Abstract
Background
One common approach to the infected total hip arthroplasty (THA) calls for a staged revision, including removal of all components. However, removal of well-fixed femoral components can result in bone loss and compromised fixation; it is not known whether it is effective to leave a well-fixed femoral component in situ, remove only the acetabular component, débride thoroughly, place a spacer, and delay reimplantation.
Questions/purposes
The purposes of this study were to determine (1) the frequency of infection recurrence; (2) the frequency of death; and (3) the Harris hip scores of patients treated with a “two-stage partial exchange” approach.
Methods
A retrospective analysis from 2000 through January 2011 revealed 19 patients with infected THA treated with partial two-stage exchange including complete acetabular component removal, aggressive soft tissue débridement, retention of the well-fixed femoral stem, placement of an antibiotic-laden cement femoral head on the trunnion of the retained stem, postoperative course of antibiotics, and delayed reimplantation. Indications for this treatment included those patients whose femoral component was determined to be well fixed and its removal would result in significant femoral bone loss and compromise of future fixation. During the study period, this represented 7% (19 of 262) of the patients whom we treated for a chronically infected THA. Minimum followup was 2 years (mean, 4 years; range, 2–11 years). None of the 19 patients in this series were lost to followup. We defined failure as recurrence of infection in the same hip or the use of long-term suppressive antibiotics.
Results
Two patients (11%), both with prior failure of two-staged treatment of infection, failed secondary to recurrence of infection at an average of 3.3 years. There were no patient deaths within 90 days. The mean Harris hip score was 68 (range, 31–100; best score is 100).
Conclusions
Insofar as 89% of patients in this series were clinically free of infection at a minimum of 2 years, we believe partial two-stage exchange may represent an acceptable option for patients with infected THA when femoral component removal would result in significant bone loss and compromise of reconstruction. Further study is required on this approach.
Level of Evidence
Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Periprosthetic infection of total joint arthroplasty is a devastating complication. Deep infection of the hip has been reported to occur in approximately 0.5% to 3% of primary and 4% to 6% of revision THAs [3, 19, 59, 60, 62].
Several treatment modalities have been described for the management of the difficult problem of periprosthetic infection after THA (Tables 1, 2). The standard of care in the United States for treatment of chronic periprosthetic joint infection (PJI) of the hip is two-stage exchange arthroplasty [20, 59, 62]. This technique includes removal of all existing hardware including both the acetabular and femoral components and placement of either a static or mobile antibiotic-laden polymethylmethacrylate cement spacer. Intravenous (IV) antibiotics are administered for a minimum of 6 weeks with delayed reimplantation taking place at 6 to 12 weeks.
Table 1.
One-stage treatment of infected THA
| Study | Year | Number of hips | Mean followup (months) | Infection eradicated |
|---|---|---|---|---|
| Drancourt et al. [11] | 1993 | 10 | 28 | 10 (100%) |
| Raut et al. [54] | 1994 | 57 | 88 | 49 (86%) |
| Raut et al. [55] | 1995 | 183 | 93 | 154 (84%) |
| Raut et al. [53] | 1996 | 15 | 96 | 14 (93%) |
| Mulcahy et al. [47] | 1996 | 15 | 53 | 15 (100%) |
| Callaghan et al. [6] | 1999 | 24 | 137 | 22 (92%) |
| Jackson and Schmalzried* [29] | 2000 | 1299 | 58 | 1077 (83%) |
| García et al. [18] | 2005 | 14 | > 24 | 14 (100%) |
| Gao and Lv [17] | 2008 | 10 | 19 | 10 (100%) |
| Rudelli et al. [58] | 2008 | 32 | 103 | 30 (94%) |
| Winkler et al. [70] | 2008 | 37 | 53 | 34 (92%) |
| Yoo et al. [72] | 2009 | 12 | 86 | 11 (92%) |
| Darley et al. [9] | 2011 | 4 | 24–36 | 4 (100%) |
| Engesæter et al. [12] | 2011 | 192 | > 24 | 170 (89%) |
| Klouche et al. [32] | 2012 | 38 | 35 | 38 (100%) |
| Combined results | 1942 | 60 | 1652 (85%) |
* Meta-analysis of 12 earlier studies not included here.
Table 2.
Two-stage treatment of infected THA
| Study | Year | Number of hips reimplanted | Mean followup (months) | Number of infections eradicated |
|---|---|---|---|---|
| McDonald et al. [45] | 1989 | 82 | 65 | 71 (87%) |
| Berry et al. [2] | 1991 | 18 | 50 | 16 (89%) |
| Lieberman et al. [40] | 1994 | 32 | 40 | 29 (92%) |
| Nestor et al. [48] | 1994 | 34 | 47 | 28 (82%) |
| Lai et al. [35] | 1996 | 39 | 48 | 34 (87%) |
| Tsukayama et al. [66] | 1996 | 34 | 46 | 29 (85%) |
| Wang and Chen [68] | 1997 | 22 | 48 | 20 (87%) |
| Younger et al. [73] | 1998 | 56 | 43 | 52 (93%) |
| Fehring et al. [15] | 1999 | 25 | 41 | 23 (88%) |
| Isiklar et al. [28] | 1999 | 10 | 23 | 10 (100%) |
| Koo et al. [33] | 2001 | 22 | 41 | 21 (95%) |
| Magnan et al. [43] | 2001 | 8 | 35 | 8 (100%) |
| Jahoda et al. [30] | 2003 | 64 | 71 | 60 (94%) |
| Karpas and Sponer [31] | 2003 | 18 | 42 | 18 (100%) |
| Yamamoto et al. [71] | 2003 | 15 | 38 | 15 (100%) |
| Evans [14] | 2004 | 23 | > 24 | 22 (96%) |
| Hsieh et al. [24] | 2004 | 128 | 59 | 122 (95%) |
| Buttaro et al. [4] | 2005 | 30 | 32 | 29 (97%) |
| Hofmann et al. [22] | 2005 | 27 | 76 | 26 (96%) |
| Hsieh et al. [25] | 2005 | 24 | 50 | 24 (100%) |
| Kraay et al. [34] | 2005 | 33 | > 24 | 28 (85%) |
| Nusem and Morgan [50] | 2006 | 18 | 108 | 17 (94%) |
| Cabrita et al. [5] | 2007 | 56 | 48 | 51 (96%) |
| Scharfenberger et al. [61] | 2007 | 26 | > 24 | 26 (100%) |
| Walter et al. [67] | 2007 | 40 | 12 | 38 (95%) |
| Stockley et al. [63] | 2008 | 114 | 74 | 100 (88%) |
| Cordero-Ampuero et al. [7] | 2009 | 20 | 53 | 20 (100%) |
| Dairaku et al. [8] | 2009 | 10 | 18 | 9 (90%) |
| Fink et al. [16] | 2011 | 40 | 35 | 40 (100%) |
| Hsieh et al. [23] | 2009 | 99 | 43 | 89 (90%) |
| Incavo et al. [27] | 2009 | 11 | NR | 9 (82%) |
| Lim et al. [41] | 2009 | 42 | 54 | 35 (83%) |
| Sanchez-Sotelo et al. [60] | 2009 | 169 | 84 | 157 (93%) |
| Toulson et al. [65] | 2009 | 84 | 65 | 80 (95%) |
| Whittaker et al. [69] | 2009 | 44 | 49 | 41 (93%) |
| Erhart et al. [13] | 2010 | 14 | > 60 | 12 (86%) |
| Romanò et al. [57] | 2010 | 102 | 48 | 98 (96%) |
| Takigami et al. [64] | 2010 | 8 | 49 | 8 (100%) |
| Engesæter et al. [12] | 2011 | 283 | > 24 | 268 (95%) |
| Lee et al. [36] | 2011 | 27 | 98 | 26 (96%) |
| Leung et al. [39] | 2011 | 47 | 58 | 30 (79%) |
| Klouche et al. [32] | 2012 | 46 | 35 | 45 (98%) |
| Neumann et al. [49] | 2012 | 43 | 67 | 43 (98%) |
| Romanò et al. [56] | 2012 | 183 | 60 | 173 (95%) |
| Berend et al. [1] | 2013 | 189 | 53 | 157 (83%) |
| Lee et al. [37] | 2013 | 17 | 48 | 15 (88%) |
| Combined results | 2476 | 52 | 2272 (92%) |
NR = not reported.
