Abstract
Background
The prevalence of pseudotumors in patients with large-head metal-on-metal (MOM) THA has been the subject of implant recalls and warnings from various regulatory agencies. To date, there is no consensus on whether ultrasound or MRI is superior for the detection of pseudotumors.
Questions/purposes
We prospectively compared ultrasound to MRI for pseudotumor detection in an asymptomatic cohort of patients with MOM THAs. We also compared ultrasound to MRI for assessment of pseudotumor growth and progressive soft tissue involvement at a 6-month interval.
Methods
We enrolled 40 patients with large-head MOM THAs in the study. The mean age was 54 years (range, 34–76 years). The mean time from surgery was 54 months (range, 40–81 months). There were 28 men and 12 women. All patients underwent ultrasound and MRI using slice encoding for metal artifact correction. The gold standard was defined as follows: if both ultrasound and MRI agreed, this was interpreted as concordant and the result was considered accurate.
Results
Ultrasound and MRI agreed in 37 of 40 patients (93%). The prevalence of pseudotumors was 31% (12 of 39) in our cohort. Twenty-three of 39 patients (59%) had completely normal tests and four (10%) had simple fluid collections. Ultrasound had a sensitivity of 100% and specificity of 96% while MRI had a sensitivity of 92% and specificity of 100%.
Conclusions
A negative ultrasound rules out pseudotumor in asymptomatic patients as this test is 100% sensitive. Given its lower cost, we recommend ultrasound as the initial screening tool for pseudotumors.
Level of Evidence
Level I, diagnostic study. See the Instructions for Authors for a complete description of levels of evidence.
Introduction
In the United States, metal-on-metal (MOM) THA had a market share of 35% in the early 2000s [5]. Numerous reports have highlighted the problem of pseudotumors in patients who have undergone large-head MOM THA [3, 4, 11, 15, 22, 26, 28]. The prevalence of pseudotumors is higher with this type of THA than with hip resurfacing, and MOM implants have been the subject of recalls and warnings from various regulatory agencies.
Both ultrasound and MRI with metal artifact reduction sequences (MARS) were identified as suitable modalities for pseudotumor detection. To date, there is no consensus on which of these imaging modalities is superior for detecting pseudotumors, following their progression, or assessing associated soft tissue damage. Furthermore, the choice of the monitoring modality is important because of the cost implication. The number of patients with MOM arthroplasties has been estimated to be 500,000. In the United States, the cost of an ultrasound scan is approximately USD 800 and that of a MRI is approximately USD 1500. For a single followup study of all patients with MOM implants, the cost differential is a staggering USD 350 million, which highlights the importance of identifying the most cost-effective screening modality for detecting and following these pseudotumors.
Ultrasound has been advocated for screening patients with MOM implants, particularly for detection of pseudotumors [9, 24]. Ultrasound has the advantage of being relatively affordable, widely available, quick to perform, noninvasive (and therefore useful for scanning the contralateral limb for comparison), and relatively unaffected by metal implants. Its detractors state that it is operator-dependent and has trouble accessing deep tissues, especially in obese patients [16]. To date, several studies have successfully used ultrasound to detect pseudotumors in both symptomatic and asymptomatic patients with large-head MOM THAs and resurfacings [20, 26, 28].
Due to its ability to image soft tissues and bone, many authors believe MRI is the best option for pseudotumor detection [10, 16]. However, when MRI is performed near a metal implant, artifacts arise, including signal loss and spatially varying displacement in both slice and in-plane directions. MRI methods tailored for metal artifact reduction have also become increasingly popular for the detection of pseudotumors [1, 14]. A number of different metal artifact reduction MRI approaches are available with different trade-offs between scan time and artifact reduction. More recently, multispectral imaging (MSI) methods (slice encoding for metal artifact correction [SEMAC], multiacquisition variable-resonance image combination [MAVRIC], warp) have been developed to further reduce displacement artifacts in both slice and in-plane directions [7, 18, 21, 27]. The main downsides of these approaches are increased scan times and reduced resolution and signal-to-noise ratio compared with standard clinical MRI. Artifacts (residual signal loss) from the metal implants are dependent on the specific metal artifact reduction technique used.
