into three main groups of genes: soluble factors, cell adhesion genes, and extracellular matrix related genes (Figure 1D). Based on the goals and hypothesis of this project, we focused on soluble factors. One gene of interest, increased in PECs and MSCs by co-culture, is a gene belonging to the TIMP family- Tissue Inhibitor of Matrix Metalloproteinase-3 (TIMP3). In our analysis of the array data, TIMP3 increased by 4.5 fold in the PCO group with p value of 1.25 × 10−7 (Figure S1D). On array, TIMP3 was not significantly changed in the MCO vs MSC groups, however qPCR revealed a significant increase in TIMP3 expression in the MCO group (Figure 1E). Although many soluble factors were changed by co-culture, our choice to focus on TIMP3 was based upon the work of others showing that TIMP3 binds to VEGFR2 and has potent inhibitory effects on VEGFR2 signaling, a key regulator of vascular endothelial permeability(14). TIMP3 has also been shown to inhibit inflammation, MMP activity and angiogenesis(8, 11, 15).
MSC-PEC Co-culture Stimulates Increased TIMP3 mRNA and Protein Levels
To re-confirm the findings from the gene expression microarrays, TIMP3 was amplified by quantitative PCR from the four groups PEC, PCO, MSC and MCO. qPCR results confirm the microarray findings of significant increase in PEC production of TIMP3 from low baseline levels (Figure 1E). In MSCs, which produce larger baseline levels of TIMP3, TIMP3 expression was significantly increased between MCO vs. MSC groups. Western blotting also reveals increased TIMP3 protein levels in both the PCO and MCO groups, with co-cultured MSCs being the largest producer of TIMP3 protein (Figure 1F). To determine if TIMP3 treatment of ECs or MSCs results in further increase of TIMP3, MSCs and PECs were treated with 0.5 and 2.0 µg of rTIMP3 for 2, 12 and 24 hours. Figures S2A–S2D, shows that rTIMP3 does not alter TIMP3 production by either cell type. To determine if non-specific cell-cell interactions may cause TIMP3 increases in PECs, we co-cultured PECs with U937, a monocytoid line. No changes were found in TIMP3 expression (Figure S4B), suggesting that TIMP3 increases may be specific to MSC-PEC interactions.
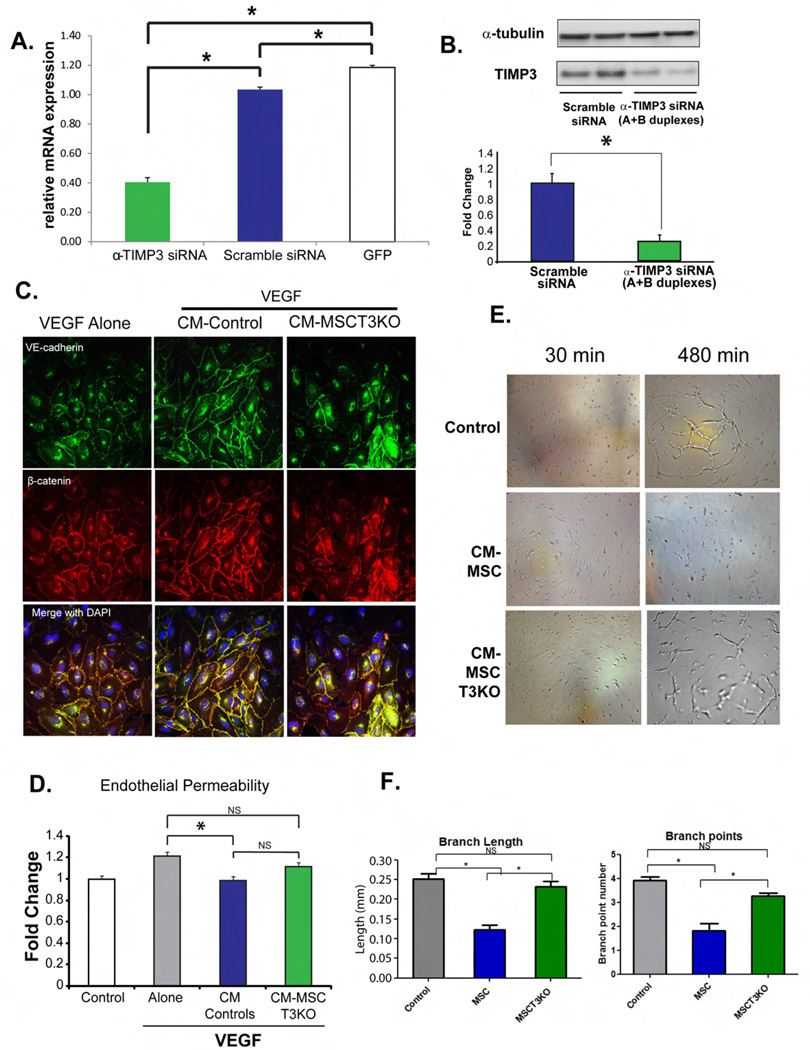
Knockdown of TIMP3 in MSCs impairs vascular stabilizing activity of CM on VEGF-A induced adherens junction breakdown, endothelial barrier compromise and Matrigel network formation
Since protein expression studies revealed that MSCs are the larger producer of TIMP3 in co-cultured cells, we sought to determine how suppression of TIMP3 production in MSCs would change the effect of MSCs on PECs. Figure 2A demonstrates by qPCR that siRNA to TIMP3 attenuates expression of TIMP3 in MSCs, which is confirmed by western blot (Figure 2B). Previously, CM collected from MSC:EC co-culture had been shown to inhibit VEGF-A induced endothelial permeability, an effect dependent on VE-Cadherin-β-catenin interactions(3). Addition of VEGF-A to PEC cultures disrupts membrane localization of VE-cadherin and β-catenin and addition of MSC:PEC CM or MSC CM prevents this disruption, while MSCs with attenuated TIMP3 (MSCT3KO) have a diminished capacity to prevent AJ disruption (Figure 2C). CM from MSCT3KO was also less effective than CM from controls in reducing VEGF-induced permeability (Figure 2D). Quantitative results support adherens junction changes (Figure S2E). PECs naturally give rise to tube-like network structures in matrigel, which are inhibited by MSC CM, but not by MSCT3KO CM (Figure 2E). These findings are depicted quantitatively using branch length and branch point number (Figure 2F). Taken together, these findings suggest that TIMP3 may be a critical mediator of the effects of MSCs on endothelial stability.
