Abstract
Background
Although its FDA-approved applications are limited, the pro-osteogenic benefits of recombinant human BMP-2 (rhBMP-2) administration have been shown in off-label surgical applications. However, the effects of rhBMP-2 on ankle fusions are insufficiently addressed in the literature, which fails to include a case-control study of adequate sample size to evaluate the efficacy of rhBMP-2 treatment.
Questions/purposes
In this study we asked whether rhBMP-2 treatment (1) would increase the rate of successful ankle fusion in complex patients (patients with comorbidities associated with poor surgical healing) compared with a control group of patients undergoing ankle fusion who did not receive rhBMP-2; (2) would reduce total time wearing a frame when compared with the control group; (3) would result in a difference in the percentage of bone bridging between the group treated with rhBMP-2 and the control group, as determined by CT scans 3 months after surgery; and (4) would encounter an equal rate of complications different from untreated patients.
Methods
A retrospective chart study was performed on 82 patients who, because of a host of comorbidities associated with poor healing, required a complex ankle arthrodesis with the Ilizarov technique. The first 40 patients did not receive rhBMP-2, whereas the subsequent 42 patients received intraoperative rhBMP-2. Time wearing the frame was determined by chart review; decision to remove the frame was made by the surgeon based on quantitative bone bridging measured using a CT scan taken 3 months after fusion.
Results
Patients treated with rhBMP-2 were more likely to obtain fusion after the initial surgery (93% versus 53%, p < 0.001; OR, 11.76; 95% CI, 3.12–44.41), spent less total time wearing the frame (124 versus 161 days, p < 0.01), and showed more bone bridging on CT scans (48% versus 32%, p < 0.05). All patients with greater than 30% bone bridging observed on CT scans 3 months postoperatively achieved successful union without further intervention.
Conclusions
Our findings suggest that rhBMP-2 is a beneficial adjunct for selected groups of patients undergoing complex ankle arthrodesis. CT is a promising modality in the assessment of bone healing in ankle fusion. A proper randomized controlled trial remains necessary to fully describe the efficacy of rhBMP-2 in accelerating bone healing.
Level of Evidence
Level III, therapeutic study. See the Instructions for Authors for a complete description of levels of evidence.
Introduction
Complex ankle fusions are often a final effort at lower limb salvage. “Complex” refers to surgery performed on patients with comorbidities associated with poor surgical healing or on patients with local healing problems who encounter a high rate of delayed and nonunions [6, 7]. Ankle fusion success was improved with the introduction of the Ilizarov technique [13, 18] which provided excellent stability, allowed immediate weightbearing, and minimized soft tissue trauma [13, 15, 22]. However, even in the controlled environment created by circular fixation, nonunion rates among patients who smoke or with neuropathy remain high [23, 24]. Based on the success of recombinant human BMP-2 (rhBMP-2) in fracture and spinal fusion healing, we began to use this material for our patients undergoing complex ankle fusion.
BMP-2 possesses proliferative and chemotactic properties, acting by inducing differentiation of noncommitted mesenchymal cells into cells of osteoblastic and chrondroblastic lineage [26]. A powerful mediator of vascular endothelial growth factor activity in animal studies [19], rhBMP-2 has been associated with several preferred clinical outcomes including increased healing rate, increased bone quality, and reduced risk of complications when administered intraoperatively [4, 25]. However, recent literature citing unexpected complications, particularly heterotopic ossification and retrograde ejaculation when used in spinal procedures in close proximity to the presacral sympathetic plexus, has slowed any push for the inclusion of rhBMP in the current standard of care [2, 5]. This reluctance also is manifest in the literature, as Garrison et al. [14], in their Cochrane review, lament the paucity of available data addressing the clinical use of rhBMP-2 in orthopaedics.
Most important to the patient base served by our service was the potential utility of rhBMP-2 in ankle fusions. This question was explored most prominently by Bibbo et al. [3], who found that 96% of patients who received rhBMP-2 achieved successful union, a rate much greater than what typically was reported for this procedure [3, 13]. Although encouraging, the lack of a control group, limited sample size, and heterogenous mode of fixation leave aspects of this question unanswered. This is particularly poignant when assessing the benefits of Ilizarov fixation on this patient group.
We therefore sought to determine whether rhBMP-2 treatment (1) would increase the rate of successful ankle fusion in complex patients compared with a control group of patients undergoing ankle fusion who did not receive rhBMP-2; (2) would reduce total time wearing a frame when compared with the control group; (3) would result in a difference in the percentage of bone bridging between the group treated with rhBMP-2 and the control group, as determined by CT scans 3 months after surgery; and (4) would be a complication rate different from those who were treated without rhBMP-2.