To date, no study has compared ultrasound to MRI directly for initial pseudotumor detection. Given the important clinical and economic considerations, we believed this was an important question to address. We therefore prospectively compared ultrasound to MRI for pseudotumor detection in an asymptomatic cohort of patients with MOM THAs. We also compared ultrasound to MRI for preliminary assessment of pseudotumor growth and progressive soft tissue involvement at a 6-month interval.
Patients and Methods
This was a prospective diagnostic study. Institutional ethics review board approval was obtained and all patients gave their informed consent before their inclusion in the study. Patients were identified from our hospital database. From 2005 to 2008, 360 primary large-head MOM THAs were performed at our institution using a Metasul® LDH® implant with a Durom® acetabular cup and an M/L Taper® stem (Zimmer Inc, Warsaw, IN, USA). Patients were invited to participate in this study if they had their MOM THAs implanted at least 2 years earlier, provided they had an uncomplicated postoperative course, a normalized WOMAC [2] score of 75 or greater (with 0 being the worst score and 100 being the best score), and no previous ultrasound or MRI for pseudotumor detection; this resulted in 122 of the 360 patients being eligible. Those patients were contacted by mail, and 63 patients (52%) responded. Of those 63, 14 were found to be ineligible (11 already had the index MOM THA revised, three had a WOMAC score of less than 75), and eight were too busy or were having other surgery. We enrolled 41 patients, but one withdrew after consenting and having blood work done, leaving 40 patients for analysis in the final study group (Table 1). The mean age of the patients was 54 years (range, 34–76 years). The mean time from surgery was 54 months (range, 40–81 months). There were 28 men and 12 women.
Table 1.
Demographic data for the study population
| Variable | Value |
|---|---|
| Number of patients | 40 |
| Number of female patients | 12 |
| Number of male patients | 28 |
| Age (at index surgery) (years)* | 54 (34–76) |
| BMI (kg/m2)* | 27 (20–43) |
| Time from surgery to scan (months)* | 54 (40–81) |
| WOMAC global score (points)*,† | 95 (75–100) |
| UCLA activity level* | 8 (5–10) |
* Values are given as the mean, with the range in parentheses; †WOMAC scores are normalized to a range of 0 to 100, with 0 being the worst and 100 being the best.
All patients underwent ultrasound (including color Doppler) and MRI using SEMAC. Both examinations were performed on the same day. All ultrasounds and MR images were read separately by one of three musculoskeletal fellowship-trained radiologists (HAO, GA, BF) who were blinded to the findings from the other modality. In cases where there was a positive finding of a pseudotumor, a second radiologist read the ultrasound/MRI to confirm this. The second radiologist was blinded to the initial positive reading of the first radiologist. No intra- or interobserver reliability testing was done.
A single sonographer (DK) experienced in musculoskeletal imaging performed the ultrasound examinations. Before the study started, this sonographer had performed ultrasound scans on approximately 220 hips with THAs: 100 hips with MOM THAs (since 2009) and 120 hips that did not have MOM THAs (since 2008). A standardized template was used to conduct the scans, which were performed using a Siemens Antares™ Ultrasound System (Siemens Medical Solutions USA, Mountain View, CA, USA). The Siemens VFX9-4 linear transducer and/or the Siemens CH6-2 curvilinear transducer was used for anterior, posterior, and/or lateral views, depending on each patient’s specific body habitus; body habitus affected the choice of transducers, but all images were obtained on all patients. Acquisition time was 20 minutes. The presence, size, and position of any fluid, cystic mass, or solid mass related to the hip were recorded, along with any involvement of neurovascular structures. The volume of any fluid or mass was calculated by multiplying the maximum recorded dimensions in millimeters in each of three planes and dividing by 1000 to convert to volume in cubic centimeters. The radiologist was asked to definitively state whether the scan was normal or abnormal, and if the latter, the radiologist categorized it as one of three possible abnormalities: (1) a solid mass, (2) a cystic mass or a complex fluid collection, or (3) a simple fluid collection. On ultrasound, a simple fluid collection was defined as anechoic with increased through-transmission and a well-defined posterior wall. A complex fluid collection/cystic mass contained debris (internal echoes), septations, or both. In cases where the first radiologist could not make a definitive classification, a second radiologist read the scan and consensus was reached by discussion on whether this abnormality was a pseudotumor or a simple fluid collection.
