Abstract
An adjuvanted recombinant subunit candidate vaccine (HZ/su) containing varicella zoster virus envelope glycoprotein E was developed for the prevention of herpes zoster and its complications. This study evaluated safety and reactogenicity of HZ/su in an ethnic Japanese population. This was a phase I, open-label and single-center study conducted between March and November of 2010 in Australia. Twenty healthy ethnic Japanese subjects, aged 18–30 y and 50–69 y (1:1) were enrolled. Subjects were administered two doses of HZ/su vaccine according to a 0, 2-mo schedule. Local and general solicited symptoms were recorded for 7 d post-vaccination. Unsolicited symptoms were recorded for 30 d post-vaccination. Serious adverse events (SAEs), new onset of autoimmune disease (NOAD), other potential immune mediated disorders and HZ cases were recorded throughout the study period. All 20 subjects were included in the according-to-protocol cohort for safety. A total of 18 subjects were included in the according-to-protocol cohort for immunogenicity: 10 in the 18–30 y age group and 8 in the 50–69 y age group. The most commonly reported local and general solicited symptoms were pain and fatigue in both groups. Back pain (in the 18–30 y age group) and chills (in the 50–69 y age group) were the most frequently reported unsolicited symptoms. There were no reports of death, SAEs, NOADs, other autoimmune mediated inflammatory disorder or suspected HZ cases. This study indicated that the two-dose regimen of HZ/su exhibited a clinically acceptable safety profile in healthy young and older ethnic Japanese adults.
Keywords: adjuvant, herpes zoster vaccine, Japanese, safety, reactogenicity, varicella-zoster virus
Introduction
Herpes zoster (HZ), or shingles, occurs as a result of the reactivation of latent varicella-zoster virus (VZV) in the dorsal or cranial root ganglia, typically many years after primary VZV infection is acquired during childhood as chickenpox.1 It is characterized by a vesicular, painful rash in a dermatomal distribution.2 In Japan, the reported incidence of HZ is 4.15 per 1000 person-years,3 similar to the incidence rates observed in the US and Europe.4-6 Consequently, approximately one in three people will develop HZ during their lifetime, and an estimate of one million HZ episodes occur annually in the United States.7 As a result, HZ is a significant public healthcare burden.8
HZ and its most common complication, postherpetic neuralgia (PHN), are characterized by acute and chronic pain, respectively, that interfere with normal day-to-day activities and lower the quality of life.9,10 Although HZ can occur at any age, the incidence of HZ and PHN increase with advancing age.1,7 HZ associated morbidity and mortality is highest in adults aged 50 y and older11 and in immunocompromised individuals.12,13
A live attenuated vaccine, Zostavax™ (Merck and Co., Inc.) is indicated against HZ and has been available since 2006 in the United States.7 The efficacy of this currently licensed vaccine is relatively low in the population most at risk (adults aged ≥ 70 y) and cannot be administered to adults who are immunocompromised due to disease or medication.14 A recombinant HZ subunit candidate vaccine containing VZV glycoprotein E (gE) adjuvanted with the proprietary GlaxoSmithKline Adjuvant System, AS01 (HZ/su), is under evaluation.15,16 VZV gE is the most abundant glycoprotein in VZV-infected cells, and is the primary prominent target of both cellular and humoral immune responses.17,18 AS01 has been evaluated in clinical trials of subunit malaria and HIV candidate vaccines and has an acceptable safety and reactogenicity profile.16
A recent phase I/II clinical trial demonstrated that HZ/su induced robust cellular and humoral immune responses in young (18–30 y) and older (50–70 y) adults and had a clinically acceptable safety profile.19 Upon the request of the Japanese regulatory authorities, this study was conducted to evaluate safety and reactogenicity of the HZ/su vaccine when administered to young (18–30 y) and older (50–69 y) Japanese adults.