Figure 2. Knockdown of TIMP3 in MSCs diminishes effect of MSCs on PECs in vitro.
(A) qPCR (B) western blot analysis of MSCs transfected with TIMP3 siRNA (MSCT3KO) shows knockdown of TIMP3 mRNA (*p<0.05). Data expressed as mean ± SEM; n =2–4. (C) Diminished protection by CM from MSCT3KO on AJs. VE-Cadherin-Green, β-Catenin-Red (D) Lack of an effect of MSCT3KO CM (*p<0.05) compared to control. Data are expressed as mean ± SEM; n =4. (E) EC network formation in matrigel shows that CM from MSCs inhibits EC network formation. Treatment of ECs with MSCT3KO CM shows only partial inhibition. Representative images shown are at 30 mins and 8 hrs. (F) Branch point length and branch point number confirms reduction in inhibition (*p<0.05) by MSCT3KO CM. Data expressed as mean ± SEM; n =4.
Recombinant TIMP3 can recapitulate the effects of MSCs and MSC CM on Adherens Junction stability, endothelial barrier function and Matrigel network formation by PECs
To determine if rTIMP3 can recapitulate the effects of MSC CM on PEC function in vitro, TIMP3’s effect on VEGF-A induced PEC permeability was assessed. rTIMP3 was added to PEC cultures prior to inducing PEC permeability. Similar to CM from MSCs, rTIMP3 also repaired AJ breakdown induced by VEGF-A (Figure 3A). Quantitative results support Adherens Junction changes (Figure S2F). The results presented in Figure 3B show that barrier performance of PEC cultures treated with 0.25–1.0 µg/ml rTIMP3 was significantly improved, but with no dose response, relative to untreated cultures. The second graph in Figure 3B shows that the effect of rTIMP3 is specific to VEGF-A induced permeability and not other inducers such as thrombin. In addition, 2D matrigel network formation was inhibited by rTIMP3 shown visually in Figure 3C and quantified in Figure 3D. To determine if the effects of rTIMP3 may be due to its ability to inhibit matrix metalloproteinase (MMPs), we tested two known inhibitors of MMPs (GM6001 and PD166793). Both of these inhibitors were incapable of inhibiting VEGF-A induced PEC permeability at concentrations known to inhibit MMPs (Figure 3E). These results suggest that TIMP3 mediates the effects of MSCs on vascular stability through inhibition of VEGF-A activity.
Figure 3. Recombinant TIMP3 (rTIMP3) recapitulates the effects of conditioned media on VEGF-treated ECs in vitro.
(A) rTIMP3 preserves EC AJs following VEGF stimulation similar to MSC-CM (B) rTIMP3 inhibits VEGF-induced EC permeability (*p<0.05) and is not concentration dependent. The effect of rTIMP3 is specific to VEGF-induced permeability, as no protection against thrombin-induced permeability is found. Data are normalized to untreated cells (Control) and expressed as mean ± SEM; n =4. (C) Similar to CM in Figure 2E, rTIMP3 (0.5 µg/mL or 1.0 µg/mL) inhibits matrigel networks. Images shown were taken at 30 mins and 8 hrs after seeding. (D) Branch point length and branch point number confirms inhibitory effect (*p<0.05) of rTIMP3. Data expressed as mean ± SEM; n =4. (E) MMP inhibition by GM6001 and PD166793 (both tested at 10 µM) cannot recapitulate the effects of rTIMP3 on VEGF-induced endothelial permeability. Data expressed as mean ± SEM; n =4.
IV MSCs increase circulating levels of TIMP3 post-TBI
We conducted experiments in a mouse model of TBI to determine if the effect of MSCs on BBB permeability post-TBI involves increased circulating TIMP3. Animals received two doses of MSCs (1 ×106/dose), 2 and 24 hours post-injury and blood was collected at 72 hours post-injury, a time when the BBB is maximally open. Western blot analysis of TIMP3 in the serum of treated animals reveals that MSCs increase circulating serum levels of TIMP3 (Figure 4A). Since the antibody used for western blot cannot distinguish between the human and mouse TIMP3, the source of the increased serum TIMP3 (human or mouse) cannot be ascertained.
Figure 4. IV MSCs alter lung and circulating TIMP3 levels in TBI mice.