The MRI examinations were performed using an eight-channel 1.5-T MR unit (Signa® HDx; GE Healthcare, St Giles Chalfont, Buckinghamshire, UK) and a torso-phased surface coil. Custom SEMAC software [21] was installed and the following sequences were obtained: axial and coronal T1 (repetition time [TR], 500 echo time [TE] minutes; echo train length [ETL], 1; receiver band width [RBW], 125; matrix, 394 × 128; field of view [FOV], 40 cm; number of excitations [NEX], 0.5; slice thickness, 5 mm] and axial and coronal proton density (TR, 3600 TE minutes; ETL, 12; RBW, 125; matrix, 384 × 256; FOV, 40 cm; slice thickness, 5 mm). Total acquisition time was 45 minutes. Gadolinium was not utilized, and therefore, strictly speaking, a focal region of signal change could not be defined as cystic or solid. An abnormality was diagnosed on MRI when a circumscribed periarticular signal abnormality was noted.
For any patient where there was a disagreement about the presence of pseudotumor between the MRI and ultrasound assessment, either repeat ultrasound and MRI or dual-energy CT scan was performed a minimum of 6 months later (range, 6–9 months) to confirm whether a pseudotumor was present, that is, to confirm the diagnosis (negative or positive for pseudotumor). The CT was performed on a Siemens Flash® 64-slice scanner (Siemens, Erlangen, Germany), using the following parameters: Kv = 120/100 (note there are two, as it is dual energy) and mAs = 140. Based on all of these tests, the two radiologists arrived at a consensus about the presence of pseudotumor in all patients.
All patients who had a detected abnormality (fluid collection or mass) on MRI or ultrasound were invited to undergo a repeat of both scans at a minimum of 6 months after the initial scans. Two radiologists interpreted each followup examination as described above. The radiologists measured changes in the size of the abnormality and commented on progression of soft tissue damage (abnormal echogenicity/atrophy on ultrasound, abnormal signal/atrophy on MRI).
Statistical Methods
To calculate sensitivities and specificities, the gold standard needs to be defined. In this study, the gold standard was defined as follows: if both ultrasound and MRI agreed, this was interpreted as concordant and the result accurate. If both tests were normal or only showed a simple fluid collection, this was taken as evidence that there was no pseudotumor. A pseudotumor was present if both tests agreed that a cystic mass, complex fluid collection, or solid mass was present. For the tests to agree, they also had to agree on location. For discordant results, additional testing was performed as described above. Needle biopsy of the suspected lesions was not undertaken as the musculoskeletal pathologist (TON) believed sampling error may have led to misdiagnosis and the real gold standard is the pathology of the intraoperative specimen.
Sensitivity of ultrasound was calculated as the proportion of those with true pseudotumors for whom pseudotumor was detected by ultrasound. Sensitivity of MRI was calculated as the proportion of those with true pseudotumors for whom pseudotumor was detected by MRI. The specificity of ultrasound was calculated as the proportion of those without true pseudotumors for whom pseudotumor was not seen on ultrasound. The specificity of MRI was calculated as the proportion of those without true pseudotumors for whom pseudotumor was not seen on MRI. Positive predictive value (PPV) and negative predictive value (NPV) of ultrasound and MRI followed the standard formulas. We calculated 95% bootstrap percentile CIs for the subset of four of the above eight measures that showed variation across replicate samples (when sample sensitivity or specificity was exactly 1, for example, no CI was computed). Finally, sensitivity and specificity for ultrasound versus MRI were compared via McNemar’s exact test.
Results
The use of ultrasound to detect pseudotumors showed a sensitivity of 100%, specificity of 96% (95% CI, 88%–100%), NPV of 100%, and PPV of 92% (95% CI, 75%–100%). SEMAC MRI had a sensitivity of 92% (95% CI, 71%–100%), specificity of 100%, PPV of 100%, and NPV of 96% (95% CI, 88%–100%). There was no significant difference between the two modalities in terms of sensitivity or specificity (p = 0.32).
