Abstract
We compared outcomes after 94 HLA-matched sibling, 168 unrelated donor bone marrow (BM) (n=81 matched and n=88 mismatched) and 86 cord blood transplantations in patients aged 1–15 years with acute lymphoblastic leukemia (ALL) in second complete remission (CR). All patients had their first bone marrow relapse within 3 years from diagnosis. Cox regression models were constructed to examine for differences in transplant-outcome by donor source. Risks of grade 2–4 acute GVHD and chronic GVHD, when compared to HLA-matched sibling transplants, were higher after matched unrelated donor BM (RR 2.42, p=0.001; RR 5.12, p<0.001, respectively), mismatched BM (RR 3.24, p<0.001; 5.19, p<0.001, respectively) and cord blood (RR 2.67, p<0.001; 2.54, p=0.024, respectively) transplants. Though non-relapse mortality was higher after transplantation of mismatched unrelated donor BM and cord blood, there were no differences in leukemia-free survival (LFS) between HLA-matched sibling and any of the unrelated donor transplantations. The 3-year probabilities of LFS were 50% after HLA-matched sibling and 44% after matched unrelated BM, 44% after mismatched unrelated BM and 43% after cord blood transplantation. Our observations support transplantation of BM or cord blood from a suitably matched unrelated donor or cord blood for patients without an HLA-matched sibling with ALL in second CR.
Keywords: acute lymphoblastic leukemia, unrelated donor transplant, early relapse
INTRODUCTION
The last 40 years have seen dramatic improvements in survival for children with acute lymphoblastic leukemia (ALL), such that cure rates with chemotherapy now approach 85% (1). Nevertheless, approximately 20% of children will relapse. The outcomes of the 70% of the children with relapsed ALL who present within three years are worse, which makes them high risk (2). With current salvage protocols, 85% of these children achieve a second complete remission (CR). However, in children with high- risk relapses who attain a second CR, durable remission is only from 0% – 25% with chemotherapy alone (4, 5). There are data to support the hypothesis that long-term leukemia-free survival (LFS) is superior after allogeneic haematopoietic stem cell transplantation. The Berlin-Frankfurt-Munster Group compared outcomes after matched unrelated donor bone marrow transplantation (BMT) and continuing chemotherapy(4). These authors observed superior LFS in children with high-risk ALL after unrelated donor BMT compared to chemotherapy alone, 44% vs. 0%, p<0.001, respectively. More recently, the Children’s Oncology Group and the Center for International Blood and Marrow Transplant Research (CIBMTR) compared LFS after HLA-matched sibling BMT and chemotherapy alone in children who relapsed within 3 years from diagnosis and attained a second CR. Higher LFS was observed after transplantation (41%) than with chemotherapy (23%) at 8-years, p<0.001 (5).
Since only 30% of patients who might benefit from transplantation have a matched sibling, identifying a suitably matched donor for transplantation remains a challenge. For Caucasians, the probability of identifying a suitably matched unrelated BM donor is about 70%, for non-Caucasians the likelihood is much less, about 30%. Many more patients are able to find a suitable donor cord blood unit because the immunologic naivety of cord blood lymphocytes allows for successful transplantation across HLA barriers not possible with unrelated donor BM grafts. This has led to the increasing use of cord blood grafts instead of BM for unrelated donor transplantation. Cord blood grafts now account for approximately 40% of pediatric allogeneic transplantations in the United States. Unrelated cord blood units are readily available, as they are already tested and banked, an advantage when considering transplantation for patients who attain a second CR after early relapse. The CIBMTR and the Eurocord Registry have compared outcomes after unrelated donor BMT and cord blood transplantation for acute leukemia in children (6, 7). Though, haematopoietic recovery is slower and non-relapse mortality higher after cord blood transplantation, long-term LFS is comparable after matched unrelated donor BM and mismatched cord blood transplantation.
Stem cell transplantation from an HLA-matched sibling is the accepted standard treatment for children with high-risk ALL in second CR. The higher risk of non-relapse mortality associated with unrelated BM and cord blood transplantation raises anxiety amongst pediatric oncologists when considering alternative donor transplantation for these patients. On the other hand, it is sometimes suggested that a more mismatched stem cell source will give better disease control, and that a matched sibling donor should not be used for the most aggressive leukemias. To address this concern, we compared transplant-outcomes after unrelated donor BM and cord blood transplantation to those after HLA-matched sibling BM transplantation in children with ALL who relapsed within 3 years from diagnosis and attained a second CR. Unrelated adult donors and cord blood grafts were selected using current selection criteria for these graft types, allowing the opportunity to evaluate and compare transplant-outcomes as practiced in the current era.
