Abstract
Background
Adverse tissue reactions associated with metal-on-metal (MOM) hips are common in resurfacing and total hip arthroplasty (THA) designs. The etiology of these reactions in painful, well-positioned arthroplasties is inconsistently described.
Questions/purposes
The purposes of this study were to compare the (1) articular wear rates; (2) histologic findings; (3) synovial response on MRI; and (4) graded intraoperative tissue damage between well-positioned, MOM hips revised for unexplained pain and MOM hips revised for other reasons and to (5) determine whether the presence of a taper junction on a MOM articulation affects these four parameters in unexplained pain.
Methods
We retrospectively studied 88 patients (94 hips) who had undergone revision of either a hip resurfacing or a large-head (> 36 mm) THA. Thirty-five hips revised for unexplained pain were compared with a control group of 59 hips revised for other causes. Articular wear was measured using three-dimensional contactless metrology and histologic analysis was performed using the aseptic lymphocyte-dominated vasculitis-associated lesion (ALVAL) score. Preoperative MRI was performed on 57 patients to determine synovial volumes and thicknesses. Tissue damage was graded from intraoperative reports.
Results
Articular wear rates in the unexplained pain group were lower than in the control group (median 2.6 μm/year versus 12.8 μm/year, p < 0.001). Sixty-six percent of patients in the unexplained pain group had histologic confirmation of ALVAL compared with 19% in the control group (p < 0.001). The synovial thickness on MRI was higher in the unexplained pain group (p = 0.04) and was highly predictive of ALVAL. Severe intraoperative tissue damage was noted in more cases in the unexplained pain group (p = 0.01). There were no differences in articular wear, histology, MRI, and tissue damage between resurfacings and THAs revised for unexplained pain.
Conclusions
Unexplained pain in patients with well-positioned MOM hips warrants further investigation with MRI to look for features predictive of ALVAL. Tissue destruction in these cases does not appear to be related to high bearing wear or the presence of a taper.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Metal-on-metal (MOM) hip bearings in THA were reintroduced in the United States on the premise that they would result in lower rates of wear and dislocation, particularly in younger patients [4]. However, reports from the Australian Orthopaedic Association National Joint Replacement Registry showed failure rates for MOM hip arthroplasties approaching 10% at 7 years [3] with metal sensitivity being an increasingly reported cause of failure [9]. The National Joint Registry for England and Wales also reported a high prevalence of failure resulting from unexplained pain in well-positioned implants, which was hypothesized to be the result of adverse soft tissue reactions [17].
The causative factors resulting in adverse tissue reactions remain unclear. Multiple authors have proposed that these reactions are associated with metallic debris and/or elevated blood metal ion levels secondary to component wear influenced by component malposition or implant design [8, 19, 21, 25, 28–30]. These data are supported with clinical outcomes at 10 years that show that acetabular component orientation determines the long-term success of a MOM hip implant [2]. Conversely, reports exist of unexplained pain and pseudotumors in low-wearing, well-positioned implants [5, 10, 11, 13, 17, 34]. The authors of these reports have speculated that patient susceptibility may be important. Histologic evaluation of the periprosthetic tissues of lesions secondary to excessive metal wear showed extensive necrosis, histiocytes, and intracytoplasmic metallic debris [26, 29, 38]. A cell-mediated type IV hypersensitivity reaction has also been described, characterized histologically by an aseptic lymphocyte-dominated vasculitis-associated lesion (ALVAL) [5, 45]. This delayed hypersensitivity reaction has been described in both low-wearing [5, 29] and high-wearing implants [38].
The modular taper junction has also been recently implicated as a cause of adverse tissue reaction [7]. To avoid the complication of neck fracture in hip resurfacings, implant companies developed resurfacing-sized heads (> 36 mm) to attach to conventional femoral stems and articulate with a monobloc resurfacing cup. In one particular design, the rate of failure of the THA version was reported as almost double that of the resurfacing with considerable damage noted at the taper interface despite low articular surface wear rates [27]. However, data from the Australian Orthopaedic Association National Joint Replacement Registry showed no difference in revision rates at 5 years between hip resurfacing and large-head THA [24]. It remains unclear as to whether the taper is responsible for the pain experienced by patients with well-positioned implants.
Given the inconsistency in the literature regarding the pathogenesis of pain in a well-positioned MOM hip, our aims were to compare the (1) articular wear rates; (2) histologic findings; (3) synovial response on MRI; (4) graded intraoperative tissue damage between well-positioned, MOM hips revised for unexplained pain and MOM hips revised for other reasons and to (5) determine whether the presence of a taper junction on a MOM articulation affects these four parameters in unexplained pain.
Patients and Methods
From our institutional retrieval database, we identified 186 MOM hip arthroplasties that had been explanted at our institution between March 2007 and March 2012. Our inclusion criteria were that the implant was a hip resurfacing or a large-head THA (> 36 mm) with a monobloc acetabular cup; the length of implantation was a minimum of 12 months to account for the running-in phase [22]; and each case had a complete minimum data set of wear of the retrieved head and cup, preoperative radiographs, serum C-reactive protein level, intraoperative findings, and postoperative histology and microbiological cultures.
Eighty-eight patients (94 hips) fulfilled these criteria and were retrospectively reviewed. These patients were divided into two groups based on the cause of revision as determined from the medical records and verified by the operating surgeon (Table 1). The unexplained pain group (35 hips) consisted of patients revised for pain originating from the hip and/or the presence of a functional impairment with components in Lewinnek’s safe zone [32] and in the absence of infection. An infection was considered ruled out only if the serum C-reactive protein level was not elevated and/or if the hip aspirate was negative after microbiologic culture. As part of an ongoing institutional initiative, all patients with a MOM hip arthroplasty presenting with pain and/or functional impairment were recommended to undergo preoperative MRI using a standard protocol optimized to reduce metallic susceptibility artifact, including the MAVRIC (multiacquisition variable-resonance image combination) sequence [42]. The presence of an adverse synovial reaction on MRI was taken into consideration when making a decision regarding revision surgery in this group. The control group (59 hips) consisted of patients revised for aseptic acetabular and femoral loosening (confirmed intraoperatively), cup malalignment defined as inclination > 70° or anteversion associated with impingement, and periprosthetic fracture. Thirty-one patients in the unexplained pain group and 26 patients in the control group underwent MAVRIC MRI preoperatively. Three patients in the unexplained pain group did not give informed consent to perform MAVRIC MRI.
Table 1.
Comparison of patient demographics, component details, and relevant preoperative data between unexplained pain and control groups
| Variable | UEP | Control | p value |
|---|---|---|---|
| Number of patients | 34 | 54 | |
| Number of hips | 35 | 59 | |
| Age (years)* | 53 (33–66) | 54 (23–80) | 0.74 |
| BMI (kg/m2)* | 27 (19–40) | 27 (16–45) | 0.13 |
| Male:female | 18:16 | 21:33 | 0.27 |
| Resurfacing:THA | 10:25 | 26:33 | 0.14 |
| Time to revision (months)* | 39 (18–103) | 30 (12–78) | 0.35 |
| Head size (mm)* | 46 (38–54) | 45 (38–54) | 0.76 |
| Acetabular inclination (°)* | 46 (33–51) | 48 (14–83) | 0.11 |
| Acetabular inclination > 30 or < 50† | 35 (100) | 30 (51) | < 0.001 |
| Acetabular anteversion (°)* | 17 (5–25) | 28 (−21 to 43) | < 0.001 |
| Implant type | 0.17 | ||
| ASR (DePuy, Warsaw, IN, USA)† | 13 (37) | 17 (29) | |
| BHR (Smith & Nephew, Memphis, TN, USA)† | 15 (43) | 14 (24) | |
| Conserve Plus (Wright Medical, Memphis, TN, USA)† | 3 (8) | 9 (15) | |
| Cormet (Corin Group, Cirencester, UK)† | 1 (3) | 5 (8) | |
| Durom (Zimmer, Warsaw, IN, USA)† | 1 (3) | 4 (7) | |
| M2a (Biomet, Warsaw, IN, USA)† | 2 (6) | 10 (17) | |
| Reason for revision | < 0.001 | ||
| Fracture† | 0 (0) | 1 (2) | |
| Loosening† | 0 (0) | 21 (35) | |
| Malalignment† | 0 (0) | 47 (63) | |
| Unexplained pain† | 35 (100) | 0 (0) | |
* Values are expressed as median, with range in parentheses; †values are expressed as number, with percentage in parentheses; UEP = unexplained pain; BMI = body mass index; ASR = Articular Surface Replacement; BHR = Birmingham Hip Resurfacing.