Removal of well-fixed components represents a significant technical challenge, especially with respect to the femoral component. Within the past several years, acetabular removal equipment has been refined to the point at which well-fixed acetabular components can be removed efficiently with minimal bone loss. However, femoral component removal remains difficult and complicated. Factors contributing to the complexity of component removal include stem length and the extent of porous coating or remaining cement. Although techniques have evolved to facilitate removal of well-fixed femoral components, in some situations, removal will sacrifice bone stock and compromise fixation of the reconstruction. In these situations, we wondered whether patients could be treated successfully for their infections without removing the well-fixed femoral components by using aggressive débridement and an antibiotic-laden acrylic cement femoral head in the acetabulum as well as a minimum of 6 weeks of IV antibiotics before reimplantation of a new acetabular component. A search of Medline, PubMed, and Medscape revealed one article on the subject of two-stage partial exchange for chronic PJI [39].
Therefore, we sought to determine (1) the frequency of infection recurrence; (2) the frequency of death; and (3) the Harris hip scores of patients treated with the two-stage partial exchange approach.
Materials and Methods
A retrospective search of our institutional database between 2000 and January 2011 revealed 262 hips that were treated for a chronic periprosthetic joint infection. Two-stage exchange was used to treat chronic infection in 243 hips. Partial two-stage exchange was used in the treatment of 19 chronically infected hips in 19 patients. Indications for using a partial two-stage exchange included those patients whose femoral component was determined to be well fixed and its removal would result in significant femoral bone loss and compromise of future fixation. A collaborative decision between the two surgeons was often made regarding these difficult cases.
Minimum followup on these 19 patients was 2 years (mean, 4 years; range, 2–11 years). No patients were lost to followup.
The criteria used for diagnosing an infection were consistent with those identified by the Musculoskeletal Infection Society, including a pathogen identified from culture from at least two separate samples or a sinus tract communicating with the prosthesis. In the absence of either of these criteria, the presence of at least four of the following criteria is required: elevated serum erythrocyte sedimentation rate (ESR) and serum C-reactive protein (CRP); elevated synovial leukocyte count; elevated synovial leukocyte percentage; one positive culture; purulence; and > 5 neutrophils per high-powered field [52].
All surgeries were performed by one of two experienced, fellowship-trained arthroplasty surgeons (AVL, KRB). All patients identified in this study had complete removal of the acetabular component and retention of the femoral component. All patients underwent aggressive débridement of the joint and mechanical cleansing with diluted povidone-iodine of exposed metal and soft tissue as part of the procedure. An antibiotic-laden acrylic cement articulating femoral head was fabricated using a pediatric ear and ulcer syringe (outer diameter: 44 mm; CR Bard, Inc, Covington, GA, USA) (Fig. 1), a bulb-type irrigation syringe (outer diameter: 52 mm; CR Bard, Inc) (Fig. 2), or, more recently, disposable cement spacer molds (StageOne Select; Biomet, Warsaw, IN, USA) (Fig. 3). High-viscosity cement, either Palacos (Zimmer, Warsaw, IN, USA) or Cobalt (Biomet), was used to fabricate the molded spacers. High-dose antibiotics as recommended by several authors, 3 to 4 g of vancomycin and 3.6 to 4.8 g of tobramycin per 40 mg bag, were added to the polymethylmethacrylate (PMMA) [22, 23, 38]. The antibiotic-laden PMMA-encapsulated unipolar femoral head was attached to the trunnion of the stem and the hip was then reduced. The wound was closed in a standard fashion.
Fig. 1A–B.
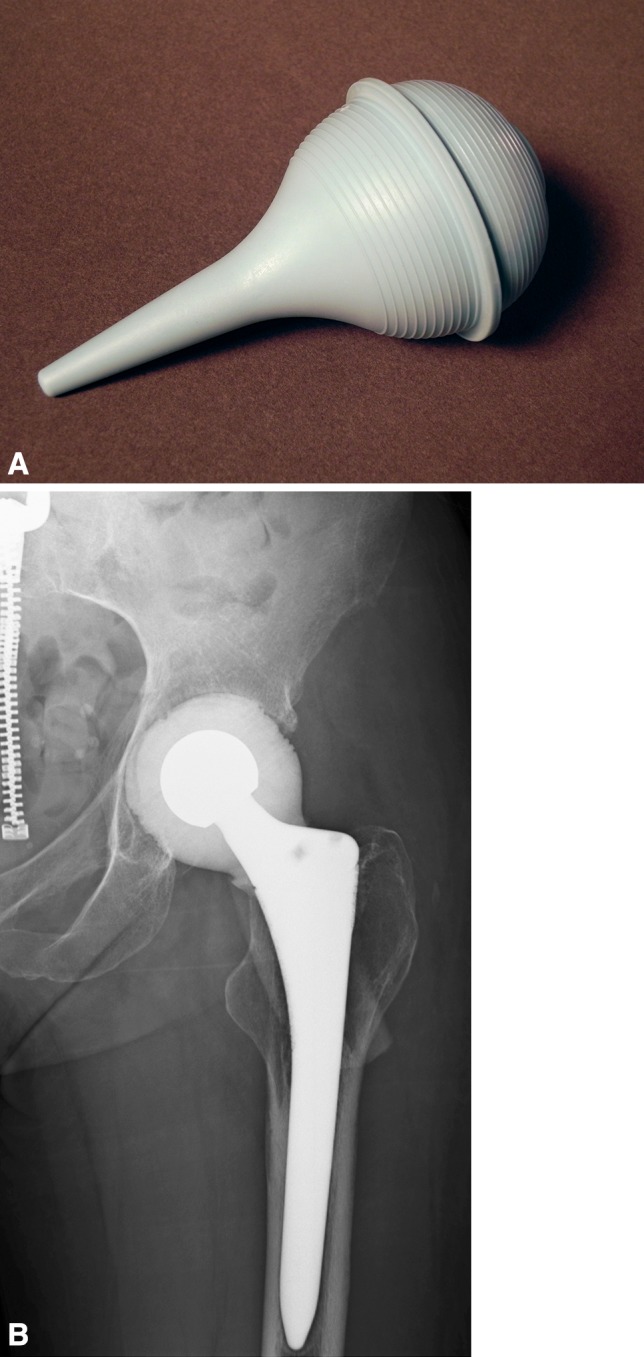
(A) One method of fabricating the antibiotic-laden acrylic cement articulating spacer was to use a pediatric ear and ulcer syringe (outer diameter: 44 mm; CR Bard, Inc, Covington, GA, USA) as a mold. (B) Radiograph reveals an interim articulating cement spacer molded with a pediatric ear and ulcer syringe.
Fig. 2A–B.
