Abstract
Background
Periprosthetic osteolysis is the leading reason for THA revision. The relationship of serum biomarkers with severe radiographic periprosthetic osteolysis has not been defined but may be important to direct future research and clinical therapeutics.
Questions/purposes
We determined whether there was an association between measurable inflammatory markers (high-sensitivity C-reactive protein [hsCRP]) or inflammatory mediators (tumor necrosis factor α [TNF-α], IL-1β, IL-6, receptor activator of nuclear factor κB ligand [RANKL], and osteoprotegerin [OPG]) and periprosthetic osteolysis.
Methods
We identified 15 patients with THAs scheduled for revision surgery because of severe periprosthetic osteolysis. For each study patient, a nonosteolytic, pain-free control patient with THAs was identified and matched for age, sex, time since initial THA, acetabular and femoral component prosthesis material, and prosthesis wear within 1.0 mm/year using a manual wear analysis technique. Overall, the study and control patients had a mean wear rate of 0.25 mm/year since index THA. There were no differences in baseline characteristics between study and control patients in age, sex, BMI, Charlson Comorbidity Index, time since initial THA, UCLA activity score, and acetabular and femoral component type. Serum hsCRP, IL-1β, IL-6, TNF-α, RANKL, and OPG were measured by ELISA in duplicate assays. Differences in values were assessed using the Wilcoxon rank-sum test.
Results
Median TNF-α levels were higher in study patients than in controls (7.1 pg/mL [SD, 11.6 pg/mL] versus 1.5 pg/mL [SD, 1.3 pg/mL]) (p < 0.01). Median IL-6 levels tended to be higher in study patients than in controls (8.9 pg/mL [SD, 13.2 pg/mL] versus 3.5 pg/mL [SD, 0.7 pg/mL]) (p = 0.09). The other serum inflammatory proteins and mediators of bone turnover were not different between groups.
Conclusions
TNF-α is elevated in patients with osteolysis compared to matched controls. The role of TNF-α and its potential as a target of nonsurgical therapy to prevent osteolysis warrant further investigation in larger, prospective studies.
Level of Evidence
Level III, diagnostic study. See Instructions for Authors for a complete description of levels of evidence.
Introduction
Periprosthetic osteolysis is among the most common indications for revision surgery after THA [12]. At present, detection of periprosthetic osteolysis relies on visualization of the osteolysis on plain film [6], CT scan [7], or MRI [8]. Thus far, there are no reliable approaches to predict which patients are at greatest risk of prosthesis failure from osteolysis, although identifying these patients at risk would facilitate the development of targeted interventions.
Inflammatory cytokines have been associated with periprosthetic osteolysis. Animal models of osteolysis have implicated both IL-1α and tumor necrosis factor α (TNF-α) in wear-induced osteolysis [2, 20], and published in vivo studies have also shown elevated levels of IL-1, IL-6, and serum TNF-α associated with prosthesis failure [5, 9, 13].
We therefore performed a case-control study to assess whether patients with THAs with severe periprosthetic osteolysis have higher levels of high-sensitivity C-reactive protein (hsCRP), a protein associated with systemic inflammation, and the inflammatory mediators TNF-α, IL-1, IL-6, receptor activator of nuclear factor κB ligand (RANKL), and osteoprotegerin (OPG), compared to matched control patients with THAs who had no pain and no radiographic evidence of osteolysis.
Patients and Methods
Identification of Study Patients
We identified patients scheduled for THA revision surgery for osteolysis at our institution. The index THA in all of these patients had been performed for osteoarthritis. Patients with inflammatory arthritis or having a repeat THA revision were excluded. For each study patient, a control patient with THA was identified from medical records. Each control was matched to a study patient for the following characteristics: age (within 5 years), sex, time since initial THA (within 5 years), and material of the acetabular cup and femoral stem, including cemented or noncemented components of the cup and stem. Controls were required to have no evidence of radiographic osteolysis at the site of the prosthesis and no prosthetic hip pain on the side of their prosthesis at the time of study enrollment.