There were 39 men and 49 women with a mean age at the time of index arthroplasty of 53 years (range, 23–80 years). Sixty-eight (72%) of the index arthroplasties in this study had been performed at outside institutions. The mean length of implant survival was 39 months (range, 12–103 months). Thirty-six hips were resurfacings and 58 were large-head THA.
Radiographic Analysis
Prerevision, standardized digital AP pelvic and cross-table lateral [36] hip radiographs were analyzed for each patient. Anteversion and inclination of the acetabular component were measured using Einzel-Bild-Roentgen-Analysis [31] software (University of Innsbruck, Innsbruck, Austria). Anteversion or retroversion of the acetabular component was confirmed by analyzing the cross-table radiograph. All measurements were performed on two occasions, 1 month apart, by an independent assessor (DHN), and mean values were used for analysis.
Articular Wear Analysis
Both femoral and acetabular components were available for all patients. Articular surface wear was measured using three-dimensional contactless metrology (RedLux Artificial Hip Profiler; RedLux Ltd, Southampton, UK). This technology uses an automated noncontact sensor to scan a whole bearing surface to a resolution of 20 nm [44]. The resulting cloud of approximately 30,000 points is meshed and compared with a best-fit sphere fitted to the unworn part of the surface. We used RedLux’s proprietary software (RedLux Ltd) to calculate linear wear (μm) by measuring the maximum deviation between the unworn sphere and the worn sphere. Volumetric wear (mm3) was measured by summing the volumes of the meshed discrete prisms located within the wear patch of the heads and cups. This technique has been validated against a roundness machine and gravimetric methods of wear measurement [44]. We determined total linear and total volumetric wear for each hip by summing femoral and acetabular component wear. Using the time to revision, we calculated the total linear wear rate and total volumetric wear rate for each hip by dividing total wear by time to revision. Hart et al. [16] have proposed a threshold for linear wear of 5 μm/year, beyond which an implant is considered to be high-wearing. This value was based on a review of the published literature on wear analysis of retrieved MOM articulations. Thus, we defined hips as high-wearing if the total linear wear rate is > 5 μm/year and low-wearing if the total linear wear rate is ≤ 5 μm/year.
Taper Wear Analysis
Taper wear analysis focused on the female taper within the modular head. If an inner sleeve was included, the interface between the sleeve and trunnion was the interface of interest. Two independent observers (DHN, NAN) visually analyzed six 11/13 tapers, 41 12/14 tapers, 10 Type 1 tapers, and one C-taper. Gross wear and deformation within the taper were subjectively graded for fretting and corrosion using a previously published scoring system [14]. We divided each taper into a proximal and distal segment and graded each segment for corrosion and fretting using a score of 1 to 4 [14]. The scores were combined and averaged between the two observers so that each taper had a mean fretting and mean corrosion score with a maximum score of 8 (4 points per region). Female tapers from 47 THA heads were assessed further using a chromatically encoded confocal measurement device (RedLux Ltd) to calculate linear taper wear. Negative molds of the tapers were created using a high-resolution (0.1 μm) replication polymer (Microset Products, Leicestershire, UK). These negative molds were then scanned. The female taper scans were analyzed similar to the heads and cups where a best fit taper was created by fitting it to the unworn surface. Linear wear (μm) was measured as the maximum deviation between the unworn female taper and the worn taper.
Histology
All tissue samples excised at revision surgery were reviewed by one experienced musculoskeletal pathologist (GP), who analyzed all slides on two separate occasions within a 1-week period before giving a final report; the results were consistent 95% of the time, suggesting high intraobserver reliability using this approach. This pathologist is responsible for reviewing tissue samples retrieved from all failed MOM hip implants revised at our institution as part of an ongoing initiative. Sampled tissue was routinely obtained from the posterior pseudocapsule and anteroinferior neck of the femur. An average of 10 tissue blocks were processed per site to avoid sampling error resulting from necrosis. Multiple tissue levels were examined at sites of chronic lymphocytic infiltrate to report on the most severe features found in at least one location. Despite doing so, two cases each in the unexplained pain and control groups were excluded as a result of the presence of widespread necrosis making it impossible to grade the tissue. The samples were serially cut and stained with hematoxylin and eosin and then examined under light microscopy without knowledge of the revision diagnosis. Sections were evaluated for the presence of fibrinous exudates, necrosis, macrophages, lymphocytes, and metallic deposits. Synovial lining, inflammatory infiltrate, and tissue organization were graded to give an ALVAL score [5]. A score of ≥ 5 out of 10 was considered as moderate to high probability for ALVAL [5] (Fig. 1). Macrophages were assessed for the presence of particulate material in the form of metallic debris (irregular black particles) and corrosion products. Metallic debris within macrophages (Fig. 2) was graded with a semiquantitative scale (0 to 4+) using the classification proposed by Natu et al. [38]. Intracytoplasmic globular greenish particles of corrosion products were not graded.
Fig. 1A–B.
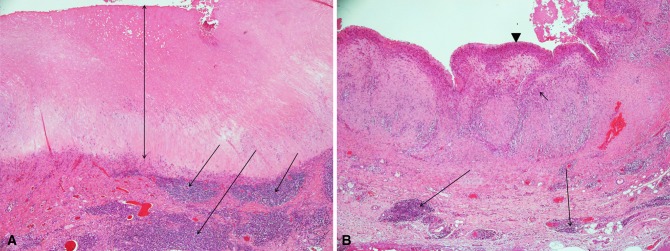
(A) Photomicrograph showing loss of the superficial synovial lining, a thick layer of necrosis (double-headed arrow), and a marked perivascular lymphocytic infiltrate located in the deep layer (arrows) (Stain, haemtoxylin and eosin, magnification ×40). This received an ALVAL score of 9 (severe ALVAL). (B) Photomicrograph showing a synovial lining with a hyperplastic superficial layer (arrowhead) with macrophages (short arrow) and small deep perivascular lymphocytic infiltrates (long arrows) (Stain, haemtoxylin and eosin, magnification ×40). This received an ALVAL score of 5 (moderate ALVAL).
Fig. 2.
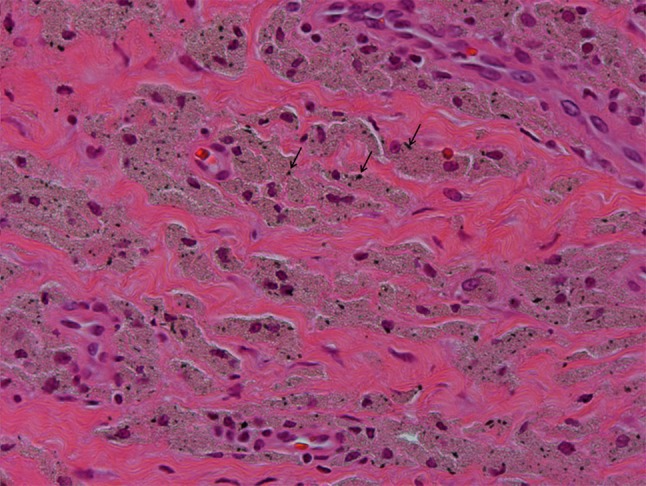
Photomicrograph showing particulate metallic debris within macrophages visualized as irregular black particles (arrows) (Stain, haemtoxylin and eosin, magnification ×400).