PATIENTS and METHODS
Data Collection
Data regarding patient, disease and transplant characteristics and outcomes were obtained from the CIBMTR, a voluntary working group of over 400 transplant centers worldwide that provide data prospectively on consecutive transplants to a Statistical Center at the Medical College of Wisconsin. All patients are followed longitudinally until death or loss to follow-up. Use of standardized data collection forms, bi-annual training of clinical research coordinators at participating centers, computerized error checks, review of submitted data by physicians and on-site audits ensure data quality. This study was approved by The Institutional Review Board of the Medical College of Wisconsin.
Inclusion criteria
Patients with ALL aged 1–15 years and in second CR at transplantation were eligible if their first relapse occurred within 3 years from diagnosis. The sites of relapse were bone marrow alone or bone marrow plus extramedullary involvement. Transplants occurred between 1997 and 2005. BM donors were HLA-matched siblings or unrelated adult donors. Cord blood grafts were from unrelated donors. There were too few recipients of peripheral blood grafts and were therefore excluded. Unrelated adult donors were matched to the patient at HLA-A, -B, -C, -DRB1 at the allele-level (8/8 HLA match) or mismatched at one- or two-loci. Patients who received cord blood units were matched for, at least 4 of 6 HLA-loci (HLA -A, -B, [antigen-level] and -DRB1 [allele-level]) and had a post-thaw total nucleated cell (TNC) dose ≥2.5×107/kg of patient body weight. All patients received myeloablative conditioning regimens and calcinurin inhibitor graft-versus-host disease (GVHD) prophylaxis.
End Points
Neutrophil recovery was defined as achieving an absolute neutrophil count ≥0.5×109/L for 3 consecutive days and platelet recovery, ≥20×109/L unsupported for 7 days. Acute and chronic GVHD were graded using standard criteria(8, 9). Non-relapse mortality was defined as death occurring in complete remission, relapse, as recurrence of disease at any site and LFS, the likelihood of surviving in complete remission after transplantation.
Statistical Analysis
Patient, disease and transplant characteristics were compared between the three groups using the Chi-square test for categorical variables (Table 1). The probability of LFS was calculated using the Kaplan-Meier estimator (10). For LFS, relapse or death from any cause, were considered events, and patients surviving in complete continuous remission were censored at the last follow-up. The cumulative incidence estimator was used to calculate the probability of neutrophil and platelet recovery, acute and chronic GVHD, relapse and non-relapse mortality(11). For haematopoietic recovery, and acute and chronic GVHD, death without an event was the competing risk; patients who were alive without the event were censored at last follow-up. For non-relapse mortality, relapse was the competing event and for relapse, non-relapse mortality was the competing event. 95% confidence intervals (CI) were calculated with the use of a log transformation.
Table 1.
Patient, disease, and transplant characteristics
| Characteristics | Matched sibling BM | Unrelated BM | Cord Blood | p-value |
|---|---|---|---|---|
| Number of Patients | 94 | 168 | 86 | |
| Age at transplantation, years, n (%) | <0.001 | |||
| 1–10 | 67 (71%) | 129 (77%) | 83 (96%) | |
| 11–15 | 27 (29%) | 3 (23%) | 3 (4%) | |
| Male sex | 52 (55%) | 104 (62%) | 49 (57%) | 0.51 |
| Race | <0.001 | |||
| Caucasian | 52 (55%) | 132 (79%) | 63 (73%) | |
| Other | 42 (45%) | 36 (21%) | 23 (27%) | |
| Performance score at transplant | 0.81 | |||
| 90 to100 | 80 (85%) | 133 (79%) | 70 (81%) | |
| < 90 | 14 (15%) | 23 (14%) | 15 (18%) | |
| Unknown | 0 | 12 (7%) | 1 (1%) | |
| Recipient CMV serostatus | 0.25 | |||
| Seropositive | 44 (47%) | 64 (38%) | 39 (46%) | |
| Seronegative | 49 (52%) | 104 (62%) | 46 (53%) | |
| Unknown | 1 (1%) | 0 (0%) | 1 (1%) | |
| ALL subtype | ||||
| B-lineage | 72 (77%) | 120 (71%) | 64 (74%) | 0.24 |
| T-lineage | 14 (15%) | 19 (11%) | 7 (8%) | |
| Unclassified/other | 8 (8%) | 29 (17%) | 15 (18%) | |
| Cytogenetics | 0.24 | |||