Western blot shows increased levels of TIMP3 in (A) serum and (B) the lungs of injured, MSC-treated mice compared to controls (*p<0.005). Data expressed as mean ± SEM; n =5. (C) qPCR demonstrates mRNA of murine TIMP3 levels in lungs is increased (*p<0.05) in MSC-treated mice. Data expressed as mean ± SEM; n =5. (D) qPCR detection of human GAPDH mRNA in MSC-treated mice indicates the presence of IV-delivered MSCs. Table shows tracking of MSCs in the spleen, liver, lung, and brain of TBI mice treated with MSCs. (+) signifies detection of human GAPDH. (E) QPCR changes in lung of TIMP3 vs. TIMP1 and TIMP2 by MSCs in TBI mice at 24 (green) and 48 (blue) hrs post-injury. MSCs cultured alone in vitro are also shown as a control (white). (F) Western blot and (G) qPCR of TIMP3 protein/mRNA in the cortex shows no difference in MSC treated mice compared to TBI alone. Data expressed as mean ± SEM; n =5.
IV MSCs increase TIMP3 levels in the lungs and spleen but not the liver post-TBI
To determine the source for the synthesis and release of TIMP3 into the circulation of MSC treated animals, TIMP3 protein and mRNA were measured in the lungs, an organ known to trap MSCs in a first- pass effect. Western Blot of lung total protein showed a significant increase in TIMP3 protein in mice receiving MSCs relative to mice receiving vehicle alone (Figure 4B). QPCR using mouse-specific primers indicates that MSC treated mice have significantly increased levels of murine TIMP3 mRNA compared to controls at 72 hours post-injury (Figure 4C). These differences are not present at 24 and 48 hours post injury (Figure S3A). Using human specific GAPDH primers, we detected GAPDH mRNA (a surrogate for tracking human MSCs) in lung tissue at 24 and 48 hr. post injury, but not 72 hr. (Figure 4D). QPCR of human TIMP3 production in the rodent lungs, reveals that human TIMP3 is increased above the production levels found in MSCs in vitro at 24 and 48 hours post-injury. Figure 4E shows that the human TIMP3 production in the lungs is increased approximately 2 and 5 fold, at 24 and 48 hours respectively, above what we find in the in vitro cultured MSCs. At 72 hours there are no MSCs (human GAPDH) detectable in the lungs. These data suggest that early increased production of TIMP3 in the lungs may come from the MSCs, but later production, after 72 hours, is most likely due to induced murine production. Figure 4E also shows that this effect is specific to TIMP3 and not human TIMP1 or 2, which remain unchanged after MSC administration. QPCR analysis of tissue from liver and spleen, two organs that also trap intravenously administered MSCs, reveals modest increases in murine TIMP3 at 24 and 48 hours but not at 72 hours in the spleen of MSC treated animals. No differences in mouse TIMP3 mRNA were detected in the liver at any time point examined (Figure S3A). Human TIMP3 was not found in the rodent liver and spleen after 24 hours, as there were no MSCs detectable in these organs one day post-injury (Figure 4D).
IV MSCs do not Increase TIMP3 expression in the brain
Measurement of TIMP3 protein in the injured cortex indicates that TBI alone increases TIMP3 protein levels in the brain (Figure 4F), however MSCs do not cause any additional increase. QPCR analysis of murine TIMP3 in the cortex also shows a slight but not significant increase in TIMP3 expression following TBI, with no change after MSC treatment (Figure 4G). Interestingly, as expected, no human GAPDH expression (no MSCs) was found in the brain of MSC treated mice (Figure 4D).
Knockdown of TIMP3 in MSCs fully abrogates the protective effects of MSCs on BBB permeability after TBI
Our findings above suggest that the production of TIMP3, both human and mouse, increase in vivo after IV MSC administration primarily in the lungs and also systemically in the serum. These findings confirm our in vitro gene expression data demonstrating a contact-mediated increase of TIMP3 in both PECs and MSCs, with MSCs being the largest producer of TIMP3. To determine if MSC derived TIMP3 is required for BBB protection by MSCs in TBI, knockdown of TIMP3 expression in MSCs was studied. Figure 5A schematically depicts the experiment. MSCs were transfected with TIMP3 siRNA (Figure 2B). 48 hours post injury, MSCs (transfected with scramble siRNA) or MSCs with attenuated TIMP3 (MSCT3KO) were injected in 2 doses, 2 and 24 hours post-injury. At 72 hours, animals were injected with Evans’s Blue (EB), an albumin-binding dye that does not pass the BBB unless it is compromised. Since the duration of the experiment is 5 days from initial transfection with TIMP3 siRNA, we confirmed that expression of TIMP3 (Figure 5B) in MSCs is attenuated for up to 5 days after transfection with TIMP3 siRNA. Figure 5C shows qualitatively that MSCs inhibit EB dye permeability post-TBI, but MSCT3KO does not. Figure 5D shows quantitatively that knockdown of TIMP3 in MSCs completely abrogates the protective effects of MSCs on BBB permeability to EB dye. To control for the possibility that effects of human MSCs on the BBB are due to an immune mediated reaction in the host, we conducted similar experiments with syngeneic C57Bl6 MSCs to determine if they could reproduce the effects of human MSCs. Murine MSCs (mMSCs) were administered IV post-TBI in the same fashion as human MSCs (Figure 5A). EB dye permeability was attenuated by mMSCs, similar to the effects found with human MSCs (Figures S4D and S4E). Further studies reveal that mMSCs produce TIMP3 (Figure S4A) and can induce enhanced TIMP3 production in co-cultures with PECs (Figure S4B). Pulmonary endothelial permeability is also inhibited by mMSC CM similar to human MSCs (Figure S4C). These data suggest the effects of human MSCs in TBI are not due to a host response to human cells and that mMSCs may mediate protective effects through production of TIMP3 as well.