Magnetic Resonance Imaging
Fifty-seven patients (59 hips) were imaged preoperatively using a standard protocol to minimize metallic susceptibility artifact [42]. Scanning was performed with 1.5-T clinical scanners (GE Healthcare, Waukesha, WI, USA) using a three-element shoulder coil (MedRad, Indianola, PA, USA) or an eight-channel cardiac coil (GE Healthcare). Two-dimensional fast spin echo images were obtained in three planes using modifications to the pulse parameters to reduce susceptibility artifact. MAVRIC was used in the coronal plane to reduce susceptibility artifact by combining multiple data sets acquired at frequency bands offset from the center proton frequency [39]. We have previously described the specific pulse sequence parameters used [39, 40].
MR images were evaluated independently by two musculoskeletal radiologists (HGP, BL) who were blinded to the patient group and radiographs. The presence and volume of a synovial response were documented. Synovitis was defined as the presence of fluid signal intensity material or solid debris, either contained by the pseudocapsule or communicating with the disrupted pseudocapsule. Volumes of synovitis (mm3) were calculated using a previously validated method of manual segmentation from the coronal MAVRIC or axial fast spin echo images [43]. The area of synovitis was calculated on each slice and the sum of the areas was multiplied by the slice thickness to obtain a volume. We have previously reported on the repeatability of our method for assessing synovial volume with an interclass correlation coefficient of 0.97 [40]. The thickness (mm) of the synovial lining was assessed on the coronal MAVRIC images and measured digitally where it was noted to be the greatest [39]. Repeatability testing for synovial thickness, performed with the radiologists specifically for this study (HGP, BL), demonstrated an intraclass correlation coefficient of 0.98 and an interclass correlation coefficient of 0.91.
Tissue Damage
Intraoperative tissue damage was subjectively graded by the operating surgeon using a previously described 4-point scale [39]: 0 = normal tissue; 1 = fluid collection ± mild synovial reaction ± pseudocapsular dehiscence; 2 = Grade 1+ moderate to severe synovial reaction ± metallosis; and 3 = Grade 2+ abductor damage and/or bone loss. A score ≥ 2 was considered to be severe soft tissue damage.
Statistical Analyses
Articular surface wear, histology, MRI, tissue damage, and taper data were compared between the unexplained pain and control groups. Within the unexplained pain group, total linear wear rate, total volumetric wear rate, ALVAL score, tissue damage score, synovial thickness on MRI, and synovial volume on MRI were compared between resurfacings and large-head THAs. The Shapiro-Wilk test showed all continuous variables except age to be nonnormally distributed; therefore, continuous data were evaluated using the Wilcoxon rank-sum test. Categorical data were analyzed using Fisher’s exact test. Spearman rank correlation coefficients were calculated to assess the relationship between wear data and histologic findings, including ALVAL score and the extent of metallic particle deposition. Similar analyses were performed to evaluate the association of synovial thickness and synovial volume on MRI with wear data and the ALVAL score. Receiver operating characteristic (ROC) curves were generated for outcomes that were significantly correlated with MRI variables to examine the ability of MRI to detect important failure modes. Significance was set at p < 0.05. All analyses were performed using SAS Version 9.2 software (SAS Institute, Cary, NC, USA).
Results
Articular Wear Analysis
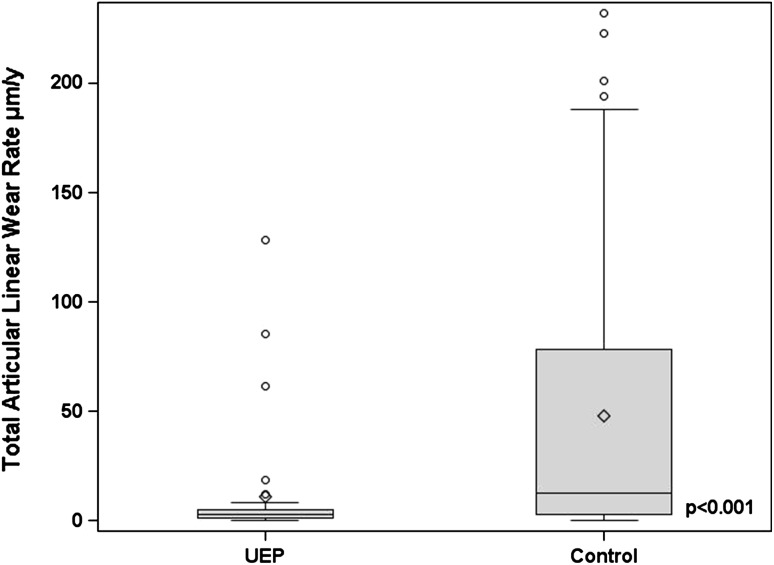
Linear and volumetric articular wear rates were lower in those hips revised for unexplained pain than in those revised for other indications (Table 2). The median total linear wear rate was 2.6 μm/year (range, 0–128.2 μm/year) in the unexplained pain group and 12.8 μm/year (range, 0–232.1 μm/year) in the control group (Fig. 3). The median total volumetric wear rate was 0.3 mm3/year (range, 0–29.3 mm3/year) in the unexplained pain group and 1.5 mm3/year (range, 0–94.3 mm3/year) in the control group. Eight of 35 hips (23%) were high-wearing (total linear wear rate > 5 μm/year) in the unexplained pain group compared with 37 of 59 hips (63%) in the control group (p < 0.001).
Table 2.
Articular wear analysis
| UEP (n = 35) | Control (n = 59) | p value | |
|---|---|---|---|
| Articular linear wear (μm) | |||
| Head* | 5.4 (0–144.8) | 10.8 (0–433.0) | 0.12 |
| Cup* | 2.0 (0–233.3) | 18.5 (0–703.8) | 0.001 |
| Total* | 8.1 (0–378.0) | 34.9 (0–954.8) | < 0.001 |
| Total linear wear rate (μm/year)* | 2.6 (0–128.2) | 12.8 (0–232.1) | < 0.001 |
| Number of hips with total linear wear rate > 5† | 8 (22.9) | 37 (62.7) | < 0.001 |
| Articular volumetric wear (mm3) | |||
| Head* | 0.8 (0–69.2) | 2.2 (0–201.0) | 0.13 |
| Cup* | 0 (0–60.1) | 1.2 (0–184.4) | 0.006 |
| Total* | 0.9 (0–129.3) | 5.0 (0–345.9) | 0.006 |
| Total volumetric wear rate (mm3/year)* | 0.3 (0–29.3) | 1.5 (0–94.3) | 0.02 |
* Values are expressed as median, with range in parentheses; †values are expressed as frequency, with percentage in parentheses; UEP = unexplained pain.
Fig. 3.
A box and whisker plot comparing total articular linear wear rate (TLWR) between the unexplained pain group (UEP) and control groups. The line inside each box represents the median value. TLWR was higher in the control group (p < 0.001).