| Normal | 29 (31%) | 36 (21%) | 19 (22%) | |
| High-risk | 5 (5%) | 18 (11%) | 6 (7%) | |
| Intermediate risk | 34 (36%) | 46 (27%) | 35 (41%) | |
| Low risk | 10 (11%) | 25 (15%) | 11 (13%) | |
| Unknown | 16 (17%) | 43 (26%) | 15 (17%) | |
| Year of transplant | 0.002 | |||
| 1997–2001 | 70 (74%) | 92 (55%) | 44 (51%) | |
| 2002–2005 | 24 (26%) | 76 (45%) | 42 (49%) | |
| Conditioning regimen | <0.001 | |||
| TBI + other | 92 (98%) | 156 (93%) | 73 (85%) | |
| Bu + other (Cy or melphalan) | 2 (2%) | 12 (7%) | 13 (15%) | |
| Graft-verses-host-disease prophylaxis | 0.005 | |||
| Cyclosporine +/− other agents | 92 (98%) | 147 (88%) | 82 (95%) | |
| Tacrolimus +/− other agents | 2 (2%) | 21 (12%) | 4 (5%) |
BM=bone marrow
Multivariate analyses were performed using Cox regression models(12). Models were built with the use of forward stepwise selection and variables that attained a p value of ≤0.05 were held in the model. We created four treatment groups that incorporated donor, graft and donor-recipient HLA match and included them in all steps of model building regardless of level of significance: HLA-matched sibling transplantation, the baseline group for all comparisons, 8/8 HLA-matched adult unrelated donor BM transplantations, HLA-mismatched adult unrelated donor BM transplantations and unrelated cord blood transplantations. Other variables considered include: patient gender (male vs. female), performance score at time of transplant (90–100 vs. <90), age at transplantation (1–10 vs. 11–15 years), recipient cytomegalovirus (CMV) serostatus (positive vs. negative), race (Caucasian vs. other), leukemia subtype (B-lineage vs. T-lineage), cytogenetics (normal vs. high-risk vs. intermediate-risk vs. low-risk), National Cancer Institute (NCI) risk group (standard vs. high), duration of first CR (<18 months vs. 18–36 months) and year of transplantation (1997–2002 vs. 2003–2007). All variables satisfied the proportional hazards assumption and there were no first order interactions detected. All P-values are two sided. All analyses were done with SAS version 9.1 (Cary, NC).
RESULTS
Patient and Transplant Characteristics
Patient-, disease-, and transplant-related characteristics are shown in Table 1. Ninety-four recipients of HLA-matched sibling donor transplantation, 168 recipients of unrelated donor BM transplantation and 86 recipients of unrelated cord blood transplantation were eligible. Most patients had B-lineage ALL. All patients had first CR duration less than 36 months and therefore are considered “high-risk”. One hundred and forty-one patients (41%) had first CR duration less than 18 months. Cytogenetic risk was defined as follows: low risk, hyperdiploid (chromosome number of 50 or higher), trisomies of 4, 10 and 17 and t (12; 21); high risk, hypodiploidy (chromosome number of 45 or less), translocation (9; 22); intermediate risk included all others abnormalities. The NCI risk groups were defined as: patients with a WBC less than 50,000 and between the ages of 1–9 years at diagnosis were classified as standard risk. The remaining patients were in the high-risk NCI risk group. Recipients of HLA-matched sibling and unrelated donor transplants were similar in gender, pre-transplant performance score, recipient CMV serostatus, cytogenetics, NCI risk group and leukemia sub-type. However, recipients of unrelated donor transplants were more likely to be Caucasian and to be transplanted more recently. Recipients of unrelated donor BM grafts were more likely to receive tacrolimus-containing GVHD prophylaxis. Though the median ages of HLA-matched sibling, adult unrelated donor BM and cord blood recipients were 8 years, 7 years and 6 years, respectively; very few cord blood recipients were older than 10 years. Eighty-one recipients (48%) of adult unrelated donor BM were matched at the allele-level at HLA-A, -B, -C, -DRB1 and 87 (52%) mismatched at one or two-loci. Eleven cord blood recipients (13%) were matched at HLA-A, -B, (antigen-level) -DRB1 (allele-level), 34 (40%) mismatched at one and 41 (48%) mismatched at two-loci. The median follow-up of patients was 6 years after HLA-matched sibling and 4 years after unrelated donor BM or cord blood transplantation.