Patients and Methods
We performed an institutional review board-approved retrospective cohort analysis of all 96 patients who underwent ankle fusion surgeries at our institution from October 2005 to June 2011. All surgeries were performed by the senior author (ATF) using an Ilizarov apparatus and a technique described previously [13]. Patients were referred to our service by fellowship-trained foot and ankle surgeons because of concerns that comorbidities precluded a successful arthrodesis using traditional internal fixation. Specific indications for use of the Ilizarov method in complex ankle arthrodesis have been described [13] and include (1) Type B hosts (as opposed to a Type A host who is a healthy person) [7]; (2) infection about or in the ankle; (3) simultaneous limb lengthening with arthrosis in patients younger than 70 years with a limb length discrepancy greater than 2.5 cm; (4) deformity of the ankle contraindicating internal fixation; and (5) osteopenia or poor skin quality suggesting a high risk for a poor outcome (Table 1). Cierny et al. defined a Type B host as an individual with systemic or local compromise such as malnutrition, smoking, renal or liver failure, diabetes, active malignancy, steroid or immunosuppression therapy, venous stasis, radiation fibrosis, or chronic hypoxia [7], whereas a Type A host is a healthy person.
Table 1.
Comorbidities characteristic of Type B hosts
| Comorbidity |
| Complex ankle arthrodesis |
| Deformity greater than 10º |
| Renal or liver failure |
| Osteomyelitis |
| Bone loss greater than 2 cm |
| Diabetes mellitus |
| Charcot neuropathy |
| Smoking |
| Rheumatoid arthritis |
| Peripheral vascular disease |
| Active malignancy |
| Immunosuppression/steroid use |
| Venous stasis |
| Radiation fibrosis |
| Chronic hypoxia |
Patients who were not deemed complex, patients who had internal fixation for the ankle arthrodesis, and patients who had inadequate followup (defined here as failure to appear for 3- and 6-month followups after frame removal) were excluded, leaving a cohort of 82 patients. These patients then were separated into two groups based on the use of rhBMP-2 at the time of fusion surgery. Demographics in these two patient groups were numerous but approximately equivalent between rhBMP-2 treatment and nontreatment groups (Table 2). All patients with Charcot neuroarthropathy had Eichenholtz Stage 3 disease [12]. Two patients in the rhBMP-2 group presented with an open fracture, but these were closed by the referring surgeon and did not require soft tissue coverage. Nine of 40 (23%) untreated patients and 12 of 42 (29%) treated with rhBMP-2 presented with at least one failed ankle arthrodesis. No patient had an active infection at the time of management. No patients were recalled specifically for this study; all data were obtained from medical records and radiographs.
Table 2.
Patient demographics
| Demographics | rhBMP-2 group | Non-rhBMP-2 group |
|---|---|---|
| Number of patients | 42 | 40 |
| Mean age (years) (range) | 57.16 (26–88) | 57.43 (21–86) |
| Mean BMI (kg/m2) (range) | 29.63 (18.8–44.3) | 29.67 (17.4–56.6) |
| Diabetes mellitus | 11 | 12 |
| Charcot neuroarthropathy | 10 | 9 |
| Smoking | 8 | 6 |
| Obesity (BMI > 30 kg/m2) | 17 | 15 |
| History of infection | 9 | 14 |
| Hypertension | 14 | 13 |
| Concurrent tibial lengthening | 8 | 6 |
rhBMP-2 = recombinant human BMP-2; comparisons between groups in all categories were not significantly different (p < .05).
Ankle fusions were performed as previously described [13] using circular fixation. The majority of patients received spinal anesthesia with intravenous sedation. Medial and lateral approaches were used. The distal fibula and medial malleolus were resected. Flat cuts were made across the distal tibia and the proximal talus. In the first 40 patients, no rhBMP-2 (Infuse; Medtronic, Minneapolis, MN, USA) was used. In the remaining 42 patients, rhBMP-2 was used. Surgical technique, weightbearing status, followup direction, and all other aspects of standard of care remained consistent. The manufacturer supplies rhBMP-2 with a collagen sponge carrier. A medium-sized kit was used, and we delivered rhBMP-2 at a concentration of 1.5 mg/cc into each of four collagen sponges. The sponge was allowed to sit for 60 to 120 minutes after preparation. This allowed for a 95% uptake of rhBMP-2, according to Dawson et al. [8]. Two of the sponges were cut into small pieces and inserted directly into the fusion site. The other two sponges were then wrapped around the fusion site. In patients with large defects, allograft was used. No other graft types or modes of intervention were used in either patient group.