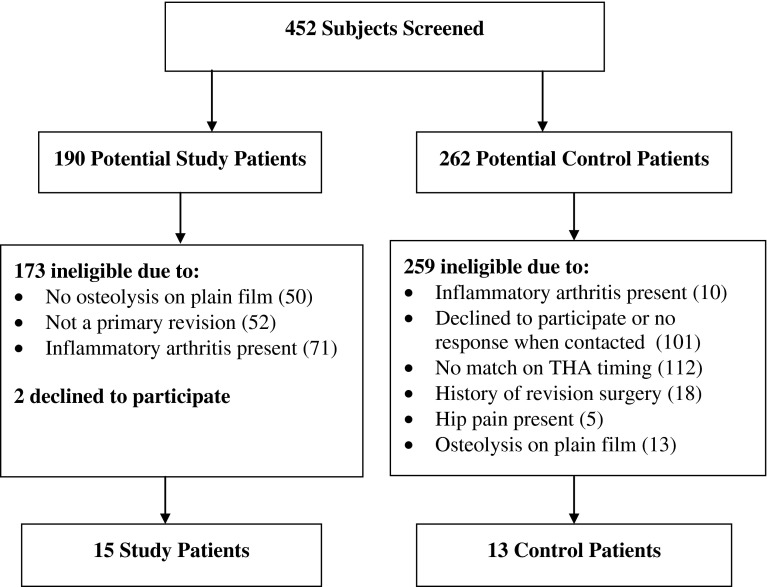
We screened 452 patients: 190 potential study patients and 262 potential controls (Fig. 1). Four hundred thirty-four patients did not meet inclusion criteria. One hundred seventy-three potential study patients were ineligible because they did not show osteolysis on their preoperative radiographs, they were having a second revision surgery, or they had inflammatory arthritis; two potential study patients declined to participate. Two hundred fifty-nine potential controls either declined to participate or did not qualify as controls due to timing of their joint arthroplasties, history of revision surgery, presence of hip pain, presence of inflammatory arthritis, or presence of osteolysis on radiographs. Controls could not be identified for two of the study patients due to (1) inability to find a cemented acetabular cup match and (2) an extremely long time (30 years) between primary THA and revision. After exclusions, there were 15 study patients and 13 control patients enrolled in the study. There were no significant differences between groups in baseline characteristics, including age, sex, BMI, race (all patients were white), prosthesis wear, Charlson Comorbidity Index, time since initial THA, UCLA activity score, and acetabular and femoral component type (Table 1).
Fig. 1.
A flowchart shows the recruitment process for study patients and controls.
Table 1.
Characteristics of study and control patients
| Variable | Study patients (n = 15) | Control patients (n = 13) | p value |
|---|---|---|---|
| Age (years)* | 70.1 (12.4) | 71.3 (9.3) | 0.77 |
| Female sex (number of patients) | 5 (33%) | 4 (31%) | 0.99 |
| BMI (kg/m2)* | 27.5 (5.1) | 24.6 (3.4) | 0.09 |
| Comorbidities (number of patients) | 0.96 | ||
| Hypertension | 8 (53%) | 5 (40%) | |
| Cardiovascular or peripheral vascular disease | 1 (10%) | 1 (13%) | |
| Gastrointestinal | 1 (13%) | 0 | |
| Time since initial THA (years) (range, 3–30 years)* | 13.0 (7.6) | 8.6 (4.7) | 0.08 |
| Prosthesis type (acetabular) (%) | 0.05 | ||
| Metal-polyethylene | 94 | 100 | |
| Prosthesis type (femoral) (%) | 0. 09 | ||
| Metal stem | 94 | 100 | |
| Cemented | 73 | 70 | |
| UCLA activity score (range, 1–10, 10 = very active)* | 5.9 (1.5) | 7.1 (2.1) | 0.10 |
| Prosthesis wear (mm)* | 0.28 (0.18) | 0.23 (0.14) | 0.40 |
| VAS score for hip pain (cm) (range, 1–10, 10 = very painful)* | 5.8 (2.8) | 0.0 (0.0) | < 0.01 |
* Values are expressed as mean, with SD in parentheses.