Neutrophil and platelet recovery
The day-42 incidence of neutrophil recovery was 98% (95% CI 91–99%) after HLA-matched sibling and 97% (95% CI 93–99%) after unrelated donor BM transplantation. Neutrophil recovery was significantly lower after cord blood transplantation, at 83% (95% CI 72–90%), p<0.001. Cumulative incidences of platelet recovery were lower after unrelated donor BM and cord blood transplantation. The day-100 incidences of platelet recovery after unrelated donor BM and cord blood transplantation were 80% (95% CI 73–86%) and 65% (95% CI 55–76%), respectively, compared to 92% (95% CI 86–97%), p<0.001, after HLA-matched sibling transplantation. There were no differences in neutrophil or platelet recovery amongst recipients of matched and mismatched unrelated donor BM transplantation.
Acute and chronic GVHD
The univariate probabilities of grades 2–4 acute and chronic GVHD are shown in Table 2. The day-100 incidence of acute GVHD was higher in matched and mismatched unrelated donor BM and cord blood transplants. In multivariate analysis, grade 2–4 acute GVHD risks were higher after any unrelated donor transplant compared to HLA-matched sibling transplantation (Table 3). Acute GVHD was more frequent in patients with performance score ≤90 and this effect was independent of donor type (RR 1.54, 95% CI 1.02 – 2.32, p=0.038). Chronic GVHD risk was also higher after any unrelated donor transplant compared to HLA-matched sibling transplantation (Table 3).
Table 2.
Univariate probabilities of GVHD, non-relapse mortality, relapse, leukemia-free and overall survival
| Outcome | Matched sibling BM (95% CI) | Unrelated matched BM (95% CI) | Unrelated mismatched BM (95% CI) | Cord Blood (95% CI) | P-value |
|---|---|---|---|---|---|
| Acute GVHD | <0.001 | ||||
| 100 days | 22 (15–31) | 46 (35–57) | 57 (47–66) | 50 (39–60) | |
| Chronic GVHD | <0.001 | ||||
| 3 years | 10 (5–17) | 36 (25–46) | 35 (25–45) | 20 (13–29) | |
| Non-relapse mortality | 0.127 | ||||
| 3 years | 13 (7–20) | 21 (13–31) | 27 (18–36) | 29 (19–38) | |
| Relapse | 0.900 | ||||
| 3 years | 37 (27–47) | 35 (24–45) | 29 (20–39) | 30 (21–40) | |
| Leukemia-free survival | 0.614 | ||||
| 3 years | 50 (40–60) | 44 (33–55) | 44 (34–55) | 43 (32–53) | |
| Overall survival | 0.724 | ||||
| 3 years | 54 (43–64) | 49 (37–59) | 47 (36–58) | 44 (33–54) |
Table 3.
Multivariable analysis of acute and chronic GVHD, non-relapse mortality, relapse, leukemia-free survival and overall survival
| Number* | Relative Risk (95% CI) | p-value | |
|---|---|---|---|
| Grade 2–4 acute GVHD | |||
| URD matched BM vs. sibling | 37/80 vs. 21/94 | 2.43 (1.42–4.16) | 0.001 |
| URD MM BM vs. sibling | 50/88 vs. 21/94 | 3.24 (1.94–5.41) | <.001 |
| Cord blood vs. sibling | 44/86 vs. 21/94 | 2.67 (1.60–4.50) | <.001 |
| Chronic GVHD | |||
| URD matched BM vs. sibling | 29/80 vs. 9/94 | 5.12 (2.42–10.84) | <.001 |
| URD MM BM vs. sibling | 31/88 vs. 9/94 | 5.19 (2.47–10.92) | <.001 |
| Cord blood vs. sibling | 17/86 vs. 9/94 | 2.54 (1.13–5.70) | 0.024 |
