Abstract
Objectives: Economic evaluations on influenza vaccination from low resource settings are scarce and have not been evaluated using a systematic approach. Our objective was to conduct a systematic review on the value for money of influenza vaccination in low- and middle-income countries.
Methods: PubMed and EMBASE were searched for economic evaluations published in any language between 1960 and 2011. Main outcome measures were costs per influenza outcome averted, costs per quality-adjusted life years gained or disability-adjusted life years averted, costs per benefit in monetary units or cost-benefit ratios.
Results: Nine economic evaluations on seasonal influenza vaccine met the inclusion criteria. These were model- or randomized-controlled-trial (RCT)-based economic evaluations from middle-income countries. Influenza vaccination provided value for money for elderly, infants, adults and children with high-risk conditions. Vaccination was cost-effective and cost-saving for chronic obstructive pulmonary disease patients and in elderly above 65 y from model-based evaluations, but conclusions from RCTs on elderly varied.
Conclusion: Economic evaluations from middle income regions differed in population studied, outcomes and definitions used. Most findings are in line with evidence from high-income countries highlighting that influenza vaccine is likely to provide value for money. However, serious methodological limitations do not allow drawing conclusions on cost-effectiveness of influenza vaccination in middle income countries. Evidence on cost-effectiveness from low-income countries is lacking altogether, and more information is needed from full economic evaluations that are conducted in a standardized manner.
Keywords: systematic review, influenza, vaccines, economic evaluation, developing countries, cost analysis
Introduction
Influenza can present as seasonal or pandemic occurrence and both are associated with severe health and economic consequences. Seasonal influenza is an important cause for morbidity and mortality globally and responsible for a significant health and economic burden.1,2 During the 2009 H1N1 influenza pandemic, approximately 414,000 pandemic influenza A (H1N1) cases and 5,000 deaths were reported to the World Health Organization (WHO) from over 207 countries, overseas territories or communities.3,4 For its recommendations on seasonal influenza vaccination, WHO mainly relied on economic evidence from high-income countries (HIC).5 However, context specific knowledge on the economic impact of influenza from low- and middle-income countries (LMICs) is important for national vaccine prioritization since the related costs affect the individual, health services, and society. Costs can result from direct costs of medical care such as medication use and hospitalization as well as from indirect costs associated with, e.g., work or school absenteeism. Vaccination can reduce these costs both in vaccinees and in their contacts within households and the community who may otherwise have been infected by them.
Inactivated and live-attenuated influenza vaccines have been available for more than 50 y and have an excellent safety profile.5 Both inactivated and live-attenuated influenza vaccines are effective in preventing severe disease and its complications and have the potential to increase societal productivity while reducing suffering and mortality during influenza epidemics.6 In response to the H1N1 pandemic, over 30 inactivated (including adjuvanted) and live-attenuated vaccines have been developed and licensed. Between September and December 2009 tens of millions of doses of the pandemic (H1N1) vaccine were administered worldwide.7 WHO recommends that countries prioritize risk groups for seasonal influenza vaccination based on disease burden, cost-effectiveness, feasibility and other considerations.5 However, country target groups selected for pandemic and seasonal vaccines as well as the general use of influenza vaccines varies greatly across nations and is less in LMICs.8,9 This may partly be because little is known about medical, contact and/or social behavior patterns and socioeconomic consequences of influenza and influenza vaccination in these regions and with regard to specific population groups. This lack of published data may have contributed to the low awareness and recognition of influenza and consequent limited vaccine coverage and uptake in these countries.5 Existing economic studies and systematic reviews of influenza vaccine cost-effectiveness and cost-benefit analysis were conducted in HICs, such as the US,10-14 England and Wales,15 France,15 Germany,15 The Netherlands16 and New Zealand.17 These evaluations found that vaccination is cost-effective and frequently cost-saving among different population groups including the elderly,12,15,16 healthy working adults,13,14 children10,11,18 and pregnant women.19,20 In contrast, fewer analyses and a review concluded that, depending on population, vaccination is not cost-saving.21,22 However, the findings of studies from HICs might not always be applicable to LMICs.
Value for money (economic value) is often assessed by decision analytic models, which include decision trees, Markov state-transmission models and dynamic transmission models.23,24 There are various types of economic evaluations that can be performed, such as cost-effectiveness analyses, cost-utility analyses and cost-benefit analyses. These analyses may be performed alongside clinical effectiveness trials or may be purely based on decision analytic models.
Cost-effectiveness analyses compare the incremental cost of an intervention to an alternative use of resources, with the incremental health outcomes of that intervention. Cost-benefit analyses convert both the costs (resources needed) for an intervention and its benefits into monetary terms, and present results in terms of a ratio of net costs to net benefits. Cost-utility analyses are a special case of cost-effectiveness analyses where outcomes are presented in terms of generic health-related utilities, such as quality adjusted life years (QALYs) or disability adjusted life years (DALYs) to allow comparability between different diseases. Costs can be considered from various perspectives, such as the health care provider, employer or society, and can include both direct and indirect costs.
Several variables impact on cost-effectiveness of influenza vaccine but are frequently not taken into account. These variables include the location where the vaccine is administered, with lower costs for vaccination in nonmedical vs. medical settings, the timing of vaccination with the earliest time point of an influenza season being the most cost-effective23 and the duration of protection achieved by vaccination.
The majority of economic evaluations on influenza vaccine have been performed in HICs and to our knowledge, no systematic review of literature on the economic analysis of influenza vaccination in LMICs has been performed. Therefore, our aim was to conduct a detailed review of literature on economic evaluations available from and for these countries. With this systematic review, we assessed whether seasonal influenza and pandemic (H1N1) 2009 influenza vaccines in vaccinated groups in LMICs is beneficial in terms of monetary value, in combination or not, with effects, consequences or utilities. The objectives were addressed for different influenza-vaccinated population groups separately and for low- vs. middle-income countries, as available.