The findings of ultrasound and MRI agreed in 37 of 40 patients (93%). The prevalence of pseudotumors was 31% (12 of 39) in this study (Fig. 1). Twenty-three of 39 patients (59%) had completely normal tests and four (10%) had simple fluid collections. Three patients (8%) had discordant readings on the first ultrasound and MRI. In the first patient, the initial ultrasound was positive for a cystic lesion posterior to the hip, but the initial MRI scan was normal. Repeat MRI and ultrasound were performed 7 months later; once again, the MRI was normal and at this time the ultrasound was read as showing a simple fluid collection or artifact. It was concluded that this was a false-positive result on initial ultrasound and that the MRI was correct. For the second patient, the initial ultrasound was read as showing a solid mass with a volume of 13 cm3 anteromedial to the hip. The initial MRI was read as normal. Repeat ultrasound and MRI at 5 months showed a mass on both examinations in the same location. In retrospect, the first MRI was reread and the mass was identified. This was deemed a false-negative result for the initial MRI. The third patient had an initial negative ultrasound with a positive initial MRI. Dual-energy CT scan revealed only a fluid collection. In this patient, the true result was indeterminate and this patient was not included in the analysis of sensitivity, specificity, PPV, and NPV.
Fig. 1A–B.
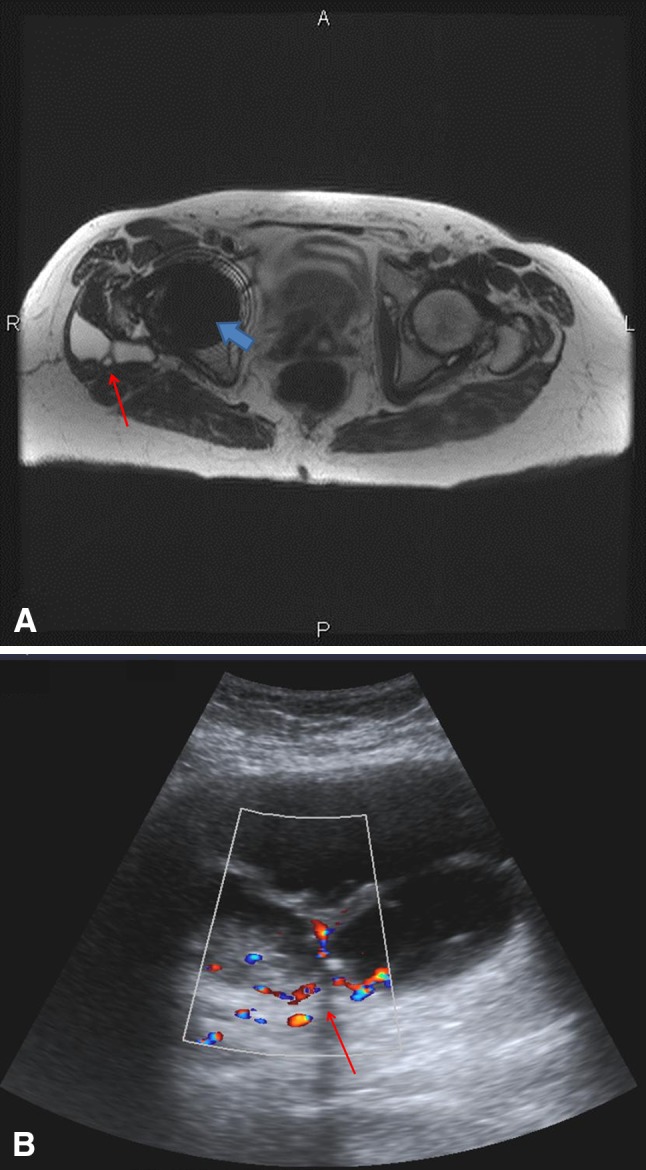
MRI and ultrasound show a large pseudotumor in a 64-year-old woman. (A) A T2-weighted axial MR image using the SEMAC protocol shows a complex high T2 signal lesion (red arrow) posterolateral to the right femoral neck component of the MOM THA. The blue arrow demonstrates residual metallic artifact from the femoral head component of the THA. (B) A color Doppler ultrasound image in the axial plane shows a septated complex fluid collection (arrow) with internal echoes and vascularity within the septations, as shown by the color flow.
Followup ultrasound and MRI were performed on eight patients with initial findings of pseudotumors at an average of 7 months after the initial scans (range, 6–9 months). Two patients (25%) had a substantial increase in size of the pseudotumor. In one patient, two masses increased on ultrasound only, from 123 and 179 cm3 (anteromedial and posterolateral to the hip) to 339 and 373 cm3, respectively, after an interval of 7 months; MRI showed no change in pseudotumor size in this patient. In the second patient, a mass anteromedial to the hip increased in size on ultrasound from 102 cm3 to 268 cm3 after an interval of 7 months; MRI showed a mild increase in pseudotumor size in this patient. No other testing was done to see if ultrasound was overestimating or MRI was underestimating this size change. SEMAC MRI detected no muscle damage at followup in these eight patients, and overall no muscle damage was seen in any patient in this study.