| Non-relapse mortality | |||
| URD matched BM vs. sibling | 17/80 vs. 13/94 | 1.61 (0.78–3.32) | 0.195 |
| URD MM BM vs. sibling | 23/88 vs. 13/94 | 1.98 (1.01–3.92) | 0.048 |
| Cord blood vs. sibling | 24/86 vs. 13/94 | 2.18 (1.11–4.30) | 0.024 |
| Relapse | |||
| URD matched BM vs. sibling | 28/80vs. 34/94 | 1.09 (0.66–1.79) | 0.745 |
| URD MM BM vs. sibling | 26/88 vs. 34/94 | 0.89 (0.53–1.48) | 0.645 |
| Cord blood vs. sibling | 25/86 vs. 34/94 | 0.95 (0.57–1.59) | 0.839 |
| Treatment failure | |||
| URD matched BM vs. sibling | 45/80 vs. 47/94 | 1.23 (0.82–1.85) | 0.322 |
| URD MM BM vs. sibling | 49/88 vs. 47/94 | 1.19 (0.80–1.78) | 0.388 |
| Cord blood vs. sibling | 49/86 vs. 47/94 | 1.30 (0.87–1.94) | 0.202 |
| Overall mortality | |||
| URD matched BM vs. sibling | 43/80 vs. 47/94 | 1.17 (0.77–1.77) | 0.458 |
| URD MM BM vs. sibling | 45/88 vs. 47/94 | 1.11 (0.74–1.68) | 0.607 |
| Cord blood vs. sibling | 46/86 vs. 47/94 | 1.257 (0.84–1.89) | 0.271 |
Numbers of events/number of evaluable patients
BM=bone marrow; URD= unrelated donor; MM=mismatched; CI=confidence interval
Non-relapse mortality and relapse
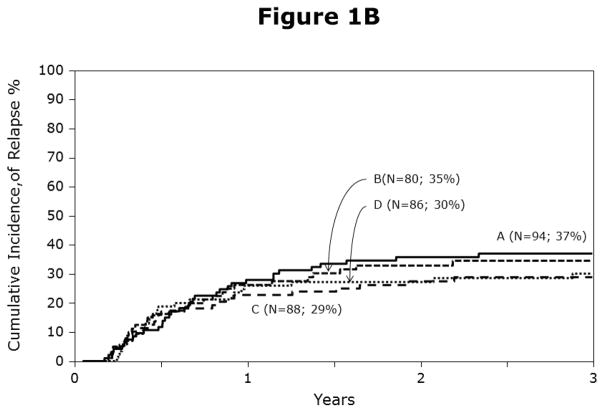
The univariate probabilities of non-relapse mortality and relapse are shown in Table 2 and Figures 1A, 1B. Compared to HLA-matched sibling transplantation, non-relapse mortality risks were higher after mismatched unrelated donor BM and cord blood transplantation but not after matched unrelated donor BM transplantation (Table 3). Leukemic recurrence occurred in 113 of 348 patients. In multivariate analysis, risks of leukemic relapse were similar after HLA-matched sibling and any unrelated donor transplantation (Table 3). Other known risk factors such as cytogenetics, NCI risk group and ALL subtype were not associated with relapse in the current analysis. We also tested for the effect of duration of first CR to determine whether relapse risks differed amongst patients with short duration first CR (<18 months) compared to those with longer duration first CR (18 – 36 months) and did not find a statistically significant difference (RR 0.78, 95% CI 0.54 – 1.14, p=0.19).
Figure 1.
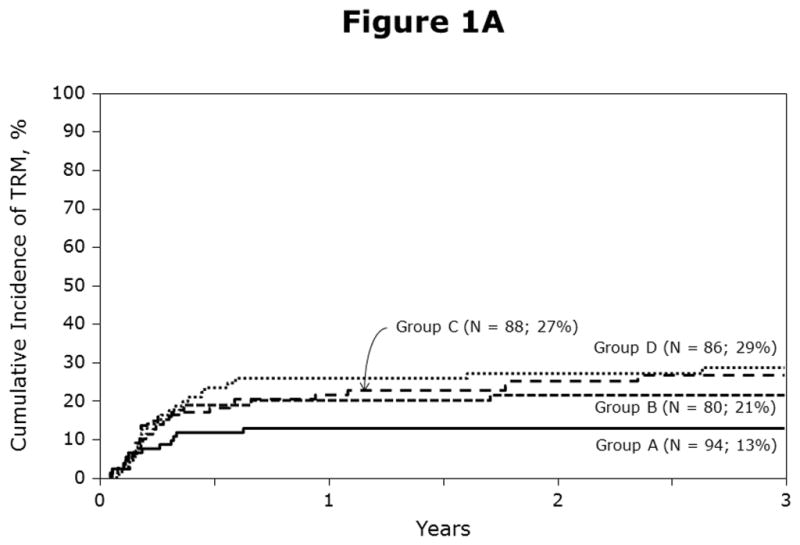
Figure 1A: Probabilities of non-relapse mortality by haematopoietic stem-cell source and donor-recipient HLA matching: A = HLA-matched sibling BM, B = matched unrelated BM, C = mismatched unrelated BM and D = cord blood transplants
Figure 1B: Probabilities of relapse by haematopoietic stem-cell source and donor-recipient HLA matching: A = HLA-matched sibling BM, B = matched unrelated BM, C = mismatched unrelated BM and D = cord blood transplants
Leukemia-free and overall survival