Figure 5. siRNA-mediated knockdown of TIMP3 in MSCs diminishes their protection of the blood-brain barrier in TBI mice.
(A) Methodology to detect BBB integrity using Evans Blue Dye (EBD). (B) TIMP3 is attenuated by transfection with TIMP3 siRNA (MSCT3KO). QPCR and western blot of TIMP3 production in MSCs transfected with siRNA confirms reduction of TIMP3 for five days post-transfection, spanning the duration of the in vivo experiment. (C) Brains show decreased EBD at the site of cortical impact with MSCs. This effect is eliminated in MSCs transfected with TIMP3 siRNA. (D) Quantitation of EBD confirms differences in (B) (*p<0.005). Data expressed as mean ± SEM; n =10. (E) Immunofluorescent staining of VE-cadherin (green), β-catenin (red), DAPI (blue) and merge(yellow) in the penumbra of sham mice, untreated TBI-injured mice (TBI), TBI-injured mice treated with MSCs (MSC), and TBI-injured mice treated with MSCs transfected with α-TIMP3 siRNA (MSC-T3KO). MSCT3KO mice show diminished restoration of AJs post-TBI. (F) Quantitation of (E) confirms attenuated VE-Cadherin expression in MSC-T3KO-treated animals (*p<0.05). Data expressed as mean ± SEM; n =4 animals, 8 sections/animal.
Knockdown of TIMP3 in MSCs abrogates effects of MSCs on Adherens Junctions in the brain
Past work has shown that MSCs preserve and reconstitute the loss of AJs and TJs post-TBI(3). To determine if this effect is altered by knockdown of TIMP3, as was found in vitro, brain tissue was sectioned and analyzed in the penumbra region of the injury (Figure 6H). Staining for Occludin (red) and VE-Cadherin (green) reveals perivascular staining of both proteins in the brain’s vasculature (Figures 6G and 5E). Qualitative analysis of Occludin-1 and VE-Cadherin expression in the penumbra shows that MSCs reconstitute depletion of VE-Cadherin post-TBI, however mice receiving MSCs with attenuated TIMP3 (MSCT3KO), do not show this protective effect. Figure 5F shows significant quantitative results of this study.
Figure 6. TIMP3 Regulates Blood-Brain Barrier Stability.
(A) Schematic with control, TBI-alone and TBI-injured mice treated with rTIMP3 at 6µg/kg. (B) Representative images of brains show decreased EBD at the site of cortical impact in rTIMP3-treated mice. (C) Quantitation of EBD confirms differences observed in (B). (*p<0.05). Data expressed as mean ± SEM; n =10. (D) Staining of Occludin (red) and VE-cadherin (green) in the penumbra of TBI alone and TBI+rTIMP3 reveal preservation of AJs by TIMP3. (E) Quantitation confirms increased expression of VE-Cadherin in rTIMP3-treated animals (*p<0.05). Data expressed as mean ± SEM; n =4, 8 sections/animal. (F) TIMP3 (red) and PDGFRβ (green) co-localize expression on vasculature. Arrows depict neuronal and vascular staining of TIMP3. (G) Confocal imaging of Occludin (Red) and VE-Cadherin (Green) shows perivascular staining of proteins in the brain vasculature. (H) Schematic of coronal brain section showing the imaged penumbral region (red).
IV recombinant TIMP3 (rTIMP3) preserves BBB function in TBI animals
The role of circulating TIMP3 is still unknown to date, and most studies on TIMP3 have generally focused on tissue expression of TIMP3(9). We sought to determine if systemically-delivered rTIMP3 could recapitulate the effects of MSCs in vivo. TIMP3 (60µg/kg) was injected in three doses to brain-injured animals (see schematic Figure 6A). EB dye extravasation into the brain was measured 48 hours post-injury as described above. Figure 6B shows qualitatively that post-TBI administration of rTIMP3 significantly reduced extravasation of EB dye into the injured brain. Quantitative analysis of EB dye extravasation reveals a significant decrease in BBB permeability after TBI in rTIMP3 treated animals (Figure 6C).
IV rTIMP3 preserves cerebral Vascular Adherens Junctions and inhibits neutrophil infiltration post-TBI
Figure 6D shows that AJs are increased in rTIMP3 treated animals. Quantitative analysis confirms this observation (Figure 6E). To determine the location in the brain of expression of TIMP3, brain sections were co-stained for TIMP3 and PDGFRβ, a vascular pericyte marker. Figure 6C shows that TIMP3 localized in both blood vessels and neurons in the brain. The close association of TIMP3 and PDGFRβ indicates that TIMP3 may be localized or bound to cerebral vascular pericytes. To determine the effects of IV rTIMP3 on neuronal inflammation, brain sections from rTIMP3 mice were stained for NIMP-R14, Iba, and GFAP, which stain for blood neutrophils, reactive microglia, and reactive astrocytes respectively. These markers of cerebral inflammation reveal significantly decreased cerebral neurtrophil infiltrates in rTIMP3 treated animals (Figure S5A and S5B), but no change in reactive microglia (Figure S5C and S5D) or astrocytosis (Figure S5E and S5F).
