Abstract
Purpose
Our aim was to investigate the causes of and treatment strategies for surgical complications of thoracic spinal stenosis.
Methods
Between May 1990 and May 2010, 283 patients with thoracic spinal stenosis were treated in our department. Three physicians were assigned to patient follow-up. Patient medical records and radiographs were reviewed. Complications were categorised as perioperative, mid- to long-term and donor-site.
Results
Follow-up was completed for 254 patients; 249 patients survived. Follow-up time ranged from one to 19 years, with a mean of six years and two months. There were 107 cases with complications an incidence rate of 42.1 %. Eleven cases were pulmonary infection, seven transient nerve-root injury, three pulmonary injury and one vertebral canal haematoma, all of which resolved. Thirteen cases of spinal cord injury postoperatively were treated using dehydration and corticosteroid therapy; eight recovered to the preoperation level, and five deteriorated. Eleven cases resulted in dural injury, and four led to cerebrospinal fluid leakage. There were five cases of wound-fat liquefaction and one of wound infection. Seven cases with deep venous thrombosis of the lower limb resolved by elevating the affected limb and administration of low-molecular-weight dextran. Seven cases of delayed wound healing recovered following change of dressings and antibiotic administration. Four cases of delayed bone-graft fusion recovered by extending the external fixation time. One case of bone-graft absorption was treated by iliac bone grafting and bracing. Two cases of internal fixation breakage were treated by removing the internal fixation.
Conclusions
Thoracic spinal stenosis surgery may result in various complications but has a good prognosis with proper treatment. The key points in reducing complications are the surgeon’s familiarity with operative imperatives and the appropriate surgical approach.
Keywords: Thoracic spinal stenosis, Complications, Surgery, Postoperative
Introduction
Thoracic spinal stenosis is not uncommon and is more often diagnosed with advances in imaging techniques. However, thoracic spinal disorders are difficult to treat, and postoperative complications are common [1–3]. We treated 283 patients with thoracic spinal stenosis in our department from May 1990 to May 2010, of these 254 were followed up; 107 patients developed complications. This study evaluated treatment methods and outcomes, including possible causes of complications.
Materials and methods
Patients
This study assessed 283 patients (175 men, 108 women) with a mean age of 51.8 (range 27–79) years. Lesion causes and distribution are shown in Table 1 and Fig. 1. Until 1995, we resected the lamina using an olecranon rongeur and a Kerrison rongeur via the standard posterior approach (original method). From 1996, we resected the lamina using a high-speed drill (uncovering method), with the approach based on lesion location and pathological type (Fig. 2).
Table 1.
The causes and lesion distributions of study patients [n (%)]
| Causes | Lesion distributions | ||
|---|---|---|---|
| Upper thoracic | Middle thoracic | Lower thoracic | |
| Ossification of the ligamentum flavum 123 | 17 (13.8) | 25 (20.3) | 81 (65.9) |
| Ossification of the posterior longitudinal ligament 73 | 29 (39.7) | 20 (27.3) | 24 (33.0) |
| Intervertebral disc herniation 54 | 4 (7.4) | 50 (92.6) | |
| Diffuse idiopathic skeletal hyperostosis 19 | 3 (15.7) | 7 (36.8) | 9 (47.5) |
| Posterior marginal intraosseous cartilaginous node 14 | 4 (28.5) | 10 (71.5) | |
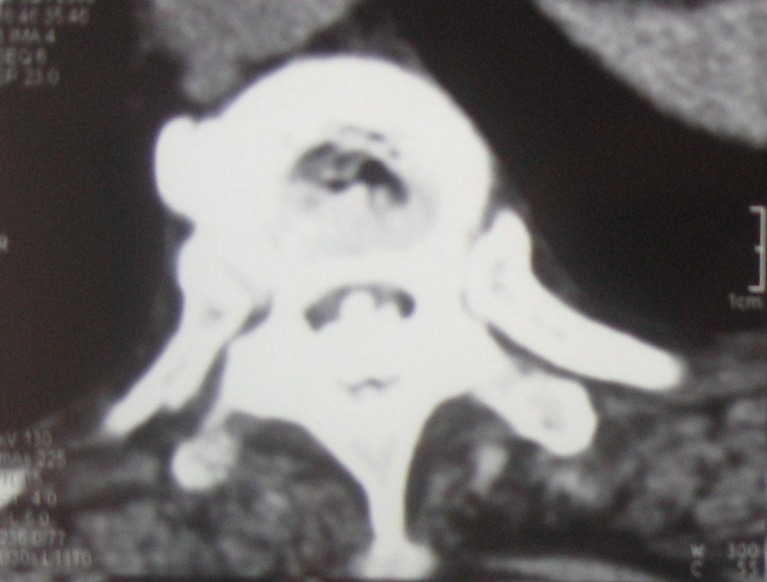
Fig. 1.
