Abstract
Background
Fixation of cementless orthopaedic implants is not always achieved, particularly in challenging scenarios such as revision surgery, trauma, and tumor reconstruction. An adjunct therapy for improving implant fixation would improve the reliability and durability of certain reconstructive procedures.
Questions/purposes
The purpose of this study was to determine the effect of local elution of the bisphosphonate alendronic acid on bone formation around porous titanium implants in an animal model.
Methods
Porous-coated cylindrical rods were coated with either 0.2 mg or 1.0 mg alendronic acid before bilateral surgical implantation into the femoral intramedullary canals of 10 experimental dogs. Twelve weeks after surgery, the femora were harvested and scanned with micro-CT to quantify the percentage volume of bone within the immediate periimplant space. Four femora from two dogs were also processed for undecalcified thin-section histology and analysis with backscattered scanning electron microscopy. Three histologic sections from each of these four femora were anatomically matched with transverse micro-CT sections to enable direct comparison of the area fraction of bone within the periimplant space.
Results
Compared with paired controls, micro-CT analysis showed that local elution of alendronic acid increased periimplant bone at both doses of 0.2 mg (+52%, p = 0.01) and 1.0 mg (+152%, p = 0.004) with 1.0 mg resulting in a 2.9-fold greater mean relative increase compared with 0.2 mg (p = 0.002). Micro-CT measurements of periimplant bone formation correlated very strongly with the backscattered scanning electron microscopy measurements (R = 0.965, p < 0.001).
Conclusions
Local elution of alendronic acid causes a dose-dependent net increase in periimplant bone formation in an animal model.
Clinical Relevance
This concept has potential to improve the biologic fixation of porous reconstructive implants.
Level of Evidence
Level II, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
There continues to be a need to enhance periimplant bone and biological fixation in and around porous-coated arthroplasty implants, especially in patients whose potential for bone healing may be less than ideal such as patients with bone cancer, poor bone stock, or osteoporosis and those undergoing revision surgery. The mechanical integrity of periimplant bone is also an increasingly important issue as the incidence of periprosthetic fractures is dramatically increasing with our aging population [5, 13].
Several means of enhancing periimplant bone around orthopaedic implants have been investigated. One approach is to use a bisphosphonate to modulate the bone healing response at the implant interface and the surrounding region. Bisphosphonates partially suppress the resorptive phase of bone remodeling and so tip the balance toward a net increase in bone formation. As such, they have potential for use in conjunction with orthopaedic devices designed for direct mechanical attachment to bone. Bisphosphonates have been shown in animal models to prevent bone resorption around an implant [18] and to increase the amount of peripheral bone and bone-implant contact [16, 20], mechanical strength of the implant-bone interface [15, 16] as well as bone ingrowth [3, 8] while allowing for greater retention of periimplant bone mineral density and cortical bone area and thickness [10]. All of these effects have contributed positively to the mechanical fixation of orthopaedic implants, in animal models, both in the short and long term.
Although systemic delivery of bisphosphonates has been demonstrated in some studies to have clinical advantages insofar as implant fixation is concerned [1, 2, 6, 17], it exposes the entire skeleton to the drug effect and by necessity subjects the patient to the associated risk of side effects or adverse events [14]. This can be avoided by locally delivering the bisphosphonate directly from the implant to surrounding bone. Prior studies have demonstrated the ability of a variety of locally delivered bisphosphonates to enhance bone formation around implants in animal models [4, 7, 8, 11–13, 16, 18, 19].
The bisphosphonate alendronic acid is of particular interest because this drug has a long history of clinical use and is widely accepted in the medical community for treatment of osteoporosis, therefore making it an excellent candidate for use in conjunction with the clinical implementation of drug-eluting orthopaedic implants. There is little or no information in the literature on the response to different alendronic acid doses in animal studies using porous implants.
The purpose of this study therefore was to determine the effect of two doses of locally delivered alendronic acid on periimplant bone formation around porous titanium implants in a canine femoral intramedullary implant model. Micro-CT was used as the primary measurement modality with secondary verification using backscattered scanning electron microscopy.