Results
Demographics. Ten subjects were enrolled in both the 18–30 and 50–69 y age groups. Among these, 18 subjects were included in the ATP cohort for immunogenicity: 10 in the 18–30 y age group and 8 in the 50–69 y age group. Two subjects were eliminated from the 50–69 y age group, one for receiving a prohibited medication prior to the second vaccine dose and the other for not complying with the vaccination schedule. The mean age of subjects at dose-1 was 26.5 y (standard deviation [SD]: 1.78) in the 18–30 y age group and 57.3 y (SD: 4.95) in the 50–69 y age group. In both age groups, 50% of subjects were female.
Safety and reactogenicity.
Overall by doses.
During the 7-d post-vaccination period, solicited local symptoms were reported after all doses (100%) of HZ/su in the 18–30 y age group and following 95% of doses in the 50–69 y age group. Solicited general symptoms were reported after all doses of HZ/su in both age groups. Grade 3 solicited local symptoms were reported after 20% of doses in the 18–30 y age group and after 32% of doses in the 50–69 y age group. Grade 3 solicited general symptoms were reported after 45% of doses in the 18–30 y age group and after 11% of doses in the 50–69 y age group.
Pain was the most frequently reported solicited local symptom in both groups (Table 1). After two doses, grade 3 pain was observed following 20% of doses in the 18–30 y age group and following 5% of doses in the 50–69 y age group. The most frequently reported solicited general symptoms in the 18–30 y and 50–69 y age groups were fatigue (after 100% of doses in both age groups), myalgia (after 95% and 84% of doses, respectively) and headache (after 70% and 58% of doses, respectively) (Table 1).
Table 1. Incidence of solicited local and general symptoms during the 7-d post-vaccination period following two doses of HZ/su (total vaccinated cohort).
| Young adults (18–30 y) (20 administered doses) |
Older adults (50–69 y) (19 administered doses) |
||||||
|---|---|---|---|---|---|---|---|
| Symptoms | n | % | 95% CI | n | % | 95% CI | |
| Local symptoms | |||||||
| Pain | Any | 20 | 100 | 83; 100 | 17 | 90 | 66; 99 |
| Grade 3 | 4 | 20 | 5; 44 | 1 | 5 | 0; 26 | |
| Redness | Any | 3 | 15 | 3; 38 | 8 | 42 | 20; 67 |
| Grade 3 | 0 | 0 | 0; 17 | 5 | 26 | 9; 52 | |
| Swelling | Any | 7 | 35 | 15; 60 | 9 | 47 | 24; 72 |
| Grade 3 | 0 | 0 | 0; 17 | 2 | 11 | 1; 34 | |
| General symptoms | |||||||
| Fatigue | Any | 20 | 100 | 83; 100 | 19 | 100 | 82; 100 |
| Grade 3 | 6 | 30 | 11; 55 | 2 | 11 | 1; 34 | |
| Gastrointestinal | Any | 6 | 30 | 11; 55 | 6 | 32 | 12; 57 |
| Grade 3 | 0 | 0 | 0; 17 | 0 | 0 | 0; 18 | |
| Headache | Any | 14 | 70 | 45; 89 | 11 | 58 | 33; 80 |
| Grade 3 | 5 | 25 | 8; 50 | 1 | 5 | 0; 26 | |
| Myalgia | Any | 19 | 95 | 75; 100 | 16 | 84 | 60; 97 |
| Grade 3 | 4 | 20 | 5; 44 | 0 | 0 | 0; 18 | |
| Temperature/(Orally) (°C) | Any | 10 | 50 | 27; 73 | 10 | 53 | 28; 76 |
| Grade 3 | 0 | 0 | 0; 17 | 0 | 0 | 0; 18 | |
n (%), number (percentage) of doses followed by at least one symptom; 95% CI, Exact 95% confidence interval
Unsolicited symptoms were reported following 45% and 32% of doses of HZ/su in the 18–30 y and 50–69 y age groups, respectively. Back pain (after 15% of doses in the 18–30 y age group) and chills (after 11% of doses in the 50–69 y age group) were the most frequently reported unsolicited symptoms. Unsolicited symptoms that were considered to be related to the study vaccine occurred following 25% and 21% of doses in the 18–30 and 50–69 y age groups, respectively. Grade 3 unsolicited symptoms were not reported.
