Abstract
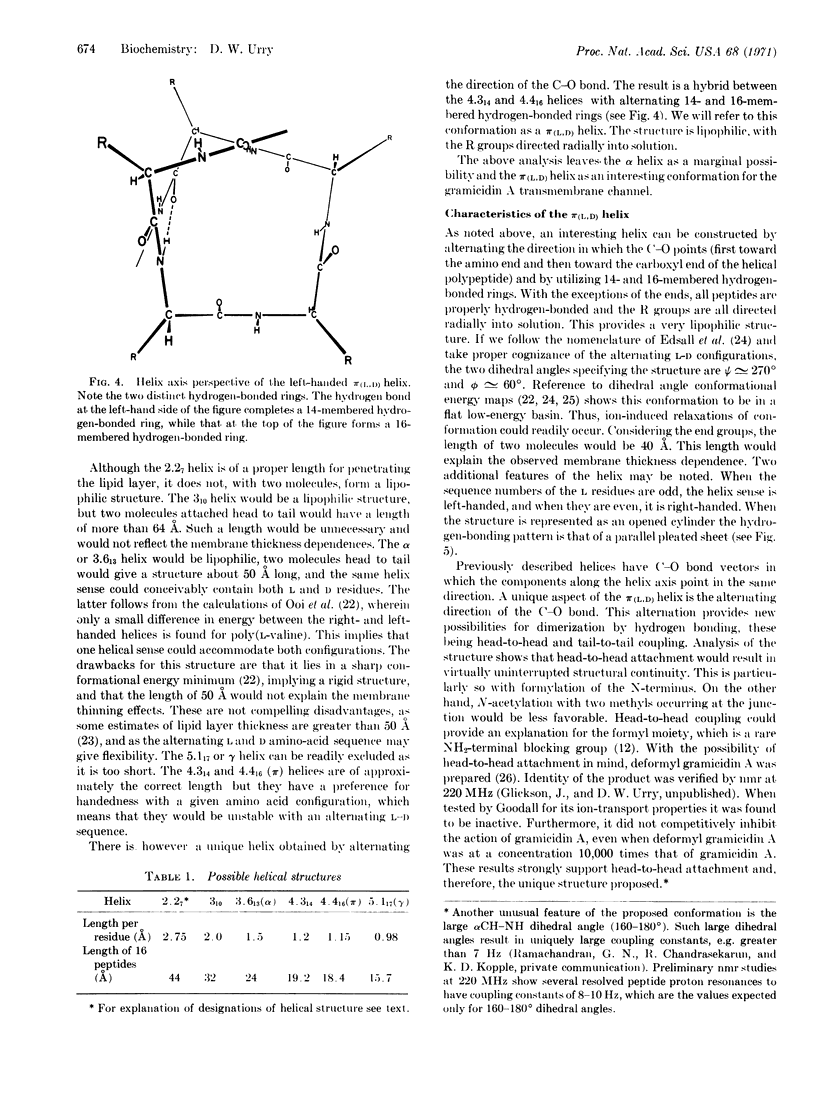
A lipophilic, left-handed helical structure is proposed for gramicidin A in which the C-O bonds alternately point toward the amino and carboxyl ends; it is a hybrid of the 4.314 and 4.416 helices. The C-O groups pointing toward the carboxyl end form part of 16-membered hydrogen-bonded rings, whereas the C-O moieties pointing toward the amino end form 14-membered hydrogenbonded rings. The proposed structure is based on conformational analysis combined with requirements for the gramicidin A transmembrane channel. Two helices combine to form the channel. The alternating C-O directions allow hydrogen-bonded dimerization by the unique possibilities of head-to-head and tail-to-tail attachment. The formyl group at the amino end allows for a favorable head-to-head attachment with no loss of structural continuity. Unpublished studies. by M. C. Goodall on the lipid bilayer conductance of deformyl gramicidin A strongly argue for head-to-head attachment. Such hydrogen-bonded association is not possible with previously described helices, as the C-O groups all point in the same direction. In relation to possible π(L,D) helices in mammalian systems, it should be noted that glycines would fill the role of D residues.
The conformation can undergo ion-induced relaxations, which provide approximate tetrahedral coordination for the ion, with facile shifting of coordinations. The ready exchange of coordinations provides the mechanism for movement of the ion along the channel. Conceivably, such transmembrane channels could have application as models for ion transport across biological membranes—an application which may be as great as, or greater than, that of carriers such as valinomycin and nonactin. Specifically, biogenic amines and drugs containing aromatic groups could control access to the channel by interactions with the two tryptophan residues at the ethanolamine end and with the negative region provided by the three oxygens.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreoli T. E., Dennis V. W., Weigl A. M. The effect of amphotericin B on the water and nonelectrolyte permeability of thin lipid membranes. J Gen Physiol. 1969 Feb;53(2):133–156. doi: 10.1085/jgp.53.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry R. J., Chapman D. Optical properties of black lecithin films. J Mol Biol. 1969 Feb 28;40(1):19–32. doi: 10.1016/0022-2836(69)90293-9. [DOI] [PubMed] [Google Scholar]
- Donohue J. Hydrogen Bonded Helical Configurations of the Polypeptide Chain. Proc Natl Acad Sci U S A. 1953 Jun;39(6):470–478. doi: 10.1073/pnas.39.6.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson K. D., Scheraga H. A. Influence of flexibility on the energy contours of dipeptide maps. Biopolymers. 1966 Jul;4(6):709–712. doi: 10.1002/bip.1966.360040611. [DOI] [PubMed] [Google Scholar]
- Hladky S. B., Haydon D. A. Discreteness of conductance change in bimolecular lipid membranes in the presence of certain antibiotics. Nature. 1970 Jan 31;225(5231):451–453. doi: 10.1038/225451a0. [DOI] [PubMed] [Google Scholar]
- Kilbourn B. T., Dunitz J. D., Pioda L. A., Simon W. Structure of the K+ complex with nonactin, a macrotetrolide antibiotic possessing highly specific K+ transport properties. J Mol Biol. 1967 Dec 28;30(3):559–563. doi: 10.1016/0022-2836(67)90370-1. [DOI] [PubMed] [Google Scholar]
- Ohnishi M., Urry D. W. Solution conformation of valinomycin-potassium ion complex. Science. 1970 May 29;168(3935):1091–1092. doi: 10.1126/science.168.3935.1091. [DOI] [PubMed] [Google Scholar]
- Ooi T., Scott R. A., Vanderkooi G., Scheraga H. A. Conformation of analysis of macromolecules. IV. Helical structures of poly-L-alanine, poly-L-valine, poly-beta-methyl-L-aspartate, poly-gamma-methyl-L-glutamate, and poly-L-tyrosine. J Chem Phys. 1967 Jun 1;46(11):4410–4426. doi: 10.1063/1.1840561. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Ivanov V. T., Evstratov A. V., Bystrov V. F., Abdullaev N. D., Popov E. M., Lipkind G. M., Arkhipova S. F., Efremov E. S., Shemyakin M. M. The physicochemical basis of the functioning of biological membranes: dynamic conformational properties of enniatin B and its K+ complex in solution. Biochem Biophys Res Commun. 1969 Nov 6;37(4):668–676. doi: 10.1016/0006-291x(69)90863-8. [DOI] [PubMed] [Google Scholar]
- Tosteson D. C., Andreoli T. E., Tieffenberg M., Cook P. The effects of macrocyclic compounds on cation transport in sheep red cells and thin and thick lipid membranes. J Gen Physiol. 1968 May;51(5 Suppl):373S+–373S+. [PubMed] [Google Scholar]