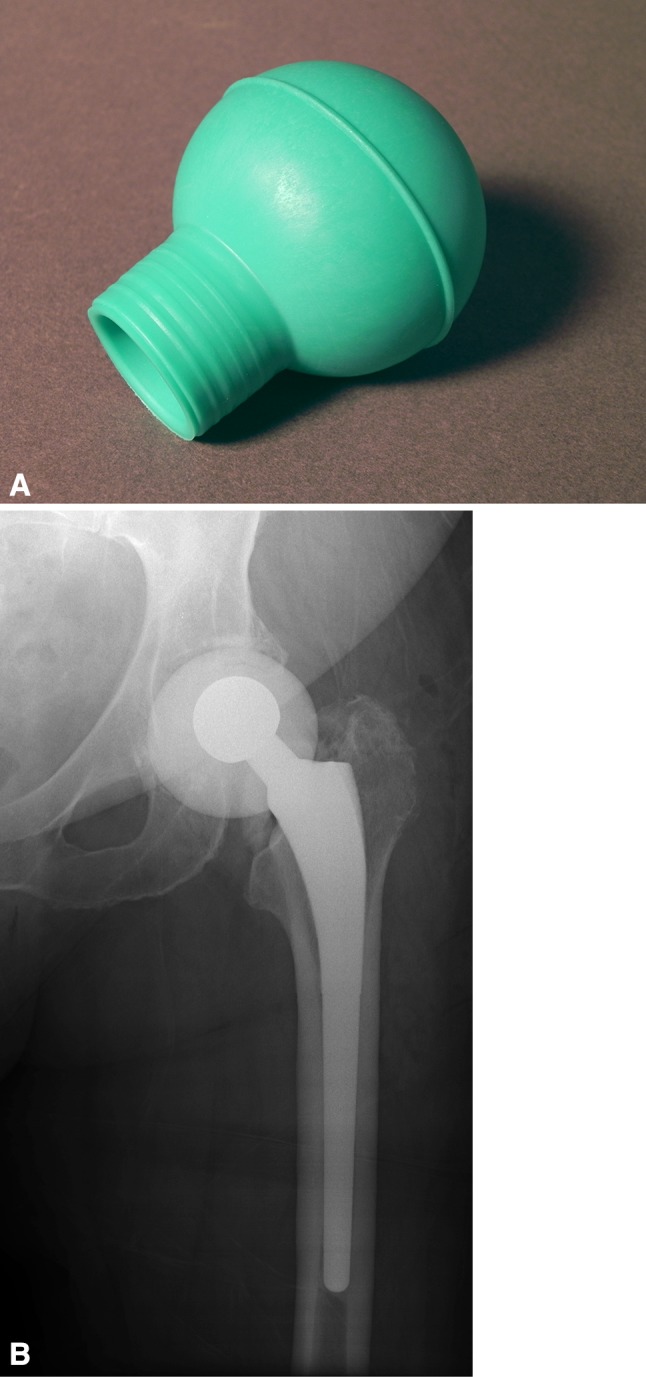
(A) To fabricate a larger articulating spacer (outer diameter, 52 mm), a bulb-type irrigation syringe (CR Bard, Inc) may be used to mold the antibiotic-laden cement. (B) Radiograph reveals an interim articulating cement spacer molded with a bulb-type irrigation syringe.
Fig. 3A–B.
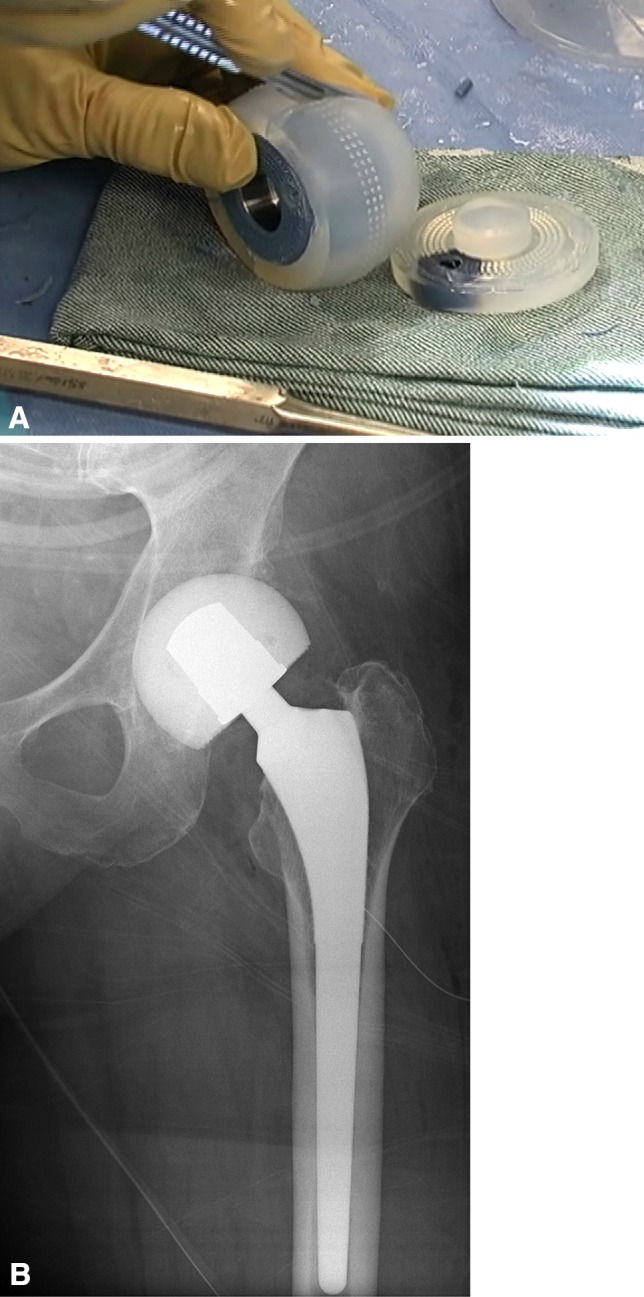
(A) More recently, disposable molds adaptable for partial radical débridement (StageOne Select; Biomet, Warsaw, IN, USA) have become available to fabricate the articulating antibiotic-laden cement spacer. (B) Radiograph reveals an interim articulating cement spacer fabricated with a disposable mold.
All patients were evaluated and managed perioperatively by the same medical consultants and infectious disease consultants. Organism-specific IV antibiotics were administered for a minimum of 6 weeks for those patients with positive cultures. For those who did not have positive cultures, we generally used a 6-week course of vancomycin as directed by the infectious disease consultant. CRP and ESR were closely monitored. The decision to perform reimplantation was made in collaboration with the medical consultant, the infectious disease consultant, and the orthopaedic surgeon. Criteria for reimplantation included stable medical condition, appropriate response to infection treatment (diminution of the ESR, return to near normal CRP, and satisfactory wound status). After reimplantation, patients were seen in followup at 6 weeks and annually thereafter or earlier if a problem arose. Clinical evaluation was performed by either the treating surgeon or a physician assistant under his direction and included the Harris hip score [21]. No patients were placed on long-term suppressive antibiotics.
In this case series, we defined failure as recurrence of infection in the same hip or the use of long-term suppressive antibiotics.
There were 10 male and nine female patients. Patient age averaged 62 years (range, 32–80 years). Patient body mass index averaged 30 kg/m2 (range, 18–51 kg/m2). Surgical procedures before infection were primary hip arthroplasty in seven patients, conversion in one, revision in eight, and reimplantation after two-stage treatment of infection in three. All patients included in this study with the exception of one had at least one medical comorbidity (Table 3). For the femoral stems left in situ with partial two-stage exchange, the design was primary in 11 and revision in eight, and the fixation was cementless in 15 and cemented in four (Table 3). Three patients had a well-functioning TKA with a long intramedullary stem in the ipsilateral femur (Fig. 4).
Table 3.