Discussion
Pseudotumors or adverse local reactions to metal debris have been well documented to occur in association with both resurfacing [6, 12, 13, 19, 26] and large-head MOM THA [3, 4, 11, 22]. Both ultrasound and MARS MRI have shown tissue reactions to occur commonly after MOM THA. In a study by Williams et al. [28], ultrasound found pseudotumors in 32% (10 of 31) of asymptomatic patients with large-head MOM THAs. In a separate study using MARS MRI, Hart et al. [15] found a prevalence of pseudotumor formation of 61% (17 of 28) in asymptomatic patients with several different large-head MOM THAs. Both the Medicines and Healthcare products Regulatory Agency [23] in the United Kingdom and the FDA in the United States have made recommendations regarding the followup imaging of patients with large-head MOM THAs. For cross-sectional imaging, MARS MRI or ultrasound is recommended in the United Kingdom, whereas MARS MRI is the imaging modality of choice in the United States. Ultrasound has the advantage of being more affordable; it also is easy and quick to perform in the hands of experienced technicians. MARS MRI has the advantage of being able to assess both the soft tissue and bone and to detect pseudotumors. To date, no study has directly compared these two tests to assess their validity in assessing asymptomatic patients with MOM THAs for adverse reactions and pseudotumors. We therefore directly compared the two, using an MRI acquisition technique (SEMAC) that has shown significant advantages over conventional MARS techniques in the presence of metallic implants [7, 18, 21, 27]. We also compared ultrasound to MRI for preliminary assessment of pseudotumor growth and progressive soft tissue involvement at a 6-month interval.
The main limitation in this study is there was no definite gold standard for presence of pseudotumor or other adverse tissue reaction. While we accepted true results as agreement/consensus on both the ultrasound and MRI scans, it is possible that both could be wrong, which would imply a higher number of false-positive and false-negative results than we have reported. Since the presence of a pseudotumor is not a random event, we believe that the approach used, though not perfect, is a reasonable approach insofar as intraoperative samples are not available on all patients. Needle biopsy itself suffers from sampling error and for this reason was not used in the study as a gold standard. Only intraoperative findings could allow more certainty and would be the only true gold standard. While our choice of gold standard is not as strong as intraoperative findings would have been, obtaining open surgical biopsies in this setting is impractical, and the approach we employed allowed the calculation of test parameters that we believe are important to define. Only one patient in this study had revision surgery. In this patient, intraoperative findings confirmed the pseudotumor that had been detected on both ultrasound and SEMAC MRI. The other limitation for both techniques is that no formal tests of reliability or reproducibility were done. The authors believe this is important. In this study, a second radiologist did confirm positive results and the authors believe, while this is not a formal test of reliability, it does show that both tests are certainly reliable for positive findings. Finally, we used a new technique for evaluation of patients with THA and possible pseudotumors (SEMAC), and because we did not also perform the MARS technique used by other authors [8, 25], the two methods cannot be directly compared.
In this study, we found that both tests performed well for initial assessment of pseudotumors in asymptomatic patients with large-head MOM THAs. The prevalence in this study was 31%, which is consistent with the literature [28]. Both tests performed equally well, with no significant difference in sensitivity or specificity found between the two. Indeed the two tests agreed in 93% of patients. Both ultrasound and SEMAC MRI showed high values for parameters associated with validity of a screening test, namely sensitivity and specificity.
Our findings support the use of ultrasound as a cost-effective screening tool. The ideal screening test should be inexpensive, easy to administer, and impose minimal discomfort on the patient. It should also be valid, reliable, and reproducible [17]. Our data suggest that ultrasound fulfills these criteria. In the United States, ultrasound costs USD 800 whereas MARS MRI costs USD 1500. For initial screening of patients, this represents a savings of USD 700,000 for every 1000 patients screened. If every patient with a positive ultrasound were to be subjected to an MRI scan, the cost savings would decrease to USD 250,000 in the same 1000-patient screening cohort, assuming a prevalence of 30%. If one takes the example of the DePuy ASR™ large-head MOM hip, it is recommended that all patients with this device have cross-sectional imaging. Given that approximately 44,000 were implanted worldwide, initial imaging with ultrasound represents a savings of USD 11,000,000 for the screening of this one implant. This highlights the importance of ultrasound as a screening tool from a cost-effectiveness standpoint. Based on our results, even a positive ultrasound does not necessitate an MRI scan in the initial screening.