Histology
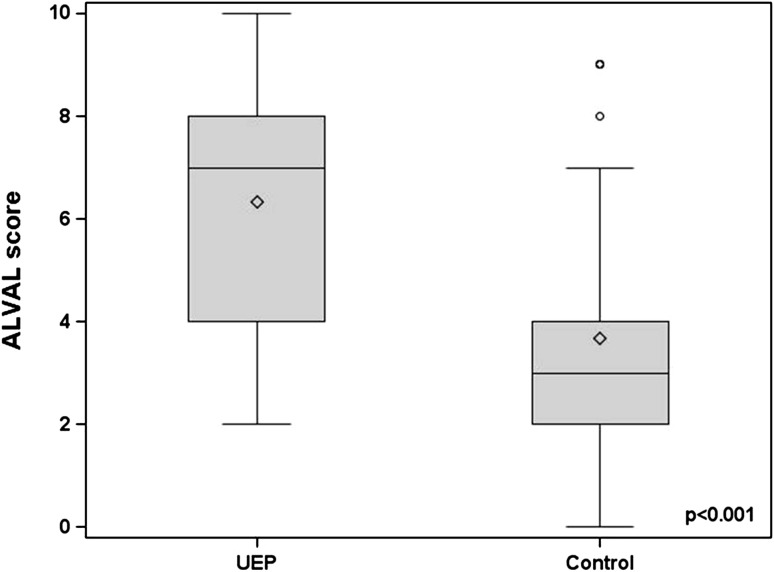
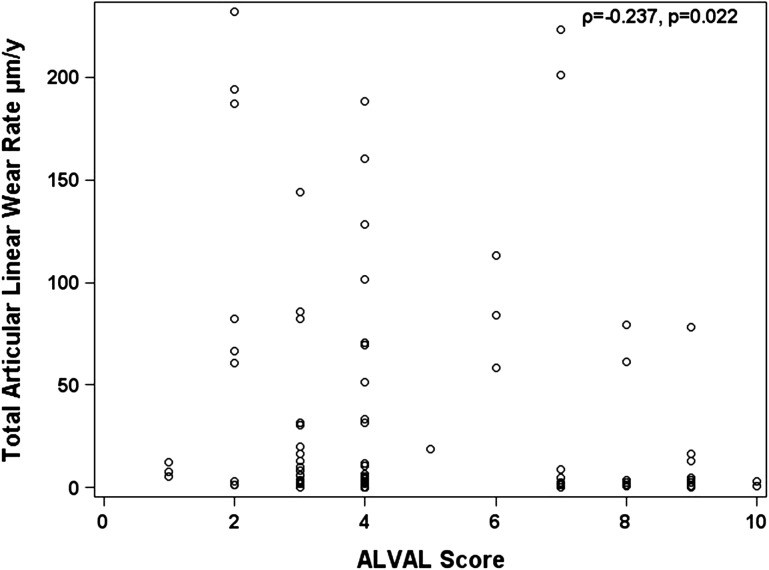
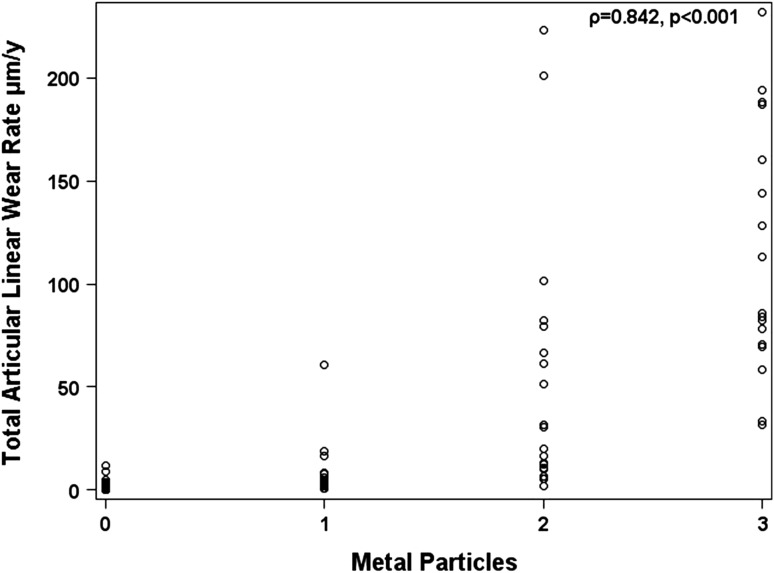
Histological findings suggestive of ALVAL were more severe and more prevalent in patients revised for unexplained pain than in those revised for other indications. The median ALVAL score was higher (p < 0.001) in the unexplained pain group compared with the control group (7 versus 3) (Fig. 4). Twenty-three cases (66%) in the unexplained pain group had an ALVAL score ≥ 5 out of 10 compared with 11 (19%) in the control group (p < 0.001). Of the remaining unexplained pain cases, three had metallosis based on intraoperative findings and nine remained unexplained with low ALVAL scores, no metallic deposits, and/or unremarkable MRI scans. Grade 2+ and 3+ metallic deposits were present in 11% of unexplained pain group cases compared with 58% of controls. The ALVAL score had a weak negative correlation with articular wear (Fig. 5). Of the 23 cases of ALVAL in the unexplained pain group, 21 had a high ALVAL score (≥ 5) and low total linear wear rate (≤ 5 μm/year) compared with only two cases with a high ALVAL score and high total linear wear rate (> 5 μm/year). The extent of metallic deposition had a strong positive correlation with wear (rho = 0.84; p < 0.001) (Fig. 6).
Fig. 4.
A box and whisker plot showing comparison of ALVAL scores between the unexplained pain group (UEP) and control groups. The line inside each box represents the median value. The median ALVAL score was higher in the UEP group (p < 0.001).
Fig. 5.
A plot of total articular linear wear rate against ALVAL score. This showed a weak negative correlation.
Fig. 6.
A plot of total articular linear wear rate and metallic particulate debris quantified histologically. This showed a strong positive correlation.
Magnetic Resonance Imaging
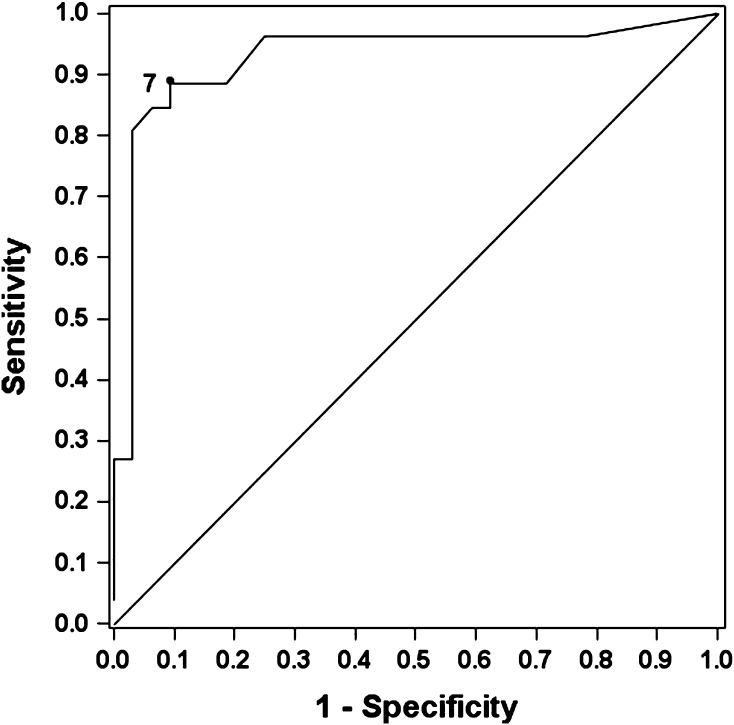
Synovial thickness on MRI was greater in patients revised for unexplained pain than in those revised for other indications. The mean synovial thickness was 10.5 mm in the unexplained pain group and 6.2 mm in the control group (p = 0.04). The mean synovial volume was 63.9 cm3 in the unexplained pain group and 49.4 cm3 in the control group (p = 0.71). Synovial thickness on MRI correlated with the ALVAL score (Table 3). An example of a representative case is given in Figure 7. Synovial volume on MRI showed a weak correlation with total volumetric wear rate (Table 3). An example of a representative case is given in Figure 8. Based on the high correlation between synovial thickness and the ALVAL score, we formulated a ROC curve to determine the value of synovial thickness that optimized sensitivity and specificity for detecting ALVAL. We found that a synovial thickness of ≥ 7 mm on MRI had a sensitivity of 88% and specificity of 90% for predicting ALVAL (Fig. 9).
Table 3.