Overall by subject.
The overall incidence of symptoms (solicited and unsolicited) during the 7-d follow-up period was 100% in both age groups. Overall, grade 3 symptoms (solicited and unsolicited) were reported by 60% and 40% of the subjects in the 18–30 y and 50–69 y age groups, respectively.
Pain was the most commonly reported solicited local symptom during the 7-d follow-up period in both age groups (Fig. 1). The most common grade 3 solicited local symptoms were pain reported by 30% of subjects in the 18–30 y age group and redness reported by 30% of subjects in the 50–69 y age group, respectively (Fig. 1). Fatigue was the most commonly reported general symptom reported by 100% of the subjects in both age groups. Other commonly reported general symptoms were myalgia (100% in the 18–30 y age group and 90% in the 50–69 y age group) and headache (90% in the 18–30 y age group and 70% in the 50–69 y age group) (Fig. 1).
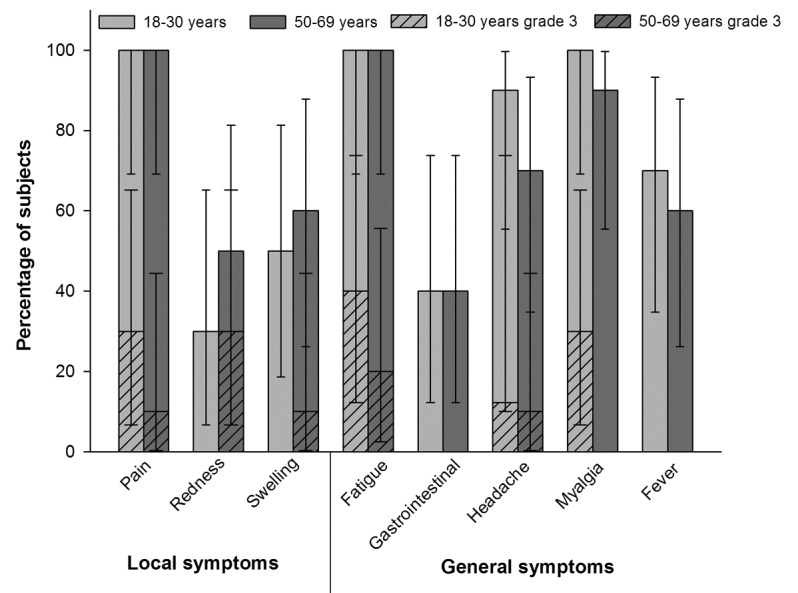
Figure 1. Percentage of subjects reporting solicited local and general symptoms during the 7-d post-vaccination period (total vaccinated cohort)
During the 30-d follow-up period, the overall incidence of unsolicited symptoms was 70% and 60% of subjects in the 18–30 y age and 50–69 y age groups, respectively. The most commonly reported unsolicited symptoms in the 18–30 y age group were back pain (20%) and upper respiratory tract infection (20%). In the 50–69 y age group, chills were the most common unsolicited symptom reported by 20% of subjects. The percentage of subjects reporting unsolicited symptoms related to vaccination were 30% in the 18–30 y age group and 40% in the 50–69 y age group. Unsolicited grade 3 symptoms were not reported during the 30-d post-vaccination period.
No deaths or SAEs were reported. NOADs or other autoimmune mediated inflammatory disorder were also not reported. There were no suspected HZ cases reported. No clinically significant hematological or biochemical abnormalities were reported.
Immunogenicity.
All subjects in the ATP cohort for immunogenicity in both age groups were seropositive for anti-gE antibodies before vaccination and remained seropositive for anti-gE antibodies post-dose-1 and post-dose-2. In the 18–30 y age group, the GMCs at baseline, post-dose-1 and post-dose-2 were 1392.1, 55064.6 and 75731.5, respectively (Fig. 2). Compared with baseline, the GMC was 54-fold higher post-dose-2 in the 18–30 y age group. Similarly, in the 50–69 y age group, the GMCs at baseline, post-dose-1 and post-dose-2 were 2122.8, 45389.4 and 65589.0, respectively (Fig. 2). The GMC compared with baseline was 31-fold higher post-dose-2 in the 50–69 y age group.