Materials and Methods
Cylindrical implants, each measuring 9 mm in diameter and 90 mm in length, were fabricated from titanium alloy (Ti-6Al-4 V) using direct metal laser sintering techniques (build tolerance of ± 10 μm). The implants had a solid core with an outer porous structure that was either grid-like or more random in geometry. The grid-like porous structure had a repeating, ordered pattern that was 2.5 mm thick (4.0-mm solid core) with a mean pore size of 330 μm and a volume porosity of 40% (Fig. 1). The random porous structure consisted of an irregular network of struts that was 1.5 mm thick (6-mm solid core) with a mean pore size of 400 μm and a volume porosity of 60% (Fig. 1). The grid-like and random porous structures resulted from a development project of the laser sintering technique as provided by industry (Pipeline Biotechnology, Cedar Knolls, NJ, USA). All implants were plasma spray-coated with a thin (10–15 μm) layer of hyaluronate acid (HA; 98% purity, 99% density, 64% crystallinity, calcium:phosphate ratio of 1.67) such that only the outermost porous structure was HA-coated, leaving the innermost pores uncoated. Commercially pure, laboratory-grade alendronic acid trihydrate (Toronto Research Chemicals, Toronto, Ontario, Canada) in 0.2-mg or 1.0-mg aliquots was dissolved in 2.0-mL aliquots of distilled deionized water. Each aliquot of alendronic acid in solution was systematically and evenly added to an implant in dropwise fashion along its length and around its circumference using a micropipette. The deposition process resulted in homogeneous saturation of the porous structure with fluid that permeated the inner pore depths through surface tension effects. This technique resulted in chemical (strong) immobilization of alendronic acid to the outer HA coating and physical (weak) deposition of alendronic acid onto the innermost non-HA-coated porous structure as described in earlier studies [4, 18]. Six grid-like implants and four random porous implants were each prepared as such with 0.2 mg and 1.0 mg alendronic acid. The implants were dried overnight in an oven at 37 °C and sterilized with ethylene oxide.
Fig. 1A–C.
(A) Photograph of the grid-like and random porous intramedullary implants (9 mm in diameter by 90 mm in length). Scanning electron micrographs illustrating the hydroxyapatite-coated grid-like porous structure (B) and random porous structure (C).
Based on earlier elution studies using the same implant model, HA coating technique, and bisphosphonate deposition technique, it was assumed that the elution profile of alendronic acid would consist of an initial burst release to the periimplant space (as a result of hydration and diffusion of bisphosphonate from the innermost, non-HA-coated porous structure) followed by a longer-term, slower release from the outermost HA coating (where the bisphosphonate was initially immobilized). The differences in pore size, geometry, and volume porosity between the grid-like and random porous implants were not considered to affect the burst release and effect of the drug on periimplant bone formation. As such, the two porous structures were grouped together according to dose for purposes of power and data analysis.
A power analysis was performed to estimate the sample size required for detecting a difference in the percentage of periimplant bone between cohorts with and without alendronic acid. A 60% increase combined with SDs of 40% were conservatively estimated based on the prior canine studies using the same implant model with zoledronic acid [4, 18]. Setting the standard alpha error level of 0.05 and beta level error of 50%, the estimated (unpaired) sample size for each alendronic acid dose was 4.
Ten healthy, skeletally mature male and female mongrel dogs weighing between 35 kg and 45 kg were used for this study. There were five dogs in each dose cohort, and within each cohort, three dogs received grid-like implants, whereas two dogs received random porous implants. Bilateral canine surgery was performed inserting the implants through the piriformis fossa and into the femoral medullary canal using the same surgical technique that is used for open intramedullary nailing. Each dog received an implant with either 0.2 mg alendronic acid or 1.0 mg alendronic on one side and a control implant with no alendronic acid on the contralateral side. The alendronic acid and control sides were randomized between left and right femurs for each dose. The animal study protocol was approved by the institution’s ethical review committee in accordance with the Canadian Council on Animal Care.