An Ilizarov/Taylor Spatial Frame™ (TSF) (Smith & Nephew, Memphis, TN, USA) external fixator then was applied to the ankle. Each ring was secured with tensioned wires and half pins. The wires were stainless steel 1.8-mm K wires tensioned to 130 kg. Hydroxyapatite-coated 6-mm tapered pins were used. The foot ring was closed anteriorly and fastened to the tibial ring block with rods. Then five wires were inserted in multiple planes through the foot and tensioned to between 90 and 130 kg. The subtalar joint was protected from compression by inserting a talus wire through the talar body, then arching it proximally and tensioning it, distracting the subtalar joint. For this construct, TSF rings were used and connected with simple threaded rods and compressed (Fig. 1). We generally allowed patients to weightbear as tolerated, although patients with neuropathy were not permitted full weightbearing throughout treatment for fear of breaking the external fixator. Pin care began on the second postoperative day and was performed once daily after that. The patients’ stay in the hospital ranged from 3 to 5 days. Patients were followed up monthly in the office to assess bone healing. A physical examination and AP, mortise, and lateral 17-inch ankle radiographs were performed at each visit. A CT scan was performed 3 months after surgery to assess bone bridging at the fusion site. Patients who did not have radiographic signs of healing 3 months after surgery were treated with repeat grafting with allograft and bone marrow aspirate. Poor healing was determined by a persistent lucency at the fusion site observed on plain radiographs or by less than 30% bone bridging on CT scans (Fig. 2). These clinical radiographic assessments were made by the operating surgeon (ATF).
Fig. 1.
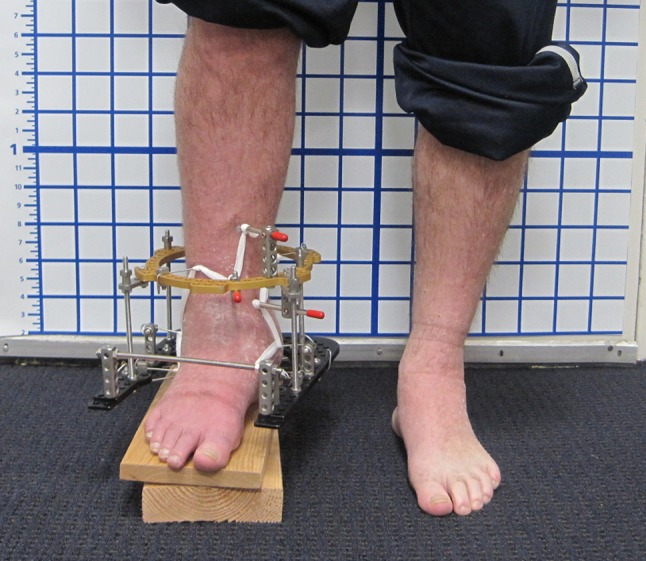
The entire construct for ankle fusion with application of the Ilizarov circular fixator with a closed foot ring and single distal tibial ring is shown. The limb shortening subsequently was remedied with tibial distraction osteogenesis.
Fig. 2A–B.
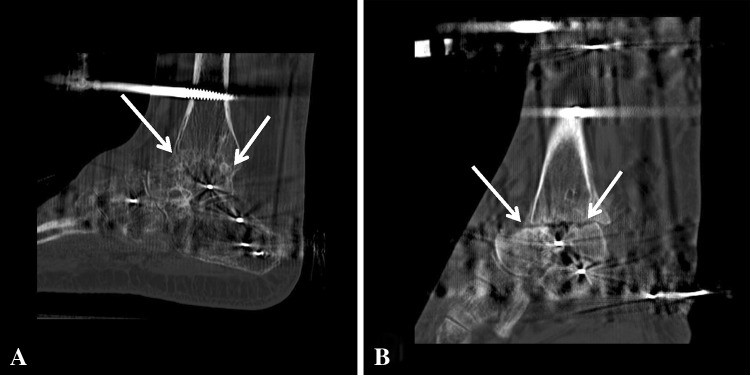
(A) The arrows highlight bridging bone across the tibiotalar fusion site as viewed on a CT scan taken 3 months after fusion. This image shows greater than 30% fusion healing, and the patient achieved successful union without further intervention. (B) No bridging bone across the tibiotalar fusion site (arrows) is seen at 3 months after fusion in a different patient.
After Ilizarov frame removal, patients wore a cast for 6 weeks followed by a cam boot for an additional 6 to 12 weeks to allow the bone defects caused by pin tracts to heal. At this point the patient was considered for a custom shoe or other long-term footwear. Normal patient followup was at 1, 3, 6, and 12 months after frame removal. Annual visits were recommended thereafter.