DISCUSSION
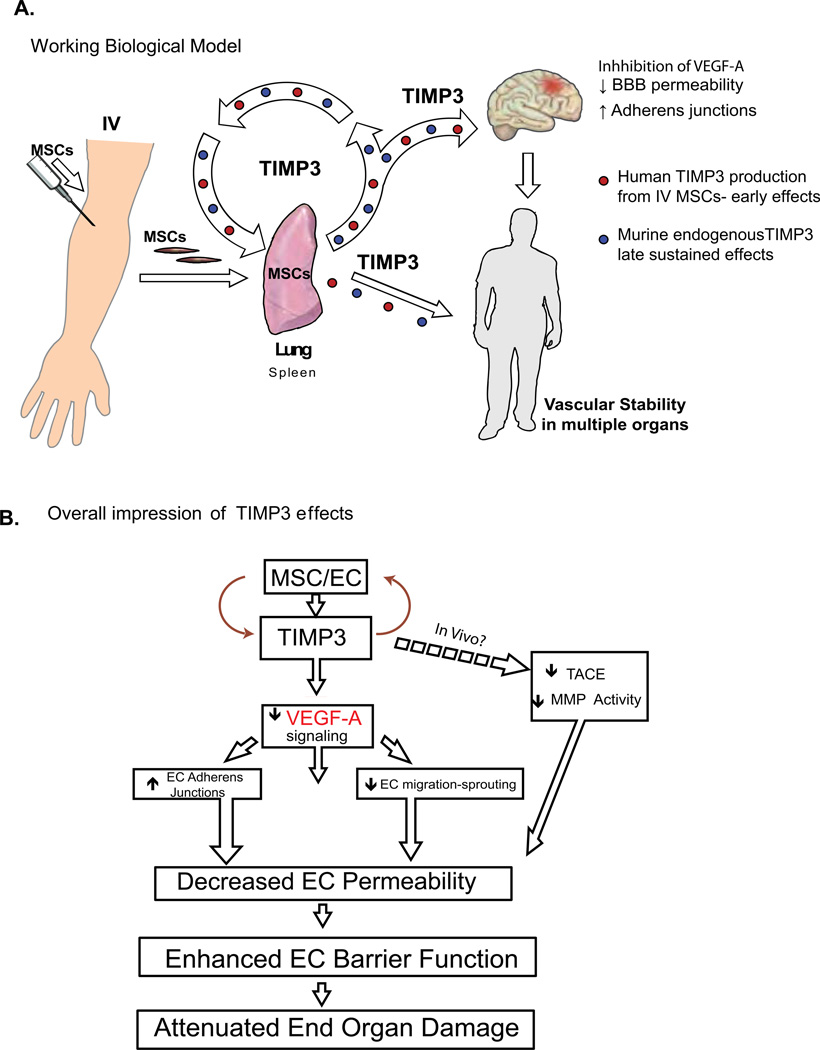
Our studies reveal that the beneficial effects of human MSCs on the BBB in TBI may be due to production of the soluble factor TIMP3. The present studies reveal three important findings: 1) MSCs increase gene and protein expression of TIMP3 in vitro and in vivo post TBI 2) siRNA-mediated knockdown of TIMP3 in MSCs abrogates the ability of MSCs to attenuate VEGF induced EC/BBB permeability in vitro and in vivo after TBI and 3) administration of rTIMP3 alone improves endothelial barrier dysfunction in vitro and in vivo after TBI. We specifically show that TIMP3 is capable of decreasing BBB permeability and restoring cerebral endothelial AJ integrity, all qualities previously reported for MSCs in TBI(3). Our data suggests that MSC interactions with PECs triggers increased TIMP3 expression, findings which are corroborated by our in vivo data showing that both murine and human TIMP3 increase in the lungs after MSC administration. Past work by our group and others has shown that 90% of IV administered MSCs are in the lungs in contact with pulmonary endothelium(2, 5). These data further support our in vitro findings that contact-mediated effects between PECs and MSCs mediate production of TIMP3 by both cell types. Interestingly, large increases in murine TIMP3 production in the liver or spleen were not found, indicating an organ (lung) specific effect. However, large increases in circulating serum TIMP3 were found in MSC treated animals. This data has led us to hypothesize that the lungs are the primary source of TIMP3, which is released systemically and has global effects on vascular endothelial stability (Figure 7a).
Figure 7. TIMP3 Enhances Blood-Brain Barrier Integrity and May Act Through Multiple Mechanisms Involving Many Organs.
(A) Biological model showing increased production and downstream effects of circulating TIMP3 following contact of intravenously-delivered MSCs with the pulmonary vasculature. (B) Schematic of the biological effects of TIMP3. IV MSCs are found primarily in the lungs in contact with pulmonary vascular endothelium. In the lungs early expression of TIMP3 is from MSCs (red) and later (72 hrs) TIMP3 production derives from the mouse. This results in systemic increases in TIMP3 and systemic vascular effects, hypothetically leading to decreased end organ damage.
Although, the mechanism by which TIMP3 modulates endothelial barrier function could be multifold, our data suggest that this effect is mediated through inhibition of VEGFR2 signaling, a known biological activity of TIMP3(11) (Figure 7b). The rapidity with which the AJs are restored by TIMP3 in PECs in vitro (30 minutes) suggests that the mechanism of action is receptor mediated. VEGF-A binding to VEGFR2 is a key inducer of vascular permeability both in vitro and in vivo after TBI(16). Inhibition of VEGFR signaling has been shown to decrease cerebral edema and BBB permeability after TBI(17). Our data demonstrate that TIMP3 inhibits VEGF-A induced endothelial permeability and compromise of AJs, both effects that regulate BBB compromise post-TBI.