Correlations between MRI variables and wear and histology
| Correlations | TLWR (μm/year) | TVWR (mm3/year) | ALVAL score |
|---|---|---|---|
| MRI synovial volume (mm3)* | Rho = 0.19 0.12 |
Rho = 0.27 0.03 |
Rho = 0.38 0.03 |
| MRI synovial thickness (mm)* | Rho = −0.02 0.87 |
Rho = −0.04 0.74 |
Rho = 0.77 < 0.001 |
* Values are expressed as Spearman’s Rho and p value; TLWR = total linear wear rate; TVWR = total volumetric wear rate; ALVAL = aseptic lymphocyte-dominated vasculitis-associated lesion.
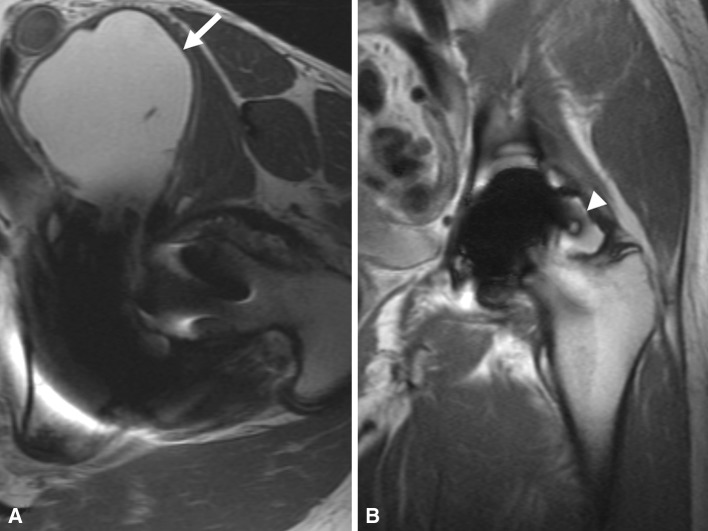
Fig. 7A–B.
(A) An axial fast spin echo MR image demonstrates a large volume of synovial fluid extending anteriorly with a thin synovial lining (white arrow) and (B) a coronal MAVRIC MR image shows distension of the pseudocapsule with a thin synovial lining (white arrowhead) in a patient who had a hip resurfacing revised for a malpositioned cup (anteversion 30.1°). The ALVAL score was 3, total linear wear rate (TLWR) 30.6 μm/year, and total volumetric wear rate (TVWR) 8.2 mm3/year.
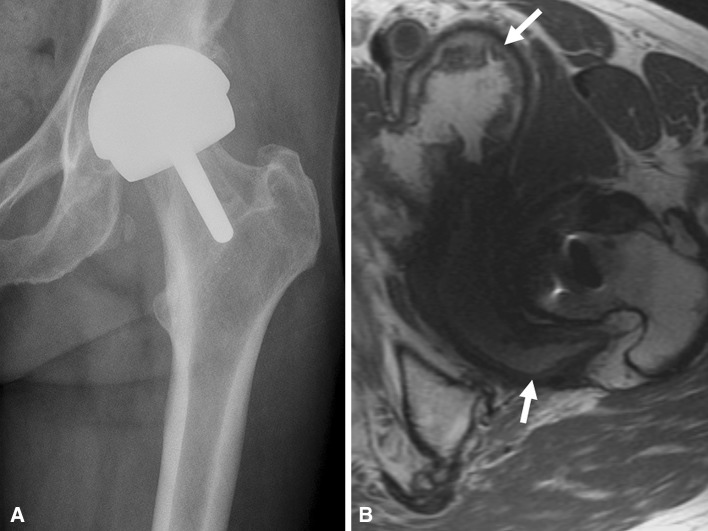
Fig. 8A–B.
(A) An AP radiograph of a patient with a hip resurfacing presenting with unexplained pain. The cup inclination was 38.9° and anteversion was 19.5°. (B) An axial MR image from the same patient demonstrates a markedly thickened synovial lining (white arrows) with distension of the pseudocapsule indicative of an adverse tissue reaction. The ALVAL score was 8, total linear wear rate (TLWR) 1.2 μm/year, and total volumetric wear rate (TVWR) 0.1 mm3/year.
Fig. 9.
A receiver operating characteristic (ROC) curve analyzing the value of synovial thickness in detecting ALVAL. We found that a synovial thickness of ≥ 7 mm on MRI had a sensitivity of 88% and specificity of 90% for diagnosing ALVAL.
Intraoperative Tissue Damage
A higher proportion of patients revised for unexplained pain had severe graded tissue damage than those revised for other indications. Twenty-three patients (68%) had severe intraoperative tissue damage scores (2 or 3) in the unexplained pain group compared with 22 (41%) in the control group (p = 0.01).
Effect of Taper Junction: Comparison Between Resurfacing and THA
No differences in articular wear rates, ALVAL score, synovial thicknesses and volumes on MRI, and proportion of patients with a severe damage score (2 or 3) were found between hip resurfacings and THAs in cases of unexplained pain (Table 4). Furthermore, we found no differences in the mean fretting score, corrosion score, or linear wear rates between THAs in the unexplained pain group and the control group (Table 5).
Table 4.
Demographics, wear, histology, MRI, and intraoperative data for hip resurfacings and THAs presenting with unexplained pain
| Variable | Hip resurfacing | THA | p value |
|---|---|---|---|
| Number of hips | 10 | 24 | |
| Age (years)* | 49 (33–60) | 54 (42–66) | 0.32 |
| BMI (kg/m2)* | 24 (22–37) | 27 (19–40) | 0.48 |
| Male:female | 3:7 | 15:9 | 0.13 |
| Time to revision (months)* | 23 (18–50) | 45 (18–103) | 0.01 |
| Head size (mm)* | 45 (40–54) | 46 (38–53) | 0.90 |
| Acetabular inclination (°)* | 47 (33–51) | 46 (33–51) | 0.56 |
| Acetabular anteversion (°)* | 16 (7–21) | 20 (5–25) | 0.14 |
| Implant type | 0.15 | ||
| ASR (DePuy, Warsaw, IN, USA)† | 1 (10) | 12 (48) | |
| BHR (Smith & Nephew, Memphis, TN, USA)† | 7 (70) | 8 (32) | |
| Conserve Plus (Wright Medical, Memphis, TN, USA)† | 1 (10) | 2 (8) | |
| Cormet (Corin Group, Cirencester, UK)† | 0 (0) | 1 (4) | |
| Durom (Zimmer, Warsaw, IN, USA)† | 0 (0) | 1 (4) | |
| M2a (Biomet, Warsaw, IN, USA)† | 1 (10) | 1 (4) | |
| TLWR (μm/year)* | 3.3 (0–128.2) | 2.2 (0–85.6) | 0.45 |
| TVWR (mm3/year)* | 0.3 (0–26.2) | 0.3 (0–29.3) | 0.83 |
| ALVAL score* | 6 (3–10) | 7 (2–10) | 0.45 |
| Metallic particles (Grade 2–3)† | 2 (20) | 2 (8) | 0.56 |
| Tissue damage score (Grade 2–3)† | 6 (60) | 17 (68) | 0.71 |
| MRI synovial volume (mm3)* | 13,917 (0–198,100) | 24,663 (0–645,575) | 0.25 |
| MRI synovial thickness (mm)* | 8 (1–29) | 9 (1–45) | 0.82 |
* Values are expressed as median, with range in parentheses; †values are expressed as number, with percentage in parentheses; BMI = body mass index; ASR = Articular Surface Replacement; BHR = Birmingham Hip Resurfacing.
Table 5.
