Abstract
Purpose
This study was undertaken to prospectively analyse, at a mean five-year follow-up, the clinical, functional, and radiographic outcomes in patients who developed postoperative acute septic knee arthritis following anterior cruciate ligament (ACL) reconstruction using hamstring autograft. We also assessed the effect of multiple arthroscopic debridement and graft retention on the functional outcomes in comparison with the matched control group.
Methods
From a consecutive case series of 2,560 ACL-injured patients who were treated with arthroscopic ACL reconstruction, we report on 24 cases with postoperative septic knee arthritis. These patients were individually matched for age, sex, comorbidity, body mass index (BMI) and preinjury Tegner activity scale in a ratio of 1/1. Clinical, laboratory, synovial fluid analysis and culture were performed. Arthroscopic debridement and graft retention was done for all cases, in addition to antibiotic therapy IV. A detailed physical examination, KT1000 laxity testing, Lysholm knee score, Tegner activity level scale, International Knee Documentation Committee (IKDC), and Knee Injury and Osteoarthritis Outcome Score (KOOS) were completed.
Results
In all cases, treatment of infection was successful after a median of three (range one to six) repeated arthroscopic graft debridement and retention, in addition to antibiotic therapy IV. At an average of five years follow-up, two patients had over five millimetres manual maximum side-to-side difference in laxity. There were no significant differences between groups regarding Lysholm score, IKDC and KOOS. Median final Tegner activity score was 5.5 versus 7 in the control group (p = 0.004). Complications included graft rupture in three patients, loss of range of motion in five, Sudeck’s atrophy in one and moderate joint narrowing in two. There were no recurrences of septic arthritis or bone infection.
Conclusion
Graft retention seems not only possible but appropriate in view of the experience presented in this article for postoperative septic knee arthritis using hamstring autograft. A potential residual complication is arthrofibrosis, which deserves maximum attention.
Keywords: Septic knee, Hamstring, ACL, Graft retention, Arthroscopic, Debridement
Introduction
Septic arthritis following anterior cruciate ligament (ACL) reconstruction occurs rarely and has been reported in 0.1–1.8 % of different retrospective studies [1–4, 9–11, 14, 15, 18, 21, 23–27] leading to an inferior postoperative activity level that appears to be related to arthrofibrosis, cartilage damage or post-infection meniscal tears [18]. Delay in diagnosis and treatment results in a worse outcome. The goals of treatment are to protect the articular cartilage and the graft [9]. Promptly initiating antibiotic therapy is the most important treatment, followed by open or arthroscopic joint decompression, debridement and lavage [4, 10]. The optimal treatment recommendation has yet to be determined; many algorithms are suggested, but there is no consensus about the best treatment modality. Earlier outcome studies of septic arthritis after ACL reconstruction included a small number of patients, and comparisons were made with historical controls [14, 25]. Additionally, functional outcomes after eradication of postoperative infection are variable [3, 4, 9, 11, 13, 14, 25]. Based on MEDLINE/PubMed searches, no previous study prospectively compares the results of postoperative infection for patients undergoing ACL reconstruction with a matched control without postoperative infection.
The aim of this study was to prospectively analyse clinical, radiographic and functional outcomes in patients who developed postoperative acute septic knee arthritis following ACL reconstruction and assess the effect of multiple arthroscopic debridement and graft retention on functional outcomes in patients compared with a matched control group, with an adequate power.
Patients and methods
From a consecutive case series of 2,560 ACL-injured patients treated with arthroscopic ACL reconstruction from March 2004 to January 2011 by the senior author, we report on 24 cases (0.94 %) with postoperative septic knee arthritis. Postoperative intra-articular infections were defined as a positive culture from a knee aspiration or a cell count consistent with intra-articular infection (over 10,000 cells/µl polymorphonuclear cells) in the synovial fluid in patients who presented with symptoms consistent with septic arthritis. Exclusion criteria were as follows:
Posterolateral rotatory instability, median cruciate ligament (MCL) tear, and posterior cruciate ligament (PCL) insufficiency
Patients on corticosteroids, and diabetic patients
Superficial wound problems that recovered with simple wound care and orally administered medication
Each patient who presented with septic knee arthritis (group 1) was individually matched for:
Gender
Age with a radius of three years for age between 18–39, and five years thereafter
Comorbidity (meniscus tear, collateral ligament sprain)
Body mass index (BMI) with a radius of three
Preinjury Tegner activity scale [22] within one level
Matching was done in a ratio of one infection to one control. The first matching consecutive partner was identified from patients with ACL reconstruction without a postoperative infection. If the case matches were somewhat older than the desired age range, selecting a match that also had a comorbidity or higher BMI was avoided. All patients gave informed consent. All patients had a detailed physical examination, including range of motion (ROM), Lachman pivot-shift test, KT1000 laxity testing and one-leg hop test. Knee functional scores determined preoperatively were the modified Lysholm knee score, Tegner activity level scale [12, 22], International Knee Documentation Committee (IKDC) ranking [8] and Knee Injury and Osteoarthritis Outcome Score (KOOS) form [17]. Radiographs were graded according to the guidelines of the IKDC [8].
Operative technique
After diagnostic arthroscopy, a three centimetre skin incision was made just medial to the tibial tubercle, semitendinosus and gracilis tendons were harvested and a four-strand graft was prepared. The graft was tensioned and fixed with biodegradable interference-fit screws of appropriate diameter.
Postoperative management
A fixed postoperative rehabilitation protocol was applied for all patients. Full extension was maintained from the first postoperative day. Immediate protected weight bearing with crutches was started as tolerated. Active closed-chain exercises were started in the first postoperative week. Patients were allowed to flex their knees 90° at the end of the first postoperative week and up to 120° at the end of the third postoperative week. Four weeks after surgery, patients returned to performing daily living activities. Noncontact sports were permitted after six to nine months.
Postoperative evaluation
Patients were followed up using the same preoperative parameters The postoperative dressing was changed at the first follow-up visit on day three, then repeated dressing until suture removal and wound healing, then office visits scheduled at two weeks for the first three months for assessment, then every month until six months, then every six months. An attempt was made to collect data during each office visit, but complete functional scores data sets were available only for preoperative assessments, one-year and final follow-up. Although data were collected during regularly scheduled follow-up visits, the frequency of missed visits resulted in some data variability. Consequently, we limited data in this study to preoperative, one-year postoperative and final follow-up for data clarity and to reduce confusion with the multiple variable data points. At an average of five years of follow-up, one patient was missing from group 1 and two patients from the control group (group 2). The final follow-up included the last recorded data for each participant.
