To The Editor
Type VII collagen (C7) is located in the skin between its two main layers, the epidermis and the dermis, where it forms structures, anchoring fibrils (AFs), that are necessary for epidermal-dermal adherence (Sakai et al., 1986; Burgeson, 1993)). The COL7A1 gene encodes for a 290 kDa alpha chain (Christiano et al., 1994). C7 is composed of three alpha chains that form a homotrimer of ∼900 kDa (1). Within the extracellular space, C7 molecules form antiparallel dimers, which aggregate laterally to form AFs. C7 has some unusual properties that make it quite distinctive from other collagens, such as its solubility in neutral buffers. Most skin-associated collagens such as type I collagen induce platelet aggregation and clot formation when exposed to the blood stream while C7 does not (Saelman et al., 1994).
Defects in both alleles of COL7A1 cause an absence or reduction of functional C7 and AFs resulting in recessive dystrophic epidermolysis bullosa (RDEB). RDEB patients have skin fragility, skin blistering, erosions, milia formation, nail loss, joint contractures, fibrotic mitten deformities of the hands and feet, mutilating scars, esophageal strictures and aggressive squamous cell carcinomas that take the patients' lives prematurely (Fine et al., 2008). Unfortunately, RDEB is incurable. Various therapeutic strategies have been envisioned for RDEB based on preclinical animal models, including the intradermal injection of allogeneic dermal fibroblasts or gene corrected RDEB fibroblasts (Oritz-Urda et al., 2003; Woodley et al., 2003), intradermal injection of lentiviral vectors expressing C7 (Woodley et al., 2004), and transplantation of gene-corrected keratinocyte autografts (Chen et al., 2002 Oritz-Urda et al., 2002). Recently, proof-of-principle clinical trials have been initiated in RDEB patients, including bone marrow/stem cell transplantation and intradermal injection of allogeneic fibroblasts (Wagner et al., 2010; Wong et al., 2008). None of these therapies have proven to be consistently effective.
We demonstrated that the intradermal injection of human recombinant C7 (rC7) into RDEB skin equivalents grafted onto immunodeficient mice or into RDEB-like, C7- knockout mice results in new C7 and AFs and reverses the RDEB skin phenotype (Woodley et al., 2004; Remington et al., 2009). Nevertheless, intradermally injected rC7 has a small diffusion radius, and is not ideal for treating large areas of skin in RDEB patients who usually have widespread lesions including the oral cavity and esophagus. In the present study, we sought to determine if we could ameliorate RDEB by intravenously (IV) administering rC7. We hypothesized that IVC7 would simultaneously home to multiple RDEB wounds and restore C7 expression and function.
We used two animal models – a full-thickness skin wound created in athymic nude mice and an RDEB skin transplantation mouse model. For the first model, 1 cm by 1 cm full-thickness wounds were made on the backs of athymic nude mice. Between 4 and 8 hours later, we injected the tail veins of the mice with rC7. As shown in Figure 1a, the IV-injected rC7 homed to wound sites and incorporated into the DEJ at two weeks after injection. In contrast, there was no detectable human rC7 in unwounded skin. None of the mice injected with PBS or laminin 111, a large non-collagenous glycoprotein within basement membranes, had rC7 in their healed skin. As shown in Figure 1b, with increasing doses of rC7, we detected a dose-dependent increase in rC7 at the mouse's dermal-epidermal junction (DEJ). In addition, IV injected rC7 was sustained at the mouse's DEJ for at least 8 weeks (data not shown).