Patient comorbidities
| Patient number | Age (years) | Sex | Comorbidities | Infectious organism | Femoral implant/characteristics |
|---|---|---|---|---|---|
| 1 | 78 | Female | History of myocardial infarction, coronary artery disease, hypertension, hypothyroid, glaucoma | Nonspecific coagulase-negative Staphylococcus | Versys (Zimmer, Warsaw, IN, USA), beaded midcoat, distal splines and flutes |
| 2 | 57 | Female | Hypertension, anxiety, insomnia, sleep apnea | Methicillin-resistant Staphylococcus aureus | Versys fiber metal taper (Zimmer), proximal fiber metal, middle grit-blasted |
| 3 | 62 | Female | Breast cancer, diabetes, arrhythmia, fibromyalgia, asthma, osteoporosis, hypothyroid, anxiety, depression | Staphylococcus epidermidis, Anaerococcus prevotii | Mallory-Head porous (Biomet, Warsaw, IN, USA), proximal plasma-sprayed, middle grit-blasted |
| 4 | 69 | Male | Hypertension, diabetes, hyperlipidemia, sleep apnea | Culture-negative | Mallory-Head porous, proximal plasma-sprayed, middle grit-blasted |
| 5 | 61 | Female | None | S epidermidis | Mallory-Head calcar (Biomet) revision stem, proximal plasma-sprayed |
| 6 | 68 | Male | History of myocardial infarction, coronary artery disease, coronary artery bypass graft, hypertension, hyperlipidemia, sleep apnea, gastroesophageal reflux disease | Streptococcus bovis | Mallory-Head calcar revision stem, proximal plasma-sprayed |
| 7 | 75 | Male | Hypertension, diabetes, chronic renal disease, sleep apnea | S epidermidis | Mallory-Head porous, proximal plasma-sprayed, middle grit-blasted; ipsilateral long-stem revision knee |
| 8 | 78 | Female | Hypertension, heart murmur, hyperlipidemia, history of acute renal insufficiency (resolved) | Culture-negative | Anatomic Medullary Locking (AML; DePuy, Warsaw, IN, USA), extensively porous coated with Porocoat®; ipsilateral long stem revision knee |
| 9 | 52 | Female | Hypertension, heart murmur, peptic ulcers, gastroesophageal reflux disease | Nonspecific coagulase-negative Staphylococcus | Mallory-Head porous, proximal plasma-sprayed, middle grit-blasted; ipsilateral long-stem revision knee |
| 10 | 44 | Male | Hypertension, benign breast mass, gastroesophageal reflux disease | Beta hemolytic Streptococcus group B, methicillin-resistant S aureus | Mallory-Head calcar revision stem, proximal plasma-sprayed |
| 11 | 78 | Female | Colon cancer, chronic anemia, chronic renal insufficiency, chronic obstructive pulmonary disorder, osteoporosis | Beta hemolytic Streptococcus group B | LINK (Lubinus) MP (manufactured by Waldemar Link GmbH, Hamburg, Germany, distributed by Wright Medical Technology, Arlington, TN, USA) modular reconstruction stem, porous coated with 70-μm microporous texture |
| 12 | 45 | Male | Anxiety, shortness of breath with mild exertion | Culture-negative | Mallory-Head modular calcar (Biomet) revision stem, proximal plasma-sprayed |
| 13 | 80 | Male | Hypertension, hyperlipidemia, prostate cancer, gastroesophageal reflux disease | Yersinia enterocolitica | Mallory-Head porous, proximal plasma-sprayed, middle grit-blasted |
| 14 | 72 | Male | Hypertension, gastroesophageal reflux disease, hyperlipidemia, sleep apnea, peptic ulcers | Culture-negative | Mallory-Head porous, proximal plasma-sprayed, middle grit-blasted |
| 15 | 55 | Female | Hypertension, sleep apnea, rheumatoid arthritis, anemia, gastroesophageal reflux disease, remote peptic ulcer, anxiety, depression | Enterococcus | Mallory-Head porous, proximal plasma-sprayed, middle grit-blasted |
| 16 | 39 | Female | Rheumatoid arthritis | S epidermidis | Mallory-Head porous, proximal plasma-sprayed, middle grit-blasted |
| 17 | 32 | Male | Juvenile rheumatoid arthritis, gastroesophageal reflux disease | S aureus | Mallory-Head calcar revision stem, proximal plasma-sprayed |
| 18 | 65 | Male | Hypertension, hyperlipidemia, abdominal aortic aneurysm, peripheral vascular disease, chronic obstructive pulmonary disorder, asthma, coronary artery disease, coronary artery bypass graft, congestive heart failure, anxiety, depression, gastroesophageal reflux disease, asbestosis | Gram-positive Streptococcus | Medallion modular (Biomet) revision stem, proximal plasma-sprayed |
| 19 | 43 | Male | Chronic pain syndrome, chronic bronchitis, anxiety, gastroesophageal reflux disease | Methicillin-resistant S aureus | Mallory-Head calcar revision stem, proximal plasma-sprayed |
Fig. 4.
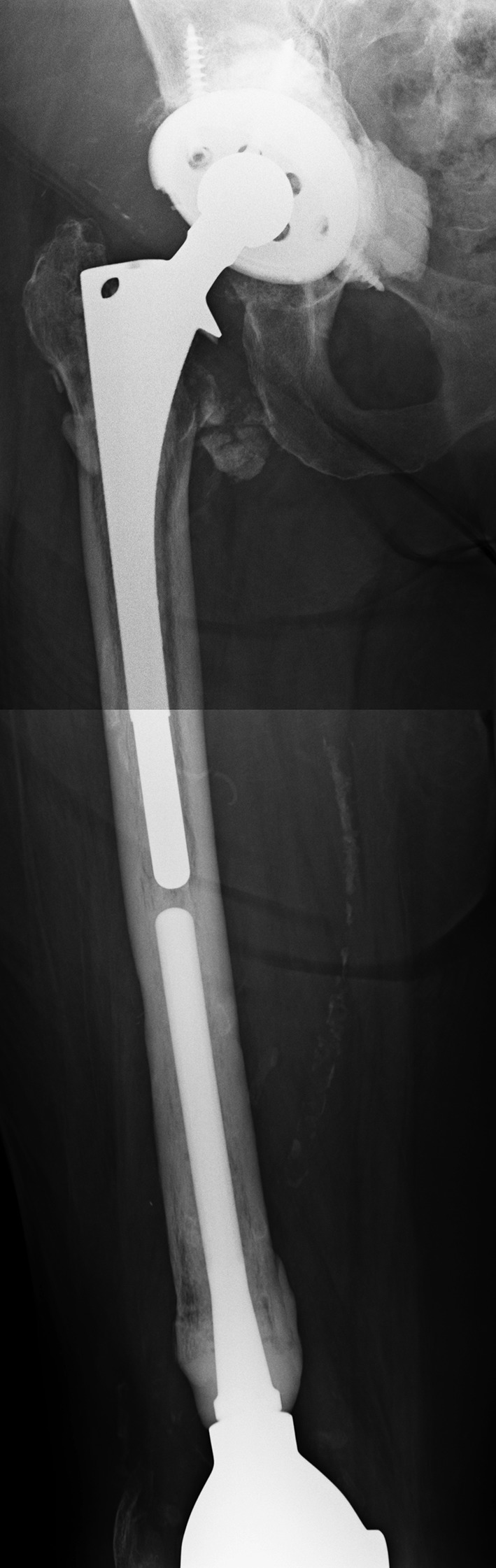
Three patients in our study presented with a well-functioning TKA with a long intramedullary stem ipsilateral to their infected THA, as shown in this example. In such scenarios, removal of the THA femoral component would create catastrophic bone loss.
Profile of Identified Organisms
One or more organisms were positively identified from aspiration in 15 of 19 patients (79%). There was no organism identified in four of 19 patients (21%). Details of the diagnosis of infection in these patients are summarized (Table 4). Gram-positive Staphylococcus and Streptococcus species accounted for the majority of organisms cultured, present in 13 patients (68%), two of whom were polymicrobial. Staphylococcus aureus was present in four (21%), three of which were methicillin-resistant (16%). Nonspecific coagulase-negative Staphylococcus was cultured in two patients (11%), Staphylococcus epidermidis in four (21%), Streptococcus species in four (21%), and one (7%) each Enterococcus, Yersinia enterocolitica, and Anaerococcus prevotii. Organisms identified at the time of partial radical débridement in the two patients who later failed secondary to recurrence were methicillin-resistant S aureus and Streptococcus species.