From this study, we cannot conclude which test is superior for longitudinal followup of patients with pseudotumors. For followup examinations, we assessed patients at an average of 7 months from initial imaging. We found substantial increases in lesion size in only two of eight patients followed. In this study, no muscle damage was detected on SEMAC MRI. This suggests that an interval of longer than 6 months may be necessary for interval cross-sectional imaging.
It should be noted that there are many MRI methods tailored to reducing metal artifacts, usually trading between short scans and extent of artifact reduction. View angle tilting (VAT) [8] is a simple pulse sequence modification to spin echo imaging to reduce in-plane displacements. For both VAT and conventional sequences, parameters may also be adjusted to reduce artifacts. For example, MARS [25] augment VAT with high-bandwidth readouts to further reduce displacement and avoid blurring. Note that the term MARS is often used more generally to describe high-bandwidth sequences with or without VAT. The use of thinner slices can also reduce slice distortion. More recent MSI techniques (SEMAC, MAVRIC, warp) build on MARS by adding additional scan time to dramatically reduce slice direction displacement artifacts [7, 18, 21, 27]. There are numerous other approaches to artifact reduction in MRI, and like the methods referenced here, these continue to be refined and improved.
In conclusion, our study demonstrated that both ultrasound and SEMAC MRI have high sensitivity and specificity for assessment of adverse reactions to metal debris in asymptomatic patients with large-head MOM THAs. Given its cost-effectiveness, we recommend ultrasound as the initial screening tool. A negative ultrasound in the hands of an experienced technician rules out pseudotumor because this test is 100% sensitive. In patients with a positive ultrasound, the next decision is which test to use for longitudinal followup: MRI or ultrasound. The data suggest that most likely the next imaging should be performed at the 1-year mark, and the choice of MARS MRI versus ultrasound remains to be clarified based on the indications for revision surgery, which are still evolving.
Acknowledgments
We thank Nelson V. Greidanus MD, MPH for contributing patients to the study; Diana Korlaet CRGS, PDDipAppSci (Medical Ultrasound) for performing the ultrasound scans; Hugue A. Ouellette MD, FRCPC, Gordon Andrews MD, FRCPC, and Borys Flak MD, FRCPC for reading the ultrasounds and MR images; Torsten O. Nielsen MD, PhD for his advice pertaining to musculoskeletal pathology; Eric C. Sayre PhD for his help with the statistical analysis; Lorna McLean BA for patient recruitment and data collection; and Daphné Savoy BA for her assistance in the preparation of this manuscript.
Footnotes
The institution of one or more of the authors (DSG, CPD, BAM, DRW, BBF) has received, during the study period, funding from Johnson and Johnson (Canada) Inc (Markham, Ontario, Canada), Zimmer, Inc (Warsaw, IN, USA), DePuy Synthes Canada Ltd (Markham, ON, Canada), Stryker Canada (Hamilton, Ontario, Canada), Mako Surgical Corp (Ft Lauderdale, FL, USA), and Bayer Inc (Toronto, Ontario, Canada). The institution of one or more of the authors (BAH) has received, during the study period, funding from GE Healthcare (Waukesha, WI, USA) and the NIH (Bethesda, MD, USA). One of the authors (DSG) certifies that he or she has received or may receive payments or benefits, during the study period, an amount of USD 10,000 to USD 100,000 from Zimmer, Inc. One of the authors (CPD) certifies that he or she has received or may receive payments or benefits, during the study period, an amount of USD 10,000 to USD 100,000 from Zimmer, Inc, and an amount of USD 10,000 to USD 100,000 from DePuy Synthes Canada Ltd. One of the authors (BAM) certifies that he or she has received or may receive payments or benefits, during the study period, an amount of USD 10,000 to USD 100,000 from Zimmer, Inc. The remaining authors (BAH, DFW, BBF) certify that they have no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that the human protocol for this investigation was approved by the appropriate ethics committee at his or her institution, that all investigations were conducted in conformity with the ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the Departments of Orthopaedics and Radiology, University of British Columbia (Vancouver, British Columbia, Canada) and at Vancouver General Hospital (Vancouver, British Columbia, Canada).