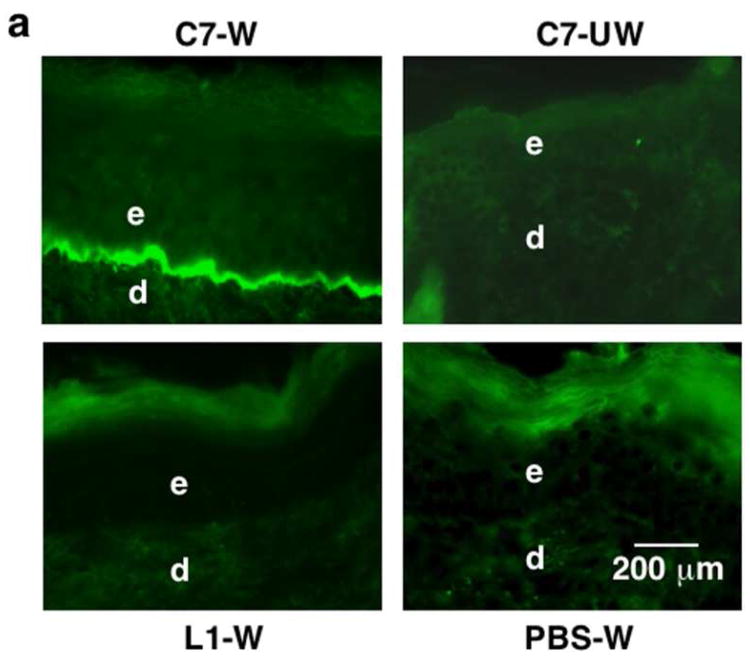
Figure 1. IV injected rC7 homes to skin wounds and incorporates into the mouse's regenerated DEJ.
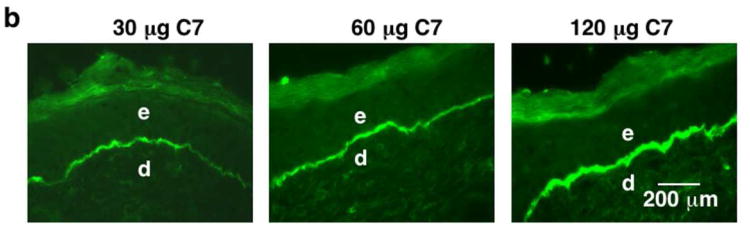
(a) Immunofluorescence staining of mouse skin (after IV injection of 100 μg of rC7 or laminin 111) was performed with antibodies specific for human C7 at 2 weeks after the injection. Note that the IV injected rC7 (n = 20 mice) homed and incorporated into the regenerated DEJ of the healed wound sites (C7-W), but was not present in un-wounded skin sites (C7-UW). No rC7 was detected in mice IV injected with laminin 111 (n = 5 mice) or PBS (n = 10 mice) (L1-W and PBS-W). (b) Dose-dependent deposition of rC7 at the mouse's DEJ after IV injection with rC7. Immunofluorescence staining with an antibody specific for human C7 was performed on healed mouse skin wounds two weeks after the animals were injected with 30 μg rC7, 60 μg rC7, or 120 μg rC7, as indicated respectively. All photographs were taken at the same exposure time.
We then examined if IV-injected rC7 trafficked to other organs. Human rC7 was only observed in healed skin wounds and not in unwounded skin or esophagus, stomach, tongue, small intestine, brain, kidney, liver, lung, spleen or heart (Supplementary Figure S1). Lastly, we did not observe any adverse effects in mice receiving IV rC7 at doses as high as 33 mg/per kg body weight. Daily weight, activity, and feeding habits were identical between experimental and control groups.
To evaluate intravenous C7 in an RDEB-like model, we transplanted skin from C7 knock out mice that have no C7 or AFs in their DEJ (Heinonen et al., 1999) onto the backs of athymic nude mice. These newborn, CO7A1-null mice exhibit extensive skin blisters and die within the first week of life. These C7-null mice recapitulate the clinical, genetic and ultrastructural features of RDEB. Because these small neonatal mice only live a few days, it is not technically feasible to inject their tail veins with C7. As an alternative, we sacrifice these mice after birth and transplant their skin onto the backs of adult athymic, nude mice. As shown in Figure 2a, RDEB skin grafts before treatment showed histological evidence of dermal-epidermal separation and entirely lacked C7 staining at the DEJ, characteristic of RDEB.