The animals were euthanized 12 weeks postoperatively and both femora were harvested and stripped of soft tissue before radiography in both AP and lateral views (Fig. 2). The radiographs were used to locate the implant within the femur and enable transverse sectioning of bone just above and below the implant to produce a sample that more easily fit inside the micro-CT chamber. The femora were scanned with a high-resolution micro-CT scanner (Model X-Tek HMX-ST 225; Nikon Metrology, Leuven, Belgium) in water at 135 kV and 65 mA with a 0.5-mm copper filter and 1000-ms exposure time to obtain an image resolution of 18 μm. Instead of the CT gantry rotating around the object to be imaged, as is the case for clinical CT imaging of humans, the scanner remained stationary and the femur was rotated. The femur was placed vertically on a rotating platform within the imaging chamber and the scan was made along the length of the femur. After each longitudinal scanning event, the platform underwent an angular rotation step and another scan was performed for the new orientation. Based on the number of projection files obtained from a complete scan, the approximate value of each rotation step was determined to be 0.7°. After a 360° revolution, an average of 2026 projection files with four frames per projection had been generated, which were then compiled to generate a three-dimensional (3-D) reconstruction of the femur. The reconstruction process was completed with CTPro software (Metris, UK), yielding images for both qualitative and quantitative analyses (Fig. 3).
Fig. 2.

Postmortem AP radiograph illustrating bilateral femoral implants.
Fig. 3A–B.

Three-dimensional micro-CT reconstructions of femora illustrating the grid-like (A) and random porous (B) types of implants.
Quantification of periimplant bone from the CT reconstruction was done using ORS Visual software (Object Research Systems, Montreal, Quebec, Canada). ORS Visual allowed for the simultaneous review and analysis of two-dimensional and 3-D CT images. Prior studies have shown that the additional bone resulting from local bisphosphonate elution was confined to a fairly narrow periimplant space [4, 18]. For this study, the image reconstruction was analyzed to quantify the amount of bone within the region between a radial distance of 0.5 mm and 2.5 mm from the implant perimeter (Fig. 4). This region was selected as a basis for measurement given that bone present in close proximity to the implant perimeter would be expected to influence mechanical support/fixation. The offset of 0.5 mm was chosen to be certain to exclude any beam hardening artifact present in the scans resulting from the metal implants. For each cross-section, the quantity of bone was expressed as the percentage of the total area within the zone of interest that was populated by bone. Quantification of this parameter was conducted only for the proximal half of the implant because the distal portion extended into the narrower diaphyseal femur where the distance between the implant and bone in some regions was variable around the implant perimeter and often less than 1 mm and sometimes less than 0.5 mm. Apart from adding complexity to the analysis, the small bone-implant space was judged to be insufficient to enable meaningful analysis. Through an integration of the cross-sectional measurements over the analyzed length of implant, the volume of periimplant bone was calculated as a percent filling of the available volume.
Fig. 4.
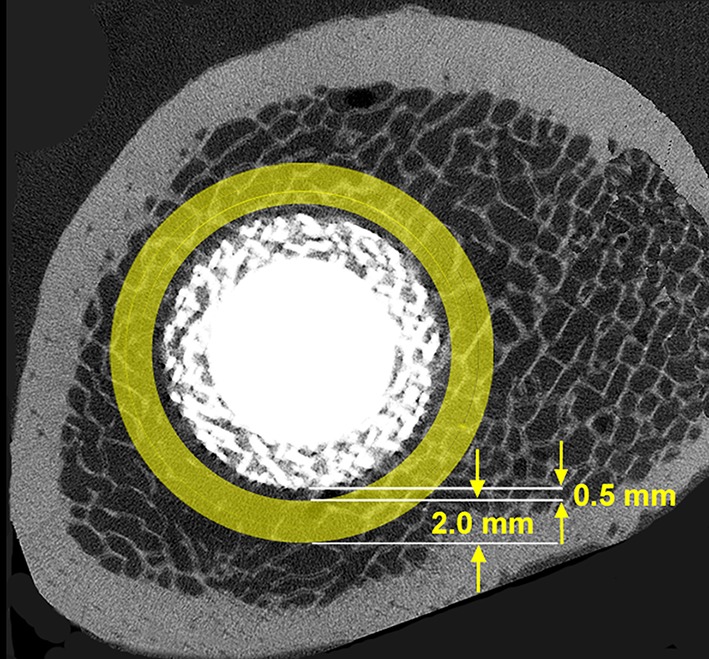
Micro-CT image illustrating the periimplant region of interest for quantification of percentage filling with bone.