The univariate probabilities of leukemia-free and overall survival are shown in Table 2. In multivariate analysis, there were no differences in treatment failure (relapse or death; inverse of LFS; Figure 2) or overall mortality after HLA-matched sibling and matched or mismatched unrelated donor BM or cord blood transplantation (Table 3). Other known risk factors such as cytogenetics, NCI risk group, duration of first CR and ALL subtype were not associated with leukemia-free and overall survival in the current analysis. One hundred and eighty-one patients died after transplantation (50% of HLA-matched sibling transplant recipients [47 of 94] and 52% of unrelated donor transplant recipients [134 of 254]). Recurrent leukemia was the most common cause of death accounting for 65% of deaths after HLA-matched sibling transplantation and 50% of deaths after unrelated donor transplantation. GVHD-related deaths were more likely after unrelated donor transplantation compared to HLA-matched sibling transplantation (13% vs. 2%). The proportion of deaths attributed to infection (10% vs. 6%), interstitial pneumonits/acute respiratory distress syndrome (12% vs. 15%), organ failure (8% vs. 7%), and other miscellaneous causes (6% vs. 4%) were not different after unrelated donor and HLA- matched sibling transplantation.
Figure 2.
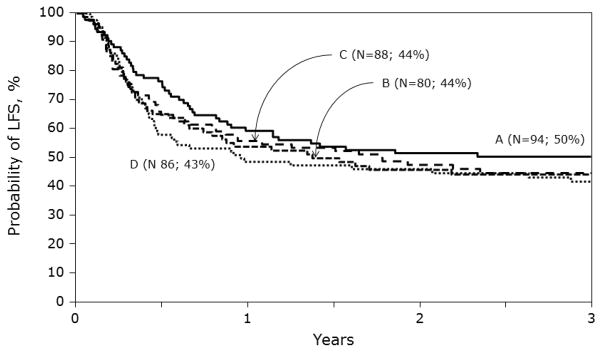
Probabilities of leukemia-free survival by haematopoietic stem-cell source and donor-recipient HLA matching: A = HLA-matched sibling BM, B = matched unrelated BM, C = mismatched unrelated BM and D = cord blood transplants
DISCUSSION
There is general agreement that for children with high-risk ALL in second CR, haematopoietic stem cell transplantation is superior to chemotherapy alone. The lack of a matched sibling for most patients and the higher risk of non-relapse mortality after mismatched unrelated donor BM or cord blood transplantation remain obstacles to referral for transplantation. Consequently, the primary objective of the current analysis was to determine whether outcomes after HLA-matched sibling BM or cord blood transplantation are comparable to those after HLA-matched sibling BM transplantation in a group of patients at very high risk of further relapse and death without transplantation. Despite higher risks of acute and chronic GVHD after unrelated donor transplantation and higher non-relapse mortality after mismatched unrelated donor BM and cord blood transplantation, the 3-year LFS was comparable after matched or mismatched unrelated donor BM, cord blood and HLA-matched sibling donor transplantation. The pattern of treatment failure differed by donor type. While non-relapse mortality was higher after unrelated donor transplantation we observed a higher, but not statistically significant risk of relapse after HLA-matched sibling donor transplantation. Others have also shown comparable LFS after matched or mismatched unrelated donor BM and cord blood transplantation for acute leukemia in children (6, 7). All patients in the current analysis experienced early relapse with duration of first CR less than 36 months. Our data does not indicate differences in rates of relapse, leukemia-free and overall survival in patients whose duration of first CR was less than 18 months compared to those with duration of first CR between 18 and 36 months.