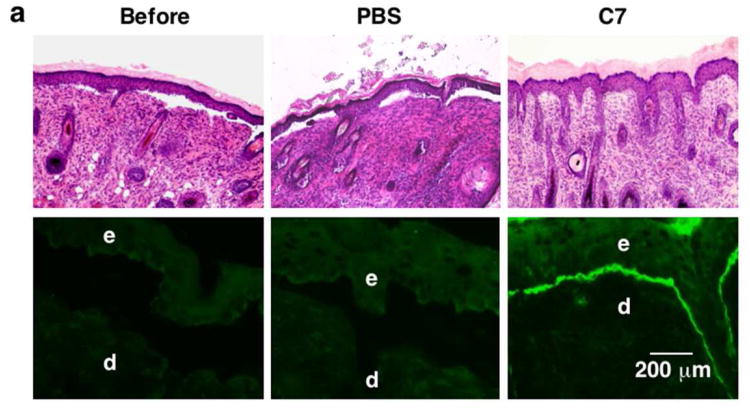
Figure 2. IV injected rC7 incorporates into the DEJ of RDEB mouse skin grafted onto athymic nude mice in vivo.
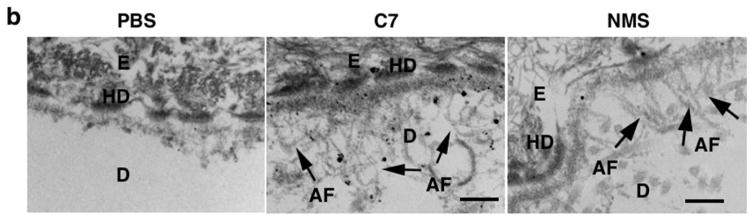
(a) Histological appearance (upper panels) and immunofluorescence staining (lower panels) of engrafted RDEB mouse skin using a monoclonal antibody specific for human C7. Left panels (Before) are skin biopsies from transplanted RDEB skin grafts taken two weeks after grafting and before treatment (n = 30 mice). Middle panels (PBS) are two-week post injection biopsies from RDEB skin grafts on athymic host nude mice that were IV injected with PBS (n = 4 mice). Right panels (C7) are two-week post injection biopsies from RDEB skin grafts on athymic host nude mice who were IV injected with 60 μg of rC7 (n = 9 mice). e, epidermis; d, dermis. (b) Immunogold labeling of engrafted murine RDEB skin injected with PBS (PBS) or 60 μg rC7 (C7) was performed with an antibody specific to human C7 (NP185, a gift of Dr. Lynn Sakai, Shriners Hospital for Children, Portland, Oregon) followed by an 1 nm gold secondary antibody. Identical IEM was performed on normal mouse skin (NMS). Note that IV injected rC7 incorporated into the RDEB skin grafts and formed AFs. Note restoration of numerous arching AFs depicted with arrows and labeled with gold particles decorating the DEJ of RDEB skin grafts received IV rC7. IEM on normal mouse skin shows numerous unlabelled AFs. HD = hemidesmosomes; D = dermis; E = epidermis; Scale bar: 100 nm.
Full-thickness, skin wounds are much different from the superficial bullous wounds of RDEB skin, which occur at the epidermal-dermal interface. Having shown that intravenous rC7 homes to full-thickness, skin wounds, we wished to evaluate if intravenous rC7 would also home to grafted RDEB mouse skin wounds. We administered intravenous rC7 to the adult athymic nude mice hosting RDEB mouse skin grafts. As shown in Figure 2a, IV-injected rC7 homed to the RDEB mouse skin, incorporated into the DEJ, and corrected the dermal-epidermal separation. In contrast, C7 null RDEB-like skin transplanted onto mice that received PBS injections revealed dermal-epidermal separation and had no rC7. These experiments show that although the characteristic RDEB wound is superficial, the wound is sufficient to allow intravenously injected rC7 to home to it, incorporate into the DEJ and improve epidermal-dermal adherence.