Selected micro-CT cross-sectional images were isolated for analysis of periimplant bone using ORS Visual. This provided discrete (local) information in addition to the volumetric (global) information about periimplant bone formation in response to alendronic acid. The analysis of individual cross-sectional images was used for eventual correlation with comparable backscattered scanning electron microscope data on periimplant bone formation.
The femora from one dog with random porous implants in each of the 0.2-mg and 1.0-mg alendronic acid cohorts were subsequently processed for undecalcified thin-section histologic analysis. This involved dehydrating in ascending ethanol solutions, defatting in a 1:1 solution of ether and acetone, and embedding in polymethylmethacrylate. Each bone-implant construct was sectioned transversely with a low-speed diamond cutoff apparatus (Buehler, Lake Bluff, IL, USA) at 2-mm intervals, the sections were contact-radiographed with a Faxitron machine (Faxitron X-Ray, Lincolnshire, IL, USA), and sections were selected to closely match the micro-CT cross-sections that had been isolated for area measurements of periimplant bone. The selected histologic sections were polished to 1200 grit using petrographic techniques, sputtercoated with gold-palladium, and imaged using backscattered scanning electron microscopy at x 20 magnification. Computerized image analysis based on gray-level discrimination was performed using ImageJ software Version 1.6.0_12 (National Institutes of Health, Bethesda, MD, USA) and a bespoke program to measure the percentage total area of bone within the radial space 0.5 mm to 2.5 mm outside the implant perimeter. This provided data for direct comparison with the analogous micro-CT data for each pair of matching sections (Fig. 5). For each of the two dogs, six histologic sections were selected for periimplant bone measurement, three from the control femur and three from the femur with the alendronic acid-dosed implant. Thus, a total of 12 histologic sections were analyzed for periimplant bone to enable direct correlation with the micro-CT data and comparison of the different measurement techniques.
Fig. 5A–B.

Micro-CT (A) and backscattered scanning electron microscopy (B) images matched for anatomic location within the femur containing a random porous type of implant dosed with 1.0 mg alendronic acid.
The evaluators for all assessments and data generation, including the micro-CT reconstructions, backscattered electron microscopy, and image analysis, were blinded to the study specifics regarding alendronic acid dose and which implant was control or dosed. The paired periimplant bone data were analyzed using paired and unpaired Student’s t-tests with p ≤ 0.05 to determine the significance of mean differences between each dose and the respective controls as well as the mean relative differences between the 0.2-mg and 1.0-mg alendronic acid doses. The data for periimplant bone from both the micro-CT and backscattered scanning electron microscope analyses were compared using Pearson’s coefficient analysis to determine the degree of correlation between the two methods.
All 10 dogs tolerated the surgery well and were observed to be ambulatory and active within 2 to 3 days after surgery. There were no infections or other postoperative complications. However, it was observed on postmortem radiography that the control random porous implant of one dog in the 1.0-mg cohort was surgically malpositioned outside of the femoral intramedullary canal. Because this removed the possibility of paired data comparison, this dog was excluded from the data analysis and all subsequent results included five dogs in the 0.2-mg cohort and four dogs in the 1.0-mg cohort.
Results
Visual comparison of AP and lateral radiographs of the control femurs and alendronic acid-dosed femurs showed no noticeable differences in the amount of periimplant bone for either of the doses (Fig. 2). The micro-CT quantitative analysis demonstrated that in each of the nine dogs, the volumetric amount of periimplant bone was greater for alendronic acid-dosed implants compared with paired controls (Table 1). The mean amount of periimplant bone for the 0.2-mg implants was 12.9% ± 6.8%, greater than the paired control mean of 9.3% ± 5.5% (p = 0.001, paired Student’s t-test). The mean amount of periimplant bone for the 1.0-mg implants was 21.9% ± 11.0%, substantially greater than the paired control mean of 9.3% ± 5.7% (p = 0.02, paired Student’s t-test). The mean relative increase with 1.0 mg alendronic acid (152% ± 36%, p = 0.004, one sample t-test) was 2.9-fold greater than the mean relative increase with 0.2 mg alendronic acid (52% ± 26%, p = 0.01, one sample t-test), a statistically significant difference (p = 0.002, unpaired Student’s t-test). The greater relative difference in the 1.0-mg cohort was readily apparent as well on inspection of paired micro-CT images (Fig. 6).