Materials and Methods
Major electronic databases including PubMed and EMBASE.com were searched (last search June 9, 2011). Two search strategies were developed in collaboration with two clinical librarians, and using economic evaluation criteria according to WHO Guidelines on standardization of economic evaluations for immunization programs.26 The first search strategy aimed to capture economic evaluations and the second strategy searched for studies on effectiveness. For the economic evaluation search the following search terms were used (with synonyms and closely related words) as thesaurus terms and free text terms: “human influenza” and “vaccination” and “economic evaluations” and terms for LMICs. Terms for LMICs were developed in a collaborative effort of the Norwegian Satellite of the Cochrane Effective Practice and Organisation of Care Group, the WHO library and volunteers outside the Cochrane collaboration.25 For the effectiveness search, thesaurus terms and free text terms included “human influenza” and “vaccination” and “effectiveness” or “influenza outcomes.” Both the economic evaluation search strategy and the effectiveness search strategy were used to identify economic evaluation studies. For more detail on the search strategies from PubMed, see Appendix 1. The full search strategies are available upon request. In addition, vaccine manufacturers and vaccine institutes were contacted for unpublished data and post marketing surveillance studies on effectiveness and cost-effectiveness of seasonal and pandemic influenza vaccines in LMICs.
Search results were managed using Reference Manager version 11. Duplicate records were removed, and first titles and then abstracts were screened by two reviewers independently. Studies selected by the two reviewers were compared, and in case of disagreement, another contributor was consulted for arbitration. Full text of citations not excluded in the first step was examined for eligibility. Studies were included when published between 1960 and 2011 and in any language. Studies were excluded based on titles when it became clear from the titles that the study was performed in a high-income country; or described vaccines other than licensed seasonal influenza vaccines or pandemic A (H1N1) 2009 influenza vaccines. The following eligibility criteria for assessing the inclusion of abstracts and full texts were set in advance:
Participants: populations of any age, sex and ethnic origin in LMICs (Appendix 2).
Intervention: licensed seasonal and pandemic A (H1N1) 2009 influenza vaccines (both inactivated and live attenuated, independent of number of doses).
Comparison: No vaccination, placebo, other influenza vaccines. Studies were excluded when influenza vaccination was compared with other influenza preventive measures.
Outcomes: (1) Costs per laboratory-confirmed influenza or influenza-like outcomes prevented (cost-effectiveness analyses). (2) Costs per quality-adjusted life years (QALYs) gained or disability-adjusted life years (DALYs) averted (cost-utility analyses). (3) Costs per benefit expressed in monetary units and cost-benefit ratios (cost-benefit analyses). Economic modeling studies were excluded when no data could be derived on the costs per influenza outcome, QALYs, DALYs or benefit.
Types of economic evaluation: Cost effectiveness analysis, cost-utility analysis or cost-benefit analysis. Partial economic evaluations were excluded.
Data were extracted on economic characteristics of the studies (e.g., evaluation type, perspective, discounting), vaccination and comparison groups, countries, vaccine type, influenza outcomes and costs assessed, assumptions made and value for money results. Results
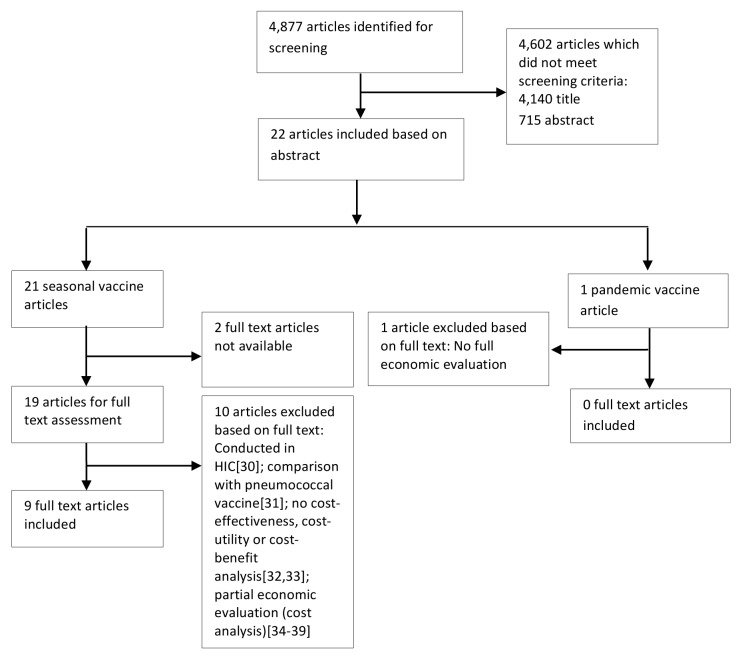
The search on effectiveness and economic evaluations of influenza vaccines in LMICs resulted in a total of 4,877 citations, of which 4,140 were excluded following review of titles. Out of 737 manuscripts left for screening, 463 did not meet the inclusion criteria. No studies were identified via vaccine manufacturers or vaccine institutes. Potentially eligible studies included economic evaluation studies on either seasonal (21) or pandemic vaccines (1). The latter was excluded since it was not a full economic evaluation and modeled the economic impact of the pandemic (H1N1) 2009 influenza on the society by means of estimating costs of a possible influenza pandemic under different scenarios and attack rates.27 Relevant economic evaluation studies of seasonal vaccines were published in the English (12), Spanish (3), Russian (3) and Chinese (3) languages. Twelve of these studies had to be excluded because the full text was not accessible in any way and the abstract did not provide sufficient information on results,28,29 because the study was conducted in a HIC,30 because the study assessed costs of influenza vaccine co-administered with pneumococcal vaccine,31 because no cost-effectiveness, cost-utility or cost-benefit analysis was performed,32,33 or because the study was a cost analysis conducted from an employer's perspective.34–39 Despite the fact that this latter group of excluded articles34–39 did not describe full economic evaluations and showed a lack of transparency regarding the information on costs, calculations or outcomes, some relevant information on cost savings of influenza vaccine including vaccine costs and/or indirect work absenteeism and presenteeism costs, was provided as summarized in Appendix 3 (Fig. 1).
Figure 1. Flow diagram of economic evaluations on seasonal and pandemic A (H1N1) 2009 influenza vaccines in LMICs.
Finally, nine studies on cost-effectiveness of seasonal influenza vaccines in LMICs were deemed eligible and included in this review. These studies varied by population group studied, methods and design [model-based (five studies) or RCT-based (four studies)] and outcomes assessed (Table 1) as well as by costs and value for money (Table 2). All eligible studies were conducted in middle-income countries, six of them were CEAs, two were CBAs, and one was a CUA. In the majority of evaluations (four), the societal perspective was chosen. Two studies were conducted from a health care provider perspective and two other studies from a third party public payer perspective. The included studies are described in detail by study design and main outcomes in the following.