Re-arthroscopy in infected patients
After joint aspiration for culture and sensitivity, patients underwent immediate arthroscopic lavage with nine to 15 litres of normal saline; samples for bacterial culture and sensitivity were obtained during arthroscopy. For infection staging at arthroscopy, the Gächter [6] classification system was used. Debridement and synovectomy with graft retention was attempted; where appropriate, local wound irrigation and debridement was done and a suction drain was placed in all knees for 48 hours. All patients were treated with antibiotics IV. Time to presentation, clinical findings, causative microorganism, synovial fluid analysis, number of procedures required and antibiotic therapy were recorded, as were detailed physical examination and functional score assessment. Arthroscopic debridement with joint lavage was repeated and guided by clinical and laboratory progression. The decision to undertake further arthroscopy and debridement was somewhat subjective; however, clinical manifestations (pain at rest, local hotness) repeat joint aspiration and organism virulence influenced the decision making. Only three patients had more than three debridements, including two at the beginning of the study and one with mixed infection. When the symptoms stabilised after initial treatment, a physical therapy program was started (in the hospital before discharge), and within three to six days after discharge, physical therapy consisting of a graded knee-strengthening program and passive motion exercises within the limits of pain was initiated at a frequency of two to three times a week.
Statistical analysis
PASW version 18 (Chicago, IL, USA) and PASS was used for analysis. Descriptive analysis was conducted to explore participant characteristics at baseline. Median, 25th and 75th interquartile ranges (IQR) of knee scores were calculated. Mean and standard deviation, number of patients (percentages) and other parameters were calculated. In a matched-pair-analysis study design with a primary end point defined as improvement of modified Lysholm score at one year, an a priori power analysis of 0.8 demonstrated 20 pairs were needed to show a difference of 10 % in Lysholm score, with a 15 % standard error (SE) at a significance level of 0.05; thus, allowing for a 20 % drop, each group would include 24 participants. Continuous variables were tested for normality. For comparing groups, variables were analysed using two-tailed unpaired t tests or Mann–Whitney U test, as appropriate. Fisher’s exact test was used for categorical data between groups, and chi-square test (by Montecarlo method for small sample) assessed categorical data across different times. The difference was considered statistically significant if at p <0.05. To compare Tegner, IKDC, radiographic IKDC, and Lysholm scores across different time periods, Friedman’s analyses were carried out. Post hoc tests were used to compare scores between a given time period and the one that preceded it. As post hoc tests were used several times, the significance level was divided by the number of planned comparisons, and each two-sample test was accordingly performed at the reduced level. A Kruskal–Wallis test was used to compare different scores between groups at different time periods. Fisher’s exact test was used to compare the number of patients who showed improvement of at least 76 % in Lysholm score at final follow-up. Finally, success was defined as IKDC ranking of A and B, plus Lysholm score >76 points. Univariate and multivariate analysis models were used to test for the preferential effect of age, BMI, duration from injury to ACL reconstruction, meniscus injury and successful outcome at one year.
Results
Infection group (group 1)
In all cases, treatment of infection was successful, and no patient had the ACL graft or screws removed. A median of three (range one to six) repeat arthroscopic debridement and synovectomy were required to eradicate infection. Most patients required arthroscopic debridement and lavage two to three times, and only three patients had more than three debridements, including two at the beginning of the study and a case of mixed infection. Acute deep knee-joint infection developed (one to six weeks after surgery) in 20 patients, and a subacute infection developed (between two weeks and two months) in four patients. Mean time to presentation of infection was 12.4 (range five to 45) days after reconstruction. Average temperature in the infection group was 38.3 °C (range 37–39.5 °C). All except one had variable degree of knee swelling, and seven complained of pain at rest. Twenty patients had local warmth, 19 of whom showed decreased ROM. Four had wound discharge, and five had inguinal lymph node enlargement. Mean peripheral white blood cell (WBC) count was 9.5 x 109/L ± 2.1 with polymorphonuclear leukocyte (PMNL) cell count of 72 ± 7. Mean erythrocyte sedimentation rate (ESR) was 59 ± 28 mm/h (range 15–124 mm/h), and the mean C-reactive protein (CRP) level was 39 mg/ml ± 18 (range, 15–84 mg/ml). Patients were categorised into four groups according to the infecting organism, as follows: seven cases 29 % presented with coagulase-negative Staphylococcus (CNS), seven had methicillin-sensitive S. aureus (MSSA), seven had other organism(s), and three presented a negative culture. Subanalysis of data according to germ type showed no significant difference between subgroups regarding the final median modified Lysholm score (p = 0.42), Tegner scale (p = 0.45) and IKDC score (p = 0.33). According to the Gächter [6] staging system of infection at arthroscopy, three patients were graded as stage 1, 21 patients as stage 2 and none as stage 3 or 4. Knee-joint aspiration resulted in the collection of turbid synovial fluid in all but three patients, in whom the fluid was apparently clear. WBC count of aspirated fluid showed a noticeable increase in WBC count, with an average of 84 ± 32 (range 34.4–165.8); >90 % of cells were of PMNL type in 20 cases. Twenty-one (87.5 %) aspirates had positive culture. Causative organisms and data of the infection group are shown in Table 1.
Table 1.