Table 1.
Volumetric periimplant bone data (%): micro-CT analysis
| Dose | Dog/sex | Control | AA | Relative difference |
|---|---|---|---|---|
| 0.2 mg AA | 1/M | 5.1 | 9.0 | 77% |
| 2/M | 2.5 | 4.6 | 82% | |
| 3/F | 8.1 | 11.4 | 41% | |
| 4/F | 17.9 | 22.1 | 24% | |
| 5/M | 12.9 | 17.3 | 34% | |
| Mean ± SD | 9.3 ± 6.2* | 12.9 ± 6.9* | 52% ± 26%‡,£ | |
| 1.0 mg AA | 6/F | 16.2 | 35.5 | 119% |
| 7/M | 6.2 | 16.9 | 173% | |
| 8/M | 3.4 | 9.9 | 191% | |
| 9/M | 11.3 | 25.2 | 123% | |
| Mean ± SD | 9.3 ± 5.7† | 21.9 ± 11.0† | 152% ± 36%‡,€ |
*p = 0.001, †p = 0.02, ‡p = 0.002, £p = 0.01, €p = 0.004; AA = alendronic acid; M = male; F = female.
Fig. 6A–B.

Paired micro-CT images of grid-like implants illustrating additional periimplant bone with 1.0 mg alendronic acid (B) compared with the matched control (A).
Qualitatively, in both the 0.2-mg and 1.0-mg cohorts, the additional bone that formed around the alendronic acid-dosed implants was structurally similar in gross appearance to native trabecular bone (Fig. 5). Neither a histological comparison or quantitative comparison of mineralization between native bone and bone exposed to alendronic acid was performed.
The quantification of periimplant bone using backscattered scanning electron microscopy and micro-CT showed very similar values for each of the 12 paired sections that were carefully matched for anatomical location within the implant-bone construct (Table 2). The Pearson’s correlation coefficient for these data was R = 0.965 (p < 0.001), indicating a very strong correlation between the two techniques for measuring periimplant bone.
Table 2.
Area measurements of periimplant bone (%): micro-CT versus backscattered scanning electron microscopy (BSEM)
| Dose | Dog | Pair | Femur | Micro-CT | BSEM |
|---|---|---|---|---|---|
| 0.2 mg | 4 | 1 | Control | 18.9 | 19.3 |
| 2 | AA | 25.8 | 34.2 | ||
| 3 | Control | 16.0 | 15.2 | ||
| 4 | AA | 20.0 | 23.5 | ||
| 5 | Control | 8.6 | 7.6 | ||
| 6 | AA | 15.1 | 11.3 | ||
| 1.0 mg | 9 | 7 | Control | 23.8 | 24.3 |
| 8 | AA | 50.7 | 50.9 | ||
| 9 | Control | 19.4 | 19.1 | ||
| 10 | AA | 50.3 | 44.2 | ||
| 11 | Control | 22.9 | 20.9 | ||
| 12 | AA | 41.0 | 39.1 |
AA = alendronic acid.
Discussion
The biologic fixation of porous implants used in reconstructive procedures is not always reliable, especially in circumstances where native bone stock and/or healing capacity are suboptimum. Using the implant to deliver a bisphosphonate compound to surrounding bone has been shown in animal studies to enhance local bone formation but the dose-response has not yet been well characterized. This investigation served to assess the effect of two doses of locally eluted alendronic acid on bone formation around porous titanium implants and to assess the use of micro-CT for such measurements. The primary finding of the study was that both doses of 0.2 mg and 1.0 mg increased periimplant bone formation 12 weeks after surgery compared with controls. As well, the 1.0-mg dose of alendronic acid was much more effective for the net formation of periimplant bone than was 0.2 mg.