Table 1. Study characteristics of included studies.
| Source | Objective | Type | Based on | Currency | Analytic horizon | Dis-counting | Influenza outcome | Impact | Uncertainty analysis | Funding source |
|---|---|---|---|---|---|---|---|---|---|---|
| Aballéa et al.40 | Economic evaluation of lowering the age threshold in various countries for influenza vaccination from currently 60 or 65 y to all people aged 50 y and older | CUA | Model | Brazilian real | Not reported | Life expectancy in unadjusted life years and quality-adjusted life years (3% per annum) | ILI; minor complications; hospitalization for pneumonia and influenza, for other respiratory complications, and other complications; and deaths | QALY gained | One-way sensitivity/ threshold analysis and PSA | IVS International Task Force |
| Chicaiza-Becerra et al.41 | Cost-effectiveness evaluation of implementing a vaccination program for health workers in contact with hospitalized oncological patients | CEA | Model | Colombian peso | Not reported | Not applicable | Days hospitalized for influenza-related complications | Cost-effectiveness ratio (additional days of hospitalization prevented) | One-way sensitivity analysis for different transmission probabilities | Not specifically mentioned |
| Dayan et al.24 | To compare from the societal perspective the costs and benefits of a general influenza vaccination program in high-risk children with no vaccination | CEA | Model | US dollars | 12 mo | Not applicable | Influenza outcomes including outpatient visits, otitis media and hospitalization | influenza episodes averted | One-way sensitivity/ threshold analysis and PSA | Not specifically mentioned |
| Gutiérrez et al.43 | Estimation of costs and health outcomes achievable by vaccination of people aged 65 y and older | CEA | Model | Mexican peso | 5 mo | Costs per life year saved by different discount rates (3% assumed as bases, estimates provided for 0%, 5%, 10%) | Pneumonia-and influenza-associated deaths and cases | Life years saved, costs per life year saved | PSA | National public health institute |
| Porras-Ramirez et al.42 | Evaluating the economic value of influenza vaccination of infants aged below 2 y and elderly aged 65 y and above | CEA | Model | US dollars | 1 y | 3% discounting | Among below 2 y old: annual number of cases of ARI, medical visits, hospitalizations and deaths from ARI Among 65 y and above: annual number of deaths and hospitalizations due to cardiocirculatory diseases. |
ARI-related hospitalization prevented, direct costs saved annually | Univariate sensitivity analysis | Ministry for social protection |
| Gao et al.46 | To assess cost benefit of vaccinating employers insured under a social-health program; results extrapolated to the whole population of the city | CBA | RCT | Chinese yuan | 5 y | Not reported | Respiratory system diseases and other chronic diseases such as cardiovascular diseases | Respiratory system disease and cardio-vascular diseases related hospitalization costs averted | Sensitivity analysis for vaccination coverage | Not specifically mentioned |
| Liu et al.47 | To compare vaccinated with non-vaccinated elderly and estimate effectiveness. | CBA | RCT | Chinese yuan | 6 mo | Not reported. | ILI incidence and related chronic diseases such as common cold, other respiratory tract or chronic disease such as diabetes | Medical consultations and number of hospital day-related costs averted | None performed | Not specifically mentioned |
| Praditsuwan et al.44 | To determine cost effectiveness of influenza vaccination in the Thai elderly living in an urban community compared with placebo | CEA | RCT | Thai baht | Not reported | Not reported | ILI, upper respiratory infection, and serologically confirmed influenza. | Unclear | None performed | National Research Council of Thailand |
| Wongsurakiat et al.45 | To determine cost- effectiveness and cost-benefit of influenza vaccination in COPD patients compared with placebo | CEA | RCT | Thai baht | 16–18 mo | Not applicable | Influenza-like outcomes including episodes of acute respiratory illness | Acute respiratory infection episodes averted | None performed | National Research Council of Thailand |
CBA, cost-benefit analysis; CUA, cost-utility analysis; CEA, cost-effectiveness analysis; QALY, quality adjusted life years; PSA, probabilistic sensitivity analysis; ILI, influenza-like illness.
Table 2. Value for money of included studies.
| Source | Participants | Country | Income category* | Perspective | Comparison | Total direct, indirect costs | Effects, consequences, utilities | Value for money | Sensitive to |
|---|---|---|---|---|---|---|---|---|---|
| Aballéa et al.40 | Elderly, 50–59 y | Brazil | uMIC | Third-party public payers | Current policy: vaccinating all elderly 55–59 y | R$ 6.5 millions | 41,006,895 QALYs | R$ 4,075 per QALY gained (US$ 1,327 based on nominal exchange rate in 2003 as reported from manuscript) |
|
| Proposed policy: vaccinating all elderly 50–59 y | R$ 16.91 millions | 41,009,449 QALYs | Incidence of ILI consultations, setting and number of work days lost | ||||||
| Societal | Current policy: vaccinating all elderly 55–59 y | R$ 56.38 millions | 41,006,895 QALYs | R$ 2,805 per QALY gained (US$ 914) |
|||||
| Proposed policy: vaccinating all elderly 50–59 y | R$ 63.55 millions | 41,009,449 QALYs | |||||||
| Chicaiza-Becerra et al.41 | Health care worker in close contact with oncological patients | Colombia | uMIC | Payers | Vaccination vs. no vaccination program | 232,000,000 pesos vaccination cost per 10,000 health care workers; 3,000,000 pesos per day of hospitalization | 10,632 d of hospitalization averted | Cost-effectiveness indicator/ratio of 2,978,000 pesos (US$ 1,324) saved per additional day of hospitalization prevented | Transmission probability |
| Dayan et al.24 | Children 6 mo to 15 y with high-risk conditions | Argentina | uMIC | Societal | Vaccination | US$ 39,286,244 | 88,856 cases | US$ 57.36 saved per influenza episode averted; net benefit to society US$ 11,894,870 or US$ 10.04 per vaccinated child | vaccine efficacy, influenza incidence and vaccine cost |
| No vaccination | US$ 51,181,114 | 296,187 cases | |||||||
| Gutiérrez et al.43 | Elderly aged 65 y and above | Mexico | uMIC | Societal | Universal vaccination program; vaccine effectiveness 20% or 30% in preventing deaths (20% in preventing cases) | Vaccine-related costs of 204,876,000 Mexican pesos;a hospitalization for pneumonia per case 12,183 Mexican pesos | 7,454–11,169 life years saved by establishing a universal vaccination program | 13,303–21,037 Mexican pesos net costs per life year saved (US$ 1210–1910) | Vaccine effectiveness, hospitalization costs |