Table 4.
Diagnosis of periprosthetic joint infection in patients with negative cultures
| Patient number | Age (years) | Sex | BMI (kg/m2) | Comorbidities | Prior hip surgery | Diagnosis of periprosthetic joint infection | Outcome |
|---|---|---|---|---|---|---|---|
| 4 | 69 | Male | 39 | Smoker, hypertension, diabetes, hyperlipidemia, Sleep apnea | Primary metal-on-metal THA | Elevated ESR, elevated CRP, elevated synovial WBC, elevated synovial PMN%, gross purulence | Success |
| 8 | 73 | Female | 33 | Hypertension, heart murmur, hyperlipidemia, history of acute renal insufficiency (resolved) | Primary metal-on-polyethylene THA | Elevated ESR, elevated CRP, elevated synovial WBC, elevated synovial PMN%, gross purulence | Success |
| 12 | 45 | Male | 31 | Anxiety, shortness of breath with mild exertion | Second revision THA | Elevated ESR, elevated CRP, elevated synovial WBC, elevated synovial PMN%, gross purulence | Success |
| 14 | 72 | Male | 32 | History of peptic ulcers, hypertension, gastroesophageal reflux disease, hyperlipidemia, sleep apnea | Primary metal-on-metal THA | Elevated ESR, elevated CRP, elevated synovial WBC, elevated synovial PMN%, gross purulence | Success |
BMI = body mass index; ESR = erythrocyte sedimentation rate; CRP = C-reactive protein; WBC = white blood cell count; PMN% = percentage of polymorphonuclear lymphocytes.
This study was approved by our institutional review board. All drugs and devices used have been approved by the US Food and Drug Administration. However, the use of high doses of antibiotics added to bone cement to form the femoral head is a clinician-directed application.
Results
Our recurrence rate with partial two-stage exchange after chronic PJI of the hip was 11% (two of 19). There have been two failures at an average time of 3.5 years. Both patients had failed prior two-stage exchange arthroplasty for infection before undergoing partial two-stage exchange. Failure 1, a 43-year-old male patient, had 15 prior hip procedures secondary to trauma and history of methicillin-resistant S aureus (MRSA) infection before partial two-stage exchange with intraoperative cultures again positive for MRSA. He required incision and débridement for infection at 1.3 years after reimplantation followed by girdlestone arthroplasty at 3.7 years. He continues on suppressive antibiotics. Failure 2, a 65-year-old man with four prior hip procedures secondary to trauma and infection, had cultures positive for Gram-positive Streptococcus species at the time of partial radical débridement. He sustained a periprosthetic femoral fracture 1.8 years after reimplantation that was treated with open reduction and internal fixation. At 3 years postoperatively, he had recurrence of infection that required complete radical débridement and reimplantation with a total femur replacement. Currently, he is doing well without recurrence of infection. There were no additional surgical procedures performed on the remaining 17 patients after reimplantation THA.
There were three patient deaths during the study period at an average of 3.5 years postoperatively (range, 1.4–6.5 years). None occurred perioperatively or within 90 days postoperatively. No patient deaths were believed to be related to the arthroplasty or as a result of infection. The time interval between partial radical débridement and reimplantation averaged 8.4 weeks (range, 5–19 weeks).
The mean Harris hip score (HHS) was 68 (range, 31–100; 0–100 possible) at most recent followup and the mean HHS pain component was 32 (range, 10–44; 0–44 possible, higher scores indicating less pain).
Discussion
Although specialized instrumentation has been developed to facilitate removal of a well-fixed acetabular component with minimal bone loss, removal of a well-fixed cemented or cementless femoral component can result in considerable bone loss rendering subsequent reconstruction extremely difficult [10, 25, 42, 44, 73]. To our knowledge only one other study has evaluated a partial two-stage method of treatment similar to the one we investigated in the current study [38]. In this report, we observed an 11% recurrence rate, no deaths believed to be associated with treatment, and a mean HHS of 68, which is in the qualitative range of “fair.”
Our study has several limitations. The first is that this study is a retrospective case series rather than a prospective, randomized comparative trial. This introduces the possibility of several kinds of bias, most notably selection bias. However, we believe that selection bias was minimal. The decision to proceed with partial radical débridement was determined by the two senior surgeons based on criteria of a well-fixed stem in which removal would compromise future fixation. These surgeons are well experienced in both primary and complex revision hip arthroplasty. Complex operative procedures are always reviewed collaboratively before surgical intervention and a treatment protocol is developed. Despite this careful consideration, other reconstructive surgeons could disagree with the difficulty of removing these femoral stems. The second shortcoming of this analysis is that the definition of well fixed and compromise of future fixation secondary to removal of the components was determined by the surgeon at the time of presentation and operative intervention. The definition used by both surgeons was cemented, grit-blasted stems without evidence of prosthetic-cement or cement-bone radiolucencies or cementless, extensively porous-coated and/or grit-blasted stems with evidence of complete osseointegration. The third limitation of this study is the small sample size. This is a result of the use of strict indications to apply this technique; larger studies from other centers would be needed to confirm these results before we could recommend it for wider use. The fourth limitation is that not all patients had positive cultures. Although all patients met the criteria established by the Musculoskeletal Infection Society for a periprosthetic infection, an organism was not identified in four patients. A recent study reported that despite extensive efforts including adequate clinical, radiographic, and intraoperative suspicion for PJI, cultures often have a high false-negative rate, and culture-negative PJI is reported to occur in 7% to 9.5% of all infected arthroplasties [26]. Even if we presume that the four culture-negative patients were not truly infected and eliminate their results, our success rate is 87% versus 89% if included.
Our infection recurrence rate of 11% was comparable to that seen in other accepted techniques for the treatment of periprosthetic joint infection (Tables 1, 2). This recurrence rate compares favorably with our previously published data on two-stage exchange in which the eradication rate at 53 months was 83% [1]. Furthermore, Engesæter et al. [12] summarized the results of treatment of 784 infected THAs as reported to the Norwegian Arthroplasty Register. The reported success rate with the end point of revision for infection was 96% for those treated by two-stage exchange, 92% for one-stage whole exchange, 74% for major partial one-stage exchange, and 80% for minor partial one-stage exchange. Our results compare favorably with their two-stage and one-stage whole exchange results and have a greater success rate than major partial or minor partial one-stage exchange. In another small clinical series by Morley et al., 15 patients underwent removal of both the femoral and acetabular components for PJI with retention of the intact femoral cement mantle [46]. An antibiotic-laden cement spacer was placed within the acetabulum and antibiotic-laden cement beads were placed in the femoral canal. Patients were treated with IV antibiotics and delayed reimplantation. The authors reported success in 14 of 15 patients (93%) and concluded the technique was appropriate and efficacious. Lastly in a recently published study by Lee et al. [37], the authors looked to determine whether infection after hip arthroplasty could be treated without removal of a well-fixed stem. They treated 17 acute hematogenous and chronic hip infections with removal of the acetabular implant and retention of the stem followed by second-stage reconstruction of the acetabulum. At mean followup of 4 years (range, 2–8 years), 15 of the 17 (88%) demonstrated no recurrence of infection. Our results in treating chronic PJI with partial two-stage exchange are comparable with those using similar methods. These studies may suggest that when attempting to eradicate infection by removal of one component, a two-stage procedure is required with local delivery of antibiotics in the soft tissues secondary to the antibiotic-laden acrylic spacer. However, further studies will be required to validate this implication.