Lesions primarily located at the disc level and caused by ossification of posterior longitudinal ligament and ligamentum flavum
Fig. 2.
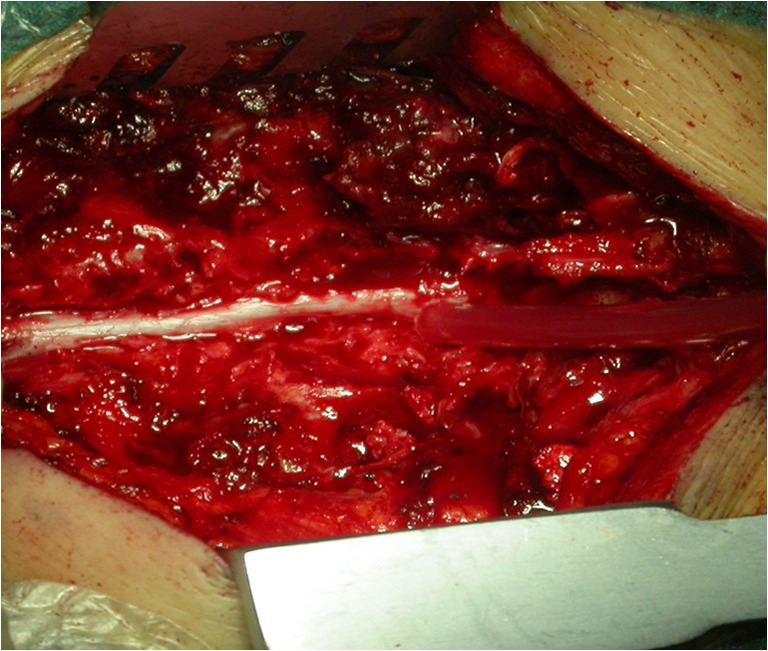
Posterior laminectomy performed using a high-speed drill (uncovering method)
Symptoms
All patients reported an insidious onset of similar symptoms lasting six months to eight years. Forty-one patients had an obvious cause, such as a traumatic fracture, bending load or preceding viral illness. Initial symptoms included low back pain radiating to the lower limbs in 96 patients, progressive numbness and weakness of the lower limbs in 47, intermittent claudication and numbness of the lower limbs in 39, thoracic and abdominal areas of numbness in 42, gait instability in 35 and dysfunction of urination and defecation in 24. Upper motor neuron injury was observed in 226 patients and lower motor neuron injury in 57.
Surgical techniques
Original method
Surgery was performed using the original method in 86 patients, including 38 with ossification of the ligamentum flavum (OLF), 26 with ossification of the posterior longitudinal ligament (OPLL), 19 with intervertebral disc herniation, two with diffuse idiopathic skeletal hyperostosis syndrome (DISH) and one with a posterior marginal intraosseous cartilaginous node. All patients were treated via the posterior approach using the olecranon rongeur and Kerrison rongeur to remove the lamina and OLF and the Kerrison rongeur to remove the medial portion of hyperplastic facet joints. Intervertebral disc excision was performed in 19 patients, and the medial half of the hyperplastic facet joint was resected in 27 patients. All patients underwent posterolateral fusion with iliac bone grafting followed by bed rest for three months.