| Porras-Ramirez et al.42 | Infants aged below 2 y and elderly aged 65 y and above | Colombia | uMIC | Societal | Vaccination | Costs associated with influenza virus infection including different direct costs: S$ 35.75–74.24 million per year | 334–1,232 ARI-related hospitalizations prevented in below 2-y-old infants; 9,747–10,263 ARI-related hospitalizations prevented in above 65 y old |
US$ 49.5–104.1 million saved implying a 59% cost reduction | Geographical region |
| No vaccination | Costs associated with influenza virus infection including different direct costs: US$ 85.29–178.44 million per year | 428–1,579 ARI-related hospitalizations in below 2-y-old infants; 16,805–34,763 in above 65 y old |
|||||||
| Gao et al.46 | 2,949 employers (employed and retired) ensured under health security program, extrapolated to a Chinese city | China | uMIC | Public health insurance/ societal | Vaccination | Av. hospitalization costs per person 358.42 Yuen (US$ 43.29); vaccine costs per person 32 Yuen (US$ 3.87); direct costs (whole city): 61,950,426 Yuen; indirect costs (whole city): 13,326,642 Yuen; total costs: 75,277,068 Yuen |
Benefit/return of investment: 63,661,724 Net benefit: 11,615,344 (total costs-return of investment) |
Cost-benefit ratio for reducing hospitalization rate 6.48 at 60% vaccine coverage; 81 million Yuen (US$ 9.78 million, reference year 2005) saved |
Vaccine coverage |
| No vaccination | Av. hospitalization costs per person: 553.21 Yuen (US$ 66.81) | ||||||||
| Liu et al.47 | Elderly | China | uMIC | Societal | Vaccination | Direct and indirect vaccination costs per person 67.45 Yuen (US$ 8.16) | Benefit/return of investment by disease category considered (p.P.): ILI: 0.0, respiratory infections: -0.28; CVD: 4.47; diabetes: 0.09 |
Cost-benefit ratio for reducing ILI and related diseases in the elderly 4.98 | No sensitivity analysis |
| No vaccination | No vaccination costs | ||||||||
| Praditsuwan et al.44 | Community-dwelling elderly aged above 60 y | Thailand | uMIC | Health care provider | Vaccination | 4,660 baht for ILId 12,885 baht for URId | Effectiveness (ILI) = 56% Effectiveness (URI) = -22.8% | Uncleare | No sensitivity analysis |
| Placebo | 4,650 baht for ILId 9,060 Baht for URId | ||||||||
| Wongsurakiat et al.45 | COPD patients | Thailand | uMIC | Health care provider | Vaccination | 37,343.6 bahtb | Effectiveness = 20.5% | 1001.6 baht (US$ 21.80, reference year 1998) per episode of ARI related to influenza per yearc | No sensitivity analysis |
| Placebo | 320,680 baht |
World Bank income categories (August 5, 2011). uMIC, upper middle-income country ($3,976 to $12,275); based on the World Bank income categories (August 5, 2011). No studies in the categories; LIC, low-income country (GNI per capita $1,005 or less) or lower MIC, lower middle-income country ($1,006 to $3,975). aCalculated as vaccination-related costs per dose (vaccine, transportation and administration) (37.8 Mexican pesos) x population above 65 y (5.42 millions). bCalculated as total reported costs (21,918 baht) + vaccine costs (15,401 baht) cCalculation based on vaccine price only. Total direct and indirect costs were measured; however, they were not included in the calculation of cost-effectiveness. dNot clear how total expenses are calculated and which costs are included.
Model-based analyses
The model-based analyses included were performed for Argentina,24 Brazil,40 Colombia41,42 and Mexico.43 Aballéa et al.40 conducted a cost-utility analysis to study the incremental costs per QALY gained when lowering the age threshold in Brazil, Germany, Italy and France for influenza vaccination among elderly aged 50 to 59 y (Brazil) and 50–64 y (other countries) compared with a policy of vaccinating elderly aged 60 y or older and 65 y or older, respectively. Direct costs considered were those of vaccination, primary care and hospitalization paid for by the third party. Costs were additionally considered from a societal perspective in which patient co-payments and working absenteeism costs were addressed. The main influenza outcomes were influenza-like illness (ILI), minor complications, hospitalizations for pneumonia and influenza, for other respiratory complications, and other complications and deaths. Value for money was assessed by comparing incremental costs per QALY gained with the value of the Gross Domestic Product (GDP) per capita of Brazil. Compared with the GDP per capita, extending the vaccination program to 50–59 y aged elderly was cost-effective in terms of utilities with a probability of 83% from a third-party payers perspective and 79% from a societal perspective, with incremental costs of R$4075 (US$ 1327, ref. year 2003, exchange rate as reported in manuscript) and R$2805 (US$ 914) per QALY gained, respectively. The costs per QALY outcomes were sensitive to incidence of ILI, number of workdays lost and setting.
The model-based cost-effectiveness study by Dayan et al.24 focused on children aged 6 mo to 15 y at high risk of influenza complications (hemodynamically significant heart disease, asthma, cystic fibrosis, HIV-infected, chemotherapy and insulin-dependent diabetes) in Argentina and compared influenza vaccination with no vaccination from a societal perspective. Direct medical costs included were vaccine costs, hospitalization costs, costs of outpatient care (including physician consultations, anti-pyretic therapy, X-rays, amantadine treatment and acute otitis media treatment) and costs for treatment of adverse events due to vaccination for which outpatient visits were needed. Sensitivity analyses included vaccine effectiveness, incidence of influenza infection, vaccine price, vaccination coverage and hospitalization costs. This study was one of the few that additionally included indirect costs including parental absence from work. Cost-effectiveness was assessed as differences in costs between the non-vaccinated and vaccinated population, and it was concluded that vaccinating high-risk children was cost-effective with US$ 57.36 saved per influenza episode averted. Net cost savings to society were lost when the vaccine price was above US$ 20 per dose, when influenza incidence was 14% or less, or when vaccine efficacy was less than 35%.
Gutiérrez et al.43 estimated net vaccine costs by including vaccination-related costs comprising of vaccine price, delivery and vaccine administration as well as costs for pneumonia-related hospitalization. Vaccination of individuals aged 65 y and older living in Mexico could save between 7454 and 11169 life years, leading to net costs per life year saved between 13,301 and 21,037 Mexican pesos (US$ 1210 and 1910, respectively). The two scenarios assessed varied by vaccine effectiveness (20% vs. 30% against deaths). The sensitivity analyses included vaccine coverage, vaccine effectiveness to prevent influenza/pneumonia deaths and cases and hospitalization costs for pneumonia.