Infection group (group 1) results
| Case number | Time to Presentation From Index Procedure | Symptoms | CRP (mg/L) | Peripheral blood | ESR (mm Hg) | Culture | Aspirate WBCs (10 3/mm3) | Arthroscopy number | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Body temperature | Clinical parameters | WBCs (109/L) | PMNL (%) | |||||||
| 1 | 9 | 37.8 | S,M,R,W | 41.5 | 7.9 | 65.1 | 30 | CNS | 57.7 | 2 |
| 2 | 12 | 38.1 | S,M,R,W | 52.6 | 11.1 | 77.2 | 64 | MSSA | 64.5 | 5 |
| 3 | 11 | 39 | S,M,P,R,D,W | 45.2 | 13.4 | 68.4 | 124 | MSSA | 94.4 | 6 |
| 4 | 28 | 37.4 | S,W | 16.1 | 8.6 | 54.0 | 37 | CNS | 64.8 | 1 |
| 5 | 22 | 37.7 | S,M | 47.3 | 6.9 | 71.7 | 15 | EF | 78.6 | 2 |
| 6 | 6 | 39 | S,M,P,R,D,W | 59.5 | 10.6 | 78.6 | 67 | MSSA | 82.5 | 3 |
| 7 | 10 | 38.5 | M,R,W | 31.0 | 9.7 | 69.1 | 38 | CNS | 125.6 | 3 |
| 8 | 5 | 38.2 | S,M,R,W | 23.4 | 8.8 | 75.3 | 89 | -ve | 83.6 | 2 |
| 9 | 12 | 39.5 | S,M,R,W | 57.5 | 13.9 | 76.9 | 118 | PS / Klebsiella | 96.8 | 4 |
| 10 | 9 | 37.8 | S,W | 22.0 | 8.3 | 73.4 | 36 | CNS | 73.8 | 3 |
| 11 | 12 | 37.6 | S,M | 17.9 | 7.4 | 69.7 | 28 | CNS | 57.0 | 2 |
| 12 | 6 | 38.5 | R,W | 32.1 | 9.9 | 82.5 | 54 | -ve | 46.8 | 2 |
| 13 | 45 | 39.4 | S,M,R,D,W | 34.6 | 12.8 | 86.3 | 47 | NHSC | 69.4 | 2 |
| 14 | 7 | 37.9 | S,M,P,R,W | 39.4 | 11.1 | 65.7 | 92 | MSSA | 34.4 | 3 |
| 15 | 11 | 37.6 | S,M,R,W | 24.0 | 8.6 | 72.3 | 66 | EC | 165.8 | 3 |
| 16 | 9 | 38.8 | S,M,P,R,W | 50.2 | 9.3 | 68.5 | 57 | P | 98.6 | 3 |
| 17 | 6 | 39.5 | S,M,P,R,D,W | 84.6 | 7.6 | 74.6 | 89 | MSSA | 42.9 | 2 |
| 18 | 6 | 38.3 | S,M,R,W | 21.5 | 8.4 | 70.8 | 44 | -ve | 83.0 | 2 |
| 19 | 20 | 38.6 | S,M,R | 43.0 | 11.7 | 85.4 | 37 | PS | 65.7 | 3 |
| 20 | 10 | 37 | S,M | 39.3 | 10.2 | 67.0 | 63 | MSSA | 71.2 | 3 |
| 21 | 12 | 38.2 | S,M,P,R,W | 46.8 | 9.6 | 72.9 | 38 | P | 74.6 | 3 |
| 22 | 8 | 38.5 | S,M,P,R,W | 72.2 | 5.4 | 74.7 | 52 | MSSA | 134.5 | 3 |
| 23 | 9 | 39.1 | S,M,R,W | 23.7 | 8.9 | 63.8 | 54 | CNS | 129.7 | 2 |
| 24 | 13 | 37.7 | S,R,W | 15.0 | 6.7 | 68.6 | 76 | CNS | 119.6 | 2 |
| 12 | 38.3 | 39 | 9.5 | 72 | 59 | 84 | 3 | |||
| (5-45) | ±0.7 | ±18 | ±2.1 | ±7 | ±28 | ±32 | (-6) | |||
Data are mean ± standard deviation, or median (range)
S, swelling; M, malaise; P, knee pain at rest; R, painful ROM; D, discharge from wound; W, warmth. CRP, C-reactive protein; WBC, white blood cell count; PMNL polymorphonuclear leukocytes, S-WBC serum white blood cell count; ESR, erythrocyte sedimentation rate.
CNS, coagulase-negative Staphylococcus; MSSA, methicillin-sensitive Staphylococcus aureus; P, Propionibacteria acnes; EC, Escherichia coli; EF, Enterococcus faecalis; NHSC nonhemolytic Streptococcus; PS, Peptostreptococcus
All patients were initially treated with cephalosporin or vancomycin IV following aspiration, and treatment was changed according to culture and sensitivity studies. Antibiotics administered IV were continued for a median of 28 (range 21–42) days; duration was determined by normalisation of laboratory values. We were unable to determine the source of contamination despite intensive surveillance of the surgical equipment and surgical team in an attempt to identify the origin of the infections. There was no cluster of infected cases, and the incidence rate was approximately four cases annually. Four patients in the infection group had previous knee arthroscopy (one medial meniscus partial resection, two plica excision, one diagnostic arthroscopy); another had a previous scar in the front of the knee from a motorcycle accident.
Comparison between groups
Average patient age at the time of operation was 26 ± 5 years in group 1 versus 27 ± 4 years in group 2. The follow-up period was 59 ± 21 and 55 ± 21 months in groups 1 and 2, respectively. No statistically significant difference was found between groups regarding patient demographics, as shown in Table 2.
Table 2.
Baseline characteristics of the patients in the infection (group 1) and control (group 2) groups
| Group 1 (19 patients) | Group 2 (23 patients) | P value | ||
|---|---|---|---|---|
| Age (years) | 26 ± 5 | 27 ± 4 | 0.78 | |
| Height (m) | 1.71 ± 0.07 | 1.73 ± 0.08 | 0.52 | |
| Weight (kg) | 76.13 ± 8.1 | 77.7 ± 6.38 | 0.45 | |
| BMI (kg/ m2) | 25.8 ± 2.6 | 26 ± 2.1 | 0.84 | |
| Duration to operation (month) | 6 ± 4.9 | 5 ± 5.1 | 0.51 | |
| Follow-up period (months) | 59 ± 21 | 55 ± 22 | 0.57 | |
| Gender (M/F) | 24 / 0 | 24 /0 | ||
| Side involved (right/left) | 15 / 9 | 14 / 10 | 0.99* | |
| Meniscal injury (No/Yes) | 20 / 4 | 20 / 4 | 0.99* | |
| Pre-operative Lachman test | Normal | 0 (0 %) | 0 (0 %) | 0.55* |
| +1 | 1 (4.1 %) | 2 (8.3 %) | ||
| +2 | 23 (95.9 %) | 22 (91.7 %) | ||
| Pre-operative pivot-shift test | Normal | 0 (0 %) | 0 (0 %) | 0.48* |
| +1 (glide) | 0 (0 %) | 0 (0 %) | ||
| +2 (clunk) | 4 (16.7 %) | 6 (25 %) | ||
| +3 (gross) | 20 (83.3 %) | 18 (75 %) | ||
| KT1000 | Normal <3 mm | 0 (0 %) | 0 (0 %) | |
| Near normal 3–5 mm | 0 (0 %) | 0 (0 %) | ||
| Abnormal > 5 mm | 24 (100 %) | 24 (100 %) | ||
Data are mean ± standard deviation or number (%) patients
BMI body mass index, KT1000 laxity test
*Fisher exact test
In both groups, Lachman test, pivot-shift test, KT1000, ROM and one-leg hop test showed no significant difference between groups at the final follow-up (Table 3).