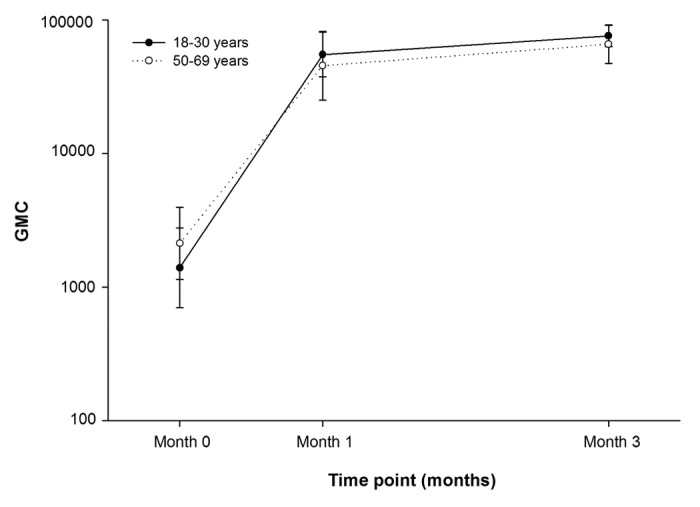
Figure 2. Geometric mean concentrations of anti-gE antibody pre-vaccination, post-dose-1 and post-dose-2 (ATP cohort for immunogenicity)
Anti-VZV antibody GMC was 13-fold higher post-dose-2 (17383.6) compared with baseline (1301.7) in the 18–30 y age group (Fig. 3). In the 50–69 y age group, the anti-VZV antibody GMC was 11-fold higher post-dose-2 (12883.3) compared with baseline (1284.4) (Fig. 3).
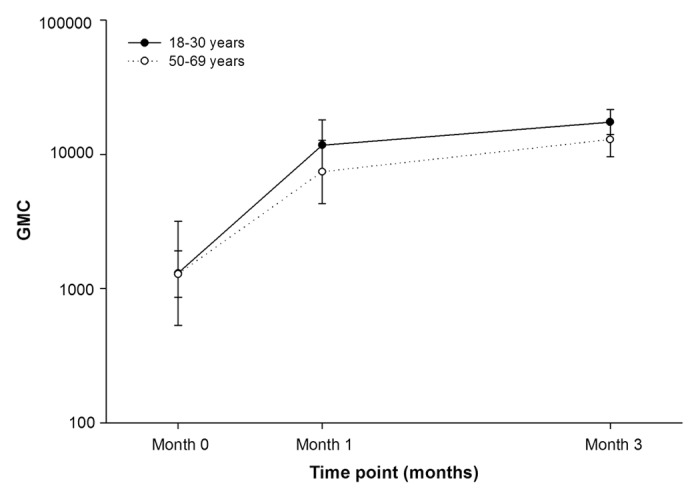
Figure 3. Geometric mean concentrations of anti-VZV antibody pre-vaccination, post-dose-1 and post-dose-2 (ATP cohort for immunogenicity)
Discussion
This is the first study to report the safety and immunogenicity profile of the candidate HZ/su vaccine when administered as a two-dose vaccination regimen in an ethnic Japanese population.