Diagnostic Evaluations
Clinical and laboratory data were collected at a single study visit for both groups. A plain AP radiograph of the hips was also performed on all potential controls to rule out osteolysis. A screening questionnaire was administered to study patients before surgery and to controls. Self-reported data included pain score at the prosthesis site on a 1- to 10-cm VAS, the UCLA activity score, and the Charlson Comorbidity Index. Height and weight were also measured.
All patients had the following serum assays performed at the time of their study visit: hsCRP, IL-1β, IL-6, TNF-α, RANKL, and OPG. Serum hsCRP assays were run immediately. Samples for all other assays were stored at −70° and batched assays were run in duplicate and with internal controls consistent in each batch. The inter- and intraassay coefficients of variation for the different laboratory assays are shown (Table 2).
Table 2.
Inter- and intraassay coefficients of variation
| Assay | Coefficients of variation (%) | |
|---|---|---|
| Interassay | Intraassay | |
| TNF-α | 3.1–8.5 | 7.3–10.6 |
| IL-1β | 8.2–19.2 | 6.4–10.2 |
| Free soluble RANKL | 6–8.3 | 4.5–7.9 |
| IL-6 | 1.6–4.2 | 3.3–6.4 |
| OPG | 3.4–7.4 | 2.4–7.0 |
TNF-α = tumor necrosis factor α; RANKL = receptor activator of nuclear factor κB ligand; OPG = osteoprotegerin.
Radiographic Definitions and Assessment of Osteolysis and Prosthesis Wear
We reviewed radiographs for all study and control patients. Assessment of periprosthetic osteolysis and prosthesis wear was determined by two independent, experienced radiologists (RS, BG) who were blinded to patient status. Periprosthetic osteolysis was defined as a nonlinear lucency of 3 mm or more around the prosthesis site, as visualized on a plain AP radiograph of the hips, based on published studies of minimum detectable osteolysis on plain film [6, 20]. Prosthesis wear was measured by the manual technique described by Wan and Dorr [21]. Both radiologists were trained in the use of this manual technique for wear measurement and experienced with its use. Wear measurements in the two groups ranged from 0.02 to 0.39 mm/year, with a mean of 0.24 mm/year (± 0.12 mm/year) across both groups and no significant difference between groups (p = 0.40). The interreader kappa for prosthesis wear measurement was 0.76.
Statistical Analysis
Baseline characteristics between groups were compared using a Wilcoxon rank-sum test (continuous variables) or a chi-square test (categorical variables). Serum concentrations of the biomarkers in the study patients versus controls were analyzed using a nonparametric paired t-test. As this was a pilot study and we did not have reliable estimates of the values of inflammatory mediators to expect, no a priori sample size calculation was performed. All statistical analyses were performed using STATA® software (Version 11.1; StataCorp LP, College Station, TX, USA).
Results
Median serum levels of TNF-α were significantly higher in study patients than in controls (7.1 pg/mL [SD, 11.6 pg/mL] versus 1.5 pg/mL [SD, 1.3 pg/mL]) (p < 0.01) (Table 3). Median IL-6 levels tended to be higher in study patients than in controls (8.9 pg/mL [SD, 13.2 pg/mL] versus 3.5 pg/mL [SD, 0.7 pg/mL]) (p = 0.09). There were no significant differences between groups in any of the other serum inflammatory proteins or mediators of bone turnover. Analysis performed excluding the two patients without matched controls showed similar results (data not shown).
Table 3.
Serum inflammatory proteins and mediators of bone resorption in study and control patients
| Variable | Study patients (n = 15) | Control patients (n = 13) | p value |
|---|---|---|---|
| TNF-α (pg/mL) | 7.1 (11.6) | 1.5 (1.3) | 0.01 |
| IL-1 (pg/mL) | 0.40 (0.37) | 0.29 (0.34) | 0.10 |
| IL-6 (pg/mL) | 8.9 (13.2) | 3.5 (0.7) | 0.09 |
| OPG (pmol/L) | 7.9 (3.0) | 7.5 (2.2) | 0.90 |
| RANKL (pmol/L) | 19.1 (23.9) | 44.8 (55.0) | 0.33 |
| hsCRP (mg/dL) | 1.86 (4.76) | 0.24 (0.19) | 0.37 |
Values are expressed as median, with SD in parentheses; TNF-α = tumor necrosis factor α; OPG = osteoprotegerin; RANKL = receptor activator of nuclear factor κB ligand; hsCRP = high-sensitivity C-reactive protein.