The perioperative mortality rate for patients treated with two-stage revision surgery of the hip for periprosthetic joint infection is both underreported and often overshadowed by infection control results after surgery. Few authors have reported the 90-day mortality rate in this subset of patients. Mortality associated with the two-stage treatment of periprosthetic hip infection appears to be high both in the perioperative period and also within the followup interval. Toulson et al. [65] reported a 26% rate of death before 2-year followup in their series of two-stage treatment of 132 infected THAs performed between 1989 and 2003. In a previous study from our center, we retrospectively reviewed and reported our results in 205 patients undergoing two-stage treatment of infected THA [1]. We reported a 90-day mortality rate of 4% with an overall mortality rate of 45% during the study period. In our current study, there were three patient deaths at an average of 3.5 years. None occurred perioperatively or within 90 days. Our 90-day mortality rate was 0% (zero of 19) and overall mortality rate was 16% (three of 19). This is considerably lower than in previously published studies; however, the current study has a smaller sample size.
The HHS is a useful tool for evaluation of outcomes of THA [21]. The mean HHS after revision for infection has been reported to average 88 for single-stage exchange and 76 for two-stage exchange arthroplasty in a single prospective study evaluating outcomes of revision THA for infection using a standard protocol [51]. The mean HHS in the current study after reimplantation was 68. Although this is lower than what is reported in the literature, it is slightly better than the mean HHS of 65 reported from our center for patients treated with full two-stage exchange arthroplasty [1].
Our data show that treatment of chronic PJI with partial two-stage exchange arthroplasty with radical débridement of the acetabular component and retention of the well-fixed cemented or cementless femoral component can be effective in eradication of infection with results comparable to other treatments used for these difficult cases. Retention of the femoral component preserves proximal femoral bone stock, decreases morbidity for the patient, and lessens reconstructive complexity at the time of the second-stage revision. Given our favorable results, we believe partial two-stage exchange may represent an acceptable option for patients with infected THA when femoral component removal would result in significant bone loss and compromise of reconstruction. However, further study is required on this method of treatment.
Footnotes
The institution (Joint Implant Surgeons, Inc, New Albany, OH, USA) of one or more of the authors has received funding from Biomet Inc (Warsaw, IN, USA) and Stryker Orthopaedics (Mahwah, NJ, USA) (TEE, KRB, MJM, JBA, AVL). One of the authors certifies that he (AVL) has or may receive payments or benefits, during the study period, an amount of more than USD 1,000,001 from Biomet Inc, and an amount of USD 10,000 to USD 100,000 from Innomed, Inc (Savannah, GA, USA). One of the authors certifies that he (KRB) has or may receive payments or benefits, during the study period, an amount of more than USD 1,000,001 from Biomet Inc. One of the authors certifies that he (MJM) has or may receive payments or benefits, during the study period, an amount of USD 10,000 to USD 100,000 from Biomet Inc.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Joint Implant Surgeons, Inc, New Albany, OH, USA.
References
- 1.Berend KR, Lombardi AV, Jr, Morris MJ, Bergeson AG, Adams JB, Sneller MA. Two-stage treatment of hip periprosthetic joint infection is associated with a high rate of infection control but high mortality. Clin Orthop Relat Res. 2013;471:510–518. doi: 10.1007/s11999-012-2595-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berry DJ, Chandler HP, Reilly DT. The use of bone allografts in two-stage reconstruction after failure of hip replacements due to infection. J Bone Joint Surg Am. 1991;73:1460–1468. [PubMed] [Google Scholar]
- 3.Bozic KJ, Ries MD. The impact of infection after total hip arthroplasty on hospital and surgeon resource utilization. J Bone Joint Surg Am. 2005;87:1746–1751. doi: 10.2106/JBJS.D.02937. [DOI] [PubMed] [Google Scholar]
- 4.Buttaro MA, Pusso R, Piccaluga F. Vancomycin-supplemented impacted bone allografts in infected hip arthroplasty. Two-stage revision results. J Bone Joint Surg Br. 2005;87:314–319. doi: 10.1302/0301-620X.87B3.14788. [DOI] [PubMed] [Google Scholar]
- 5.Cabrita HB, Croci AT, Camargo OP, Lima AL. Prospective study of the treatment of infected hip arthroplasties with or without the use of an antibiotic-loaded cement spacer. Clinics (Sao Paulo). 2007;62:99–108. doi: 10.1590/S1807-59322007000200002. [DOI] [PubMed] [Google Scholar]
- 6.Callaghan JJ, Katz RP, Johnston RC. One-stage revision surgery of the infected hip. A minimum 10-year followup study. Clin Orthop Relat Res. 1999;369:139–143. doi: 10.1097/00003086-199912000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Cordero-Ampuero J, Esteban J, García-Cimbrelo E. Oral antibiotics are effective for highly resistant hip arthroplasty infections. Clin Orthop Relat Res. 2009;467:2335–2342. doi: 10.1007/s11999-009-0808-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dairaku K, Takagi M, Kawaji H, Sasaki K, Ishii M, Ogino T. Antibiotics-impregnated cement spacers in the first step of two-stage revision for infected totally replaced hip joints: report of ten trial cases. J Orthop Sci. 2009;14:704–710. doi: 10.1007/s00776-009-1406-z. [DOI] [PubMed] [Google Scholar]
- 9.Darley ES, Bannister GC, Blom AW, Macgowan AP, Jacobson SK, Alfouzan W. Role of early intravenous to oral antibiotic switch therapy in the management of prosthetic hip infection treated with one- or two-stage replacement. J Antimicrob Chemother. 2011;66:2405–2408. doi: 10.1093/jac/dkr277. [DOI] [PubMed] [Google Scholar]
- 10.Della Valle CJ, Paprosky WG. Classification and an algorithmic approach to the reconstruction of femoral deficiency in revision total hip arthroplasty. J Bone Joint Surg Am. 2003;85(Suppl 4):1–6. [DOI] [PubMed]
- 11.Drancourt M, Stein A, Argenson JN, Zannier A, Curvale G, Raoult D. Oral rifampin plus ofloxacin for treatment of Staphylococcus-infected orthopedic implants. Antimicrob Agents Chemother. 1993;37:1214–1218. doi: 10.1128/AAC.37.6.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engesæter LB, Dale H, Schrama JC, Hallan G, Lie SA. Surgical procedures in the treatment of 784 infected THAs reported to the Norwegian Arthroplasty Register. Acta Orthop. 2011;82:530–537. doi: 10.3109/17453674.2011.623572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erhart J, Jaklitsch K, Schurz M, Vécsei V, Ehall R. Cementless two-staged total hip arthroplasty with a short term interval period for chronic deep periprosthetic infection. Technique and long-term results. Wien Klin Wochenschr. 2010;122:303–310. doi: 10.1007/s00508-010-1372-7. [DOI] [PubMed] [Google Scholar]
- 14.Evans RP. Successful treatment of total hip and knee infection with articulating antibiotic components: a modified treatment method. Clin Orthop Relat Res. 2004;427:37–46. doi: 10.1097/01.blo.0000143739.07632.7c. [DOI] [PubMed] [Google Scholar]