Outcomes include initial union, final union, and total time wearing the frame. An initial union is defined here as a union that occurred 3 months after surgery as a result of the initial fusion procedure. These patients did not require additional grafting surgery. Final union was defined as bony union at the time of frame removal regardless of the need for further intervention. Total time wearing the frame was measured from the date of frame placement at the initial surgery to the date of final frame removal. Mean followup from the date of frame removal was 43 months (range, 16–84 months).
CT imaging was available at 2.5 to 3.5 months after the index procedure in 34 of 63 (54%) patients. All measurements were performed by a radiologist who was blinded to patient identity or treatment group. CT examinations were performed of the ankles at 1-mm slice intervals with 0.5-mm overlap. A soft tissue and bone algorithm were obtained using a filtered back projection technique with a low iterative dose component to help reduce the overall dose imparted to the patient. Images were obtained at 140 kVp and 300 to 340 mAs to optimize evaluation in the setting of hardware and for accommodation to patient size. Using the axial source images, sagittal and coronal reformations were obtained at 1- to 2-mm slice intervals. Each sagittal image obtained through the area of fusion then was viewed using a routine bone window and an extended bone window to help further reduce streak artifact. On each image, the amount of bony bridging was quantified as was the overall joint space. Overall healing was calculated by taking the cumulative sum of bone bridging measurements and calculating ossification as a percentage of the sum of all joint spaces [20].
Of patients excluded from this study, eight were deemed noncomplex (three who received BMP-2, five non-BMP-2), four had a prior internal fixation (one with BMP-2 and three non-BMP-2), and two were lost to followup (one with BMP-2, one non-BMP-2). Statistical analysis was performed using Prism 5.0 (GraphPad Software, La Jolla, CA, USA). Statistical significance in initial and final unions between BMP-2 and untreated groups was determined using the Fisher’s exact test. Significance in time wearing the frame and CT healing was determined using the unpaired two-way t-test. For groups with fewer than six members, the Mann-Whitney test was used to account for nonGaussian distributions.
Results
The proportion of patients who achieved initial union was greater in the rhBMP-2 group than in the control group (39 of 42, 92%, versus 21 of 40, 53%; p < 0.001; OR, 11.76; 95% CI, 3.12–44.41). The initial union rate for patients with Charcot neuropathy treated with rhBMP-2 was nine of 10 (90%). This was greater than (p = 0.005) the rate of initial union of two of nine (22%) for patients not treated with rhBMP-2. An improvement in initial union rate also was observed among patients with diabetes, hypertension, obesity, and a history of infection (Table 3). The final union rate of rhBMP-2-treated patients was 40 of 42 (92%). This was similar to (p = 0.08) the final union rate of non-rhBMP-2-treated patients (33 of 40 [82%]) (Table 4). No differences were observed in the rate of initial or final union in patients who received allograft in untreated (78% versus 100% with allograft; n = 7, p = 0.30), or rhBMP-2 (94% versus 100% with allograft; n = 10 with allograft, p = 1) groups. Similarly, time wearing the frame was not affected by administration of allograft in either untreated (172 ± 12 days versus 131 ± 20 days, p = 0.10) or rhBMP-2 (130 ± 8 days versus 117 ± 8 days with autograft, p = 0.37) groups.
Table 3.
Initial union success rate
| Comorbidity | rhBMP-2 (%) | Sample size | Untreated (%) | Sample size | p value |
|---|---|---|---|---|---|
| Composite | 93 | 42 | 53 | 40 | < 0.0001 |
| Diabetics | 82 | 11 | 33 | 12 | 0.0361 |
| Charcot | 90 | 10 | 22 | 9 | 0.0055 |
| Smokers | 88 | 8 | 50 | 6 | 0.2448 |
| Obese | 88 | 17 | 53 | 15 | 0.0491 |
| Infection | 100 | 9 | 50 | 14 | 0.0189 |
| Hypertension | 93 | 14 | 46 | 13 | 0.0128 |
| Concurrent lengthening | 100 | 8 | 50 | 6 | 0.0549 |
rhBMP-2 = recombinant human BMP-2.
Table 4.
Final union success rate
| Comorbidity | rhBMP-2 (%) | Sample size | Untreated (%) | Sample size | p value |
|---|---|---|---|---|---|
| Composite | 95 | 42 | 83 | 40 | 0.0844 |
| Diabetics | 82 | 11 | 92 | 12 | 0.5901 |
| Charcot | 90 | 10 | 89 | 9 | 1.0000 |
| Smokers | 100 | 8 | 67 | 6 | 0.1250 |
| Obese | 94 | 17 | 87 | 15 | 0.5887 |
| Infection | 100 | 9 | 79 | 14 | 0.2530 |
| Hypertension | 93 | 14 | 92 | 13 | 1.0000 |
| Concurrent lengthening | 100 | 8 | 67 | 6 | 0.1648 |
rhBMP-2 = recombinant human BMP-2.