Discussion
Periprosthetic osteolysis is one of the leading causes of revisions after THA [12]. The association of serum biomarkers with severe radiographic periprosthetic osteolysis has not been consistently defined. We therefore determined whether there was an association between measurable inflammatory markers or inflammatory mediators and periprosthetic osteolysis.
There are several limitations to our study, including a small sample size and the lack of ethnic diversity among our subjects. For these reasons, our data should be considered preliminary and may be best suited as pilot data for future studies that would include larger numbers of patients with osteolysis and control patients. Other limitations are that only patients with severe osteolysis were included; the relationship of early disease to TNF-α levels was not examined. It is also worth noting that some evidence suggests that TNF-α varies with circadian rhythms [17] and also across monthly menstrual cycles [3]. Future, larger studies should consider exploring these potential associations and consider accounting for the time of serum TNF-α measurement.
In addition, plain radiographs were used as a screen for osteolysis, and it is possible that controls could have had mild or posterior osteolysis that was missed, as plain films have been shown to be less sensitive than either CT or MRI for identifying the presence and extent of periprosthetic osteolysis [6, 14]. Also, our methods for measuring prosthesis wear were based on a plain AP view, rather than more precise three-dimensional CT, which may have contributed to variance in biomarker measurements between study patients and controls. Future studies of the potential association of serum inflammatory proteins and osteolysis should ideally include more precise methods of measuring both the presence and extent of osteolysis and the degree of prosthesis wear, perhaps by a more precise imaging technique such as CT or MRI.
With these limitations in mind, this study found that TNF-α, a serum inflammatory protein that has been associated with bone resorption [2, 9], was significantly elevated in patients with THAs with periprosthetic osteolysis compared to matched control patients with THAs with no radiographic signs of osteolysis. IL-6 was also elevated, although with the numbers available the difference was not significant, and this finding must be interpreted with particular caution. TNF-α is one of the inflammatory mediators of osteolysis, along with IL-1 and IL-6 [5, 15, 16]. In vitro studies have shown that TNF-α and its downstream effect on osteoclastogenesis are an important part of the mechanism of osteolysis [4]. Recent studies suggest that the carriage of the TNF-238A allele was associated with an increased association of osteolysis after THA (odds ratio = 1.7; 95% CI: 1–2.9), independent of other risk factors, underscoring the important role that TNF-α may play as a mediator of osteolysis [10].
Serum TNF-α has been shown to be elevated in patients with osteolysis [2, 9], although these results have not been consistent across studies [13]. Fiorito et al. [9] conducted a case-control study of eight patients with postarthroplasty osteolysis compared to 10 control patients postarthroplasty without osteolysis and found that serum TNF-α levels were elevated in those patients with osteolysis compared to those patients without (mean ± SD: 4.32 ± 5.2 versus 3.84 ± 1.1.3), although the difference was not statistically significant (p = 0.62). In an earlier study, Hernigou et al. [13] compared TNF-α levels in six patients with periprosthetic osteolysis and 10 patients postarthroplasty without osteolysis and found no detectable levels of TNF-α in either group. Our study has slightly larger numbers of matched patients, which is perhaps why we were able to detect significant differences in TNF-α levels.
Osteolysis is a leading cause of THA failure [12]. Currently, there is no effective method for predicting which patients with THAs are most likely to develop osteolysis, and the condition can only be identified after bony damage has occurred. Moreover, even if early detection were to be possible, at this time, there is no proven therapy to stop the progression of osteolysis. In a canine model of periprosthetic osteolysis, bisphosphonates were shown to prevent radiolucencies after implantation [19], but this strategy has not been successfully replicated in humans. Given the availability of therapies that effectively lower TNF-α levels, it is intriguing to speculate on their potential use in preventing osteolysis. TNF-α inhibition has been widely used in rheumatoid arthritis therapy, where it is extremely effective in preventing erosions [1]. Gene therapy with a soluble inhibitor of TNF-α reduced wear-induced bone resorption in a mouse model of osteolysis [5]. However, while anti-TNF-α therapy has been administered to a small number of human subjects with periprosthetic osteolysis [18], the results are not definitive, and the potential for infection may be significant [11]. Larger randomized trials are needed to evaluate whether TNF-α inhibition might play a role in the prevention or modification of wear-induced periprosthetic osteolysis.