- 15.Fehring TK, Calton TF, Griffin WL. Cementless fixation in 2-stage reimplantation for periprosthetic sepsis. J Arthroplasty. 1999;14:175–181. doi: 10.1016/S0883-5403(99)90122-5. [DOI] [PubMed] [Google Scholar]
- 16.Fink B, Rechtenbach A, Büchner H, Vogt S, Hahn M. Articulating spacers used in two-stage revision of infected hip and knee prostheses abrade with time. Clin Orthop Relat Res. 2011;469:1095–1102. doi: 10.1007/s11999-010-1479-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao H, Lv H. [One-stage revision operations for infection after hip arthroplasty] [in Chinese] Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2008;22:5–8. [PubMed] [Google Scholar]
- 18.García S, Soriano A, Esteban P, Almela M, Gallart X, Mensa J. [Usefulness of adding antibiotic to cement in one stage exchange of chronic infection in total hip arthroplasty] [in Spanish] Med Clin (Barc). 2005;125:138–139. doi: 10.1157/13076942. [DOI] [PubMed] [Google Scholar]
- 19.Hanssen AD, Rand JA. Evaluation and treatment of infection at the site of a total hip or knee arthroplasty. Instr Course Lect. 1999;48:111–122. [PubMed] [Google Scholar]
- 20.Hanssen AD, Spangehl MJ. Treatment of the infected hip replacement. Clin Orthop Relat Res. 2004;420:63–71. doi: 10.1097/00003086-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed] [Google Scholar]
- 22.Hofmann AA, Goldberg TD, Tanner AM, Cook TM. Ten-year experience using an articulating antibiotic cement hip spacer for the treatment of chronically infected total hip. J Arthroplasty. 2005;20:874–879. doi: 10.1016/j.arth.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh PH, Huang KC, Lee PC, Lee MS. Two-stage revision of infected hip arthroplasty using an antibiotic-loaded spacer: retrospective comparison between short-term and prolonged antibiotic therapy. J Antimicrob Chemother. 2009;64:392–397. doi: 10.1093/jac/dkp177. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh PH, Shih CH, Chang YH, Lee MS, Shih HN, Yang WE. Two-stage revision hip arthroplasty for infection: comparison between the interim use of antibiotic-loaded cement beads and a spacer prosthesis. J Bone Joint Surg Am. 2004;86:1989–1997. [PubMed] [Google Scholar]
- 25.Hsieh PH, Shih CH, Chang YH, Lee MS, Yang WE, Shih HN. Treatment of deep infection of the hip associated with massive bone loss: two-stage revision with an antibiotic-loaded interim cement prosthesis followed by reconstruction with allograft. J Bone Joint Surg Br. 2005;87:770–775. doi: 10.1302/0301-620X.87B6.15411. [DOI] [PubMed] [Google Scholar]
- 26.Huang R, Hu CC, Adeli B, Mortazavi J, Parvizi J. Culture-negative periprosthetic joint infection does not preclude infection control. Clin Orthop Relat Res. 2012;470:2717–2723. doi: 10.1007/s11999-012-2434-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Incavo SJ, Russell RD, Mathis KB, Adams H. Initial results of managing severe bone loss in infected total joint arthroplasty using customized articulating spacers. J Arthroplasty. 2009;24:607–613. doi: 10.1016/j.arth.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 28.Isiklar ZU, Demirörs H, Akpinar S, Tandogan RN, Alparslan M. Two-stage treatment of chronic staphylococcal orthopaedic implant-related infections using vancomycin impregnated PMMA spacer and rifampin containing antibiotic protocol. Bull Hosp Jt Dis. 1999;58:79–85. [PubMed] [Google Scholar]
- 29.Jackson WO, Schmalzried TP. Limited role of direct exchange arthroplasty in the treatment of infected total hip replacements. Clin Orthop Relat Res. 2000;381:101–105. doi: 10.1097/00003086-200012000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Jahoda D, Sosna A, Landor I, Vavrík P, Pokorný D, Hudec T. [Two-stage reimplantation using spacers—the method of choice in treatment of hip joint prosthesis-related infections. Comparison with methods used from 1979 to 1998] [in Czech] Acta Chir Orthop Traumatol Cech. 2003;70:17–24. [PubMed] [Google Scholar]
- 31.Karpas K, Sponer P. Management of the infected hip arthroplasty by two-stage reimplantation. Acta Medica (Hradec Kralove). 2003;46:113–115. [PubMed] [Google Scholar]
- 32.Klouche S, Leonard P, Zeller V, Lhotellier L, Graff W, Leclerc P, Mamoudy P, Sariali E. Infected total hip arthroplasty revision: one- or two-stage procedure? Orthop Traumatol Surg Res. 2012;98:144–150. doi: 10.1016/j.otsr.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 33.Koo KH, Yang JW, Cho SH, Song HR, Park HB, Ha YC, Chang JD, Kim SY, Kim YH. Impregnation of vancomycin, gentamicin, and cefotaxime in a cement spacer for two-stage cementless reconstruction in infected total hip arthroplasty. J Arthroplasty. 2001;16:882–892. doi: 10.1054/arth.2001.24444. [DOI] [PubMed] [Google Scholar]
- 34.Kraay MJ, Goldberg VM, Fitzgerald SJ, Salata MJ. Cementless two-staged total hip arthroplasty for deep periprosthetic infection. Clin Orthop Relat Res. 2005;441:243–249. doi: 10.1097/01.blo.0000194312.97098.0a. [DOI] [PubMed] [Google Scholar]
- 35.Lai KA, Shen WJ, Yang CY, Lin RM, Lin CJ, Jou IM. Two-stage cementless revision THR after infection. 5 recurrences in 40 cases followed 2.5-7 years. Acta Orthop Scand. 1996;67:325–328. doi: 10.3109/17453679609002324. [DOI] [PubMed] [Google Scholar]
- 36.Lee PT, Clayton RA, Safir OA, Backstein DJ, Gross AE. Structural allograft as an option for treating infected hip arthroplasty with massive bone loss. Clin Orthop Relat Res. 2011;469:1016–1023. doi: 10.1007/s11999-010-1673-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee YK, Lee KH, Nho JH, Ha YC, Koo KH. Retaining well-fixed cementless stem in the treatment of infected hip arthroplasty. Good results in 19 patients followed for mean 4 years. Acta Orthop. 2013;84:260–264. doi: 10.3109/17453674.2013.795830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leone JM, Hanssen AD. Management of infection at the site of a total knee arthroplasty. J Bone Joint Surg Am. 2005;87:2335–2348. doi: 10.2106/00004623-200510000-00026. [DOI] [PubMed] [Google Scholar]
- 39.Leung F, Richards CJ, Garbuz DS, Masri BA, Duncan CP. Two-stage total hip arthroplasty: how often does it control methicillin-resistant infection? Clin Orthop Relat Res. 2011;469:1009–1015. doi: 10.1007/s11999-010-1725-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lieberman JR, Callaway GH, Salvati EA, Pellicci PM, Brause BD. Treatment of the infected total hip arthroplasty with a two-stage reimplantation protocol. Clin Orthop Relat Res. 1994;301:205–212. [PubMed] [Google Scholar]
- 41.Lim SJ, Park JC, Moon YW, Park YS. Treatment of periprosthetic hip infection caused by resistant microorganisms using 2-stage reimplantation protocol. J Arthroplasty. 2009;28:1264–1269. doi: 10.1016/j.arth.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 42.Lombardi AV, Jr, Berend KR. The shattered femur: radical solution options. J Arthroplasty. 2006;21(Suppl 1):107–111. doi: 10.1016/j.arth.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Magnan B, Regis D, Biscaglia R, Bartolozzi P. Preformed acrylic bone cement spacer loaded with antibiotics: use of two-stage procedure in 10 patients because of infected hips after total replacement. Acta Orthop Scand. 2001;72:591–594. doi: 10.1080/000164701317269003. [DOI] [PubMed] [Google Scholar]