The results of this study demonstrate that the HZ/su vaccine has an acceptable safety profile in both young and older Japanese adults. Injection site pain was the most commonly reported solicited local symptom while fatigue, headache and myalgia were the commonly reported general symptoms, in both age groups. Most symptoms were of mild or moderate intensity. The observation that mild to moderate local and general reactions are common following HZ/su vaccination is in agreement with those previously reported in young (18–30 y) and older (≥ 50 y) adults, although grade 3 reactions appeared to be more common in this population, in particular for reported scores on pain, fatigue, headache, and myalgia.19,20 We consider that this incongruence may be due to chance findings in a small sample size, but it may also stem from a cultural difference in pain perception. Contrasting the higher grade 3 reactogenicity, no subjects withdrew from the study due to adverse events or intolerable reactogenicity. No vaccine-related safety issues, such as immune-mediated disorders or new onset of autoimmune disease, arose during the study. Therefore, the safety profile of the HZ/su vaccine in ethnic Japanese adults appears acceptable and consistent with that observed in other adult populations.16 Nonetheless, although beyond the scope of this study, there is scientific merit in using serological markers to further explore immune-mediated disorders after vaccination.
A humoral immune response was elicited by two doses of HZ/su in both young and older adults. The magnitude of these responses were comparable to those elicited by HZ/su in earlier studies, indicating that HZ/su immunogenicity is similar in ethnic Japanese compared with non-Japanese populations.19
Recombinant subunit vaccines are attractive alternatives to live vaccines given their potential advantage in eliciting a stronger immune response, a greater likelihood of an acceptable safety profile in immunocompromised individuals and their ease of production.21
This study should be interpreted in light of certain limitations. The modest sample size was based on expected findings concerning the primary objective, but restricts the generalizability of the study results to a larger Japanese population. Similarly, the inclusion of a placebo-group may have better explained the inconsistency between findings between this and other studies using the HZ/su vaccine candidate.19,20 Additionally, this study did not evaluate safety and immunogenicity of the vaccine in Japanese adults aged ≥ 70 y, although considerable data in this age group from non-Japanese populations have been generated, and previous data suggest that reactogenicity may be reduced in this population.20 Nonetheless, the results of the present study suggests that the two-dose regimen of HZ/su exhibited a clinically acceptable safety profile in healthy young and older ethnic Japanese adults.
Materials and Methods
Study design and subjects.
This phase I, open-label, single-center study was conducted between March and November of 2010, in Australia. Healthy ethnic Japanese subjects (who were born in Japan, spoke Japanese and had four ethnic Japanese grandparents), aged 18–30 y and 50–69 y (1:1 ratio) were enrolled. Subjects were administered two doses of HZ/su vaccine according to a 0, 2-mo schedule. Persons were not enrolled if they had received any investigational drug/vaccine 30 d prior to study vaccine administration, or if they had received any immunosuppressants or immune-modifying drugs six months prior to the first study vaccine dose, had a history of HZ or had previously been vaccinated against HZ, or had an allergy likely to be aggravated by any vaccine component. Persons were also excluded if they had symptoms of acute illness, or if they had an axillary temperature ≥ 37.5°C.
The guidelines of Good Clinical Practice, the Declaration of Helsinki and other applicable regulatory requirements were adhered to during the conduct of the study. All study-related documents were reviewed and approved by an Australian ethics committee. All adults provided written informed consent prior to performing any study-related procedures.
Study vaccine and administration.
HZ/su (GlaxoSmithKline Vaccines) is composed of the lyophilized form of VZV-gE antigen and a liquid formulation of the adjuvant system AS01 stored in separate vials and reconstituted within two hours before administration. All subjects were administered 0.5 ml of the reconstituted vaccine intramuscularly in the deltoid region.
Assessment of safety and reactogenicity.
Local solicited symptoms (pain, redness and swelling) at the injection site and general solicited symptoms (fatigue, fever, gastrointestinal symptoms [nausea, vomiting, diarrhea, and/or abdominal pain], headache and myalgia) were recorded during the 7-d post-vaccination follow-up period. Unsolicited adverse events (AEs) were recorded during the 30-d post-vaccination follow-up period. Serious adverse events (SAEs), new onset of autoimmune disease (NOAD), other potential immune mediated disorders and HZ cases were recorded throughout the study period (list provided in supplement). Intensity of symptoms was graded on a scale of 0–3. Grade 3 pain was defined as significant pain that prevented normal daily activity; grade 3 redness and swelling were defined as injection site surface diameter > 100 mm; grade 3 fever was defined as body temperature > 39.0°C. Other solicited general symptoms (headache, fatigue, gastrointestinal symptoms, myalgia) and unsolicited symptoms were graded as severe if they prevented normal daily activity. Hematological and biochemical parameters were recorded at pre-vaccination and at one month after each dose of HZ/su.