Our study has several strengths, including the incorporation of wear measurement and type of prosthesis material used in the matching of study patients and controls to control for the effect of prosthesis materials on rates of wear and osteolysis. We believe there is a clinical need for biomarkers that can predict the risk of developing osteolysis before irreversible bony destruction and prosthesis failure. Targeted interventions then may be developed and tested on those most at risk. While our findings show that TNF-α levels are significantly elevated in the serum of patients with end-stage osteolysis, the temporal relationship of TNF-α to the development of osteolysis is unknown. It is intriguing to speculate that serial monitoring might pick up rising TNF-α levels, allowing the preclinical identification of patients with THAs developing osteolysis.
Acknowledgments
The authors thank Robert Schneider MD and Bernard Ghelman MD in the Department of Radiology, Hospital for Special Surgery, for their assistance in the radiographic measurements.
Footnotes
The institution of one or more of the authors (RKC, LAM) has received, during the study period, funding from the American College of Rheumatology (Atlanta, GA, USA), The Arthritis Foundation (Atlanta, GA, USA), and the NIH (Bethesda, MD, USA). One of the authors (RKC) has received funding, during the study period, from the American College of Rheumatology (Physician-Scientist Development Award). One of the authors (LAM) has received funding, during the study period, from the NIH/NIAMS (Grant K23AR050607) and The Arthritis Foundation (Investigator Award).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Hospital for Special Surgery, New York, NY, USA.
References
- 1.Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, Genovese MC, Wasko MC, Moreland LW, Weaver AL, Markenson J, Finck BK. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000;343:1586–1593. doi: 10.1056/NEJM200011303432201. [DOI] [PubMed] [Google Scholar]
- 2.Blaine TA, Rosier RN, Puzas JE, Looney RJ, Reynolds PR, Reynolds SD, O’Keefe RJ. Increased levels of tumor necrosis factor-alpha and interleukin-6 protein and messenger RNA in human peripheral blood monocytes due to titanium particles. J Bone Joint Surg Am. 1996;78:1181–1192. doi: 10.2106/00004623-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Brännström M, Fridén BE, Jasper M, Norman RJ. Variations in peripheral blood levels of immunoreactive tumor necrosis factor alpha (TNFalpha) throughout the menstrual cycle and secretion of TNFalpha from the human corpus luteum. Eur J Obstet Gynecol Reprod Biol. 1999;83:213–217. doi: 10.1016/S0301-2115(99)00003-2. [DOI] [PubMed] [Google Scholar]
- 4.Braun T, Zwerina J. Positive regulators of osteoclastogenesis and bone resorption in rheumatoid arthritis. Arthritis Res Ther. 2011;13:235. doi: 10.1186/ar3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Childs LM, Goater JJ, O’Keefe RJ, Schwarz EM. Effect of anti-tumor necrosis factor-alpha gene therapy on wear debris-induced osteolysis. J Bone Joint Surg Am. 2001;83:1789–1797. doi: 10.2106/00004623-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Claus AM, Engh CA, Jr, Sychterz CJ, Xenos JS, Orishimo KF, Engh CA., Sr Radiographic definition of pelvic osteolysis following total hip arthroplasty. J Bone Joint Surg Am. 2003;85:1519–1526. doi: 10.2106/00004623-200308000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Claus AM, Totterman SA, Sychertz CJ, Tamez-Pena JG, Looney RJ, Engh CA. Computed tomography to assess pelvic lysis after hip replacement. Clin Orthop Relat Res. 2004;422:167–174. doi: 10.1097/01.blo.0000129345.22322.8a. [DOI] [PubMed] [Google Scholar]