References
- 1.Anderson H, Toms AP, Cahir JG, Goodwin RW, Wimhurst J, Nolan JF. Grading the severity of soft tissue changes associated with metal-on-metal hip replacements: reliability of an MR grading system. Skeletal Radiol. 2011;40:303–307. doi: 10.1007/s00256-010-1000-7. [DOI] [PubMed] [Google Scholar]
- 2.Bellamy N, Buchanan W, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or the knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 3.Berend KR, Morris MJ, Adams JB, Lombardi AV., Jr Metal-on-metal hip arthroplasty: going, going, gone…–affirms. J Bone Joint Surg Br. 2012;94(11 suppl):75–77. doi: 10.1302/0301-620X.94B11.30745. [DOI] [PubMed] [Google Scholar]
- 4.Bosker BH, Ettema HB, Boomsma MF, Kollen BJ, Maas M, Verheyen CC. High incidence of pseudotumour formation after large-diameter metal-on-metal total hip replacement: a prospective cohort study. J Bone Joint Surg Br. 2012;94:755–761. doi: 10.1302/0301-620X.94B6.28373. [DOI] [PubMed] [Google Scholar]
- 5.Bozic KJ, Kurtz S, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ. The epidemiology of bearing surface usage in total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:1614–1620. doi: 10.2106/JBJS.H.01220. [DOI] [PubMed] [Google Scholar]
- 6.Canadian Hip Resurfacing Study Group A survey on the prevalence of pseudotumors with metal-on-metal hip resurfacing in Canadian academic centers. J Bone Joint Surg Am. 2011;93(suppl 2):118–121. doi: 10.2106/JBJS.J.01848. [DOI] [PubMed] [Google Scholar]
- 7.Chen CA, Chen W, Goodman SB, Hargreaves BA, Koch KM, Lu W, Brau AC, Draper CE, Delp SL, Gold GE. New MR imaging methods for metallic implants in the knee: artifact correction and clinical impact. J Magn Reson Imaging. 2011;33:1121–1127. doi: 10.1002/jmri.22534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho ZH, Oh CH, Mun CW, Kim YS. Some results of high-flow-velocity NMR imaging using selection gradient. Magn Reson Med. 1986;3:857–862. doi: 10.1002/mrm.1910030606. [DOI] [PubMed] [Google Scholar]
- 9.Douis H, Dunlop DJ, Pearson AM, O’Hara JN, James SL. The role of ultrasound in the assessment of post-operative complications following hip arthroplasty. Skeletal Radiol. 2012;41:1035–1046. doi: 10.1007/s00256-012-1390-9. [DOI] [PubMed] [Google Scholar]
- 10.Fang CS, Harvie P, Gibbons CL, Whitwell D, Athanasou NA, Ostlere S. The imaging spectrum of peri-articular inflammatory masses following metal-on-metal hip resurfacing. Skeletal Radiol. 2008;37:715–722. doi: 10.1007/s00256-008-0492-x. [DOI] [PubMed] [Google Scholar]
- 11.Gill IP, Webb J, Sloan K, Beaver RJ. Corrosion at the neck-stem junction as a cause of metal ion release and pseudotumour formation. J Bone Joint Surg Br. 2012;94:895–900. doi: 10.1302/0301-620X.94B7.29122. [DOI] [PubMed] [Google Scholar]
- 12.Glyn-Jones S, Pandit H, Kwon YM, Doll H, Gill HS, Murray DW. Risk factors for inflammatory pseudotumour formation following hip resurfacing. J Bone Joint Surg Br. 2009;91:1566–1574. doi: 10.1302/0301-620X.91B12.22287. [DOI] [PubMed] [Google Scholar]
- 13.Grammatopolous G, Pandit H, Kwon YM, Gundle R, McLardy-Smith P, Beard DJ, Murray DW, Gill HS. Hip resurfacings revised for inflammatory pseudotumour have a poor outcome. J Bone Joint Surg Br. 2009;91:1019–1024. doi: 10.1302/0301-620X.91B8.22562. [DOI] [PubMed] [Google Scholar]
- 14.Hart AJ, Sabah S, Henckel J, Lewis A, Cobb J, Sampson B, Mitchell A, Skinner JA. The painful metal-on-metal hip resurfacing. J Bone Joint Surg Br. 2009;91:738–744. doi: 10.1302/0301-620X.91B6.21682. [DOI] [PubMed] [Google Scholar]