Assessment of immunogenicity.
Blood samples were collected before vaccination and one month after each dose of the HZ/su vaccine. Antibodies against gE were measured using enzyme-linked immunosorbent assay (ELISA) (assay cut-off was 18 mIU/ml). Antibodies against VZV were measured using a commercial ELISA kit (Enzygnost™, Dade Behring, Marburg, Germany) (assay cut-off was 25 mIU/ml).
Statistical analysis.
All statistical analyses were performed using SAS® version 9.1.3 and StatsXACT version 7.0. No formal sample size estimation was performed for the present study. A sample size of 20 subjects was defined, with 10 subjects each in the two age groups (18–30 y and 50–69 y).
Safety and reactogenicity analyses were performed on the total vaccinated cohort which included subjects who were vaccinated at least once with HZ/su vaccine. The immunogenicity analysis was performed on the according-to-protocol (ATP) cohort which included subjects who complied with all the study procedures (including meeting the exclusion and elimination criteria) and for whom the assay results were available at post-vaccination time-points. Seropositivity rates and the corresponding geometric mean concentrations (GMCs) for anti-gE and anti-VZV antibodies were described. A seropositive subject was a subject whose anti-gE antibody concentration and anti-VZV antibody concentration was ≥ 18 mIU/ml and ≥ 25 mIU/ml, respectively. The GMCs were calculated by taking the anti-log of the mean of the log concentrations transformations.
Trademarks
Zostavax is a registered trademark of Merck and Co., Inc. Enzygnost is a registered trademark of Dade Behring, Marburg, Germany.
Supplementary Material
Acknowledgments
This study (eTrack: 113819/NCT01086449) was sponsored and funded by GlaxoSmithKline Biologicals SA, Belgium. GlaxoSmithKline Biologicals SA also took charge of all costs associated with the development and publishing of the manuscript. The authors thank Jennifer Yuan for carrying out the clinical trial, Ashmita Ravishankar for medical writing and Jarno Jansen for editorial assistance and coordination in the preparation of this manuscript.
Glossary
Abbreviations:
- AE
adverse event
- ATP
according-to-protocol, ELISA, enzyme-linked immunosorbent assay
- gE
glycoprotein E
- HZ
herpes zoster
- mIU
milli international unit
- NOAD
new onset of autoimmune disease
- PHN
postherpetic neuralgia
- SAE
serious adverse event
- VZV
varicella-zoster virus
- HZ/su
AS01B-adjuvanted glycoprotein E vaccine
Disclosure of Potential Conflicts of Interest
All authors are employed by the GlaxoSmithKline group of companies. Thomas Heineman owns GSK stock and stock options.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/24269
References
- 1.Eshleman E, Shahzad A, Cohrs RJ. Varicella zoster virus latency. Future Virol. 2011;6:341–55. doi: 10.2217/fvl.10.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varicella vaccines. WHO position paper. Wkly Epidemiol Rec. 1998;73:241–8. [PubMed] [Google Scholar]
- 3.Toyama N, Shiraki K, Society of the Miyazaki Prefecture Dermatologists Epidemiology of herpes zoster and its relationship to varicella in Japan: A 10-year survey of 48,388 herpes zoster cases in Miyazaki prefecture. J Med Virol. 2009;81:2053–8. doi: 10.1002/jmv.21599. [DOI] [PubMed] [Google Scholar]
- 4.Chapman RS, Cross KW, Fleming DM. The incidence of shingles and its implications for vaccination policy. Vaccine. 2003;21:2541–7. doi: 10.1016/S0264-410X(03)00034-3. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez Chiappe S, Sarazin M, Turbelin C, Lasserre A, Pelat C, Bonmarin I, et al. Herpes zoster: Burden of disease in France. Vaccine. 2010;28:7933–8. doi: 10.1016/j.vaccine.2010.09.074. [DOI] [PubMed] [Google Scholar]