Table 3.
Laxity assessments, range of motion (ROM) and one-leg hop test at final follow-up evaluation in infection (group 1) and control (group 2) groups
| Group 1 | Group 2 | P value* | ||
|---|---|---|---|---|
| Lachman test | Normal | 18(75 %) | 20(83.3 %) | 0.62 |
| +1 | 4(16.7 %) | 4(16.7 %) | ||
| +2 | 2(8.3 %) | 0(0 %) | ||
| Pivot-shift test | Normal | 16 (66.7 %) | 17 (70.8 %) | 0.99 |
| +1 (glide) | 5 (20.8 %) | 4 (16.7 %) | ||
| +2 (clunk) | 1 (4.2 %) | 2 (8.3 %) | ||
| +3 (gross) | 2 (8.3 %) | 1(4.2 %) | ||
| KT1000 | Normal <3 mm | 17 (70.8 %) | 19 (79.2 %) | 0.79 |
| Near normal 3–5 mm | 5 (20.8 %) | 4 (16.7 %) | ||
| Abnormal > 5 mm | 2 (8.3 %) | 1 (4.2 %) | ||
| ROM (extension) | Normal ≤ 2 ° | 16 (66.7 %) | 19(79.2 %) | 0.41 |
| Near normal 3–5° | 3 (12.5 %) | 4 (16.7 %) | ||
| Abnormal 6–10° | 3 (12.5 %) | 1 (4.2 %) | ||
| Severely abnormal >10° | 2 (8.3 %) | 0 | ||
| ROM (flexion) |
Normal ≤ 5° | 14 (58.3 %) | 19 (79.2 %) | 0.15 |
| Near normal 6–15° | 5 (20.8 %) | 3 (12.5 %) | ||
| Abnormal 16–25° | 1 (4.2 %) | 2 (8.3 %) | ||
| Severely abnormal > 25° | 4 (16.7 %) | 0 | ||
| One-leg hop test | Normal ≥ 90 % opposite side | 11 (45.8 %) | 15 (62.5 %) | 0.63 |
| Near normal 89–76 % opposite side | 7 (29.2 %) | 6 (25 %) | ||
| Abnormal 75–50 % opposite side | 3 (12.5 %) | 2 (8.3 %) | ||
| Severely abnormal < 50 % opposite side | 3 (12.5 %) | 1 (4.2 %) | ||
Data are number (%) of patients
ROM range of motiot, KT1000 laxity test
*Chi-square test (Monte Carlo method)
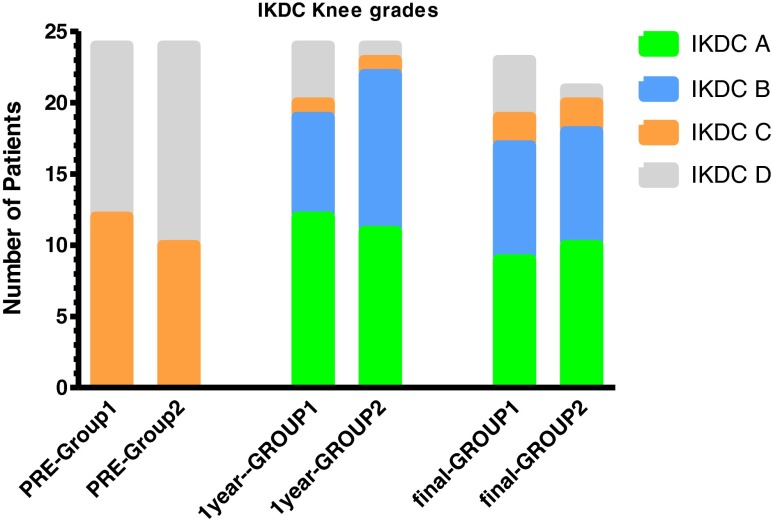
Mean operative tourniquet time and median modified Lysholm, Tegner, IKDC, radiographic IKDC and KOOS scores are presented in Table 4. Significant improvement was recorded in the final modified Lysholm score, and the IKDC ranking (Fig. 1) compared with the corresponding preoperative data in both groups (p value <0.025). At final follow-up, 16/24 patients in group 1 achieved at least 76 points in the Lysholm score versus 21/24 patients in group 2 [Fisher’s exact test p = 0.17; relative risk (RR) = 0.60; 95 % confidence interval (CI) 1.01–2.82].
Table 4.
Results for infection (group 1) and control (group 2) groups
| Group 1 (24 patients) | Group 2 (24 patients) | P value* | ||
|---|---|---|---|---|
| Follow-up duration in months (range) | 59 ± 21 (18–96) | 55 ± 21 (18–96) | 0.57 | |
| Operative duration (min) | 74 ± 14 | 71 ± 11 | 0. 42 | |
| Tegner activity acale | Preoperative | 8 (7–9) | 8 (7–8) | 0.48 |
| Postinjury | 3 (2–4)*** | 3 (2.25-4)*** | 0.89 | |
| 1 year | 5.5 (4–7)*** | 7 (6–8)*** | 0.001 | |
| Final | 5.5 (4–7) | 7 (6–8) | 0.004 | |
| P value** | <0.017 | <0.017 | ||
| IKDC ranking | Preoperative | 3.5 (3–4) | 4 (3–4) | 0.57 |
| 1 year | 2 (1–2)*** | 2 (1–2)*** | 0.82 | |
| Final | 2 (1–2.75) | 1.5 (1–2) | 0.35 | |
| P value** | <0.025 | <0.025 | ||
| Radiographic IKDC | Preoperative | 1 (1–1) | 1 (1–1) | 0.99 |
| 1 year | 1 (1–1) | 1 (1–1) | 0.99 | |
| Final | 1 (1–2)*** | 1 (1–1) | 0.09 | |
| P value** | <0.025 | <0.025 | ||
| Modified Lysholm score | Preoperative | 61(55–69) | 60 (57–63) | 0.68 |
| 1 year | 87.5(76–95)*** | 89 (85–95)*** | 0.08 | |
| Final | 85(72–93) | 90 (83–95) | 0.10 | |
| P value** | <0.025 | <0.025 | ||
| KOOS Score | KOOS symptoms | 86 (69–89) | 87 (76–95) | 0.39 |
| KOOS pain | 82 (75–93) | 89 (84–97) | 0.07 | |
| KOOS daily | 96 (82–99) | 96 (91–99) | 0.28 | |
| KOOS sports | 90 (65–99) | 95 (86–99) | 0.45 | |
| KOOS QOL | 84 (63–100) | 100 (77–100) | 0.20 | |
| Percentage(%) of improvement of modified Lysholm score | 32.4 % ± 33 | 47.4 % ± 19.8 | 0.07 | |
Data are median (25–75th percentile) or mean ± standard deviation, except where stated
IKDC International Knee Documentation Committee, KOOS Knee Injury and Osteoarthritis Outcome Score, QOL quality of life
* Student t test, Mann–Whitney U test, or Kruskall–Wallis test used as appropriate
** Friedman test with post hoc analysis
*** Significantly different from the preceding time period
Fig. 1.