Other possible regulators of BBB permeability by TIMP3 could be MMP inhibition or the anti-inflammatory effects of TIMP3. TBI causes a biphasic increase in BBB permeability: a rapid phase that occurs within minutes and a more delayed phase that peaks between 24–72 hrs after injury. The rapid phase is thought to arise from physical injury to brain microvessels and the mechanisms that underlie the delayed phase involve a number of processes including MMP inactivation and inflammation. Our data in vitro suggest that the immediate effects of TIMP3 on endothelial barrier function are not due to either of these activities since MMP inhibition in vitro could not recapitulate the effects of TIMP3 on VEGF-A induced permeability, however we cannot rule out this possibility in vivo. TIMP3 has also been shown to have anti-inflammatory effects through inhibition of the tumor necrosis factor-a (TNF-α) convertase (TACE/ADAM-17)(7). Further studies are needed to assess the contribution of TACE inhibition to the effects of TIMP3 on BBB post-TBI.
The mechanism by which MSCs increase TIMP3 expression in PECs and vice-versa is unclear at this time. We have ruled out the possibility that TIMP3 regulates TIMP3 expression in PECs and MSCs (Figure S2). Other possibilities include direct promoter regulation by MSCs. A number of studies have shown TIMP3 promoter methylation plays an important role in TIMP3 expression. Translation of TIMP3 mRNA can also be regulated by several microRNAs including miR-21, miR-221 and miR-222(18), some of which are known to be down regulated after TBI(19). Further investigation is required as to whether MSC modulate any of these processes.
Perhaps the most interesting and convincing data supporting the role of TIMP3 in BBB stability is our finding that a selected dose of recombinant TIMP3 is capable of significantly inhibiting BBB permeability. From a therapeutic standpoint the dose of rTIMP3 used would be a critical issue to determine since TIMP3 has been shown to induce apoptosis and most likely would have a defined therapeutic window(20). Interestingly, one might hypothesize that the role of circulating TIMP3 is to maintain systemic vascular stability and homeostasis. TIMP3 has been found to have genetic variants that are associated with macular degeneration(21) and Sorsby’s Fundus dystrophy(22). Both of these ocular diseases are associated with vascular changes and instability, suggesting a role for TIMP3 in regulating vascular stability in multiple organs.
From a translational standpoint, it is clear that the future role of stem cells in disease is unclear. There are a number of logistical and ethical issues surrounding the clinical therapeutic use of stem cells in human disease. As our group and many others in the field are finding, much of the demonstrated potential of MSCs in human disease models has been shown to be due to the production of soluble factors(3, 13, 23, 24).There are multiple advantages to identifying these factors considering the possibility of a “cell free” therapeutic that can be used instead of cells. There is a clear unmet need for effective therapeutics in traumatic brain injury, the leading cause of death in children and now a rising concern in military personnel wounded in combat. Treatment options for brain swelling are few and often include drastic measures such as craniotomy to relieve intracranial pressures. These studies demonstrate that TIMP3 may have a translational role as a “cell free” therapeutic for the acute treatment of cerebral edema after TBI.
Experimental Methods
Primary Cells and Cell Lines
First-passage human MSCs and PECs were purchased from Lonza (Walkersville, Maryland). MSCs were found to have undetectable levels of MHC class II and very low levels of MHC class I; intermediate levels of the cellular adhesion proteins ICAM; and high levels of CD105, CD29, and CD44(2;6). Cells were used at passages 2–7.
MSC-PEC Co-culture and MACs Cell Separation
PECs were co-cultured in contact with MSCs or with MSCs in transwells. The ratio of MSCs to PECs was 1:5. Cells were harvested by trypsinization after 24 hr. and sorted by magnetic cell sorting (MACS) using CD31 beads (Miltenyi Biotec, Germany). The CD44− population of PECs was used in subsequent assays of endothelial function. An aliquot of this population of cells was confirmed to be CD31+ by flow cytometry.
siRNA Transfection of MSCs
About 5×105 MSCs were transfected with 2 µg anti-TIMP3 siRNA (OriGene Technologies, Inc, Rockville, MD.) or pBlast empty vector using the Amaxa MSC Nucleofection system (Lonza). Transfection efficiencies were estimated using a green fluorescent protein plasmid to be about 80%.
Endothelial Cell Permeability Assay and Matrigel Assay
PEC monolayer permeability was tested by adding 10 µL of 10mg/mL 40-kDa flourescein isothiocyanate (FITC)-Dextran (Sigma-Aldrich, St. Louis, MO) as described previously(3). MMP inhibitors used include GM6001(Millipore Billerica,MA) at 10µM and PD166793 (Santa Cruz Santa Cruz,CA) at 10µM. Alternate inducers of PEC permeability include TNFα (50 ng/ml) and Thrombin (10 ng/ml). For matrigel assay, PECs were treated with rTIMP3 or media. 1×104 cells were seeded and endothelial network formation was monitored at 1, 2, 3, 6, and 8 hr after seeding and images were captured on an Olympus CKX41 light microscope (Center Valley, PA). Tube length and branch point numbers were assessed by manual measurements and branch point counts were taken from 3 individual wells for each treatment group.