To determine whether IV rC7 could restore AFs at the DEJ of the engrafted RDEB mouse skin in vivo, we carried out immuno-electron microscopy using a monoclonal antibody that is specific to human C7 and recognizes an epitope within NC1 domain of C7, as described (Sakai et al., 1986 Sakai et al., 1996). As shown in Figure 2b, the injected rC7 incorporated into the DEJ of the engrafted RDEB mouse skin and formed AF structures, thus demonstrating correction of the major ultrastructural abnormality of RDEB mouse skin. In contrast, there were no detectable AFs at the DEJ of RDEB mouse skin grafts transplanted onto mice that received intravenous PBS. These data indicate that protein-based therapy by IV injection of C7 can correct the abnormal RDEB dermal-epidermal separation and restore C7 expression and AF formation at the DEJ in vivo.
In summary, we showed that rC7 administered intravenously to mice homed to engrafted RDEB mouse skin, incorporated into the DEJ of the grafts, and restored C7, AFs and epidermal-dermal adherence. These data suggest that intravenously administered C7 could simultaneously migrate to the DEJ throughout the RDEB patient's skin, reverse the “sub-clinical”, microscopic epidermal-dermal separation and prophylactically prevent frank skin blisters and erosions from forming. We believe that protein therapy via intravenous C7 may be a valid therapeutic strategy for patients with RDEB who currently have few therapeutic options.
Supplementary Material
Tissue distribution of IV injected rC7. Two weeks after IV injection of human 100 μg rC7, necropsies were performed on the mice (n = 10 mice) and tissues obtained from esophagus (Eso), stomach, tongue, brain, kidney, liver, lung, spleen, heart, small intestine (SI), unwounded skin (Un) and wounded healed skin (Wn) were subjected to immunostaining using an antibody specific for human C7. Note that IV injected rC7 was readily detected in wounded skin but not in any internal organs or unwounded skin.
Acknowledgments
This work was supported by grants (NIH RO1 AR47981 to M.C, RO1 AR33625 to M.C. and D.T.W., Sponsored Research Project from Lotus Tissue Repair. Inc. to M.C. and D.T.W.). We thank Sara Tufa for technical support of immuno-EM.
Abbreviations
- AFs
anchoring fibrils
- C7
type VII collagen
- DEJ
dermal-epidermal junction
- IV
intravenous
- RDEB
recessive dystrophic epidermolysis bullosa
- rC7
human recombinant C7
Footnotes
Conflict of interest: Drs. Mei Chen and David Woodley are consultants for Lotus Tissue Repair, Inc. and hold stock in the company. Dr. Mei Chen, Dr. David T. Woodley and the University of Southern California hold patents for recombinant type VII collagen and have filed a Conflict of Interest Declaration with Dr. Randoph W. Hall, Vice Provost for Research Advancement at the University of Southern California.