- 44.Maurer SG, Baitner AC, Di Cesare PE. Reconstruction of the failed femoral component and proximal femoral bone loss in revision hip surgery. J Am Acad Orthop Surg. 2000;8:354–363. doi: 10.5435/00124635-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 45.McDonald DJ, Fitzgerald RH, Jr, Ilstrup DM. Two-stage reconstruction of a total hip arthroplasty because of infection. J Bone Joint Surg Am. 1989;71:828–834. [PubMed] [Google Scholar]
- 46.Morley JR, Blake SM, Hubble MJ, Timperley AJ, Gie GA, Howell JR. Preservation of the original femoral cement mantle during the management of infected cemented total hip replacement by two-stage revision. J Bone Joint Surg Br. 2012;94:322–327. doi: 10.1302/0301-620X.94B3.28256. [DOI] [PubMed] [Google Scholar]
- 47.Mulcahy DM, O’Byrne JM, Fenelon GE. One stage surgical management of deep infection of total hip arthroplasty. Ir J Med Sci. 1996;165:17–19. doi: 10.1007/BF02942793. [DOI] [PubMed] [Google Scholar]
- 48.Nestor BJ, Hanssen AD, Ferrer-Gonzalez R, Fitzgerald RH., Jr The use of porous prostheses in delayed reconstruction of total hip replacements that have failed because of infection. J Bone Joint Surg Am. 1994;76:349–359. doi: 10.2106/00004623-199403000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Neumann DR, Hofstaedter T, List C, Dorn U. Two-stage cementless revision of late total hip arthroplasty infection using a premanufactured spacer. J Arthroplasty. 2012;27:1397–1401. doi: 10.1016/j.arth.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 50.Nusem I, Morgan DA. Structural allografts for bone stock reconstruction in two-stage revision for infected total hip arthroplasty: good outcome in 16 of 18 patients followed for 5–14 years. Acta Orthop. 2006;77:92–97. doi: 10.1080/17453670610045740. [DOI] [PubMed] [Google Scholar]
- 51.Oussedik SI, Dodd MB, Haddad FS. Outcomes of revision total hip replacement for infection after grading according to a standard protocol. J Bone Joint Surg Br. 2010;92:1222–1226. doi: 10.1302/0301-620X.92B9.23663. [DOI] [PubMed] [Google Scholar]
- 52.Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469:2992–2994. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raut VV, Orth MS, Orth MC, Siney PD, Wroblewski BM. One stage revision arthroplasty of the hip for deep Gram negative infection. Int Orthop. 1996;20:12–14. doi: 10.1007/s002640050019. [DOI] [PubMed] [Google Scholar]
- 54.Raut VV, Siney PD, Wroblewski BM. One-stage revision of infected total hip replacements with discharging sinuses. J Bone Joint Surg Br. 1994;76:721–724. [PubMed] [Google Scholar]
- 55.Raut VV, Siney PD, Wroblewski BM. One-stage revision of total hip arthroplasty for deep infection. Long-term followup. Clin Orthop Relat Res. 1995;321:202–207. [PubMed] [Google Scholar]
- 56.Romanò CL, Romanò D, Albisetti A, Meani E. Preformed antibiotic-loaded cement spacers for two-stage revision of infected total hip arthroplasty. Long-term results. Hip Int. 2012;22(Suppl 8):S46–S53. doi: 10.5301/HIP.2012.9570. [DOI] [PubMed] [Google Scholar]
- 57.Romanò CL, Romanò D, Logoluso N, Meani E. Long-stem versus short-stem preformed antibiotic-loaded cement spacers for two-stage revision of infected total hip arthroplasty. Hip Int. 2010;20:26–33. doi: 10.1177/112070001002000104. [DOI] [PubMed] [Google Scholar]
- 58.Rudelli S, Uip D, Honda E, Lima AL. One-stage revision of infected total hip arthroplasty with bone graft. J Arthroplasty. 2008;23:1165–1177. doi: 10.1016/j.arth.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 59.Salvati EA, González Della Valle A, Masri BA, Duncan CP. The infected total hip arthroplasty. Instr Course Lect. 2003;52:223–245. [PubMed] [Google Scholar]
- 60.Sanchez-Sotelo J, Berry DJ, Hanssen AD, Cabanela ME. Midterm to long-term followup of staged reimplantation for infected hip arthroplasty. Clin Orthop Relat Res. 2009;467:219–224. doi: 10.1007/s11999-008-0480-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scharfenberger A, Clark M, Lavoie G, O’Connor G, Masson E, Beaupre LA. Treatment of an infected total hip replacement with the PROSTALAC system. Part 1: Infection resolution. Can J Surg. 2007;50:24–28. [PMC free article] [PubMed] [Google Scholar]
- 62.Spangehl MJ, Younger AS, Masri BA, Duncan CP. Diagnosis of infection following total hip arthroplasty. Instr Course Lect. 1998;47:285–295. [PubMed] [Google Scholar]
- 63.Stockley I, Mockford BJ, Hoad-Reddick A, Norman P. The use of two-stage exchange arthroplasty with depot antibiotics in the absence of long-term antibiotic therapy in infected total hip replacement. J Bone Joint Surg Br. 2008;90:145–148. doi: 10.1302/0301-620X.90B2.19855. [DOI] [PubMed] [Google Scholar]
- 64.Takigami I, Ito Y, Ishimaru D, Ogawa H, Mori N, Shimizu T, Terabayashi N, Shimizu K. Two-stage revision surgery for hip prosthesis infection using antibiotic-loaded porous hydroxyapatite blocks. Arch Orthop Trauma Surg. 2010;130:1221–1226. doi: 10.1007/s00402-009-0991-9. [DOI] [PubMed] [Google Scholar]
- 65.Toulson C, Walcott-Sapp S, Hur J, Salvati E, Bostrom M, Brause B, Westrich GH. Treatment of infected total hip arthroplasty with a 2-stage reimplantation protocol: update on ‘our institution’s’ experience from 1989 to 2003. J Arthroplasty. 2009;24:1051–1060. doi: 10.1016/j.arth.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 66.Tsukayama DT, Estrada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78:512–523. doi: 10.2106/00004623-199604000-00005. [DOI] [PubMed] [Google Scholar]
- 67.Walter G, Bühler M, Hoffmann R. [Two-stage procedure to exchange septic total hip arthroplasties with late periprosthetic infection. Early results after implantation of a reverse modular hybrid endoprosthesis] [in German] Unfallchirurg. 2007;110:537–546. doi: 10.1007/s00113-007-1238-2. [DOI] [PubMed] [Google Scholar]
- 68.Wang JW, Chen CE. Reimplantation of infected hip arthroplasties using bone allografts. Clin Orthop Relat Res. 1997;335:202–210. doi: 10.1097/00003086-199702000-00001. [DOI] [PubMed] [Google Scholar]
- 69.Whittaker JP, Warren RE, Jones RS, Gregson PA. Is prolonged systemic antibiotic treatment essential in two-stage revision hip replacement for chronic Gram-positive infection? J Bone Joint Surg Br. 2009;91:44–51. doi: 10.1302/0301-620X.91B1.20930. [DOI] [PubMed] [Google Scholar]
- 70.Winkler H, Stoiber A, Kaudela K, Winter F, Menschik F. One stage uncemented revision of infected total hip replacement using cancellous allograft bone impregnated with antibiotics. J Bone Joint Surg Br. 2008;90:1580–1584. doi: 10.1302/0301-620X.90B12.20742. [DOI] [PubMed] [Google Scholar]
- 71.Yamamoto K, Miyagawa N, Masaoka T, Katori Y, Shishido T, Imakiire A. Clinical effectiveness of antibiotic-impregnated cement spacers for the treatment of infected implants of the hip joint. J Orthop Sci. 2003;8:823–828. doi: 10.1007/s00776-003-0722-y. [DOI] [PubMed] [Google Scholar]
- 72.Yoo JJ, Kwon YS, Koo KH, Yoon KS, Kim YM, Kim HJ. One-stage cementless revision arthroplasty for infected hip replacements. Int Orthop. 2009;33:1195–1201. doi: 10.1007/s00264-008-0640-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Younger AS, Duncan CP, Masri BA. Treatment of infection associated with segmental bone loss in the proximal part of the femur in two stages with use of an antibiotic-loaded interval prosthesis. J Bone Joint Surg Am. 1998;80:60–69. doi: 10.2106/00004623-199801000-00011. [DOI] [PubMed] [Google Scholar]