Animals and Traumatic Brain Injury Model
All procedures were approved by the University of Texas Health Science Center Institutional Animal Care and Use Committee (HSC-AWC-10-037) and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. A well-established model of controlled cortical impact injury (CCI) (3, 25, 26) was used to cause TBI in the animals as previously described(3). A single impact at a velocity of 4 m/s with a deformation depth of 1.6 mm, was delivered- a severe injury. At 2 and 24 h after injury, 1×106MSCs, siRNA-transfected MSCs, pBlast empty vector MSCs, or vehicle control (phosphate-buffered saline, PBS) were administered via tail vein injections in a total final volume of 125 µL. Evans Blue dye (EB) studies are described previously(3). For rTIMP3- 60.0 µg/kg rTIMP3 or vehicle control (PBS) was administered at 2, 24, and 48 h after injury, via tail vein injections in a total volume of 125 µL.
Affymetrix Arrays and Biostastical Analysis
The Affymetrix Human Genome U133 Plus 2.0 GeneChip® (Santa Clara, CA) was used for gene expression profiling. The raw data was quantified using Robust Multiarray Analysis (RMA) algorithm(27). To identify differentially expressed genes between two groups, two-sample t-test was utilized on probe set-by-probe set basis; the p-value for each probe set was computed based on the test statistic applied(28). Supervised two-way hierarchical clustering technique was utilized to illustrate findings. To produce two-way clustering heat map, the Pearson correlation was used for distance matrix calculation and the Ward’s method was applied as linkage rule. The expression data processing and analyses were performed using R (version 2.10.0) and Bioconductor packages (http://www.bioconductor.org/). To better understand genes differentially expressed between PECs cultured alone or co-cultured with MSCs and then separated, we used clustering to group genes with appropriate p-values (FDR < 1%) into sets that exhibited similar expression patterns. Gene annotation enrichment analysis of clustered genes was performed using the NIH DAVID annotation tool (http://david.abcc.ncifcrf.gov).
Statistical analysis
Data in all graphs are shown as mean+/−SEM. Where applicable, data were analyzed using a Student’s t-test for 2 group comparisons.
Supplementary Material
Acknowledgements
The authors thank Scott Holmes for excellent graphic design. The authors thank Angela Beeler and Michelle Sauer for manuscript assistance.
Funding: This work is funded in part by Mission Connect and the Texas Institute for Rehabilitation and Research. Funding for this work also received from an NHLBI K18 award (K18HL102256-01) and Bentsen Foundation Houston, TX.
Footnotes
Author Contributions: TM designed and executed the majority of the in vitro experiments. KW, YZ, JZ, JZ, PL and MG designed and performed the in vivo experiments. RK, CSC, JBH, and PKD provided insight into project design and data analysis. JR assisted with the manuscript. JW and LS conducted bioinformatics analysis. AYK assisted with study design and execution. SP designed, analyzed, and interpreted the data of the experiments and wrote the manuscript.
Competing Interests: AYK is a full-time employee of AMGEN, which holds patents related to TIMP3.
Data and materials: The array data for this study has been deposited in the Geo database http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE40613. Ascension number is GSE40613.
References
- 1.Matthay MA, Goolaerts A, Howard JP, Lee JW. Mesenchymal stem cells for acute lung injury: preclinical evidence. Crit Care Med. 2010 Oct;38:S569. doi: 10.1097/CCM.0b013e3181f1ff1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pati S, Gerber MH, Menge TD, Wataha KA, Zhao Y, Baumgartner JA, Zhao J, Letourneau PA, Huby MP, Baer LA, Salsbury JR, Kozar RA, Wade CE, Walker PA, Dash PK, Cox CS, Jr, Doursout MF, Holcomb JB. Bone marrow derived mesenchymal stem cells inhibit inflammation and preserve vascular endothelial integrity in the lungs after hemorrhagic shock. PLoS One. 2011;6:e25171. doi: 10.1371/journal.pone.0025171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pati S, Khakoo AY, Zhao J, Jimenez F, Gerber MH, Harting M, Redell JB, Grill R, Matsuo Y, Guha S, Cox CS, Reitz MS, Holcomb JB, Dash PK. Human mesenchymal stem cells inhibit vascular permeability by modulating vascular endothelial cadherin/beta-catenin signaling. Stem Cells Dev. 2011 Jan;20:89. doi: 10.1089/scd.2010.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Picinich SC, Mishra PJ, Glod J, Banerjee D. The therapeutic potential of mesenchymal stem cells. Cell- & tissue-based therapy. Expert Opin Biol Ther. 2007 Jul;7:965. doi: 10.1517/14712598.7.7.965. [DOI] [PubMed] [Google Scholar]
- 5.Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA, Cox CS., Jr Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009 Jun;18:683. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, Nguyen AT, Malide D, Combs CA, Hall G, Zhang J, Raffeld M, Rogers TB, Stetler-Stevenson W, Frank JA, Reitz M, Finkel T. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma. J Exp Med. 2006 May 15;203:1235. doi: 10.1084/jem.20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black RA. TIMP3 checks inflammation. Nat Genet. 2004 Sep;36:934. doi: 10.1038/ng0904-934. [DOI] [PubMed] [Google Scholar]