There were several limitations to this study. First, it would have been preferable to have studied more implants of each of the grid-like and random porous structures despite the fact that they were made using the same manufacturing technique, had similar overall pore characteristics, were coated with the same HA, and had alendronic acid added using the same deposition technique. The study evolved from the grid-like implants to the more suitable random porous implants as the novel metal laser sintering technique was further developed by the implant manufacturer; as such, the study was intended from the outset to represent a pilot study that would provide guidance for subsequent, more in-depth dose-response studies. The implants themselves served as carriers or vehicles for local delivery of the bisphosphonate and as such were not the primary focus of the study. More important was to assess the differences in periimplant bone formation compared with paired controls as a function of alendronic acid dose. In this respect it is of note that in all nine dogs that provided useful data, the femur containing the implant with added alendronic acid showed substantially greater net bone formation independent of dose and independent of type of porous structure. Thus, although relative differences may have existed between the different porous structures with regard to bisphosphonate elution and the effect on periimplant bone formation, the fundamental finding was the same for both. Related to this limitation is that only four random porous implants were evaluated with scanning electron microscopy for comparison of periimplant bone values with micro-CT. However, in view of the very strong correlation between the techniques on the basis of the 12 matched section pairs, further such analysis was not indicated.
A second limitation is that the micro-CT and scanning electron microscopy techniques used to quantify periimplant bone did not provide information about the histological appearance or physical nature of the bone that formed in response to local alendronic acid elution. Thus, only the gross presence or absence of bone, without details of bone cells, inflammatory cells, morphology, vascularity, or mineral content, was identified and quantified. Future studies that would address these issues could include decalcified paraffin histology after removal of the implant from the bone and quantification of bone mineral levels using techniques of backscattered electron microscopy. Third, the intramedullary implant model was not clinically realistic in that it did not represent a fully functioning joint replacement with attendant physiological loading. As such, the bone-implant interface did not experience the forces that can influence bone remodeling. However, in studies of this type, it is important to eliminate as many confounding variables as possible so that the results can be properly interpreted. The static implant model eliminated the variable of loading and the clinical problems that can occur after joint replacement (eg, dislocation, implant loosening). Another limitation is that bone immediately at the implant surface was not measured; the effect of the additional periimplant bone on the actual strength of the bone-implant interface would have to be ascertained with direct mechanical testing. Finally, the use of mongrel dogs precluded controlling for age, size, breed, and possible health issues. In this regard it would have been preferable to used pure bred animals confined to a single sex within a relatively narrow age range to better eliminate variables that might influence the bone healing response in the presence of bisphosphonate.
The finding of greater amounts of periimplant bone with alendronic acid elution was expected given earlier reports of such results using other bisphosphonates in other animal models [4, 7, 8, 12, 13, 15, 18, 19]. Because alendronic acid had not previously been used in the same type of HA-coated porous intramedullary implant model, it was not known what dose would elicit a response similar to that described by Tanzer et al. [18] and Bobyn et al. [4] using zoledronic acid. The 0.2-mg and 1.0-mg alendronic acid doses were selected based on the less potent nature of alendronic acid compared with zoledronic acid on information from the study of Garbuz et al. [8] and on calculations of maximum alendronic acid concentration relative to published data on the threshold for cell toxicity [9]. Given that the bone that formed around the implants with both doses appeared normal in overall structure and density indicates that 0.2 mg and 1.0 mg were both within acceptable limits. Of note is that increased periimplant bone consistently formed with both types of alendronic acid-dosed porous structures, both of which were coatings on a solid substrate, in contrast to the fully porous implants previously studied using zoledronic acid [4, 18]. This indicates the versatility of the concept, that it has applicability to various implant designs. Also of interest, of course, is that the periimplant bone formation response was substantially and statistically significantly greater with 1.0 mg alendronic acid. This provides an excellent starting point for future dose-response studies of local alendronic acid elution from porous implants. The potential advantage of additional periimplant bone is that it could increase mechanical fixation of the implant, as shown by Jakobsen et al. [12] and Peter et al. [15] in animal studies of local bisphosphonate treatment. Some clinical studies have suggested that systemic bisphosphonate therapy can offer benefits to patients undergoing total joint arthroplasty, including reduction of periprosthetic bone loss [1, 2], reduction of implant migration [6], and reduction in revision rate [17]. These potential benefits need to be balanced against the risk of adverse events related to bisphosphonate therapy, as alluded to earlier [14]. The rationale for local delivery is hopefully to provide the same types of benefits with a single, relatively low bisphosphonate dose while avoiding the need for chronic, systemic dosing and its attendant risks.