Taper data for retrieved large-head THA implants
| Variable | UEP | Control | p value |
|---|---|---|---|
| Number of hips | 25 | 33 | |
| Fretting score* | 6 (2–6.5) | 6 (2–8.0) | 0.87 |
| Corrosion score* | 5.3 (2–7.5) | 5.0 (2–8.0) | 0.88 |
| Linear wear rate (μm/year)* | 1.4 (0–18.1) | 0 (0–30.6) | 1 |
| Head size (mm)* | 46 (38–53) | 45 (38–53) | 0.68 |
| Implant type | 0.001 | ||
| ASR (DePuy, Warsaw, IN, USA)† | 12 (48) | 17 (52) | |
| BHR (Smith & Nephew, Memphis, TN, USA)† | 8 (32) | 0 (0) | |
| Conserve Plus (Wright Medical, Memphis, TN, USA)† | 2 (8) | 3 (9) | |
| Cormet (Corin Group, Cirencester, UK)† | 1 (4) | 0 (0) | |
| Durom (Zimmer, Warsaw, IN, USA)† | 1 (4) | 4 (12) | |
| M2a (Biomet, Warsaw, IN, USA)† | 1 (4) | 9 (27) | |
| Taper geometry | 0.001 | ||
| 11/13† | 0 (0) | 6 (18) | |
| 12/14† | 23 (92) | 18 (55) | |
| C-Taper† | 1 (4) | 0 (0) | |
| Type 1† | 1 (4) | 9 (27) | |
* Values are expressed as mean, with range in parentheses; †values are expressed as number, with percentage in parentheses; UEP = unexplained pain; ASR = Articular Surface Replacement; BHR = Birmingham Hip Resurfacing.
Discussion
The literature regarding the underlying etiology of periprosthetic soft tissue lesions around MOM hip arthroplasties is inconsistent. The interchangeable use of terms such as adverse local tissue reactions [1], adverse reactions to metal debris [29], ALVAL [45], and pseudotumor [41] has contributed to the lack of consensus. Some authors suggest that these soft tissue reactions arise as a result of an adverse reaction to a metallic wear burden and/or elevated blood metal ion levels [19, 28, 41] and may be dose-dependent [26]. This theory has been supported by recent evidence showing failure of well-positioned MOM THAs as a result of fretting and corrosion at the head-neck taper junction [6, 27]. However, others have suggested that adverse tissue reactions can occur in well-functioning hips [20] with well-positioned components [10, 34], even in the absence of symptoms [46]. Given the severe tissue damage that can result from an adverse tissue reaction, an urgent need exists to accurately identify a failing implant from a group of well-positioned MOM hips. This study has shown that well-positioned MOM hips revised for unexplained pain most commonly demonstrate ALVAL. Synovial thickness on MRI appears to be highly predictive of ALVAL. The tissue destruction seen in cases of ALVAL does not appear to be related to bearing wear or the presence of a taper.
We acknowledge several limitations to our study. First, we studied a heterogeneous group of implants that included resurfacings and large-head THAs of different designs. The heterogeneity could not be avoided because we are a tertiary referral center, and over 70% of the hips in this study were referred from outside centers. Second, we did not include any metal ion data. Over the period of this study, testing for metal ion levels was not routine at our institution. Published data suggest that metal ions have a sensitivity of approximately 60% at a cutoff level of 7 μg/L of cobalt or chromium to detect an adverse reaction [18, 33]. Because this threshold is lowered to increase sensitivity, specificity decreases. Metal ion testing, however, is important for the surveillance of MOM hips and may prove to be very useful in longitudinal followup. Since the completion of this study, our institution has begun routinely monitoring patients with metal ion levels. Third, as a result of the retrospective nature of our study, not all patients underwent an MRI before being revised for unexplained pain. We therefore found some patients in the unexplained pain group who did not have an adverse tissue reaction. However, we were still able to draw useful conclusions as a result of the stark differences in causes for revision between the two study groups. Fourth, although the ALVAL score has been reported to have an interobserver and intraobserver variability of 0.71 and 0.68, respectively [5], we did not conduct a double-blind evaluation of our histology samples. All our slides were read twice on two different days within a 1-week period by our expert pathologist (GP) to decrease intraobserver variability. For any instances of a discrepancy, a consensus was reached with an independent pathologist in the department. Finally, the intraoperative tissue damage score is subjective as has been previously acknowledged [39]. The inclusion of some objectivity in grading tissue damage intraoperatively has been helpful in reporting the extent of damage encountered by surgeons when revising hips for unexplained pain.
We found that articular wear rates were lower in those hips revised for unexplained pain. This is in agreement with Hart et al. [17] who found that a large number of patients revised for unexplained pain had a low rate of bearing wear. In contrast, a recent study [15] correlating wear with histology after failed hip resurfacing concluded that the majority of pseudotumors are associated with increased implant wear. The pseudotumor group in their study was not controlled for cup position and therefore any comparison to our study becomes difficult to make.
The majority of cases (66%) in the unexplained pain group had histologic evidence of ALVAL. We found no strong correlation between the ALVAL score and wear. Furthermore, only a small proportion of cases with unexplained pain (two of 35) had a combination of a high ALVAL score and high wear. These findings are in agreement with those of Campbell et al. [5], who found that high ALVAL scores were associated with low component wear. Their study did not have a control group and simply looked at revisions for pseudotumor-like reactions. It was unclear whether these cases had been diagnosed on MRI.
We have previously reported on the use of a prototype pulse sequence to further reduce susceptibility artifact and permit the accurate and reproducible measurement of the volume of an adverse synovial response [40] and the thickness of the synovial lining [39]. In the current study, we have shown that synovial thickness was greater in patients with unexplained pain. We found that synovial thickness correlated strongly with the ALVAL score, and the ROC curve analysis showed that a ≥ 7-mm synovial thickness has an 88% sensitivity and 90% specificity for diagnosing ALVAL. We also found a positive correlation between synovial volume on MRI and volumetric wear (Table 3). This finding is in agreement with Kwon et al. [13, 25] and Grammatopoulos et al. [15] who noted that pseudotumors are associated with high-wearing implants. Numerous other authors have reported the association between wear and pseudotumors [8, 19, 21, 28, 30], and therefore the correct use of nomenclature becomes important. We found that component malposition and high wear tend to cause large-volume synovial responses, whereas unexplained pain in well-positioned components is most commonly the result of ALVAL that causes synovial thickening. Both may be described as pseudotumors on MRI but are quite different in their pathology.
We also found a larger proportion of patients (68%) with a severe intraoperative tissue damage score (2 or 3) in the unexplained pain group compared with the control group (41%). The use of this damage score was previously reported and, like with the ALVAL score, does correlate with synovial thickness on MRI [39]. We speculate that the presence of a more thickened and solid adverse response as seen in cases with a high ALVAL score results in more necrotic damage to the soft tissue envelope and abductor mechanism compared with a large fluid-based collection that may be seen in the setting of a low ALVAL score (Fig. 7).
We found no differences in articular wear, histology, MRI, or intraoperative findings between hip resurfacings and large-head THAs revised for unexplained pain. Furthermore, when analyzing the taper junction of the large-head THAs, no difference was noted in wear and corrosion between cases of unexplained and explained pain. The potential adverse effect of the taper in large-head THAs was recently highlighted [6, 23, 37], especially for the ASR (DePuy, Warsaw, IN, USA) [27] and Durom (Zimmer, Warsaw, IN, USA) [12] designs. This finding was also noted in metal-on-polyethylene THAs with smaller head sizes [7]. However, in agreement with our findings, a large retrieval study comprising 240 MOM hip components, comparing hip resurfacings with large-head THAs, found component wear rates to be similar [35]. Further work is clearly required to clarify the roles of taper design and patient susceptibility in taper-induced failure.
Failure of a painful, well-positioned MOM hip occurs most commonly as a result of a delayed hypersensitivity reaction, namely ALVAL. This is typically associated with low component wear. Synovial thickness on MRI has a high sensitivity and specificity to diagnose ALVAL in cases of unexplained pain, and this finding is associated with a higher incidence of severe intraoperative tissue damage. Regardless of the presence of a taper junction, a well-positioned MOM hip device associated with unexplained pain should be promptly investigated with modern MRI artifact-reduction sequences to guide the surgeon toward timely intervention before more severe tissue damage develops.