- 8.Cooper HJ, Ranawat AS, Potter HG, Foo LF, Jawetz ST, Ranawat CS. Magnetic resonance imaging in the diagnosis and management of hip pain after total hip arthroplasty. J Arthroplasty. 2009;24:661–667. doi: 10.1016/j.arth.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Fiorito S, Magrini L, Goalard C. Pro-inflammatory and anti-inflammatory circulating cytokines and periprosthetic osteolysis. J Bone Joint Surg Br. 2003;85:1202–1206. doi: 10.1302/0301-620X.85B8.12799. [DOI] [PubMed] [Google Scholar]
- 10.Gallo J, Mrazek F, Petrek M. Variation in cytokine genes can contribute to severity of acetabular osteolysis and risk for revision in patients with ABG 1 total hip arthroplasty: a genetic association study. BMC Med Genet. 2009;10:109. doi: 10.1186/1471-2350-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilson M, Gossec L, Mariette X, Gherissi D, Guyot MH, Berthelot JM, Wendling D, Michelet C, Dellamonica P, Tubach F, Dougados M, Salmon D. Risk factors for total joint arthroplasty infection in patients receiving tumor necrosis factor alpha-blockers: a case-control study. Arthritis Res Ther. 2010;12:R145. doi: 10.1186/ar3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris WH. Wear and periprosthetic osteolysis: the problem. Clin Orthop Relat Res. 2001;393:66–70. doi: 10.1097/00003086-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Hernigou P, Intrator L, Bahrami T, Bensussan A, Farcet JP. Interleukin-6 in the blood of patients with total hip arthroplasty without loosening. Clin Orthop Relat Res. 1999;366:147–154. doi: 10.1097/00003086-199909000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Kitamura N, Pappedemos PC, Duffy PR, 3rd, Stepniewski AS, Hopper RH, Jr, Engh CA, Jr, Engh CA. The value of anteroposterior pelvic radiographs for evaluating pelvic osteolysis. Clin Orthop Relat Res. 2006;453:239–245. doi: 10.1097/01.blo.0000246554.41058.8d. [DOI] [PubMed] [Google Scholar]
- 15.Maloney WJ, James RE, Smith RL. Human macrophage response to retrieved titanium alloy particles in vitro. Clin Orthop Relat Res. 1996;322:268–278. doi: 10.1097/00003086-199601000-00032. [DOI] [PubMed] [Google Scholar]
- 16.Merkel KD, Erdmann JM, McHugh KP, Abu-Amer Y, Ross FP, Teitelbaum SL. Tumor necrosis factor-alpha mediates orthopedic implant osteolysis. Am J Pathol. 1999;154:203–210. doi: 10.1016/S0002-9440(10)65266-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muc-Wierzgoń M, Baranowski MM, Madej K, Wierzgoń J, Kokot T, Brodziak AJ. Dynamics of diurnal changes in serum concentration of TNF-alpha soluble receptors in gastrointestinal cancer patients. Biol Regul Homeost Agents. 2000;14:204–208. [PubMed] [Google Scholar]
- 18.Schwarz EM, Campbell D, Totterman S, Boyd A, O’Keefe RJ, Looney RJ. Use of volumetric computerized tomography as a primary outcome measure to evaluate drug efficacy in the prevention of peri-prosthetic osteolysis: a 1-year clinical pilot of etanercept vs. placebo. J Orthop Res. 2003;21:1049–1055. doi: 10.1016/S0736-0266(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 19.Shanbhag AS, Hasselman CT, Rubash HE. The John Charnley Award. Inhibition of wear debris mediated osteolysis in a canine total hip arthroplasty model. Clin Orthop Relat Res. 1997;344:33–43. doi: 10.1097/00003086-199711000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Walde TA, Weiland DE, Leung SB, Kitamura N, Sychertz CJ, Engh CA, Jr, Claus AM, Potter HG, Engh CA., Sr Comparison of CT, MRI, and radiographs in assessing pelvic osteolysis: a cadaveric study. Clin Orthop Relat Res. 2005;437:138–144. doi: 10.1097/01.blo.0000164028.14504.46. [DOI] [PubMed] [Google Scholar]
- 21.Wan Z, Dorr LD. Natural history of femoral focal osteolysis with proximal ingrowth smooth stem implant. J Arthroplasty. 1996;11:718–725. doi: 10.1016/S0883-5403(96)80011-8. [DOI] [PubMed] [Google Scholar]