The secondary finding of the study was that micro-CT was a valuable tool for quantifying periimplant bone, heretofore not reported for studying the effect of bisphosphonate elution from porous implants. Inevitable beam scatter was compensated for by excluding the immediate 0.5-mm radius around each implant during quantitative analysis. Although this avoided artifact in the periimplant bone measurements, it by necessity excluded the bone in closest proximity to the implant that would also play a role in overall implant fixation. The micro-CT data were supplemented with higher resolution backscattered scanning electron microscope data on periimplant bone that considered the same periimplant space as did the areal micro-CT analysis on matched transverse sections. The strong correlation between the two techniques provided important validation of the micro-CT technique for quantifying bone in very close vicinity to relatively large titanium implants. Micro-CT is far less time-consuming than undecalcified thin-section histology and provides very rapid, detailed information about the local bone response to bisphosphonate elution from porous implants.
The results of this study provide valuable information on the alendronic acid dose-response to guide subsequent studies that further characterize the concept of local bisphosphonate elution and its effect on porous implant fixation. Alendronic acid in doses of 0.2 mg and 1.0 mg significantly increased periimplant bone formation in an animal model compared with controls, the 1.0-mg dose resulting in the greater relative mean increase of 136%, in keeping with the results reported in similar experiments using zoledronic acid [4, 18]. It would be helpful in future work to examine additional alendronic acid doses at different time periods using a single type of porous-structured implant and to conduct elution studies for comparison with published information on HA-coated fully porous tantalum implants doped with zoledronic acid. It would also be of value to measure the mechanical strength of the bone-implant interface resulting from the additional periimplant bone formed in the presence of bisphosphonate.
Acknowledgments
We thank Pipeline Biotechnology for providing the implants.
Footnotes
The institution of one or more of the authors (JDB, MT) has received, during the study period, funding from the Canadian Institutes of Health Research and Pipeline Biotechnology. Two of the authors (JDB, MT) certify that they each received payments or benefits, during the study period, an amount of USD 10,000 to USD 100,000 from Pipeline Biotechnology (Cedar Knolls, NJ, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Arabmotlagh M, Pilz M, Warzecha J, Rauschmann M. Changes of femoral periprosthetic bone mineral density 6 years after treatment with alendronate following total hip arthroplasty. J Orthop Res. 2009;27:183–188. doi: 10.1002/jor.20748. [DOI] [PubMed] [Google Scholar]
- 2.Bhandari M, Bajammal S, Guyatt GH, Griffith L, Busse JW, Schünemann H, Einhorn TA. Effect of bisphosphonates on periprosthetic bone mineral density after total joint arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2005;87:293–301. doi: 10.2106/JBJS.D.01772. [DOI] [PubMed] [Google Scholar]
- 3.Bobyn JD, Hacking SA, Krygier JJ, Harvey EJ, Little DG, Tanzer M. Zoledronic acid causes enhancement of bone growth into porous implants. J Bone Joint Surg Br. 2005;87:416–420. doi: 10.1302/0301-620X.87B3.14665. [DOI] [PubMed] [Google Scholar]
- 4.Bobyn JD, McKenzie K, Karabasz D, Krygier JJ, Tanzer M. Locally delivered bisphosphonate for enhancement of bone formation and implant fixation. J Bone Joint Surg. 2009;91(Suppl 6):23–31. doi: 10.2106/JBJS.I.00518. [DOI] [PubMed] [Google Scholar]