Acknowledgments
We thank Giorgio Perino MD, Hospital for Special Surgery, for grading the histology samples, Brett Lurie MD, Hospital for Special Surgery, for analyzing the MRI scans, and Stephanie Gold BA, for assistance with data collection. Danyal H. Nawabi MD, thanks the British Hip Society Charnley Latta Fund for supporting his fellowship training.
Footnotes
One or more of the authors certifies that he (EPS) or she, or a member of his or her immediate family, has or may receive payments or benefits, during the study period, an amount less than USD 100,000 from Smith & Nephew (Memphis, TN, USA). One or more of the authors certifies that he (TW) or she, or a member of his or her immediate family, has or may receive payments or benefits, during the study period, an amount less than USD 10,000, from Mathys ABG (Bettlach, Switzerland), and an amount USD 10,000 to USD 100,000, from Exactech (Great Neck, NY, USA). One or more of the authors certifies that he or she (HGP), or a member of his or her immediate family, has or may receive payments or benefits, during the study period, an amount less than USD 10,000, from Regentis Biomaterials Ltd (Or-Akiva, Israel), and receives institutional research support from General Electric Healthcare (Waukesha, WI, USA). One or more of the authors certifies that he (DEP) or she, or a member of his or her immediate family, has or may receive payments or benefits, during the study period, an amount less than USD 100,000 from MAKO (Fort Lauderdale, FL, USA) and an amount less than USD 10,000 from Stryker (Mahwah, NJ, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Amstutz HC, Le Duff MJ, Campbell PA, Wisk LE, Takamura KM. Complications after metal-on-metal hip resurfacing arthroplasty. Orthop Clin North Am. 2011;42:207–230. doi: 10.1016/j.ocl.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Amstutz HC, Le Duff MJ, Johnson AJ. Socket position determines hip resurfacing 10-year survivorship. Clin Orthop Relat Res. 2012;470:3127–3133. doi: 10.1007/s11999-012-2347-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Australian Orthopedic Association. National Joint Replacement Registry Report. Annual Report. Adelaide: AOA; 2011. Available at: www.aoa.org.au. Accessed June 15, 2013.
- 4.Bozic KJ, Kurtz S, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ. The epidemiology of bearing surface usage in total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:1614–1620. doi: 10.2106/JBJS.H.01220. [DOI] [PubMed] [Google Scholar]
- 5.Campbell P, Ebramzadeh E, Nelson S, Takamura K, De Smet K, Amstutz HC. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res. 2010;468:2321–2327. doi: 10.1007/s11999-010-1372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chana R, Esposito C, Campbell PA, Walter WK, Walter WL. Mixing and matching causing taper wear: corrosion associated with pseudotumour formation. J Bone Joint Surg Br. 2012;94:281–286. doi: 10.1302/0301-620X.94B2.27247. [DOI] [PubMed] [Google Scholar]
- 7.Cooper HJ, Della Valle CJ, Berger RA, Tetreault M, Paprosky WG, Sporer SM, Jacobs JJ. Corrosion at the head-neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J Bone Joint Surg Am. 2012;94:1655–1661. doi: 10.2106/JBJS.K.01352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Haan R, Campbell PA, Su EP, De Smet KA. Revision of metal-on-metal resurfacing arthroplasty of the hip: the influence of malpositioning of the components. J Bone Joint Surg Br. 2008;90:1158–1163. doi: 10.1302/0301-620X.90B9.19891. [DOI] [PubMed] [Google Scholar]
- 9.De Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL acetabular system and the ASR hip resurfacing system. An analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am. 2011;93:2287–2293. doi: 10.2106/JBJS.J.01727. [DOI] [PubMed] [Google Scholar]
- 10.Donell ST, Darrah C, Nolan JF, Wimhurst J, Toms A, Barker TH, Case CP, Tucker JK. Norwich Metal-on-Metal Study Group. Early failure of the Ultima metal-on-metal total hip replacement in the presence of normal plain radiographs. J Bone Joint Surg Br. 2010;92:1501–1508. doi: 10.1302/0301-620X.92B11.24504. [DOI] [PubMed] [Google Scholar]
- 11.Ebramzadeh E, Campbell PA, Takamura KM, Lu Z, Sangiorgio SN, Kalma JJ, De Smet KA, Amstutz HC. Failure modes of 433 metal-on-metal hip implants: how, why, and wear. Orthop Clin North Am. 2011;42:241–250. doi: 10.1016/j.ocl.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Garbuz DS, Tanzer M, Greidanus NV, Masri BA, Duncan CP. The John Charnley Award: Metal-on-metal hip resurfacing versus large-diameter head metal-on-metal total hip arthroplasty: a randomized clinical trial. Clin Orthop Relat Res. 2010;468:318–325. doi: 10.1007/s11999-009-1029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glyn-Jones S, Roques A, Taylor A, Kwon YM, McLardy-Smith P, Gill HS, Walter W, Tuke M, Murray D. The in vivo linear and volumetric wear of hip resurfacing implants revised for pseudotumor. J Bone Joint Surg Am. 2011;93:2180–2188. doi: 10.2106/JBJS.J.01206. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg JR, Gilbert JL, Jacobs JJ, Bauer TW. A multicenter retrieval study of the hip prostheses. Clin Orthop Relat Res. 2002;401:149–161. doi: 10.1097/00003086-200208000-00018. [DOI] [PubMed] [Google Scholar]
- 15.Grammatopolous G, Pandit H, Kamali A, Maggiani F, Glyn-Jones S, Gill HS, Murray DW, Athanasou N. The correlation of wear with histological features after failed hip resurfacing arthroplasty. J Bone Joint Surg Am. 2013;95:e811-10. [DOI] [PubMed]
- 16.Hart AJ, Ilo K, Underwood R, Cann P, Henckel J, Lewis A, Cobb J, Skinner J. The relationship between the angle of version and rate of wear of retrieved metal-on-metal resurfacings: a prospective, CT-based study. J Bone Joint Surg Br. 2011;93:315–320. doi: 10.1302/0301-620X.93B3.25545. [DOI] [PubMed] [Google Scholar]
- 17.Hart AJ, Matthies A, Henckel J, Ilo K, Skinner J, Noble P. Understanding why metal-on-metal hip arthroplasties fail. A comparison between patients with well-functioning and revised Birmingham Hip Resurfacing arthroplasties. J Bone Joint Surg Am. 2012;94:1–10. doi: 10.2106/JBJS.K.01266. [DOI] [PubMed] [Google Scholar]
- 18.Hart AJ, Sabah SA, Bandi AS, Maggiore P, Tarassoli P, Sampson B, Skinner JA. Sensitivity and specificity of blood cobalt and chromium metal ions for predicting failure of metal-on-metal hip replacement. J Bone Joint Surg Br. 2011;93:1308–1313. doi: 10.1302/0301-620X.93B10.26249. [DOI] [PubMed] [Google Scholar]
- 19.Hart AJ, Sabah SA, Henckel J, Lewis A, Cobb J, Sampson B, Mitchell A, Skinner JA. The painful metal-on-metal hip resurfacing. J Bone Joint Surg Br. 2009;91:738–744. doi: 10.1302/0301-620X.91B6.21682. [DOI] [PubMed] [Google Scholar]
- 20.Hart AJ, Satchithananda K, Liddle AD, Sabah SA, McRobbie D, Henckel J, Cobb JP, Skinner JA, Mitchell AW. Pseudotumors in association with well-functioning metal-on-metal hip prostheses: a case-control study using three-dimensional computed tomography and magnetic resonance imaging. J Bone Joint Surg Am. 2012;94:317–325. doi: 10.2106/JBJS.J.01508. [DOI] [PubMed] [Google Scholar]