Studies from Columbia also concluded that influenza vaccination would be cost-effective, however, focusing on different groups being vaccinated and from different perspectives. Chicaiza-Becerra et al.41 evaluated the implementation of a vaccination program for health workers in close contact with hospitalized oncological patients. Vaccination costs per 10,000 vaccinated health care personnel and average costs for hospitalization of oncological patients that have contracted influenza virus, were included. The input data were transmission probabilities, number of health personnel in contact with oncological patients, annual numbers of hospitalized cancer patients and days of hospitalization per cancer patient plus additional days due to influenza complications. From a payer's point of view, the vaccine program introduction could result in cost savings up to 2,978,000 Colombian pesos (US$ 1,324) due to additional days of hospitalization averted. In consequence, such a program could avert 10,632 d of hospitalization, although it was not clearly stated how this result was obtained. The second Colombian study42 analyzed the incremental costs-effectiveness ratio for vaccinating children below 2 y of age and elderly above 65 y. Influenza outcomes and direct cost components included varied by target group studied. For children, the annual number of acute respiratory infection (ARI) cases, medical visits, hospitalizations and deaths from ARI were considered together with ARI-related hospitalization and medical visit costs. For the elderly, the annual number of deaths and hospitalizations due to cardiocirculatory diseases were considered as well as ARI-related hospitalization costs. It was found that influenza infection associated costs could be reduced by 59% if vaccination for the target groups was implemented. Vaccination appeared to be highly cost-effective for the infant age-group and cost-saving for the age-group above 65 y. The incremental cost-effectiveness ratio for influenza vaccination in the infant age group ranged from US$ 1,900 to 2,967 per averted death. The number of preventable ARI-related hospitalizations among infants below 2 y of age was estimated to be between 334 and 1232 and among elderly between 9747 and 10263. Results from the sensitivity analysis indicate robustness of all parameters studied between two different geographical scenarios.
RCT-based analyses
The RCT-based cost-effectiveness and cost-benefit analyses included were conducted in Thailand44,45 and China.46,47
Wongsurakiat et al.45 compared chronic obstructive pulmonary disease (COPD) patients vaccinated with inactivated influenza vaccine to a group receiving placebo. Discounting and sensitivity analyses were not performed, and cost-effectiveness was evaluated by means of cost differences between vaccinated and placebo group with regard to episodes of ARI averted. In the cost-effectiveness analysis only the vaccine price was included and no hospitalization or outpatient costs. The conclusion was that the vaccine is cost-effective and cost-saving for COPD patients; however, it is not obvious which costs were compared with the costs per episode prevented.
The second RCT-based cost-effectiveness analysis was conducted for inactivated influenza vaccination of community-dwelling elderly aged 60 y or older compared with a placebo group.44 Outcomes assessed were ILI, upper respiratory infection and serologically confirmed influenza. No discounting was reported, and sensitivity analyses were not performed. By including vaccine price, total expenses of ILI and of upper respiratory infections into the costs, the authors concluded that it may not be cost-effective to recommend vaccination of community-dwelling elderly aged 60 y and above.
Both Chinese RCTs were cost-benefit analyses. Gao et al.46 looked at cost-benefits of vaccinating employees insured under a social health program and extrapolated the effects to the population of a Chinese city. Liu et al.,47 on the other hand, focused on cost-benefits of vaccinating elderly. The trials considered outcomes such as ILI and several other chronic diseases including cardio-vascular diseases (CVD) and diabetes. In Gao et al.46 direct costs included were hospitalization costs, vaccine and vaccine-related costs for cold chain, administration and transportation, as well as treatment costs for adverse vaccine events. Indirect costs of work absenteeism, such as average salary per day of absence, were also considered. At vaccine coverage of 60%, Gao et al.46 found a cost-benefit ratio for reducing hospitalization related to respiratory system diseases and CVD of 6.48 with costs saved of 81 million Yuen (US$ 9.78 million in 2005). Vaccine coverage above 60% did not significantly impact on the cost-benefit ratio.
By comparing differences in rates of ILI and ILI-related clinic visits, respiratory diseases and chronic diseases (CVD, diabetes) between vaccinated and non-vaccinated elderly in relation to total cost estimates, Liu et al.47 reported a summary cost-benefit ratio of 4.98 achieved by vaccination. However, a miscalculation ignoring the negative benefit for respiratory infections is likely. Looking at disease-specific cost-benefit ratios, the highest benefit was achieved for CVD (4.47), but there was none for ILI (0.00) and respiratory infections (-0.28). The total cost estimate was based on vaccine-related costs, such as vaccine price, administration and transportation, as well as costs for treatment of adverse vaccine events (Table 1; Table 2).
Discussion
This comprehensive systematic review provides findings on the value for money of seasonal influenza vaccination in LMICs as obtained from nine studies conducted in upper middle-income countries. One additional article on pandemic (A/H1N1) influenza vaccination was available,20 but this only described a partial economic evaluation providing costs of a possible influenza pandemic, and was therefore excluded.
Overall, the articles included indicate cost-effectiveness of seasonal influenza vaccination in elderly, children with high-risk conditions, infants, health care personnel and COPD patients in MICs. Out of the nine economic evaluations of seasonal influenza vaccine in LMICs, five were model-based economic studies and suggest that, compared with different alternative scenarios or current situation, seasonal influenza vaccination could provide value for money for elderly aged 50–59 y compared with elderly of 55–59 y, elderly above 65 y of age, children with high-risk conditions of six mo to 15 y compared with no vaccination, infants below 2 y of age and health care personnel in contact with oncological patients. The evidence on value for money is in line with investigations from some high-income countries, where influenza vaccination was found to be cost-effective among elderly, healthy adults, children and pregnant women.10-20 Vaccination even resulted in cost savings per influenza episode averted instead of expenditures, which has also been shown for the US.14 Among the RCT-based economic evaluations from Thailand, one reported influenza vaccination to be cost-effective and cost-saving for COPD patients; the other one concluded that it might not be cost-effective for community-dwelling elderly aged 60 y and above. RCTs from China found that influenza vaccination was cost-beneficial for elderly and healthy adults with regard to influenza-related and non-related outcomes (e.g., CVD).
Six of the excluded studies were cost analyses conducted from an employer's perspective.34–39 Despite their exclusion, we considered it important to report characteristics and the overall finding from these investigations, which was that influenza vaccination is cost-saving (Appendix 3). All of these studies need to be interpreted with care since calculation procedures for economic loss as well as cost components and financial interest were not well reported and limitations were rarely discussed.