Patients treated with rhBMP-2 spent less time wearing the frame than patients who did not receive rhBMP-2. The mean total time wearing the frame for rhBMP-2-treated patients was 124 days (SEM 6.53, compared with the 161-day mean SEM 13.3) prescribed for non-rhBMP-2-treated patients (p = 0.011). Patients with Charcot neuropathy treated with rhBMP-2 had a mean time wearing the frame of 132.0 days (SEM, 10.52), which was less than (p = 0.01) the mean time wearing the frame of 206 days (SEM, 20) for patients with Charcot neuropathy not treated with rhBMP-2 (Table 5).
Table 5.
Total time wearing frame to final union
| Comorbidity | rhBMP-2 (days) | Untreated (days) | p value |
|---|---|---|---|
| Composite | 124 ± 6.5 | 161 ± 13 | 0.0117 |
| Diabetics | 131 ± 11 | 178 ± 24 | 0.1179 |
| Charcot | 132 ± 11 | 206 ± 20 | 0.0104 |
| Smokers | 147 ± 61 | 170 | N/A |
| Obese | 129 ± 14 | 144 ± 19 | 0.5199 |
| Infection | 124 ± 29 | 173 ± 18 | 0.1881 |
| Hypertension | 129 ± 26 | 169 ± 23 | 0.2836 |
Values are mean ± SD; rhBMP-2 = recombinant human BMP-2; N/A = not applicable.
Patients treated with rhBMP-2 showed more bone bridging than the patients not treated with this product, as assessed on CT scans obtained 3 months after the index procedure. Patients treated with rhBMP-2 showed a mean of 48% (SD, 4.18) bone bridging, which was greater than the 32% (± 5.90) bridging seen in the non-rhBMP-2-treated patients (p = 0.036). Regarding CT analysis and clinical outcome, 100% of patients who were measured to have 30% or greater bone bridging at the fusion site 3 months after the index procedure achieved a successful initial union without intervention.
There were no differences in the frequency of complications between the groups. Six of 42 rhBMP-2-treated patients (14.3%) and five of 40 untreated patients (12.5%) were treated for pin site infections during the time they wore the frame. One patient in the non-rhBMP-2 group underwent a below knee amputation owing to a deep infection. No heterotopic ossification, deep vein thrombosis, compartment syndrome, wound breakdown, or focal neurologic deficiency was observed in either patient group.
Discussion
Although the Ilizarov method of circular fixation has been associated with strong ankle fusion outcomes, recent work suggests that patients with Charcot neuropathy, smokers, and postinfection patients continue to have substantial nonunion rates [13, 17, 24]. Attention has turned to the potential benefit of rhBMP-2 to improve surgical outcome in complex patients [10, 14]. We asked whether rhBMP-2 treatment could (1) increase the rate of successful ankle fusion in complex patients; and (2) reduce total time wearing a frame when compared with non-rhBMP-2-treated patients. We also asked (3) whether CT scans performed 3 months after surgery could confirm a difference in the percentage of bone bridging between the two groups; and (4) whether rhBMP-2 treated patients exhibited short- or long-term complications as compared with untreated patients.
As with any retrospective study, we encountered several limitations. We were limited to the number of patients available to us at the start of the study. The limited sample size presented here includes patients with multiple overlapping comorbidities. Nearly every patient who was included in one comorbidity category fell into several. This presents a confounding risk, although the groups that were associated with significance were profoundly different from the remainder of the study population. The experimental and control groups were not randomized. The use of rhBMP-2 came about as a change in practice. Therefore, the earlier ankle fusions were done without rhBMP-2 and the more recent ankle surgeries were performed with rhBMP-2. This introduces bias. Although our preoperative and postoperative standard of care remained consistent throughout the patient group, we could not control for the exact surgical technique performed and any improvement in surgical skill with time. Placement of the rhBMP-2 soaked collagen sponges was variable in many cases based on the dimensions of the fusion site. There has been no study, to our knowledge, that definitively determines ideal placement for the collagen sponge, and therefore we cannot confirm an equal scale of effect in all rhBMP-2 treated patients. However, it is likely that this would have decreased rhBMP-2 efficacy, and therefore the significance we present would be increased. Further technical variation includes use of allograft in some patients with larger defects, which represents a potential source of skewed findings. However, we believe this be minimal based on the nonsignificance measured in intragroup analysis. The use of a 3-month point for assessing initial fusion was clinically based. Treatment was not allowed to fail in patients who had poor healing, and these patients underwent an additional surgical procedure of bone grafting. By intervening we do not know how many of the patients with poor healing would have achieved fusion or had nonunion. However, as our revision modality was consistent, we believe that the comparative final union rate between rhBMP-2 and untreated groups was not affected by this. The treatment course, defined as time wearing the frame, had end points demarcated by the surgeon based on standardized radiologic criteria of using CT scans to detect greater than 30% healing as measured by the surgeon, and persistent lucency of the fusion site. As this was not blinded, it is impossible to say with certainty that the surgeon’s opinion of patient healing was not biased based on his intervention. However, this potential for bias was minimized by adhering to the previously noted criterion. Further, retrospective quantitative validation using a blinded radiologist and a previously used CT analysis algorithm supports our clinical findings. Although variability in morbidity subgroups was less than that observed between subgroups, the reduced sample size and sparse literature available prevented us from performing a formal power analysis. The availability of CT data in the appropriate reference range of 2.5 to 3.5 months after fusion from only 53% of the total cohort compromised a comprehensive assessment of the efficacy of this analysis technique. The remainder of patients had this scan later during their clinical course owing to scheduling variability. These data were removed owing to the difficulty encountered in comparing such a wide temporal range of quantitative measurements. Loss to followup was similar between rhBMP-2 and non-rhBMP-2 groups (n = 1 each).