Comparison of preoperative and postoperative International Knee Documentation Committee (IKDC) ranking: *Sample size 24 in both groups preoperatively and at 1 year follow-up and 23 in group 1 and 21 in group 2 at final follow-up
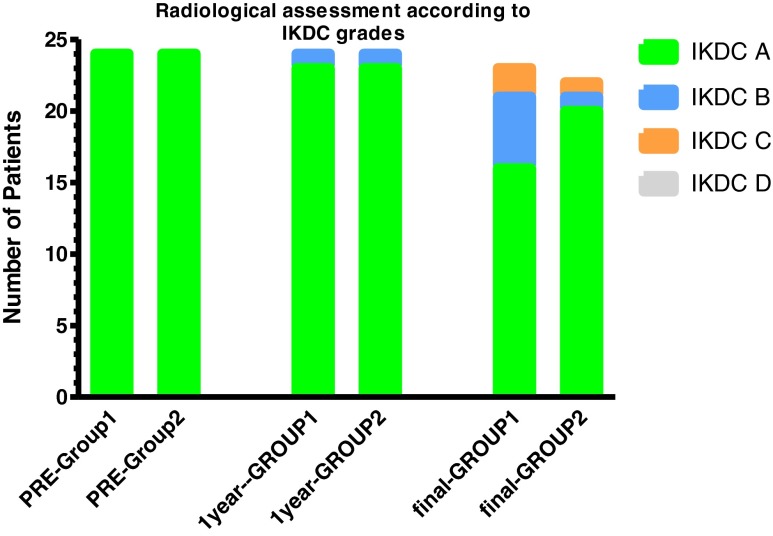
Tegner activity scale improved significantly at final review in both groups compared with preoperative status (p < 0.017), with significant intergroup difference at final follow-up (p = 0.004). All patients except four in group 1 achieved satisfactory daily activity levels; the remaining four had KOOS quality of life (QOL) subscale less than 44 points, which is considered unsatisfactory [23]. Fifteen patients (62.5 %) in group 1 and 21 (87.5 %) in group 2 returned to recreational sporting activities [Fisher’s exact test p = 0.09; RR = 0.56; 95% CI = 0.33–0.92]. Median ROM (125° and 130°) in both groups was less than the preoperative median ROM; however, this difference was statistically insignificant (p = 0.27) at final follow-up. Radiographic evaluation demonstrated moderate joint narrowing (IKDC ranking C) in two patients with concomitant partial medial meniscectomy in group 1 versus only one patient in group 2. At final follow-up, deterioration of radiographic osteoarthritis grade by one grade was noted in 7/23 cases in group 1 (30 %) and in 2/21 in group 2 (10 %) [Fisher’s exact test p = 0.14; RR = 1.7; 95% CI = 01.03–2.8] (Table 5 and Fig. 2). No findings consistent with the presence of osteomyelitis were recorded.
Table 5.
Preoperative and postoperative distribution of patients according to International Knee Documentation Committee (IKDC) grading scale of knee osteoarthritis in the infection (group 1) and control (group 2) groups
| Group | Grade | Preoperative* | I-year Follow-up* | Final Follow-up** |
|---|---|---|---|---|
| Group 1 (24 patients) | 0 | 24 | 23 | 18 |
| Mild | 0 | 1 | 3 | |
| Moderate | 0 | 0 | 2 | |
| Severe | 0 | 0 | 0 | |
| Group 2 (24 patients) | 0 | 24 | 23 | 19 |
| Mild | 0 | 1 | 1 | |
| Moderate | 0 | 0 | 1 | |
| Severe | 0 | 0 | 0 | |
| Total | 48 | |||
* Sample size 24 in both groups
** Sample size 23 in group1 and 2 in group 2
Fig. 2.