Improved method
Surgery was performed using the improved method in 197 patients, including 85 with OLF, 47 with OPLL, 35 with disc herniation, 17 with DISH and 13 with posterior marginal intraosseous cartilaginous nodes. Thirty-five patients with disc herniation and 51 with single-segment OLF were treated via the transthoracic or extraperitoneal approach, with resection of the disc or osteophyte and interbody fusion with iliac bone grafting. The remaining patients were treated via the posterior approach, with resection of the lamina using a high-speed drill (uncovering method). These 135 patients with disc herniation or lesions in the cervicothoracic or thoracolumbar junction underwent internal fixation and fusion with iliac bone grafting.
Complications
Complications were categorised as perioperative, mid- to long-term or donor-site related. Perioperative complications included spinal cord injury, nerve-root injury, dural injury, cerebrospinal fluid (CSF) leakage, fat liquefaction, pulmonary injury, haematoma, infection (pulmonary or wound) and intestinal obstruction. Mid- to long-term complications included delayed wound healing, low back pain, deep venous thrombosis (DVT), delayed bone-graft fusion and disruption of internal fixation. Donor-site complications included haematoma, pain and infection.
Results
Three physicians performed postoperative assessments. Of the 254 patients who were followed up, 249 survived, three died of cerebrovascular accidents, one died of heart disease and one died of accidental trauma. The mean follow-up period was six years and two months (range one to 19 years). The complication rate was 42.1 % (107 patients), including 88 patients (34.6 %) with surgical-site and 19 (10.5 %, 19/181) with donor-site complications: 58 men, 49 women; mean age 52.4 (range 38–76) years. Thirty-eight patients had OLF, 26 had OPLL, 28 had disc herniation, seven had DISH and eight had posterior marginal intraosseous cartilaginous nodes.
Original method
Of the 86 patients who underwent surgery by the original method, 69 were followed up and 49 had complications (complication rate 71.0 %), including 45 patients (65.2 %) with surgical-site complications and four (8.7 %, 4/46) with donor-site complications (Table 2 and 3).
Table 2.
Surgical-site complications in operation groups (n, %)
| Complications | Original method (69) | Improved method (185) |
|---|---|---|
| Perioperative | ||
| Spinal cord injury | 9 (13.0) | 4 (2.2) |
| Dural injury | 6 (8.7) | 5 (2.7) |
| Transient nerve-root injury | 4(5.8) | 3 (1.6) |
| Pulmonary infection | 4(5.8) | 7 (3.7) |
| Cerebrospinal fluid leakage | 3(4.3) | 2 (1.1) |
| Wound-fat liquefaction | 2(2.9) | 3 (1.6) |
| Pulmonary injury | 3(1.6) | |
| Wound infection | 1(1.4) | 1(0.5) |
| Vertebral canal haematoma | 1(1.4) | |
| Intestinal obstruction | 1(0.5) | |
| Mid- to long-term | ||
| Delayed wound healing | 3(4.3) | 4(2.2) |
| Deep venous thrombosis | 2(2.9) | 5(2.7) |
| Aggravated neurological symptoms | 5(8.7) | |
| Delayed bone-graft fusion | 2(2.9) | 2(1.1) |
| Low back pain | 2(2.9) | 1(0.5) |
| Internal fixation breakage | 2(1.1) | |
| Bone graft absorption | 1 (1.4) | |
| Total | 45 (65.2) | 43(23.2) |
Table 3.
Donor-site complications in different operation groups [n (%)]
| Complications | Original method (46) | Improved method (135) |
|---|---|---|
| Haematoma | 1 (2.1) | 3 (2.2) |
| Pain | 2 (4.3) | 8 (5.9) |
| Numbness | 1 (2.1) | 4 (3.0) |
Improved method
Of the 197 patients who underwent surgery by the improved method, 185 were followed up. Fifty-eight patients developed complications (31.2 %), including 43 (23.2 %) with surgical-site and 15 (11.1 %, 15/135) with donor-site complications (Tables 2 and 3).