Our findings suggest that the rate of initial union of ankle arthrodesis at 3 months was improved by rhBMP-2 administration. This effect was noted in multiple patient subgroups (Charcot, diabetes, postinfection, and smokers) throughout the rhBMP-2 cohort but was most profound in patients with Charcot neuroarthropathy. The patients who did not achieve union by 3 months were not considered to have nonunion. These patients had delayed unions, and some may have achieved healing without further intervention. This is why our initial union rate of 52% in the non-rhBMP-2 group was low compared the rate in another study [13]. The rate of final union was similar in the two patient groups. This supports the use of CT assessment of union at 3 months postoperatively and aggressive intervention for patients with inadequate healing. The potential for rhBMP-2 to improve ankle fusion outcomes has been covered, to our knowledge, in only two previous studies. Bibbo et al. [3] found a 96% ankle fusion rate with the use of rhBMP-2 (n = 110 in 69 patients); however, there was no control group. DeVries et al. [9] attempted to compare the rate of fusion and time until radiographic union for rhBMP-2- and untreated groups in an analysis of tibiotalocalcaneal fusions after failed initial arthrodesis, but as a result of the small sample size (n = 7 in rhBMP-2 group and n = 16 in untreated), their study failed to show significance.
Time wearing the frame is not just a measure of clinical outcome, but also a metric for the risk of morbidity sustained by the wearer. This morbidity includes the increased risk of superficial and deep infection, pin loosening or failure, tibial fracture from the stress riser produced by the frame, and pain from the frame [21, 27]. Logically, it is in the best interest of the surgeon and the patient to use modalities that reduce the total time wearing the frame. Our untreated patient group had a median time wearing the frame of 24.1 weeks (range, 7–39.1 weeks), a range comparable to that in previous studies [7, 13]. In contrast, the rhBMP-2-treated patient group had a median time wearing the frame of 17.3 weeks (range, 10.1–35.7 weeks), an improvement of nearly 2 months. Patients with Charcot neuropathy showed the most profound improvement in time wearing the frame, a reduction from 29 weeks to 18.4 weeks—an improvement of 3 months. The fragile bone structure of patients with Charcot neuropathy in particular presents a particular reconstructive challenge. Herscovici et al. [17] reported that even pantalar fusion in these patients resulted in a 50% complication rate and a 25% reoperation rate. The combination of increased salvage rate for patients and decreased costs secondary to reoperation would suggest the potential for rhBMP-2 to become the standard of practice in this patient group [1]. The low incidence of complications in our patients also may be attributed to the decreased time wearing the frame allowed by rhBMP-2 use.
Our CT findings provide quantitative support to our clinical observations. Current clinical management of patients undergoing Ilizarov frame treatment is purely dependent on clinical judgment aided by plain film readings to assess cortices of healing and bone alignment [13, 16, 22]. This system is highly dependent on the experience of the surgeon and, regardless of skill, can result in premature or late frame removal, increasing patient morbidity and/or nonunion. A difficulty in establishing CT-dependent criteria for frame removal is our lack of patients with adverse complications and poor healing. Prior work suggests that a threshold of 32% is correlated with good fracture healing [11]. Our study is hindered by a large proportion of our patient population that did not receive CT, making firm conclusions on this end point difficult. Therefore we can state only that 100% of patients who were measured to have achieved 30% or greater healing observed on CT scans 3 months postoperatively achieved successful union without grafting; it is possible that some of the patients who were lost to followup had results different from the results of patients who returned for followup, and those results likely were not as good. Future prospective, randomized, blinded trials will allow us to substantially increase sample size and thereby allow us to more precisely associate CT measurement thresholds with clinical outcomes.