Comparison of preoperative and postoperative radiological International Knee Documentation Committee (IKDC) scale. *Sample size 24 in both groups preoperatively and at 1-year follow-up and 23 in group1 and 21 in group 2 at final follow-up
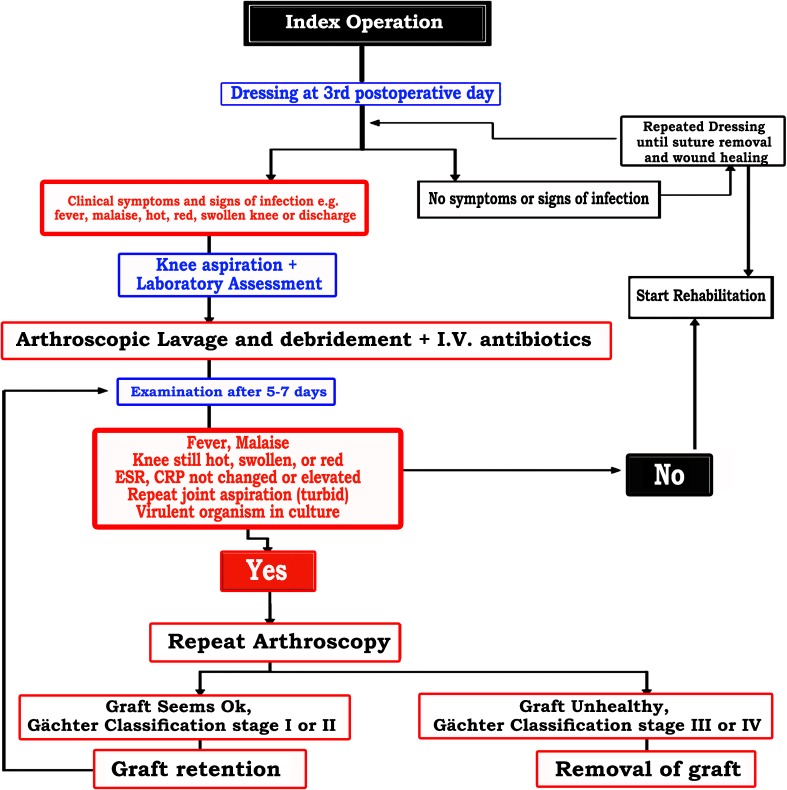
Four patients had meniscus injury that was managed by partial meniscectomy, none of whom had meniscal repair. No patient had collateral ligament repair. Multivariate statistical analysis indicated that age, BMI, and duration to operation had no statistically significantly effect on the success rate. Patients who had associated meniscus injury had worse outcomes than those who did not have meniscus injury (OR 0.08; p = 0.004; 95 % CI = 0.015–0.459). Finally, an algorithm is proposed for managing patients with postoperative intra-articular infection following ACL reconstruction (Fig. 3).
Fig. 3.
Algorithm for managing patients with postoperative intra-articular infection following anterior cruciate ligament (ACL) reconstruction
Complications
Three patients in group 1 and one in group 2 were diagnosed as having a failed ACL reconstruction due to graft rupture. Revision was done for one patient in each group using patellar tendon allograft, which achieved good results at follow-up; the others refused a further procedure. Four patients (three in group 1) had arthroscopic partial meniscectomy three to six years after ACL reconstruction following another injury. Sudeck’s atrophy was recorded in one patient in group 1. Loss of range of flexion and extension occurred in 5/24 patients for an average incidence of 21 % in group 1 versus 0/24 in group 1 and an average incidence of 1 % in our overall series. For patients with arthrofibrosis, arthroscopic arthrolysis was done; final ROM for these patients was included in the final result. No cases with vascular injuries, deep vein thrombosis (DVT), peroneal nerve injuries, recurrence of septic arthritis or bone infection were recorded.
Discussion
Results of our study prove that multiple arthroscopic debridement and graft retention achieved successful functional outcome in patients who developed postoperative ACL infection when compared with a carefully matched control group when early diagnosis, prompt IV administration of antibiotics and aggressive management is done. At an average of five years of follow-up, there was no statistically significant difference between groups regarding clinical and functional scores. The lack of statistical significance is not attributable to the absence of a suitable sample size or type 2 errors. However, the infected patients reported an average Tegner score 1.5 levels lower compared with controls (median 5.5 vs 7, p = 0.004)., which compares with lower levels of 1.86–3 reported in the literature [4, 14, 18]. Infection after arthroscopic ACL reconstruction is uncommon and has been reported in 0.14–1.8 % of cases [1, 3, 4, 9, 10, 14, 15, 18, 21, 23–27]. Overall, the authors of these 14 studies, in addition to our study (Table 6), reported 182 infections after 28,115 ACL reconstruction, most of which succeeded in preserving the graft after an average of 1.8 arthroscopies.
Table 6.
Literature review of septic arthritis after anterior cruciate ligament (ACL) reconstruction and results of this study
| Study | Number (incidence) | Number of ACL reconstructions | Graft | Average age in years | Average days to presentation | Average number of treatments* | Follow-up months |
|---|---|---|---|---|---|---|---|
| Williams et al. 1997 [27] | 7 (0.3 %) | 2,500 | 4 BPTB 3 Hamstring | 31 (17–50) | 21 (3–79) | 1.6 (1–2) | 29 (7–71) |
| McAllister et al. 1999 [14] | 4 (0.48 %) | 831 | 3 BPTB 1 Hamstring | 26 (20–34) | 11.2 (8–18) | 2.7 (2–4) | 36 (28–42) |
| Viola et al. 2000 [25] | 14 (0.78 %) | 1,794 | 14 BPTB | 21 (17–29) | 7.7 (2–20) | 1** | 14 (5–43) |
| Indelli et al. 2002 [9] | 6 (0.14 %) | 3,500 | 4 BPTB 2 Allograft Achillis | 32 (20–51) | 20 (9–34) | 2.3 (1–4) | 36 (24–96) |
| Schollin-Borg et al. 2003 [18] | 10 (1.7 %) | 575 | 6 BPTB 4 Hamstring | 28 (19–39) | 15.4 (4–20) | 1 (+ continuous irrigation) | 36* |
| Fong et al. 2004 [4] | 7 (1.4 %) | 472 | 7 Hamstring | 23 (19–30) | 24 (7–56) | 1.4 (1–3) | 12 (5–26) |
| Jude et al. 2006 [10] | 11 (0.62 %) | 1,615 | 11 Hamstring | 28 (22–35) | 14.2 (6–45) | 2.4 (1–4) | 22 (10–48) |
| Van Tongel et al. 2007 [24] | 15 (0.51 %) | 1,736 | 12 hamstring 2 BPTB 1 pes anserinus + Achilles | 33 (17–50) | 10.9 (2–455) | 1.9 (1–4) | 58 (9–99) |
| Binnet &Basarir 2007 [2] | 6 (0.49 %) | 1,231 | 4 BPTB 2 Hamstring | 25 (20–30) | 22 (14–35) | 2.6 (1–5) | 102 (30–196) |
| Wang et al. 2009 [26] | 21 (0.52 %) | 4,068 | 1 BPTB 20 Hamstring | 29 (16–58) | 13.5 (3–29) | Conservative (6) 1 scope (19 patients) | NA |
| Monaco et al. 2010 [15] | 12 (0.97 %) | 1,232 | 12 Hamstring | 24 (16–43) | 16 (10–20) | Irrigation (ambulatory) 1 scope (4 patients) | 38 (6–54) |
| Barker et al. 2010 [1] | 18 (0.58 %) | 3,126 | 7 BPTB 5 Hamstring6 Allograft | 34 (16–52) | 32 (5–205) | 1.5 (1–3) 5 grafts removed | NA |
| Sonnery-Cottet et al. 2011 [21] | 12 (0.61 %) | 1,975 | 7 BPTB 4 hamstring 1 Quadriceps | 29 (18–49) | 16 (2–37) | 1.25 (1–2) | NA |
| Torres-Claramunt et al. 2012 [23] | 15 (1.8) | 810 | 13 Hamstring 2 BPTB | 34 (±7) | 24 (±14) | 1.3 (±0.6) | 39 (±13) |
| Current Study | 24 (0.94 %) | 2,650 | 24 Hamstring | 26 (19–35) | 12 (5–45) | 3 (1–6) | 59 (18–96) |
| Total | 182 (0.14–1.8 %) | 28,115 | 118 Hamstring 54 BPTB9 Allograft 1 Quadriceps | 28.2 (16–58) | 17.3 (2–455) | 1.8 (1–6) | 40 (6–196) |
Except where stated, numbers in parenthesis indicate (range).