Given the limited number of investigations and the lack of standardized definitions used in the studies, this review points to the shortage of transparent economic evaluations being available for LMICs and to the challenges associated with providing a statement on cost-effectiveness or cost saving achieved by influenza vaccination. First of all, we only identified studies that were conducted in urban regions of upper middle-income countries, meaning the results cannot be generalized to rural regions, particularly for low and lower middle-income countries. Second, the identified studies suffer from several methodological limitations: (1) The study outcome was not clearly defined and framed, i.e., influenza was assessed unspecifical and not laboratory confirmed and reported as ILI, ARI, or pneumonia.40,42-44,46,47 In one trial, CVD and diabetes was additionally considered, and highest cost-benefit was, controversially, achieved for CVD, but no cost-benefit was obvious for respiratory infections and ILI.47 Different definitions and imprecise detection of influenza could impact on projected cost savings and benefits. (2) Certain variables were not reported, defined or considered, e.g., timing of vaccination, the interaction between timing of vaccine administration and seasonal variability as well as some cost components. The assumptions and definitions reported vary greatly ranging from vaccine effectiveness and whether or not the same QALY weights can be used across different countries24,40 to variations in influenza incidence and costs included. Mostly, only certain direct costs were considered, e.g., no treatment costs for influenza, which may have particularly affected cost-effectiveness estimation in populations such as cancer patients,41 and no direct costs for treatment of adverse vaccine events.40 Indirect costs were only addressed in a few studies24,40,46 but also not to a full extent; thus, other factors, e.g., costs of secondary transmission of influenza to household contacts, were ignored.24 (3) In the few cases where important outcomes such as QALYs were reported, stratification by factors potentially impacting on costs per QALY gained, e.g., the setting in which the vaccine was administered, was not done.40 (4) The transparency of information was affected as reporting of precise values, costs covered, and presenting of threshold analyses was incomplete in some instances or it was not clear where cost estimates were derived from.40-44,47 Missing crucial information on funding source, analytical horizon and discount rate also impacted on credibility and transparency of the results presented. Thus, it could be that future benefits and costs were included without stating it and that conflicts of interest arose from the funding source, which would impact on the conclusion drawn.
Results from the RCT-based analyses should be interpreted with even more caution than those from the model-based studies, because mainly only direct medical costs of influenza vaccination were reported, economic calculations and the data on which the studies based their conclusions were not transparent or well described, and no uncertainty analyses were performed. For example, Wongsurakiat et al.45 did not indicate to which costs they compared the costs per ARI prevented, which makes a comprehensive statement on costs saved impossible. Praditsuwan et al.44 on the other hand did not clearly describe which expenses were included, and it was not possible to extract a value for money outcome from that study. The result from that RCT-based evaluation in elderly in Thailand suggests that influenza vaccination of all older persons living in the community was not cost-effective. However, value for money outcomes from these RCTs might be influenced by factors potentially impacting on effectiveness such as antigenic match between vaccine strain and circulating virus strains14,48 and seasonality of influenza within Thailand, which may experience influenza presence during the whole year or in a biannual pattern instead of annual epidemics.2,48 Major limitations in the Chinese RCT among elderly derive from focusing on diseases not related to influenza-vaccine, such as CVD and diabetes, and from the result that the cost-benefit ratio was highest for CVD, which makes it likely that other factors than vaccination may play a role.47 In addition, the summary cost benefit presented for vaccinating elderly might be misleading since it suggests a benefit for all diseases considered which is not obvious from the individual data presented.
One of the limitations of our review is related to the fact that the first screen of studies was based on titles. Of the titles that were possibly relevant, all abstracts were examined for eligibility. Since only a few studies described economic aspects and since we looked at all of them, we believe not to have missed relevant published information. Our search focused on medical databases and it is possible that there are publications listed in economic databases only, which are not covered by our review. Given the low number of studies and inconsistencies in definitions and outcomes used, we did not perform a quality appraisal in a meta-analytic manner. However, study quality issues were assessed descriptively and interpreted accordingly.
Conclusion
In this systematic review, we identified information on seasonal influenza vaccination indicating that influenza vaccine provides value for money in certain population groups in MICs. However, the number of studies included was limited and their quality rather low with a lack of reporting important parameters. We only retrieved nine economic evaluations from MICs, all of them based in urban regions of upper middle-income countries. Part of these studies was funded by industry or did not state the funding source, which might have induced a risk of potential conflict of interest. Future work should include more transparent information and context-specific data on economic benefits in middle and especially low-income countries, particularly for at risk populations recommended for vaccination by WHO. This also includes work to better estimate influenza-attributable morbidity and mortality, which is to be taken into account when assessing health care expenditure and benefits. In addition, broader economic benefits of vaccination beyond mortality and morbidity reduction are also relevant to decision makers. Hence, better evidence is needed about some of these benefits such as intersectoral macroeconomic benefits or intergenerational tax transfers.49
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We greatly acknowledge the support and efforts of Tomas Allen, senior librarian at the WHO, and Johannes C.F. Ket, Medical Information Specialist at the Medical Library of the VU University Amsterdam, for assisting in development of a search strategy and guiding through the search process. We thank Sayyora Esser and Dr. Natasha Shapovalova, for their support in translating information from the Russian language studies. The authors alone are responsible for the views expressed in this publication, which does not necessarily reflect the views of the World Health Organization.
This study was funded by the United States of America Center for Disease Control and Prevention Grant 5U50C1000748, Project 49 through the World Health Organization.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/24704
References
- 1.Nichol KL. Cost-effectiveness and socio-economic aspects of childhood influenza vaccination. Vaccine. 2011;29:7554–8. doi: 10.1016/j.vaccine.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 2.Simonsen L. The global impact of influenza on morbidity and mortality. Vaccine. 1999;17(Suppl 1):S3–10. doi: 10.1016/S0264-410X(99)00099-7. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO) Pandemic influenza A (H1N1) 2009 virus vaccine - conclusions and recommendations from the October 2009 meeting of the immunization Strategic Advisory Group of Experts. Wkly Epidemiol Rec. 2009;84:505–8. [PubMed] [Google Scholar]
- 4.World Health Organization (WHO). Acute respiratory infections. Update September 2009. The A/2009 H1N1 influenza virus pandemic. Available from: http://www.who.int/vaccine_research/diseases/ari/en/index5.html [Accessed October 8, 2012].