References
- Burgeson RE. Type VII collagen, anchoring fibrils, and epidermolysis bullosa. J Invest Dermatol. 1993;101:252–55. doi: 10.1111/1523-1747.ep12365129. [DOI] [PubMed] [Google Scholar]
- Christiano AM, Greenspan DS, Lee S, et al. Cloning of human type VII collagen: complete primary sequence of the α1(VIII) chain and identification of intragenic polymorphisms. J Biol Chem. 1994;269:20256–262. [PubMed] [Google Scholar]
- Chen M, Kasahara N, Keene DR, et al. Restoration of type VII collagen expression and function in dystrophic epidermolysis bullosa. Nat Genet. 2002;32(4):670–675. doi: 10.1038/ng1041. [DOI] [PubMed] [Google Scholar]
- Fine JD, Eady RA, Bauer EA, et al. The classification of inherited epidermolysis bullosa (EB): Report of the Third International Consensus Meeting on Diagnosis and Classification of EB. J Am Acad Dermatol. 2008;58:931–50. doi: 10.1016/j.jaad.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Heinonen S, Männikkö M, Klement JF, et al. Targeted inactivation of the type VII collagen gene (Col7a1) in mice results in severe blistering phenotype: a model for recessive dystrophic epidermolysis bullosa. J Cell Sci. 1999;112:3641–48. doi: 10.1242/jcs.112.21.3641. [DOI] [PubMed] [Google Scholar]
- Ortiz-Urda S, Lin Q, Green CL, et al. Injection of genetically engineered fibroblasts corrects regenerated human epidermolysis bullosa skin tissue. J Clin Invest. 2003;111:251–55. doi: 10.1172/JCI17193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Urda S, Thyagarajan B, Keene DR, et al. Stable nonviral genetic correction of inherited human skin disease. Nat Med. 2002;8:1166–70. doi: 10.1038/nm766. [DOI] [PubMed] [Google Scholar]
- Remington J, Wang X, Hou Y, et al. Injection of recombinant human type VII collagen corrects the disease phenotype in a murine model of dystrophic epidermolysis bullosa. Mol Ther. 2009;17:26–33. doi: 10.1038/mt.2008.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai LY, Keene DR, Morris NP, et al. Type VII collagen is a major structural component of anchoring fibrils. J Cell Biol. 1986;103:1577–86. doi: 10.1083/jcb.103.4.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai LY, Keene DR. Fibrillin: monomers and microfibrils. In: Ruoslahti E, Engvall E, editors. Methods in Enzymology. Vol. 245. Academic Press; 1994. pp. 47–50. [Google Scholar]
- Saelman EU, Nieuwenhuis HK, Hese KM, et al. Platelet adhesion to collagen types I through VIII under conditions of stasis and flow is mediated by GPIa/IIa (alpha 2 beta 1-integrin) Blood. 1994;83:1244–50. [PubMed] [Google Scholar]
- Woodley DT, Krueger GG, Jorgensen CM, et al. Normal and gene-corrected dystrophic epidermolysis bullosa fibroblasts alone can produce type VII collagen at the basement membrane zone. J Invest Dermatol. 2003;121:1021–28. doi: 10.1046/j.1523-1747.2003.12571.x. [DOI] [PubMed] [Google Scholar]
- Woodley DT, Keene DR, Atha T, et al. Injection of recombinant human type VII collagen restores collagen function in dystrophic epidermolysis bullosa. Nat Med. 2004;10:693–95. doi: 10.1038/nm1063. [DOI] [PubMed] [Google Scholar]
- Woodley DT, Keene DR, Atha T, et al. Intradermal injection of lentiviral vectors corrects regenerated human dystrophic epidermolysis bullosa skin tissue in vivo. Mol Ther. 2004;10:318–26. doi: 10.1016/j.ymthe.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Wong T, Gammon L, Liu L, et al. Potential of fibroblast cell therapy for recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 2008;128:2179–89. doi: 10.1038/jid.2008.78. [DOI] [PubMed] [Google Scholar]
- Wagner JE, Ishida-Yamamoto A, McGrath JA, et al. Bone marrow transplantation for recessive dystrophic epidermolysis bullosa. N Engl J Med. 2010;363:629–39. doi: 10.1056/NEJMoa0910501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tissue distribution of IV injected rC7. Two weeks after IV injection of human 100 μg rC7, necropsies were performed on the mice (n = 10 mice) and tissues obtained from esophagus (Eso), stomach, tongue, brain, kidney, liver, lung, spleen, heart, small intestine (SI), unwounded skin (Un) and wounded healed skin (Wn) were subjected to immunostaining using an antibody specific for human C7. Note that IV injected rC7 was readily detected in wounded skin but not in any internal organs or unwounded skin.