- 8.Gill SE, Huizar I, Bench EM, Sussman SW, Wang Y, Khokha R, Parks WC. Tissue inhibitor of metalloproteinases 3 regulates resolution of inflammation following acute lung injury. Am J Pathol. 2010 Jan;176:64. doi: 10.2353/ajpath.2010.090158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kishnani NS, Staskus PW, Yang TT, Masiarz FR, Hawkes SP. Identification and characterization of human tissue inhibitor of metalloproteinase-3 and detection of three additional metalloproteinase inhibitor activities in extracellular matrix. Matrix Biol. 1995 Feb;14:479. doi: 10.1016/0945-053x(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 10.Kwak HI, Mendoza EA, Bayless KJ. ADAM17 co-purifies with TIMP-3 and modulates endothelial invasion responses in three-dimensional collagen matrices. Matrix Biol. 2009 Oct;28:470. doi: 10.1016/j.matbio.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Qi JH, Ebrahem Q, Moore N, Murphy G, Claesson-Welsh L, Bond M, Baker A, Anand-Apte B. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat Med. 2003 Apr;9:407. doi: 10.1038/nm846. [DOI] [PubMed] [Google Scholar]
- 12.Marmarou A. A review of progress in understanding the pathophysiology and treatment of brain edema. Neurosurg Focus. 2007;22:E1. doi: 10.3171/foc.2007.22.5.2. [DOI] [PubMed] [Google Scholar]
- 13.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009 Jul 2;5:54. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weis SM, Cheresh DA. Pathophysiological consequences of VEGF-induced vascular permeability. Nature. 2005 Sep 22;437:497. doi: 10.1038/nature03987. [DOI] [PubMed] [Google Scholar]
- 15.Amour A, Slocombe PM, Webster A, Butler M, Knight CG, Smith BJ, Stephens PE, Shelley C, Hutton M, Knauper V, Docherty AJ, Murphy G. TNF-alpha converting enzyme (TACE) is inhibited by TIMP-3. FEBS Lett. 1998 Sep 11;435:39. doi: 10.1016/s0014-5793(98)01031-x. [DOI] [PubMed] [Google Scholar]
- 16.Nag S, Eskandarian MR, Davis J, Eubanks JH. Differential expression of vascular endothelial growth factor-A (VEGF-A) and VEGF-B after brain injury. J Neuropathol Exp Neurol. 2002 Sep;61:778. doi: 10.1093/jnen/61.9.778. [DOI] [PubMed] [Google Scholar]
- 17.Koyama J, Miyake S, Sasayama T, Kondoh T, Kohmura E. Effect of VEGF receptor antagonist (VGA1155) on brain edema in the rat cold injury model. Kobe J Med Sci. 2007;53:199. [PubMed] [Google Scholar]
- 18.Garofalo M, Di Leva G, Romano G, Nuovo G, Suh SS, Ngankeu A, Taccioli C, Pichiorri F, Alder H, Secchiero P, Gasparini P, Gonelli A, Costinean S, Acunzo M, Condorelli G, Croce CM. miR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell. 2009 Dec 8;16:498. doi: 10.1016/j.ccr.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Redell JB, Liu Y, Dash PK. Traumatic brain injury alters expression of hippocampal microRNAs: potential regulators of multiple pathophysiological processes. J Neurosci Res. 2009 May 1;87:1435. doi: 10.1002/jnr.21945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JK, Shin JH, Suh J, Choi IS, Ryu KS, Gwag BJ. Tissue inhibitor of metalloproteinases-3 (TIMP-3) expression is increased during serum deprivation-induced neuronal apoptosis in vitro and in the G93A mouse model of amyotrophic lateral sclerosis: a potential modulator of Fas-mediated apoptosis. Neurobiol Dis. 2008 May;30:174. doi: 10.1016/j.nbd.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Kaur I, Rathi S, Chakrabarti S. Variations in TIMP3 are associated with age-related macular degeneration. Proc Natl Acad Sci U S A. 2010 Jul 13;107:E112. doi: 10.1073/pnas.1007476107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobson SG, Cideciyan AV, Bennett J, Kingsley RM, Sheffield VC, Stone EM. Novel mutation in the TIMP3 gene causes Sorsby fundus dystrophy. Arch Ophthalmol. 2002 Mar;120:376. doi: 10.1001/archopht.120.3.376. [DOI] [PubMed] [Google Scholar]
- 23.Block GJ, Ohkouchi S, Fung F, Frenkel J, Gregory C, Pochampally R, DiMattia G, Sullivan DE, Prockop DJ. Multipotent stromal cells are activated to reduce apoptosis in part by upregulation and secretion of stanniocalcin-1. Stem Cells. 2009 Mar;27:670. doi: 10.1002/stem.20080742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang X, Neyrinck AP, Matthay MA, Lee JW. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J Biol Chem. 2010 Aug 20;285:26211. doi: 10.1074/jbc.M110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991 Oct;39:253. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- 26.Zhao J, Pati S, Redell JB, Zhang M, Moore AN, Dash PK. Caffeic Acid phenethyl ester protects blood-brain barrier integrity and reduces contusion volume in rodent models of traumatic brain injury. J Neurotrauma. 2012 Apr 10;29:1209. doi: 10.1089/neu.2011.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003 Feb 15;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pounds S, Morris SW. Estimating the occurrence of false positives and false negatives in microarray studies by approximating and partitioning the empirical distribution of p-values. Bioinformatics. 2003 Jul 1;19:1236. doi: 10.1093/bioinformatics/btg148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.