In the current analysis, for the unrelated donor BM group, significant differences in non-relapse mortality risks were seen only after mismatched unrelated donor BM, not in the matched unrelated BM, compared to HLA-matched sibling transplantation. Donor-recipient HLA matching, when considering transplantation of BM from an unrelated adult donor, is important and currently the ideal donor is one matched to the recipient at a minimum of HLA A, B, C, and DRB1 using high resolution (allele-level) typing (13). In the absence of a matched adult unrelated donor, cord blood grafts are an acceptable option. Compared to HLA-matched sibling BM transplantation, LFS is similar after unrelated cord blood transplantation, and consistent with a smaller series from a single institution (19). In the current analysis, we included only transplants performed with cord blood units that contained a minimum infused total nucleated cell dose ≥2.5 × 107/kg, the current clinical standard for this type of graft. Despite this, neutrophil recovery was slower, and the likelihood of recovery lower, compared to HLA-matched sibling transplantation. In general early non-relapse mortality is higher after mismatched cord blood transplantation. Strategies to overcome the cell dose limitation include: transplantation of two cord blood units to achieve the desired cell dose, ex-vivo expansion of cord blood cells and co-infusion of haplo-identical cells (15–21). Additionally, the larger inventory and preferential banking of larger units allow for selection of cord blood units that are matched or one-locus mismatched to the recipient. Non-relapse mortality rates after matched and 1-locus mismatched cord blood units with cell dose >3 × 107/kg have been shown to be similar (7).
We did not observe significant differences in relapse risks after unrelated donor and HLA-matched sibling transplantation. This is not surprising since others have shown post-transplant adoptive immunotherapy with donor leukocyte infusion or natural killer cells are disappointing for treating relapsed ALL. A strong graft-versus-leukemia effect may be absent or minimal for ALL using these techniques (22, 23).
The relative efficacy of unrelated donor and HLA-matched sibling transplantation did not differ by patient age or other known prognostic factors such as ALL subtype, cytogenetics and NCI risk group. The current analysis includes only patients with ALL in second CR who had had a bone marrow relapse within 3 years from diagnosis. Further, all patients received T-cell replete grafts, myeloablative conditioning regimens and calcineurin inhibitor GVHD prophylaxis. While the homogenous study population was an advantage with respect to studying transplant-outcomes the homogeneity may have prevented us from identifying disease-specific factors known to influence long-term leukemia-free survival. It is plausible that factors predictive of long-term survival at diagnosis may no longer be relevant after first relapse. Nevertheless, these known risk factors and chemotherapy received prior to transplantation may have influenced the likelihood of achieving and sustaining second CR.
Though randomized clinical trials are the “gold standard” for comparing different treatment strategies including donor selection, in the absence of such a trial, we have used data collected by a large transplant registry to perform a carefully controlled analysis. A randomized controlled trial is unlikely to be performed. The logistics of randomizing patients to different donor sources is formidable because different processes are needed to identify and procure a related donor, an unrelated adult donor or a cord blood graft. Because very few patients will have a suitably matched unrelated adult donor and a banked cord blood unit available to them, accrual to such a trial would take many years. If a biologic randomization were to be carried out such that any patient with an HLA-matched sibling would be assigned to that arm, ethical considerations such as the rationale of subjecting a patient with a matched unrelated adult donor to mismatched cord blood transplant could be an impediment to accrual. Our findings suggest that all patients with ALL with early relapse who attain a second CR should proceed to BMT with the best available donor source.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pui CH, Sandlund JT, Pei D, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Total Therapy Study XIIIB at St Jude Children’s Research Hospital. Blood. 2004 Nov 1;104(9):2690–2696. doi: 10.1182/blood-2004-04-1616. [DOI] [PubMed] [Google Scholar]
- 2.Pui CH, Campana D, Evans WE. Childhood acute lymphoblastic leukaemia--current status and future perspectives. Lancet Oncol. 2001 Oct;2(10):597–607. doi: 10.1016/S1470-2045(01)00516-2. [DOI] [PubMed] [Google Scholar]