To our knowledge, our study is the largest retrospective analysis of rhBMP-2 on ankle fusions, and the first to suggest, in the setting of a comparative study, that rhBMP-2 improves clinical results (fusion, time wearing a frame, and bone bridging) in patients undergoing ankle fusion. A prospective randomized clinical trial with sufficient sample size should be performed before conclusive recommendations regarding the use of rhBMP-2 in patients with complex ankle fusions can be made. Future studies will focus on the development of such a trial standardized by CT scan-mediated cutoffs for clinical course.
Acknowledgments
We thank the staff and fellows at the Institute for Limb Lengthening and Complex Reconstruction at Hospital for Special Surgery for their assistance in gathering the patient list and charts necessary for the composition of this study, and for participating in the care of the patients included in the study population.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the Division of Limb Lengthening and Complex Reconstruction, Hospital for Special Surgery, New York, NY, USA.
References
- 1.Alt V, Heissel A. Economic considerations for the use of recombinant human bone morphogenetic protein-2 in open tibial fractures in Europe: the German model. Curr Med Res Opin. 2006;22(suppl 1):S19–S22. doi: 10.1185/030079906X80602. [DOI] [PubMed] [Google Scholar]
- 2.Anderson CL, Whitaker MC. Heterotopic ossification associated with recombinant human bone morphogenetic protein-2 (infuse) in posterolateral lumbar spine fusion: a case report. Spine (Phila Pa 1976). 2012;37:E502–E506. doi: 10.1097/BRS.0b013e318238870b. [DOI] [PubMed] [Google Scholar]
- 3.Bibbo C, Patel DV. Haskell MD Recombinant bone morphogenetic protein-2 (rhBMP-2) in high-risk ankle and hindfoot fusions. Foot Ankle Int. 2009;30:597–603. doi: 10.3113/FAI.2009.0597. [DOI] [PubMed] [Google Scholar]
- 4.Bishop GB, Einhorn TA. Current and future clinical applications of bone morphogenetic proteins in orthopaedic trauma surgery. Int Orthop. 2007;31:721–727. doi: 10.1007/s00264-007-0424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burkus JK, Dryer RF, Peloza JH. Retrograde ejaculation following single-level anterior lumbar surgery with or without recombinant human bone morphogenetic protein-2 in 5 randomized controlled trials: clinical article. J Neurosurg Spine. 2013;18:112–121. doi: 10.3171/2012.10.SPINE11908. [DOI] [PubMed] [Google Scholar]
- 6.Chahal J, Stephen DJ, Bulmer B, Daniels T, Kreder HJ. Factors associated with outcome after subtalar arthrodesis. J Orthop Trauma. 2006;20:555–561. doi: 10.1097/01.bot.0000211156.13487.6a. [DOI] [PubMed] [Google Scholar]
- 7.Cierny G, 3rd, Mader JT, Penninck JJ. A clinical staging system for adult osteomyelitis. Clin Orthop Relat Res. 2003;414:7–24. doi: 10.1097/01.blo.0000088564.81746.62. [DOI] [PubMed] [Google Scholar]
- 8.Dawson E, Bae HW, Burkus JK, Stambough JL, Glassman SD. Recombinant human bone morphogenetic protein-2 on an absorbable collagen sponge with an osteoconductive bulking agent in posterolateral arthrodesis with instrumentation: a prospective randomized trial. J Bone Joint Surg Am. 2009;91:1604–1613. doi: 10.2106/JBJS.G.01157. [DOI] [PubMed] [Google Scholar]
- 9.DeVries JG, Nguyen M, Berlet GC, Hyer CF. The effect of recombinant bone morphogenetic protein-2 in revision tibiotalocalcaneal arthrodesis: utilization of the Retrograde Arthrodesis Intramedullary Nail database. J Foot Ankle Surg. 2012;51:426–432. doi: 10.1053/j.jfas.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 10.DiGiovanni CW, Petricek JM. The evolution of rhPDGF-BB in musculoskeletal repair and its role in foot and ankle fusion surgery. Foot Ankle Clin. 2010;15:621–640. doi: 10.1016/j.fcl.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Dorsey ML, Liu PT, Roberts CC, Kile TA. Correlation of arthrodesis stability with degree of joint fusion on MDCT. AJR Am J Roentgenol. 2009;192:496–499. doi: 10.2214/AJR.08.1254. [DOI] [PubMed] [Google Scholar]