BPTB bone–patellar tendon–bone, NA not available.
*Arthroscopic and/or open joint irrigation and debridements and, where appropriate, local wound irrigation and debridement
**Antibiotics started initially: 6 patients failed to improve and were treated with arthroscopic incision and drainage after 2 weeks of antibiotics
Previous knee surgery or concomitant surgical procedures performed during initial ACL reconstruction were reported to be a risk factor for septic knee arthritis either because of increased operative time, additional or larger incisions, lengthy tourniquet inflation or the use of suture material acting as a foreign body [27]. In this series, four patients in group 1 (16 %) had had previous knee arthroscopy and another four patients had an additional meniscus injury. Multivariate statistical analysis indicated that age, BMI and time from injury to operation had no statistically significantly effect on the success rate. Patients with associated meniscus injury had worse outcome than those without.
The goals of treatment for septic arthritis after ACL reconstruction are to protect the articular cartilage and the graft [14]. However, optimal clinical management guidelines have not yet been completely established because of the rarity and heterogeneity of this complication. Published recommendations differ for graft retention, open versus arthroscopic treatment, type and duration of antibiotics and time to revision [16]. This lack of agreement reflects the challenging nature of this complication.
The presence of compromised tissue, such as the avascular graft and the presence of hardware, promote biofilm formation by pathogens and may preclude infection control [7]. Radical debridement with graft and hardware removal facilitates treatment of persistent infection; however, the majority of surgeons are reluctant to do this because it destabilises the knee joint and necessitates a repeat reconstructive procedure [5–8, 12, 13, 16, 17, 22, 28]. Schulz et al. [20] reported that early infection can be managed arthroscopically and satisfactory results can be expected. In advanced or chronic infection, a more radical approach seems favourable, although the authors reported no comparative results to statistically support their idea. Schub et al. [19] reported long-term results of four septic arthritis cases of 831 consecutive patients who underwent ACL reconstruction surgery. When compared with their earlier follow-up, the long-term results in these four patients showed declines in pain-related subjective measures likely related to pain from arthritis, but both functional testing and activity-related subjective scales either remained stable or improved after an average follow-up of 17.9 years. Also, their radiographic studies revealed progression of arthritis; one of the four had an ACL graft rupture.
Antibiotic therapy should be started for every patient who has strong clinical evidence of infection, and treatment should be continued even if synovial fluid cultures are negative [18, 25]. In addition, an initial arthroscopic procedure with debridement and lavage using normal saline is an effective therapeutic intervention to minimise the severity of sequelae, including osteoarthritis, osteomyelitis and arthrofibrosis [14]. Arthroscopy allows direct access to the knee joint and shorter postoperative recovery time with less morbidity than does open arthrotomy [4]. Furthermore, aggressive synovectomy lowers the number of bacteria in the infected joint [2].
S. aureus and CNS are the two most common bacteria found in septic arthritis after ACL reconstruction [3, 9, 14, 21, 25]. In the series we report here, the infecting organisms were 29 % CNS and 29 % S. aureus. These two micro-organisms represent the majority of this type of infection [1, 24, 26]. Negative synovial cultures despite the infection was present in three cases (12.5 %), which could be due to the short interval (under one week) between ACL reconstruction and time of infection presentation, as also reported by Viola [25].
In our study, functional assessment was done with the widely used various knee scores: the modified Lysholm knee score, Tegner scale, IKDC ranking and KOOS score, to allow data comparison; however, a limitation of our study is that translation of these systems has not been cross-culturally adapted. Another limitation is that none of our infected patients were professional athletes or had preoperative arthritic changes. All but three infected particpants were stage 2 Gächter classification, and there were no cases with marked infection. These factors may justify our overall good results.
To our knowledge, this is the first study to prospectively compare the results of postoperative infection for patients undergoing ACL reconstruction with a matched control. We can thus conclude that our protocol is advantageous, as we present a prospective case–control series with a control group with sufficient power. This protocol achieved successful clinical and functional outcomes; however, confirming the arthritis risk will require a longer follow-up period. A randomised controlled trial with sufficient power will best determine other treatment protocols; however, the low prevalence of this complication precludes the accumulation of large series and thus hinders undertaking such an investigation at a single institution.