- 6.Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82:1341–9. doi: 10.4065/82.11.1341. [DOI] [PubMed] [Google Scholar]
- 7.Harpaz R, Ortega-Sanchez IR, Seward JF, Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC) Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2008;57(RR-5):1–30, quiz CE2-4. [PubMed] [Google Scholar]
- 8.Gershon AA, Gershon MD, Breuer J, Levin MJ, Oaklander AL, Griffiths PD. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J Clin Virol. 2010;48(Suppl 1):S2–7. doi: 10.1016/S1386-6532(10)70002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmader KE, Levin MJ, Gnann JW, Jr., McNeil SA, Vesikari T, Betts RF, et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50-59 years. Clin Infect Dis. 2012;54:922–8. doi: 10.1093/cid/cir970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray AV, Reisinger KS, Kerzner B, Stek JE, Sausser TA, Xu J, et al. Safety and tolerability of zoster vaccine in adults ≥60 years old. Hum Vaccin. 2011;7:1130–6. doi: 10.4161/hv.7.11.17982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. 2009;84:274–80. doi: 10.4065/84.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrei G, Snoeck R. Emerging drugs for varicella-zoster virus infections. Expert Opin Emerg Drugs. 2011;16:507–35. doi: 10.1517/14728214.2011.591786. [DOI] [PubMed] [Google Scholar]
- 13.Oxman MN. Zoster vaccine: current status and future prospects. Clin Infect Dis. 2010;51:197–213. doi: 10.1086/653605. [DOI] [PubMed] [Google Scholar]
- 14.Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, et al. Shingles Prevention Study Group A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–84. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 15.Garçon N, Chomez P, Van Mechelen M. GlaxoSmithKline Adjuvant Systems in vaccines: concepts, achievements and perspectives. Expert Rev Vaccines. 2007;6:723–39. doi: 10.1586/14760584.6.5.723. [DOI] [PubMed] [Google Scholar]
- 16.Garçon N, Van Mechelen M. Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert Rev Vaccines. 2011;10:471–86. doi: 10.1586/erv.11.29. [DOI] [PubMed] [Google Scholar]
- 17.Grose C. Glycoproteins encoded by varicella-zoster virus: biosynthesis, phosphorylation, and intracellular trafficking. Annu Rev Microbiol. 1990;44:59–80. doi: 10.1146/annurev.mi.44.100190.000423. [DOI] [PubMed] [Google Scholar]
- 18.Grahn A, Studahl M, Nilsson S, Thomsson E, Bäckström M, Bergström T. Varicella-zoster virus (VZV) glycoprotein E is a serological antigen for detection of intrathecal antibodies to VZV in central nervous system infections, without cross-reaction to herpes simplex virus 1. Clin Vaccine Immunol. 2011;18:1336–42. doi: 10.1128/CVI.05061-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leroux-Roels I, Leroux-Roels G, Clement F, Vandepapelière P, Vassilev V, Ledent E, et al. A phase 1/2 clinical trial evaluating safety and immunogenicity of a varicella zoster glycoprotein e subunit vaccine candidate in young and older adults. J Infect Dis. 2012;206:1280–90. doi: 10.1093/infdis/jis497. [DOI] [PubMed] [Google Scholar]
- 20.Chlibek R, Smetana J, Pauksens K, Van den Hoek A, Richardus JH, Wachter J, et al. Safety and immunogenicity of three different formulations of an adjuvanted varicella-zoster virus subunit candidate vaccine in older adults. Presented at the 37th Annual International Herpesvirus Workshop (IHW), Canada, August 4–9, 2012. Available at: http://www.herpesvirusworkshop.com/2012/ Accessed: November 2nd, 2012.
- 21.Clark TG, Cassidy-Hanley D. Recombinant subunit vaccines: potentials and constraints. Dev Biol (Basel) 2005;121:153–63. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