- 12.Eichenholtz SN. Congenital pseudarthrosis of tibia. N Y State J Med. 1970;70:1049–1058. [PubMed] [Google Scholar]
- 13.Fragomen AT, Borst E, Schachter L, Lyman S, Rozbruch SR. Complex ankle arthrodesis using the Ilizarov method yields high rate of fusion. Clin Orthop Relat Res. 2012;470:2864–2873. doi: 10.1007/s11999-012-2470-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garrison KR, Shemilt I, Donell S, Ryder JJ, Mugford M, Harvey I, Song F, Alt V. Bone morphogenetic protein (BMP) for fracture healing in adults. Cochrane Database Syst Rev. 2010;6:CD006950. doi: 10.1002/14651858.CD006950.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gessmann J, Citak M, Fehmer T, Schildhauer TA, Seybold D. Ilizarov external frame technique for Pirogoff amputations with ankle disarticulation and tibiocalcaneal fusion. Foot Ankle Int. 2013;34:856–864. doi: 10.1177/1071100713475612. [DOI] [PubMed] [Google Scholar]
- 16.Hawkins BJ, Langerman RJ, Anger DM, Calhoun JH. The Ilizarov technique in ankle fusion. Clin Orthop Relat Res. 1994;303:217–225. [PubMed] [Google Scholar]
- 17.Herscovici D, Sammarco GJ, Sammarco VJ, Scaduto JM. Pantalar arthrodesis for post-traumatic arthritis and diabetic neuroarthropathy of the ankle and hindfoot. Foot Ankle Int. 2011;32:581–588. doi: 10.3113/FAI.2011.0581. [DOI] [PubMed] [Google Scholar]
- 18.Ilizarov GA. The tension-stress effect on the genesis and growth of tissues: Part II. The influence of the rate and frequency of distraction. Clin Orthop Relat Res. 1989;239:263–285. [PubMed] [Google Scholar]
- 19.Jacobsen KA, Al-Aql ZS, Wan C, Fitch JL, Stapleton SN, Mason ZD, Cole RM, Gilbert SR, Clemens TL, Morgan EF, Einhorn TA, Gerstenfeld LC. Bone formation during distraction osteogenesis is dependent on both VEGFR1 and VEGFR2 signaling. J Bone Miner Res. 2008;23:596–609. doi: 10.1359/jbmr.080103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones CP, Coughlin MJ, Shurnas PS. Prospective CT scan evaluation of hindfoot nonunions treated with revision surgery and low-intensity ultrasound stimulation. Foot Ankle Int. 2006;27:229–235. doi: 10.1177/107110070602700401. [DOI] [PubMed] [Google Scholar]
- 21.Jorge LS, Chueire AG, Rossit AR. Osteomyelitis: a current challenge. Braz J Infect Dis. 2010;14:310–315. [PubMed] [Google Scholar]
- 22.Khanfour AA. Versatility of Ilizarov technique in difficult cases of ankle arthrodesis and review of literature. Foot Ankle Surg. 2013;19:42–47. doi: 10.1016/j.fas.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Kugan R, Aslam N, Bose D, McNally MA. Outcome of arthrodesis of the hindfoot as a salvage procedure for complex ankle pathology using the Ilizarov technique. Bone Joint J. 2013;95:371–377. doi: 10.1302/0301-620X.95B3.29885. [DOI] [PubMed] [Google Scholar]
- 24.Myers TG, Lowery NJ, Frykberg RG, Wukich DK. Ankle and hindfoot fusions: comparison of outcomes in patients with and without diabetes. Foot Ankle Int. 2012;33:20–28. doi: 10.3113/FAI.2012.0020. [DOI] [PubMed] [Google Scholar]
- 25.Pimenta L, Marchi L, Oliveira L, Coutinho E, Amaral R. A prospective, randomized, controlled trial comparing radiographic and clinical outcomes between stand-alone lateral Interbody lumbar fusion with either silicate calcium phosphate or rh-BMP2. J Neurol Surg A Cent Eur Neurosurg. 2013 Feb 26. [Epub ahead of print]. [DOI] [PubMed]
- 26.Rauch F, Lauzier D, Croteau S, Travers R, Glorieux FH, Hamdy R. Temporal and spatial expression of bone morphogenetic protein-2, -4, and -7 during distraction osteogenesis in rabbits. Bone. 2000;27:453–459. doi: 10.1016/S8756-3282(00)00337-9. [DOI] [PubMed] [Google Scholar]
- 27.Saw A, Chua YP, Hossain G, Sengupta S. Rates of pin site infection during distraction osteogenesis based on monthly observations: a pilot study. J Orthop Surg (Hong Kong). 2012;20:181–184. doi: 10.1177/230949901202000209. [DOI] [PubMed] [Google Scholar]