Discussion
Thoracic spinal stenosis is common, but few studies have reported on the complications associated with surgical treatment.
Perioperative complications and treatment
Intraoperative spinal cord injury is relatively frequent during thoracic spinal surgery because of the local anatomical characteristics. Takahata et al. [4] reported 33 % incidence of postoperative neurological deterioration among 30 patients. Li et al. [5] reported a retrospective clinical study of 31 thoracic myelopathy cases; postoperative paralysis occurred in five (16.1 %). Yamazaki et al. [6] reported six patients with spinal cord injury among 51 patients treated with posterior decompression for thoracic OPLL. In our series, the injury rate was 5.1 % (13/254). The most common causes of spinal cord injury during surgery are direct injury, limited range of decompression and reperfusion injury. In our series, spinal cord injury occurred in four patients with disc herniation who underwent surgery via the posterior approach (21.1 %). After dehydration and corticosteroid therapy, neurological function returned to the preoperative level in one of these patients. Spinal cord injury from the olecranon rongeur or Kerrison rongeur during laminectomy occurred in two patients with OLF. Deterioration of a spinal cord injury occurred five hours after surgery in two patients with OPLL and OLF. This was considered to be caused by supine postoperative positioning with a cervicothoracic kyphotic angle more than 45°, resulting in spinal cord compression. After three hours of lateral positioning, dehydration, and corticosteroid therapy, the aggravation resolved. One patient with OPLL (T6–T9) and OLF (T7–T9) who underwent laminectomy and decompression (T7–T9) developed worsening of paralysis three days after surgery. Postoperative magnetic resonance imaging (MRI) showed that the spinal cord was angled backwards at the upper end of the decompression. The patient was treated by emergency decompression to the level of C5, dehydration, and corticosteroid therapy and the paresis resolved after seven days. One patient developed deteriorating neurological symptoms eight hours after surgery. Postoperative MRI showed a haematoma in the vertebral canal. The patient was treated by surgical evacuation of the haematoma and dehydration and corticosteroid therapy and recovered to the preoperative level after ten days.
Decompression is the only effective treatment for thoracic spinal stenosis [3]. Posterior discectomy is a relatively easy operation, but traction on the spinal cord is inevitable, which increases the risk of spinal cord injury [7–9]. Mulier et al. [10] reported postoperative aggravation of spinal cord dysfunction in 28 % of 129 patients who underwent this procedure. We stopped using this technique because of the high risk of intraoperative complications and poor results and now use the encroachment method to perform laminectomy in patients with OLF [11]. Use of the Kerrison rongeur often results in spinal cord injury due to irritation of the spinal cord and its blood supply [12, 13]. Intraoperative spinal cord injury occurred in two patients in our series who underwent surgery by the original method for these reasons. The uncovering method was developed to minimise the risk of spinal cord injury. Use of a high-speed drill can decrease the risk of spinal cord injury. Two patients in our series developed spinal cord ischemia–reperfusion injury, both of which were successfully treated by dehydration. Since we started routine intraoperative and postoperative administration of dexamethasone and mannitol, we have not experienced any further cases of this particular complication. Two patients developed aggravation of a spinal cord injury due to postoperative local compression. In patients with a large kyphotic angle or who had undergone upper thoracic spinal surgery, we subsequently placed them in the lateral position postoperatively. Many previous reports have discussed the optimal range of decompression, and we recommend that the range should include more than one vertebral level and both sides of the medial facet. To avoid injury to the blood supply to the spinal cord, the microcirculation around the intervertebral foramen should be protected when ligating the vertebral segmental vessels. The Adamkiewicz artery should be carefully protected. This artery lies on the left side at T7–L4 in about 80 % of cases and usually supplies the nutrient arteries to the spinal cord at T9–L1 [14–16]. Injury to the Adamkiewicz artery may be disastrous. Intraoperative evoked-potential monitoring can reduce the risk of spinal cord injury.