- 5.Cook RE, Jenkins PJ, Walmsley PJ, Patton JT, Robinson CM. Risk factors for periprosthetic fractures of the hip: a survivorship analysis. Clin Orthop Relat Res. 2008;466:1652–1656. doi: 10.1007/s11999-008-0289-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedl G, Radl R, Stihsen C, Rehak P, Aigner R, Windhager R. The effect of a single infusion of zoledronic acid on early implant migration in total hip arthroplasty. A randomized, double-blind, controlled trial. J Bone Joint Surg Am. 2009;91:274–281. doi: 10.2106/JBJS.G.01193. [DOI] [PubMed] [Google Scholar]
- 7.Gao Y, Zou S, Liu X, Bao C, Hu J. The effect of surface immobilized bisphosphonates on the fixation of hydroxyapatite-coated titanium implants in ovariectomized rats. Biomaterials. 2009;30:1790–1796. doi: 10.1016/j.biomaterials.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 8.Garbuz DS, Hu Y, Kim WY, Duan K, Masri BA, Oxland TR, Burt H, Wang R, Duncan CP. Enhanced gap filling and osteoconduction associated with alendronate-calcium phosphate-coated porous tantalum. J Bone Joint Surg Am. 2008;90:1090–1100. doi: 10.2106/JBJS.G.00415. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Moreno C, Serrano S, Nacher M, Farré M, Díez A, Mariñoso ML, Carbonell J, Mellibovsky L, Nogués X, Ballester J, Aubía J. Effect of alendronate on cultured normal human osteoblasts. Bone. 1998;22:233–239. doi: 10.1016/S8756-3282(97)00270-6. [DOI] [PubMed] [Google Scholar]
- 10.Goodship AE, Blunn GW, Green J, Coathup MJ. Prevention of strain-induced osteopenia in aseptic loosening of hip prostheses using perioperative bisphosphonate. J Orthop Res. 2008;26:693–703. doi: 10.1002/jor.20533. [DOI] [PubMed] [Google Scholar]
- 11.Hilding M, Aspenberg P. Local preoperative treatment with a bisphosphonate improves the fixation of total knee prostheses. Acta Orthop. 2007;78:795–799. doi: 10.1080/17453670710014572. [DOI] [PubMed] [Google Scholar]
- 12.Jakobsen T, Baas J, Kold S, Bechtold JE, Elmengaard B, Søballe K. Local bisphosphonate treatment increases fixation of hydroxyapatite-coated implants inserted with bone compaction. J Orthop Res. 2009;27:189–194. doi: 10.1002/jor.20745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindahl H. Epidemiology of periprosthetic femur fracture around a total hip arthroplasty. Injury. 2007;38:651–654. doi: 10.1016/j.injury.2007.02.048. [DOI] [PubMed] [Google Scholar]
- 14.Papapetrou PD. Bisphosphonate-associated adverse events. Hormones. 2009;8:96–110. doi: 10.14310/horm.2002.1226. [DOI] [PubMed] [Google Scholar]
- 15.Peter B, Gauthier O, Laib S, Bujoli B, Guicheux J, Janvier P, van Lenthe GH, Müller R, Zambelli PY, Bouler JM, Pioletti DP. Local delivery of bisphosphonate from coated orthopedic implants increases implant mechanical stability in osteoporotic rats. J Biomed Mater Res A. 2006;76:133–143. doi: 10.1002/jbm.a.30456. [DOI] [PubMed] [Google Scholar]
- 16.Peter B, Pioletti DP, Laib S, Bujoli B, Pilet P, Janvier P, Guicheux J, Zambelli PY, Bouler JM, Gauthier O. Calcium phosphate drug delivery system: influence of local zoledronate release on bone implant osteointegration. Bone. 2005;36:52–60. doi: 10.1016/j.bone.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Prieto-Alhambra B, Javaid MK, Judge A, Murray D, Carr A, Cooper C, Arden NK. Association between bisphosphonate use and implant survival after primary total arthroplasty of the knee or hip: population based retrospective cohort study. BMJ. 2011;343:1–9. doi: 10.1136/bmj.d7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanzer M, Karabasz D, Krygier JJ, Cohen R, Bobyn JD. Bone augmentation around and within porous implants by local bisphosphonate elution. Clin Orthop Relat Res. 2005;441:30–39. doi: 10.1097/01.blo.0000194728.62996.2d. [DOI] [PubMed] [Google Scholar]
- 19.Tengvall P, Skoglund B, Askendal A, Aspenberg P. Surface immobilized bisphosphonate improves stainless-steel screw fixation in rats. Biomaterials. 2004;25:2133–2138. doi: 10.1016/j.biomaterials.2003.08.049. [DOI] [PubMed] [Google Scholar]
- 20.Yoshinari M, Oda Y, Inoue T, Matsuzaka K, Shimono M. Bone response to calcium phosphate-coated and bisphosphonate-immobilized titanium implants. Biomaterials. 2002;23:2879–2885. doi: 10.1016/S0142-9612(01)00415-X. [DOI] [PubMed] [Google Scholar]