- 21.Hart AJ, Skinner JA, Henckel J, Sampson B, Gordon F. Insufficient acetabular version increases blood metal ion levels after metal-on-metal hip resurfacing. Clin Orthop Relat Res. 2011;469:2590–2597. doi: 10.1007/s11999-011-1930-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heisel C, Streich N, Krachler M, Jakubowitz E, Kretzer JP. Characterization of the running-in period in total hip resurfacing arthroplasty: an in vivo and in vitro metal ion analysis. J Bone Joint Surg Am. 2008;90:125–134. doi: 10.2106/JBJS.H.00437. [DOI] [PubMed] [Google Scholar]
- 23.Huber M, Reinisch G, Trettenhahn G, Zweymüller K, Lintner F. Presence of corrosion products and hypersensitivity-associated reactions in periprosthetic tissue after aseptic loosening of total hip replacements with metal bearing surfaces. Acta Biomaterialia. 2009;5:172–180. doi: 10.1016/j.actbio.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 24.Jack CM, Walter WL, Shimmin AJ, Cashman K, de Steiger RN. Large diameter metal on metal articulations. Comparison of total hip arthroplasty and hip resurfacing arthroplasty. J Arthroplasty. 2013;28:650–653. doi: 10.1016/j.arth.2012.07.032. [DOI] [PubMed] [Google Scholar]
- 25.Kwon YM, Glyn-Jones S, Simpson DJ, Kamali A, McLardy-Smith P, Gill HS, Murray DW. Analysis of wear of retrieved metal-on-metal hip resurfacing implants revised due to pseudotumours. J Bone Joint Surg Br. 2010;92:356–361. doi: 10.1302/0301-620X.92B3.23281. [DOI] [PubMed] [Google Scholar]
- 26.Kwon YM, Xia Z, Glyn-Jones S, Beard D, Gill HS, Murray DW. Dose-dependent cytotoxicity of clinically relevant cobalt nanoparticles and ions on macrophages in vitro. Biomed Mater. 2009;4:025018. doi: 10.1088/1748-6041/4/2/025018. [DOI] [PubMed] [Google Scholar]
- 27.Langton DJ, Jameson SS, Joyce TJ, Gandhi JN, Sidaginamale R, Mereddy P, Lord J, Nargol AV. Accelerating failure rate of the ASR total hip replacement. J Bone Joint Surg Br. 2011;93:1011–1016. doi: 10.1302/0301-620X.93B8.26040. [DOI] [PubMed] [Google Scholar]
- 28.Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AV. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: a consequence of excess wear. J Bone Joint Surg Br. 2010;92:38–46. doi: 10.1302/0301-620X.92B1.22770. [DOI] [PubMed] [Google Scholar]
- 29.Langton DJ, Joyce TJ, Jameson SS, Lord J, Van Orsouw M, Holland JP, Nargol AV, De Smet KA. Adverse reaction to metal debris following hip resurfacing: the influence of component type, orientation and volumetric wear. J Bone Joint Surg Br. 2011;93:164–171. doi: 10.1302/0301-620X.93B2.25099. [DOI] [PubMed] [Google Scholar]
- 30.Langton DJ, Sprowson AP, Joyce TJ, Reed M, Carluke I, Partington P, Nargol AV. Blood metal ion concentrations after hip resurfacing arthroplasty: a comparative study of articular surface replacement and Birmingham Hip Resurfacing arthroplasties. J Bone Joint Surg Br. 2009;91:1287–1295. doi: 10.1302/0301-620X.91B10.22308. [DOI] [PubMed] [Google Scholar]
- 31.Langton DJ, Sprowson AP, Mahadeva D, Bhatnagar S, Holland JP, Nargol AV. Cup anteversion in hip resurfacing: validation of EBRA and the presentation of a simple clinical grading system. J Arthroplasty. 2010;25:607–613. doi: 10.1016/j.arth.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 32.Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60:217–220. [PubMed] [Google Scholar]
- 33.Malek IA, King A, Sharma H, Malek S, Lyons K, Jones S, John A. The sensitivity, specificity and predictive values of raised plasma metal ion levels in the diagnosis of adverse reaction to metal debris in symptomatic patients with a metal-on-metal arthroplasty of the hip. J Bone Joint Surg Br. 2012;94:1045–1050. doi: 10.1302/0301-620X.94B8.27626. [DOI] [PubMed] [Google Scholar]
- 34.Matthies A, Skinner JA, Osmani H, Henckel J, Hart AJ. Pseudotumors are common in well-positioned low-wearing metal-on-metal hips. Clin Orthop Relat Res. 2012;470:1895–1906. doi: 10.1007/s11999-011-2201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matthies A, Underwood R, Cann P, Ilo K, Nawaz Z, Skinner J, Hart AJ. Retrieval analysis of 240 metal-on-metal hip components, comparing modular total hip replacement with hip resurfacing. J Bone Joint Surg Br. 2011;93:307–314. doi: 10.1302/0301-620X.93B3.25551. [DOI] [PubMed] [Google Scholar]
- 36.McArthur B, Cross M, Geatrakas C, Mayman D, Ghelman B. Measuring acetabular component version after THA: CT or plain radiograph? Clin Orthop Relat Res. 2012;470:2810–2818. doi: 10.1007/s11999-012-2292-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer H, Mueller T, Goldau G, Chamaon K, Ruetschi M, Lohmann CH. Corrosion at the cone/taper interface leads to failure of large-diameter metal-on-metal total hip arthroplasties. Clin Orthop Relat Res. 2012;470:3101–3108. doi: 10.1007/s11999-012-2502-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Natu S, Sidaginamale RP, Gandhi J, Langton DJ, Nargol AV. Adverse reactions to metal debris: histopathological features of periprosthetic soft tissue reactions seen in association with failed metal on metal hip arthroplasties. J Clin Pathol. 2012;65:409–418. doi: 10.1136/jclinpath-2011-200398. [DOI] [PubMed] [Google Scholar]
- 39.Nawabi DH, Gold S, Lyman S, Fields K, Padgett DE, Potter HG. MRI predicts ALVAL and tissue damage in metal-on-metal hip arthroplasty. Clin Orthop Relat Res. 2013 Jan 26 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 40.Nawabi DH, Hayter CL, Su EP, Koff MF, Perino G, Gold SL, Koch KM, Potter HG. Magnetic resonance imaging findings in symptomatic versus asymptomatic subjects following metal-on-metal hip resurfacing arthroplasty. J Bone Joint Surg Am. 2013;95:895–902. doi: 10.2106/JBJS.K.01476. [DOI] [PubMed] [Google Scholar]
- 41.Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gibbons CL, Ostlere S, Athanasou N, Gill HS, Murray DW. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2008;90:847–851. doi: 10.1302/0301-620X.90B7.20213. [DOI] [PubMed] [Google Scholar]
- 42.Potter HG, Foo LF. Magnetic resonance imaging of joint arthroplasty. Orthop Clin North Am. 2006;37:361–373. doi: 10.1016/j.ocl.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Potter HG, Nestor BJ, Sofka CM, Ho ST, Peters LE, Salvati EA. Magnetic resonance imaging after total hip arthroplasty: evaluation of periprosthetic soft tissue. J Bone Joint Surg Am. 2004;86:1947–1954. doi: 10.2106/00004623-200409000-00013. [DOI] [PubMed] [Google Scholar]
- 44.Tuke M, Taylor A, Roques A, Maul C. 3D Linear and volumetric wear measurement on artificial hip joints—validation of a new methodology. Precision Engineering. 2010;34:777–783. doi: 10.1016/j.precisioneng.2010.06.001. [DOI] [Google Scholar]
- 45.Willert HG, Buchhorn GH, Fayyazi A, Flury R, Windler M, Koster G, Lohmann CH. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg Am. 2005;87:28–36. doi: 10.2106/JBJS.A.02039pp. [DOI] [PubMed] [Google Scholar]
- 46.Williams DH, Greidanus NV, Masri BA, Duncan CP, Garbuz DS. Prevalence of pseudotumor in asymptomatic patients after metal-on-metal hip arthroplasty. J Bone Joint Surg Am. 2011;93:2164–2171. doi: 10.2106/JBJS.J.01884. [DOI] [PubMed] [Google Scholar]