Nerve-root injury is comparatively rare during surgical treatment of thoracic spinal stenosis, and single nerve-root injury does not have serious consequences [17, 18]. Nerve-root injury occurred in seven patients in our series: two were due to traction during posterior discectomy, three to irritation caused by the Kerrison rongeur, one to incorrect use of the drill and one to incorrect use of the bone knife during partial resection of the facet joint. These patients all recovered from the intraoperative injury within six months. When resecting the facet joint with a rongeur, surgeons should be aware of nerve-root distribution and use a small dissector when necessary to protect the roots. Nerve-root injury may also occur during placement of vertebral pedicle screws. Preoperative imaging is necessary to determine optimal screw entry point and direction. It is easy to injure the dura during surgery because of adhesions between osteophytes and the dura, especially in patients with OLF and OPLL. Li et al. [5] reported four patients with CSF leakage in 31 thoracic myelopathy cases who underwent decompression surgery. Yamazaki et al. [6] reported eight patients with CSF leakage among 51 patients treated with posterior decompression for thoracic OPLL. Takahata et al. [4] reported 12 patients (40 %) with dural tear during thoracic myelopathy surgery. In our series, dural injury occurred in 11 patients and CSF leakage in five. In five patients with a longitudinal dural injury, the dura was closed using a 1–0 silk suture, and one of these patients developed CSF leakage. In four patients with an irregular dural injury, the dura was repaired with thoracolumbar fascia, and two of these patients developed CSF leakage. Two patients with a small, unrepaired dural injury developed CSF leakage. In one patient, the dura developed mesh-like holes after surgery because of the long-term severe compression, even though the dura was not damaged during surgery. Factors causing a slight increase in intracranial pressure such as coughing and defecation could cause CSF leakage. In our experience, use of the uncovering method greatly reduced the incidence of dural injury. Use of the drill swing can cause direct injury to the dura, and both elbows should be supported by a stable fulcrum when using the drill. All dural injuries should be repaired. Many different methods of dural repair using various materials have been reported. After repair, anaesthetists should ask the patient to perform a few Valsalva manoeuvres to confirm absence of CSF leakage. Use of fibrin glue is not recommended because it can prolong adhesion time of surrounding tissues. In patients at risk of CSF leakage, it is important to ensure tight suturing of the muscle and fascia layers.
Postoperative haematoma can occur within or outside the vertebral canal, usually on the day of surgery, and often results from incomplete intraoperative haemostasis, blocked drainage tubes or coagulopathy. Haematoma in the vertebral canal can injure the spinal cord with catastrophic consequences if it is not treated urgently. In this series, one patient developed a haematoma in the vertebral canal. This patient was treated by emergency surgery to drain the haematoma and ensure haemostasis and to place a drainage catheter. Subcutaneous haematoma causes fever and local pain and swelling. No patient in this series developed subcutaneous haematoma, but surgeons should be aware of this potential complication.
Mid- to long-term complications and treatment
Delayed bone-graft fusion occurred in four patients who were subsequently treated by prolonged external fixation, and bone-graft absorption occurred in one patient who was subsequently treated by further surgery. These complications may occur because of insufficient preparation of the bone-graft bed, unstable internal fixation, or lack of strong external fixation [19–21]. Breakage of the internal fixation occurred in two patient three years after thoracolumbar fixation. This may have been because of movement of the thoracolumbar spine, poor fusion or incorrect elastic modulus of the internal fixation. Osteoporosis and heavy workload may also cause screw loosening. These two patients were asymptomatic after removal of the internal fixation.