- 15.Hart AJ, Satchithananda K, Liddle AD, Sabah SA, McRobbie D, Henckel J, Cobb JP, Skinner JA, Mitchell AW. Pseudotumors in association with well-functioning metal-on-metal hip prostheses: a case-control study using three-dimensional computed tomography and magnetic resonance imaging. J Bone Joint Surg Am. 2012;94:317–325. doi: 10.2106/JBJS.J.01508. [DOI] [PubMed] [Google Scholar]
- 16.Hayter CL, Potter HG, Su EP. Imaging of metal-on-metal hip resurfacing. Orthop Clin North Am. 2011;42:195–205. doi: 10.1016/j.ocl.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Hennekins CH, Buring JE. Epidemiology in Medicine. Boston, MA: Little, Brown and Co; 1987. [Google Scholar]
- 18.Koch KM, Lorbiecki JE, Hinks RS, King KF. A multispectral three-dimensional acquisition technique for imaging near metal implants. Magn Reson Med. 2009;61:381–390. doi: 10.1002/mrm.21856. [DOI] [PubMed] [Google Scholar]
- 19.Kwon YM, Glyn-Jones S, Simpson DJ, Kamali A, McLardy-Smith P, Gill HS, Murray DW. Analysis of wear of retrieved metal-on-metal hip resurfacing implants revised due to pseudotumours. J Bone Joint Surg Br. 2010;92:356–361. doi: 10.1302/0301-620X.92B3.23281. [DOI] [PubMed] [Google Scholar]
- 20.Kwon YM, Ostlere SJ, McLardy-Smith P, Athanasou NA, Gill HS, Murray DW. “Asymptomatic” pseudotumors after metal-on-metal hip resurfacing arthroplasty: prevalence and metal ion study. J Arthroplasty. 2011;26:511–518. doi: 10.1016/j.arth.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 21.Lu W, Pauly KB, Gold GE, Pauly JM, Hargreaves BA. SEMAC: slice encoding for metal artifact correction in MRI. Magn Reson Med. 2009;62:66–76. doi: 10.1002/mrm.21967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthies AK, Skinner JA, Osmani H, Henckel J, Hart AJ. Pseudotumors are common in well-positioned low-wearing metal-on-metal hips. Clin Orthop Relat Res. 2012;470:1895–1906. doi: 10.1007/s11999-011-2201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medicines and Healthcare products Regulatory Agency. MDA/2010/033 Medical Device Alert: all metal-on-metal (MoM) hip replacements. Available at: http://www.mhra.gov.uk/Publications/Safetywarnings/MedicalDeviceAlerts/CON079157. Accessed February 13, 2012.
- 24.Nishii T, Sakai T, Takao M, Yoshikawa H, Sugano N. Ultrasound screening of periarticular soft tissue abnormality around metal-on-metal bearings. J Arthroplasty. 2012;27:895–900. doi: 10.1016/j.arth.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Olsen RV, Munk PL, Lee MJ, Janzen DL, MacKay AL, Xiang QS, Masri B. Metal artifact reduction sequence: early clinical applications. Radiographics. 2000;20:699–712. doi: 10.1148/radiographics.20.3.g00ma10699. [DOI] [PubMed] [Google Scholar]
- 26.Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gibbons CL, Ostlere S, Athanasou N, Gill HS, Murray DW. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2008;90:847–851. doi: 10.1302/0301-620X.90B7.20213. [DOI] [PubMed] [Google Scholar]
- 27.Sutter R, Ulbrich EJ, Jellus V, Nittka M, Pfirrmann CW. Reduction of metal artifacts in patients with total hip arthroplasty with slice-encoding metal artifact correction and view-angle tilting MR imaging. Radiology. 2012;265:204–214. doi: 10.1148/radiol.12112408. [DOI] [PubMed] [Google Scholar]
- 28.Williams DH, Greidanus NV, Masri BA, Duncan CP, Garbuz DS. Prevalence of pseudotumor in asymptomatic patients after metal-on-metal hip arthroplasty. J Bone Joint Surg Am. 2011;93:2164–2171. doi: 10.2106/JBJS.J.01884. [DOI] [PubMed] [Google Scholar]