References
- 1.Barker JU, Drakos M, Maak TG, Warren RF, Williams RJ, Allen AA. Effect of graft selection on the incidence of postoperative infection in anterior cruciate ligament reconstruction. Am J Sports Med. 2010;38(2):281–286. doi: 10.1177/0363546509346414. [DOI] [PubMed] [Google Scholar]
- 2.Binnet M, Basarir K. Risk and outcome of infection after different arthroscopic anterior cruciate ligament reconstruction techniques. Arthroscopy. 2007;23(8):862–868. doi: 10.1016/j.arthro.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Burks R, Friederichs M, Fink B, Luker M, West H, Greis P. Treatment of postoperative anterior cruciate ligament infections with graft removal and early reimplantation. Am J Sports Med. 2003;31:414–418. doi: 10.1177/03635465030310031501. [DOI] [PubMed] [Google Scholar]
- 4.Fong SY, Tan JL. Septic arthritis after arthroscopic anterior cruciate ligament reconstruction. Ann Acad Med Singap. 2004;33(2):228–234. [PubMed] [Google Scholar]
- 5.Frobell RB, Roos EM, Roos HP, Ranstam J, Lohmander LS. A randomized trial of treatment for acute anterior cruciate ligament tears. N Engl J Med. 2010;363:331–342. doi: 10.1056/NEJMoa0907797. [DOI] [PubMed] [Google Scholar]
- 6.Gächter A. The joint infection [in German] Inform Arzt. 1985;6:35–43. [Google Scholar]
- 7.Gristina AG, Costerton JW. Bacterial adherence and the glycocalyx and their role in musculoskeletal infection. Orthop Clin North Am. 1984;15:517–535. [PubMed] [Google Scholar]
- 8.Hefti F, Muller W, Jakob RP, Staubli HU. Evaluation of knee ligament injuries with the IKDC form. Knee Surg Sports Traumatol Arthrosc. 1993;1:226–234. doi: 10.1007/BF01560215. [DOI] [PubMed] [Google Scholar]
- 9.Indelli P, Dillingham M, Fanton G, Schurman D. Septic arthritis in postoperative anterior cruciate ligament reconstruction. Clin Orthop Relat Res. 2002;398:182–188. doi: 10.1097/00003086-200205000-00026. [DOI] [PubMed] [Google Scholar]
- 10.Judd MA, Bottoni LT, Kim D, Burke CP, Hooker MA. Infections following arthroscopic anterior cruciate ligament reconstruction. Arthroscopy. 2006;22(4):375–384. doi: 10.1016/j.arthro.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Kohn D. Unsuccessful arthroscopic treatment of pyarthrosis following anterior cruciate ligament reconstruction. Arthroscopy. 1988;4:287–289. doi: 10.1016/S0749-8063(88)80047-1. [DOI] [PubMed] [Google Scholar]
- 12.Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10:150–154. doi: 10.1177/036354658201000306. [DOI] [PubMed] [Google Scholar]
- 13.Matava M, Evans T, Wright R, Shively R. Septic arthritis of the knee following anterior cruciate ligament reconstruction: Results of a survey of sports medicine fellowship directors. Arthroscopy. 1998;14:717–725. doi: 10.1016/S0749-8063(98)70098-2. [DOI] [PubMed] [Google Scholar]
- 14.McAllister D, Parker R, Cooper A, Recht M, Abate J. Outcomes of post-operative septic arthritis after anterior cruciate ligament reconstruction. Am J Sports Med. 1999;27:562–570. doi: 10.1177/03635465990270050301. [DOI] [PubMed] [Google Scholar]
- 15.Monaco E, Masteri B, Labianca L, Speranza A, Vadala A, Iorio R, Ferritti A. Clinical and radiological outcomes of postoperative septic arthritis after anterior cruciate ligament reconstruction. J Orthop Sci. 2010;15:198–203. doi: 10.1007/s00776-009-1447-3. [DOI] [PubMed] [Google Scholar]
- 16.Mouzopoulos G, Fotopoulos VC, Tzurbakis M. Septic knee arthritis following ACL reconstruction: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1033–1042. doi: 10.1007/s00167-009-0793-1. [DOI] [PubMed] [Google Scholar]
- 17.Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;8:439–448. doi: 10.1186/1477-7525-1-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schollin-Borg M, Michaelsson K, Hans R. Presentation, outcome, and cause of septic arthritis after anterior cruciate ligament reconstruction: a case control study. Arthroscopy. 2003;19(9):941–947. doi: 10.1016/j.arthro.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Schub DL, Schmitz LM, Sakamoto FA, Winalski CS, Parker RD. Long-term Outcomes of Postoperative Septic Arthritis After Anterior Cruciate Ligament Reconstruction. Am J Sports Med. 2012;40(12):2764–2770. doi: 10.1177/0363546512461903. [DOI] [PubMed] [Google Scholar]
- 20.Schulz AP, Götze S, Schmidt HG, Jürgens C, Faschingbauer M. Septic arthritis of the knee after anterior cruciate ligament surgery: a stage-adapted treatment regimen. Am J Sports Med. 2007;35(7):1064–1069. doi: 10.1177/0363546507299744. [DOI] [PubMed] [Google Scholar]
- 21.Sonnery-Cottet B, Archbold P, Zayni R, Bortolletto J, Thaunat M, Prost T, Padua V, Chambat P. Prevalence of septic arthritis after anterior cruciate ligament reconstruction among professional athletes. Am J Sports Med. 2011;39(11):2371–2376. doi: 10.1177/0363546511417567. [DOI] [PubMed] [Google Scholar]
- 22.Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;198:43–49. [PubMed] [Google Scholar]
- 23.Torres-Claramunt R, Pelfort X, Erquicia J, Gil-Gonzalez S, Gelber P E, Puig L, Monllau JC (2012) Knee joint infection after ACL reconstruction: prevalence, management and functional outcomes. Knee Surg Sports Traumatol Arthrosc 2012. doi:10.1007/s00167-012-2264-3 [DOI] [PubMed]
- 24.Van Tongel A, Stuyck J, Bellemans J, Vandenneucker H. Septic arthritis after arthroscopic anterior cruciate ligament reconstruction, a retrospective analysis of incidence, management and outcome. Am J Sports Med. 2007;35:1059–1063. doi: 10.1177/0363546507299443. [DOI] [PubMed] [Google Scholar]
- 25.Viola R, Marzano N, Vianello R. An unusual epidemic of Staphylococcus-negative infections involving anterior cruciate ligament reconstruction with salvage of the graft and function. Arthroscopy. 2000;16:173–177. doi: 10.1016/S0749-8063(00)90032-X. [DOI] [PubMed] [Google Scholar]
- 26.Wang C, Ao Y, Wang J, Hu Y, Cui G, Yu J. Septic Arthritis after arthroscopic anterior cruciate ligament reconstruction: A Retrospective Analysis of Incidence, Presentation, Treatment, and Cause. Arthroscopy. 2009;25(3):243–248. doi: 10.1016/j.arthro.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Williams R, Laurencin C, Warren R, Speciale A, Brause B, O’Brien S. Septic arthritis after arthroscopic anterior cruciate ligament reconstruction: Diagnosis and management. Am J Sports Med. 1997;25:261–267. doi: 10.1177/036354659702500222. [DOI] [PubMed] [Google Scholar]
- 28.Zalavras CC, Patzakis MJ, Tibone J, Weisman N, Holtom P. Treatment of persistent infection after anterior cruciate ligament surgery. Clin Orthop Relat Res. 2005;439:52–55. doi: 10.1097/01.blo.0000181499.49740.e5. [DOI] [PubMed] [Google Scholar]