The overall reported rate of DVT after spinal surgery is 14 %, but the rate may be as high as 70 % in paraplegic patients [22]. DVT usually occurs between five days and one year after surgery. In our series, DVT occurred in seven patients with oedema of both lower limbs over six months after surgery. These patients were treated by bed rest, warm compresses, elevation of the affected limb and low-molecular-weight dextran, resulting in resolution within 20 days. The reasons for DVT development in these patients may have been older age, paralysis of both lower limbs before surgery, prolonged bed rest after surgery and lack of exercise. The risk of DVT can be reduced by limb exercises, use of graded elastic stockings and administration of low-molecular-weight dextran. Active exercise was encouraged in postoperative patients, and passive movement and muscle massage were provided for paralysed patients.
Donor-site complications and treatment
The most common donor-site complications are haematoma, pain, numbness and infection. Four patients with haematoma were treated by evacuation of the haematoma and pressure dressings. Ten patients experienced donor-site pain, which resolved in seven patients at six months to two years after surgery. The other three patients have ongoing mild pain. Five patients experienced donor-site numbness, which resolved after physical therapy in four patients but continued after physical therapy in one. There were no cases of donor-site infection.
Conclusion
In conclusion, thoracic spinal stenosis surgery may result in various complications but has a good prognosis through proper treatment. The key points to reduce the complications are total familiarity with operative skills required and the correct surgical approach.
Acknowledgments
This research was supported by grants from the National Nature Science Foundation of China (No. 81071486). There is no actual or potential conflict of interest in relation to this article.
References
- 1.Hulme A. The surgical approach to thoracic intervertebral disc protrusions. J Neurol Neurosurg Psychiatry. 1960;23:133–137. doi: 10.1136/jnnp.23.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Currier BL, EisMont FJ, Green BA. Transthoracic disc excision and fusion for herniated thoracic discs. Spine. 1994;19:323–328. doi: 10.1097/00007632-199402000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Akhaddar A, Mansouri A, Zrara I, Gazzaz M, Maftah M, Mostarchid B, Benomar S, Boucetta M. Thoracic spinal cord compression by ligamentum flavum ossifications. Joint Bone Spine. 2002;69:319–323. doi: 10.1016/S1297-319X(02)00400-1. [DOI] [PubMed] [Google Scholar]
- 4.Takahata M, Ito M, Abumi K, Kotani Y, Sudo H, Minami A. Clinical results and complications of circumferential spinal cord decompression through a single posterior approach for thoracic myelopathy caused by ossification of posterior longitudinal ligament. Spine (Phila Pa 1976) 2008;33:1199–1208. doi: 10.1097/BRS.0b013e3181714515. [DOI] [PubMed] [Google Scholar]
- 5.Li M, Meng Z, Du J, Tao H, Luo Z, Wang Z. Management of thoracic myelopathy caused by ossification of the posterior longitudinal ligament combined with ossification of the ligamentum flavum-a retrospective study. Spine J. 2012;12:1093–1102. doi: 10.1016/j.spinee.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 6.Yamazaki M, Mochizuki M, Ikeda Y, Sodeyama T, Okawa A, Koda M, Moriya H. Clinical results of surgery for thoracic myelopathy caused by ossification of the posterior longitudinal ligament: operative indication of posterior decompression with instrumented fusion. Spine (Phila Pa 1976) 2006;31:1452–1460. doi: 10.1097/01.brs.0000220834.22131.fb. [DOI] [PubMed] [Google Scholar]