- 5.World Health Organization Vaccines against influenza WHO position paper—November 2012. Wkly Epidemiol Rec. 2012;47:461. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) Influenza and pneumococcal vaccination coverage levels among persons aged > or = 65 years--United States, 1973-1993. MMWR Morb Mortal Wkly Rep. 1995;44:506–7, 513-5. [PubMed] [Google Scholar]
- 7.World Health Organization (WHO). Statement from WHO global Advisory Committee on Vaccine Safety about the safety profile of pandemic influenza A (H1N1) 2009 vaccines. Available from: http://www.who.int/csr/resources/publications/swineflu/cp164_2009_1612_gacvs_h1n1_vaccine_safety.pdf [Accessed September 20, 2012]
- 8.Fedson DS, Hirota Y, Shin HK, Cambillard PE, Kiely J, Ambrosch F, et al. Influenza vaccination in 22 developed countries: an update to 1995. Vaccine. 1997;15:1506–11. doi: 10.1016/S0264-410X(97)00091-1. [DOI] [PubMed] [Google Scholar]
- 9.Ng S, Wu P, Nishiura H, Ip DK, Lee ES, Cowling BJ. An analysis of national target groups for monovalent 2009 pandemic influenza vaccine and trivalent seasonal influenza vaccines in 2009-10 and 2010-11. BMC Infect Dis. 2011;11:230. doi: 10.1186/1471-2334-11-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen GM, Nettleman MD. Economic impact of influenza vaccination in preschool children. Pediatrics. 2000;106:973–6. doi: 10.1542/peds.106.5.973. [DOI] [PubMed] [Google Scholar]
- 11.Luce BR, Zangwill KM, Palmer CS, Mendelman PM, Yan L, Wolff MC, et al. Cost-effectiveness analysis of an intranasal influenza vaccine for the prevention of influenza in healthy children. Pediatrics. 2001;108:E24. doi: 10.1542/peds.108.2.e24. [DOI] [PubMed] [Google Scholar]
- 12.Mullooly JP, Bennett MD, Hornbrook MC, Barker WH, Williams WW, Patriarca PA, et al. Influenza vaccination programs for elderly persons: cost-effectiveness in a health maintenance organization. Ann Intern Med. 1994;121:947–52. doi: 10.7326/0003-4819-121-12-199412150-00008. [DOI] [PubMed] [Google Scholar]
- 13.Nichol KL. Cost-benefit analysis of a strategy to vaccinate healthy working adults against influenza. Arch Intern Med. 2001;161:749–59. doi: 10.1001/archinte.161.5.749. [DOI] [PubMed] [Google Scholar]
- 14.Nichol KL, Mallon KP, Mendelman PM. Cost benefit of influenza vaccination in healthy, working adults: an economic analysis based on the results of a clinical trial of trivalent live attenuated influenza virus vaccine. Vaccine. 2003;21:2207–17. doi: 10.1016/S0264-410X(03)00029-X. [DOI] [PubMed] [Google Scholar]
- 15.Scuffham PA, West PA. Economic evaluation of strategies for the control and management of influenza in Europe. Vaccine. 2002;20:2562–78. doi: 10.1016/S0264-410X(02)00154-8. [DOI] [PubMed] [Google Scholar]
- 16.Postma MJ, Baltussen RP, Palache AM, Wilschut JC. Further evidence for favorable cost-effectiveness of elderly influenza vaccination. Expert Rev Pharmacoecon Outcomes Res. 2006;6:215–27. doi: 10.1586/14737167.6.2.215. [DOI] [PubMed] [Google Scholar]
- 17.Scott WG, Scott HM. Economic evaluation of vaccination against influenza in New Zealand. Pharmacoeconomics. 1996;9:51–60. doi: 10.2165/00019053-199609010-00006. [DOI] [PubMed] [Google Scholar]
- 18.Meltzer MI, Neuzil KM, Griffin MR, Fukuda K. An economic analysis of annual influenza vaccination of children. Vaccine. 2005;23:1004–14. doi: 10.1016/j.vaccine.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 19.Jit M, Cromer D, Baguelin M, Stowe J, Andrews N, Miller E. The cost-effectiveness of vaccinating pregnant women against seasonal influenza in England and Wales. Vaccine. 2010;29:115–22. doi: 10.1016/j.vaccine.2010.08.078. [DOI] [PubMed] [Google Scholar]
- 20.Skedgel C, Langley JM, MacDonald NE, Scott J, McNeil S. An incremental economic evaluation of targeted and universal influenza vaccination in pregnant women. Can J Public Health. 2011;102:445–50. doi: 10.1007/BF03404197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitzner KA, Shortridge KF, McGhee SM, Hedley AJ. Cost-effectiveness study on influenza prevention in Hong Kong. Health Policy. 2001;56:215–34. doi: 10.1016/S0168-8510(00)00140-8. [DOI] [PubMed] [Google Scholar]
- 22.Gatwood J, Meltzer MI, Messonnier M, Ortega-Sanchez IR, Balkrishnan R, Prosser LA. Seasonal influenza vaccination of healthy working-age adults: a review of economic evaluations. Drugs. 2012;72:35–48. doi: 10.2165/11597310-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 23.Myers ER, Misurski DA, Swamy GK. Influence of timing of seasonal influenza vaccination on effectiveness and cost-effectiveness in pregnancy. Am J Obstet Gynecol. 2011;204(Suppl 1):S128–40. doi: 10.1016/j.ajog.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Dayan GH, Nguyen VH, Debbag R, Gómez R, Wood SC. Cost-effectiveness of influenza vaccination in high-risk children in Argentina. Vaccine. 2001;19:4204–13. doi: 10.1016/S0264-410X(01)00160-8. [DOI] [PubMed] [Google Scholar]
- 25.Available at epocoslo.cochrane.org/ lmic-databases, last update November 2010, accessed at May 5, 2011
- 26.Walker DG, Hutubessy R, Beutels P. WHO Guide for standardisation of economic evaluations of immunization programmes. Vaccine. 2010;28:2356–9. doi: 10.1016/j.vaccine.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 27.Yoldascan E, Kurtaran B, Koyuncu M, Koyuncu E. Modeling the economic impact of pandemic influenza: a case study in Turkey. J Med Syst. 2010;34:139–45. doi: 10.1007/s10916-008-9225-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zubareva LA, Evdoshenko VG, Turovskaia SI, Krivchemko EI, Shall’ AIa. [Epidemiological and economic evaluation of the effectiveness of immunization with live influenza vaccine during the influenza epidemic of 1971-1973 in the city of Frunze] Zdravookhr Kirg. 1976;3:21–4. [PubMed] [Google Scholar]