- 3.Buchanan GR, Rivera GK, Pollock BH, et al. Alternating drug pairs with or without periodic reinduction in children with acute lymphoblastic leukemia in second bone marrow remission: a Pediatric Oncology Group Study. Cancer. 2000 Mar 1;88(5):1166–1174. doi: 10.1002/(sici)1097-0142(20000301)88:5<1166::aid-cncr29>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 4.Borgmann A, von Stackelberg A, Hartmann R, et al. Unrelated donor stem cell transplantation compared with chemotherapy for children with acute lymphoblastic leukemia in a second remission: a matched-pair analysis. Blood. 2003 May 15;101(10):3835–3839. doi: 10.1182/blood.V101.10.3835. [DOI] [PubMed] [Google Scholar]
- 5.Eapen M, Raetz E, Zhang MJ, et al. Outcomes after HLA-matched sibling transplantation or chemotherapy in children with B-precursor acute lymphoblastic leukemia in a second remission: a collaborative study of the Children’s Oncology Group and the Center for International Blood and Marrow Transplant Research. Blood. 2006 Jun 15;107(12):4961–4967. doi: 10.1182/blood-2005-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rocha V, Cornish J, Sievers EL, et al. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood. 2001 May 15;97(10):2962–2971. doi: 10.1182/blood.v97.10.2962. [DOI] [PubMed] [Google Scholar]
- 7.Eapen M, Rubinstein P, Zhang MJ, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007 Jun 9;369(9577):1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 8.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995 Jun;15(6):825–828. [PubMed] [Google Scholar]
- 9.Flowers ME, Kansu E, Sullivan KM. Pathophysiology and treatment of graft-versus-host disease. Hematol Oncol Clin North Am. 1999 Oct;13(5):1091–1112. viii–ix. doi: 10.1016/s0889-8588(05)70111-8. [DOI] [PubMed] [Google Scholar]
- 10.Klein JPMM. Survival Analysis: Techniques or censored and truncated data. 2. Springer-Verlag; New York, NY: 2003. [Google Scholar]
- 11.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999 Mar 30;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 12.Cox DR. Regression models and life-tables (with discussion) JR Statist Soc. 1972;B(34):187–220. [Google Scholar]
- 13.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007 Dec 15;110(13):4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 14.Smith AR, Baker KS, Defor TE, Verneris MR, Wagner JE, Macmillan ML. Hematopoietic cell transplantation for children with acute lymphoblastic leukemia in second complete remission: similar outcomes in recipients of unrelated marrow and umbilical cord blood versus marrow from HLA matched sibling donors. Biol Blood Marrow Transplant. 2009 Sep;15(9):1086–1093. doi: 10.1016/j.bbmt.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bautista G, Cabrera JR, Regidor C, et al. Cord blood transplants supported by co-infusion of mobilized hematopoietic stem cells from a third-party donor. Bone Marrow Transplant. 2009 Mar;43(5):365–373. doi: 10.1038/bmt.2008.329. [DOI] [PubMed] [Google Scholar]
- 16.Jaroscak J, Goltry K, Smith A, et al. Augmentation of umbilical cord blood (UCB) transplantation with ex vivo-expanded UCB cells: results of a phase 1 trial using the AastromReplicell System. Blood. 2003 Jun 15;101(12):5061–5067. doi: 10.1182/blood-2001-12-0290. [DOI] [PubMed] [Google Scholar]
- 17.Rocha V. Broxymeyer H. New approaches for improving engraftment after Cord Blood Transplantation. Biol Blood Marrow Transplant. 2009 Nov;2 doi: 10.1016/j.bbmt.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Brunstein CG, Baker KS, Wagner JE. Umbilical cord blood transplantation for myeloid malignancies. Curr Opin Hematol. 2007 Mar;14(2):162–169. doi: 10.1097/MOH.0b013e32802f7da4. [DOI] [PubMed] [Google Scholar]
- 19.Robinson SN, Ng J, Niu T, et al. Superior ex vivo cord blood expansion following co-culture with bone marrow-derived mesenchymal stem cells. Bone Marrow Transplant. 2006 Feb;37(4):359–366. doi: 10.1038/sj.bmt.1705258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delaney C, Varnum-Finney B, Aoyama K, Brashem-Stein C, Bernstein ID. Dose-dependent effects of the Notch ligand Delta1 on ex vivo differentiation and in vivo marrow repopulating ability of cord blood cells. Blood. 2005 Oct 15;106(8):2693–2699. doi: 10.1182/blood-2005-03-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frassoni F, Gualandi F, Podesta M, et al. Direct intrabone transplant of unrelated cord-blood cells in acute leukaemia: a phase I/II study. Lancet Oncol. 2008 Sep;9(9):831–839. doi: 10.1016/S1470-2045(08)70180-3. [DOI] [PubMed] [Google Scholar]
- 22.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002 Mar 15;295(5562):2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 23.Collins RH, Jr, Shpilberg O, Drobyski WR, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997 Feb;15(2):433–444. doi: 10.1200/JCO.1997.15.2.433. [DOI] [PubMed] [Google Scholar]