- 7.Taher F, Lebi DR, Cammisa FP, Pinter DW, Sun DY, Girardi FP. Transient neurological deficit following midthoracic decompression for severe stenosis: a series of three cases. Eur Spine J. 2013;22:S416–S420. doi: 10.1007/s00586-013-2829-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Dostenburegge RJ, Herpers MJ, De Kruijik JR. Spinal cord compression caused by unusual location and extension of ossified ligamenta flavum in a Caucasian male: a case report and literature. Spine. 1999;24:486–488. doi: 10.1097/00007632-199903010-00019. [DOI] [PubMed] [Google Scholar]
- 9.Kato S, Murakami H, Demura S, Yoshioka K, Hayashi H, Tsuchiya H. Novel surgical technique for ossification of posterior longitudinal ligament in the thoracic spine. J Neurosurg Spine. 2012;17:525–529. doi: 10.3171/2012.9.SPINE12617. [DOI] [PubMed] [Google Scholar]
- 10.Mulier S, Debois V. Thoracic disc herniations: transthoracic, lateral, or posterolateral approach? A review. Surg Neurol. 1998;49:599–608. doi: 10.1016/S0090-3019(98)00008-1. [DOI] [PubMed] [Google Scholar]
- 11.Dimar JR, 2nd, Bratcher KR, Glassman SD, Howard JM, Carreon LY. Identification and surgical treatment of primary thoracic spinal stenosis. Am J Orthop (Belle Mead NJ) 2008;37:564–568. [PubMed] [Google Scholar]
- 12.Coppes MH, Bakker NA, Metzemakers JD, Groen RJ. Posterior transdural discectomy: a new approach for the removal of a central thoracic disc herniation. Eur Spine J. 2012;21:623–628. doi: 10.1007/s00586-011-1990-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eggspuehler A, Sutter MA, Grob D, Porchet F, Jeszenszky D, Dvorak J. Multimodal intraoperative monitoring (MIOM) during surgical decompression of thoracic spinal stenosis in 36 patients. Eur Spine J. 2007;16:S216–S220. doi: 10.1007/s00586-007-0425-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang UK, Choe WJ, Chung CK, Kim HJ. Surgical treatment for thoracic spinal stenosis. Spinal Cord. 2001;39:362–369. doi: 10.1038/sj.sc.3101174. [DOI] [PubMed] [Google Scholar]
- 15.Epstein NE, Schwall G. Thoracic spinal stenosis: diagnostic and treatment challenges. J Spinal Disord. 1994;7:259–269. doi: 10.1097/00002517-199407030-00011. [DOI] [PubMed] [Google Scholar]
- 16.Yu S, Wu D, Li F, Hou T. Surgical results and prognostic factors for thoracic myelopathy caused by ossification of ligamentum flavum: posterior surgery by laminectomy. Acta Neurochir (Wien) 2013;155:1169–1177. doi: 10.1007/s00701-013-1694-0. [DOI] [PubMed] [Google Scholar]
- 17.Yoon SH, Kim WH, Chung SB, Jin YJ, Park KW, Lee JW, Chung SK, Kim KJ, Yeom JS, Jahng TA, Chung CK, Kang HS, Kim HJ. Clinical analysis of thoracic ossified ligamentum flavum without ventral compression lesion. Eur Spine J. 2011;20:216–223. doi: 10.1007/s00586-010-1515-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen XQ, Yang HL, Wang GL, Gu Y, Pan WM, Dong RB, Qiu ZJ, Wu JB, Mei X. Surgery for thoracic myelopathy caused by ossification of the ligamentum flavum. J Clin Neurosci. 2009;16:1316–1320. doi: 10.1016/j.jocn.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 19.Wang VY, Kanter AS, Mummaneni PV. Removal of ossified ligamentum flavum via a minimally invasive surgical approach. Neurosurg Focus. 2008;25:E7. doi: 10.3171/FOC/2008/25/8/E7. [DOI] [PubMed] [Google Scholar]
- 20.Hrabálek L, Kalita O, Langová K. Surgical treatment of thoracic disc herniation. Acta Chir Orthop Traumatol Cech. 2010;77:312–319. [PubMed] [Google Scholar]
- 21.Nasser MJ. Standard fenestration approach to thoracic disc herniation. Br J Neurosurg. 2009;23:418–421. doi: 10.1080/02688690802716145. [DOI] [PubMed] [Google Scholar]
- 22.Takahata M, Ito M, Abumi K, Kotani Y, Sudo H, Minami A. Clinical results and complications of circumferential spinal cord decompression through a single posterior approach for thoracic myelopathy caused by ossification of posterior longltudinal ligament. Spine. 2008;33:1199–1208. doi: 10.1097/BRS.0b013e3181714515. [DOI] [PubMed] [Google Scholar]