- 29.Xing LH, Ge CM, Xing LJ. Cost-benefit analysis of influenza vaccination in Rizhao City, Shangdong Province, China. Chin J Biol. 2008;21:692–4. [Google Scholar]
- 30.Nichol KL. Complications of influenza and benefits of vaccination. Vaccine. 1999;17(Suppl 1):S47–52. doi: 10.1016/S0264-410X(99)00105-X. [DOI] [PubMed] [Google Scholar]
- 31.Wang MC, Wang TP, Yang SM. [Effectiveness of 23-valent penumococcal polysaccharide and split-virus influenza vaccines to prevent respiratory diseases for elderly people] Zhongguo Yi Miao He Mian Yi. 2010;16:229–32. [PubMed] [Google Scholar]
- 32.Apisarnthanarak A, Puthavathana P, Kitphati R, Auewarakul P, Mundy LM. Outbreaks of influenza A among nonvaccinated healthcare workers: implications for resource-limited settings. Infect Control Hosp Epidemiol. 2008;29:777–80. doi: 10.1086/588162. [DOI] [PubMed] [Google Scholar]
- 33.Wood SC, Alexseiv A, Nguyen VH. Effectiveness and economical impact of vaccination against influenza among a working population in Moscow. Vaccine. 1999;17(Suppl 3):S81–7. doi: 10.1016/S0264-410X(99)00299-6. [DOI] [PubMed] [Google Scholar]
- 34.Ivannikov IuG, Efimenko IB, Marinich IG, Luk’ianov IuV, Naĭkhin AN. [Evaluation of mass influenza prevention effectiveness using an inactivated chromatographic vaccine in Leningrad] Zh Mikrobiol Epidemiol Immunobiol. 1980;11:18–27. [PubMed] [Google Scholar]
- 35.Sharipova IS, Kuz’minykh SI, Fel’dblium IV. [An evaluation of the epidemiological and economic efficacy of immunizing adults with the Vaxigrippe vaccine] Zh Mikrobiol Epidemiol Immunobiol. 1998;4:46–9. [PubMed] [Google Scholar]
- 36.At’kov OY, Azarov AV, Zhukov DA, Nicoloyannis N, Durand L. Influenza vaccination in healthy working adults in Russia: observational study of effectiveness and return on investment for the employer. Appl Health Econ Health Policy. 2011;9:89–99. doi: 10.2165/11538680-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Burckel E, Ashraf T, de Sousa Filho JP, Forleo Neto E, Guarino H, Yauti C, et al. Economic impact of providing workplace influenza vaccination. A model and case study application at a Brazilian pharma-chemical company. Pharmacoeconomics. 1999;16:563–76. doi: 10.2165/00019053-199916050-00012. [DOI] [PubMed] [Google Scholar]
- 38.Morales A, Martinez MM, Tasset-Tisseau A, Rey E, Baron-Papillon F, Follet A. Costs and benefits of influenza vaccination and work productivity in a Colombian company from the employer’s perspective. Value Health. 2004;7:433–41. doi: 10.1111/j.1524-4733.2004.74006.x. [DOI] [PubMed] [Google Scholar]
- 39.Samad AH, Usul MH, Zakaria D, Ismail R, Tasset-Tisseau A, Baron-Papillon F, et al. Workplace vaccination against influenza in Malaysia: does the employer benefit? J Occup Health. 2006;48:1–10. doi: 10.1539/joh.48.1. [DOI] [PubMed] [Google Scholar]
- 40.Aballéa S, Chancellor J, Martin M, Wutzler P, Carrat F, Gasparini R, et al. The cost-effectiveness of influenza vaccination for people aged 50 to 64 years: an international model. Value Health. 2007;10:98–116. doi: 10.1111/j.1524-4733.2006.00157.x. [DOI] [PubMed] [Google Scholar]
- 41.Chicaíza-Becerra LA, García-Molina M, Ballesteros M, Gamboa O, Díaz J, Vega R. [Economic evaluation of influenza vaccine applied to health personnel attending hospitalised oncological patients] Rev Salud Publica (Bogota) 2008;10:756–66. doi: 10.1590/s0124-00642008000500008. [DOI] [PubMed] [Google Scholar]
- 42.Porras-Ramírez A, Alvis-Guzmán N, Rico-Mendoza A, Alvis-Estrada L, Castañeda-Orjuela CA, Velandia-González MP, et al. [Cost effectiveness of influenza vaccination in children under 2 years old and elderly in Colombia] Rev Salud Publica (Bogota) 2009;11:689–99. doi: 10.1590/s0124-00642009000500002. [DOI] [PubMed] [Google Scholar]
- 43.Gutiérrez JP, Bertozzi SM. [Influenza vaccination in the elderly population in Mexico: economic considerations] Salud Publica Mex. 2005;47:234–9. doi: 10.1590/s0036-36342005000300007. [DOI] [PubMed] [Google Scholar]
- 44.Praditsuwan R, Assantachai P, Wasi C, Puthavatana P, Kositanont U. The efficacy and effectiveness of influenza vaccination among Thai elderly persons living in the community. J Med Assoc Thai. 2005;88:256–64. [PubMed] [Google Scholar]
- 45.Wongsurakiat P, Lertakyamanee J, Maranetra KN, Jongriratanakul S, Sangkaew S. Economic evaluation of influenza vaccination in Thai chronic obstructive pulmonary disease patients. J Med Assoc Thai. 2003;86:497–508. [PubMed] [Google Scholar]
- 46.Gao JM, Yu Q, Tang GH. [Cost-benefit analysis on the strategy of social health insurance regarding vaccination against influenza in Xi’an city] Zhonghua Liu Xing Bing Xue Za Zhi. 2008;29:17–22. [PubMed] [Google Scholar]
- 47.Liu M, Liu GF, Wang Y, Zhao W, Wang L, Shi W, et al. [Study on the effectiveness and cost-benefit of influenza vaccine on elderly population in Beijing city] Zhonghua Liu Xing Bing Xue Za Zhi. 2005;26:412–6. [PubMed] [Google Scholar]
- 48.Palache B. New vaccine approaches for seasonal and pandemic influenza. Vaccine. 2008;26:6232–6. doi: 10.1016/j.vaccine.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 49.Deogaonkar R, Hutubessy R, van der Putten I, Evers S, Jit M. Systematic review of studies evaluating the broader economic impact of vaccination in low and middle income countries. BMC Public Health. 2012;12:878. doi: 10.1186/1471-2458-12-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.