Abstract
Background
Rheumatic disease and heart disease share common underpinnings involving inflammation. The high levels of inflammation that characterize rheumatic diseases provide a “natural experiment” to help elucidate the mechanisms by which inflammation accelerates heart disease. Rheumatoid arthritis (RA) is the most common of the rheumatic diseases and has the best studied relationships with heart disease.
Methods
Review of current literature on heart disease and rheumatoid arthritis
Results
Patients with RA have an increased risk of developing heart disease that is not fully explained by traditional cardiovascular risk factors. Therapies used to treat RA may also affect the development of heart disease; by suppressing inflammation, they may also reduce the risk of heart disease. However, their other effects, as in the case of steroids, may increase heart disease risk.
Conclusions
Investigations of the innate and adaptive immune responses occurring in RA may delineate novel mechanisms in the pathogenesis of heart disease, and help identify novel therapeutic targets for the prevention and treatment of heart disease.
Introduction
The role of inflammation in the development of heart disease has only been recognized relatively recently. Rheumatic disease can be viewed as a ‘natural experiment’ in the interplay between chronic inflammation and heart disease, which could elucidate the fundamental mechanisms by which inflammation accelerates development of atherosclerosis and heart disease. Rheumatoid arthritis (RA), systemic lupus erythematosus, Sjögren’s syndrome, systemic scleroderma, inflammatory myositis and psoriatic arthritis are characterized by chronic inflammation in various body systems, most often the joints, skin, eyes, lungs, and kidneys, but also in the heart and vascular system. RA is the most common and best studied of the autoimmune rheumatic diseases, and will be the primary focus of this overview.
The immune underpinnings of heart disease and RA share many similarities. In addition, circulating acute phase reactants, such as C-reactive protein (CRP), are substantially elevated in RA and are risk markers for heart disease in the general population. Understanding the factors responsible for heart disease in patients with RA, such as abnormal immunity and chronic inflammation, may lead to novel therapeutic targets in the prevention of heart disease.
Epidemiology of heart disease in RA
Patients with RA have a 1.5–2.0 fold increased risk of developing coronary artery disease (CAD) compared with the general population (1,2), similar in magnitude to the risk imparted by diabetes mellitus (3). This increased CAD risk is evident even before the clinical recognition of RA: at diagnosis, individuals with RA were over three times as likely to have had a prior myocardial infarction (MI) than subjects without RA (2). An expert committee of the European League Against Rheumatism has recommended that CV risk scores (e.g., Framingham) be multiplied by 1.5 in some patients with RA to reflect their increased risk of heart disease (4).
Patients with RA also have twice the risk of developing heart failure (5). This risk is more pronounced in the RA patients who are rheumatoid factor positive than among seronegative patients. Patients with RA are less likely to have typical signs and symptoms of heart failure, tend to be managed less aggressively and have poorer outcomes (6). Importantly, patients with RA and heart failure are more likely to have a preserved ejection fraction (>50%), and less likely to have clinical evidence of CAD. Patients with RA may have a reduced likelihood of developing heart failure after MI (7). Collectively, these findings suggest patients with RA are more likely to have heart failure due to diastolic dysfunction, which may be related to systemic inflammation.
The possible effect of RA on the risk of non-cardiac vascular disease is less clear. Some reports have noted increased risks of cerebrovascular disease in RA (8), while others have not (9). Overt and subclinical peripheral vascular disease appears to be increased in patients with RA, and is associated with severity, particularly extraarticular systemic disease (10–12) The risk of venous thromboembolism appears to be increased 2–3 fold in RA compared with the general population (9,13).
CV risk factor profile in RA
Patients with RA tend to have a different profile of cardiac risk factors, including a higher frequency of smoking and an altered lipid profile, compared with the general population. The lipid profile in RA is characterized by suppression of total and LDL cholesterol levels during periods of high-grade inflammation, with a proportionately greater suppression of high-density lipoprotein (HDL) levels, yielding an unfavorable ratio of total to HDL cholesterol. Lipid levels have a paradoxical relationship with CAD risk in RA, since lower lipid levels are associated with more severe systemic inflammation, which in turn is associated with increased CAD risk (14). Inflammation in RA also appears to alter lipoprotein structure and function (15); the serum amyloid A load carried by HDL increases and Apolipoprotein A-I decreases, altering the usual anti-atherogenic effects of HDL and resulting in pro-atherogenic effects (16).
Patients with RA are more likely to have lower muscle mass and low body mass index, which may result from uncontrolled inflammation, limitations of physical activity, or both. Low body mass in RA appears to be associated with a worsened prognosis (17). Cachexia, characterized by low muscle and fat mass, is now uncommon in RA, but the combination of low muscle mass and high fat mass is more prevalent in patients with RA and may be even more problematic from a heart disease perspective (18). Visceral adiposity in RA is associated with insulin resistance, hypertension, metabolic syndrome, and a greater inflammatory load (18).
Hypertension is common and it appears to be underdiagnosed and undertreated in RA (19). Hypertension in RA may be exacerbated by inflammation and medications.
Increased risk of heart disease in patients with RA is associated with elevation of inflammatory markers, including CRP, erythrocyte sedimentation rate, rheumatoid factor, anti-citrullinated protein antibodies, and with more active or severe RA (20). Rheumatoid factor and anti-nuclear antibodies have been associated with heart disease and overall mortality, even in patients without rheumatic diseases (21).
Heart Disease Management/Outcome in RA
Patients with RA are typically managed by several physicians, and coordination of care may be suboptimal. Smoking cessation and control of standard risk factors are all indicated in patients with RA, but may be underused because of the understandable focus on management of RA itself. Despite the well understood benefits of exercise on general and cardiovascular health, the majority of patients with RA do not pursue a regular exercise program (22,23). Both aerobic and resistance exercise training for patients with RA has been shown to be efficacious in improving overall well being, the muscle mass loss associated with RA, and markedly improving physical function without exacerbating disease activity and is likely to reduce cardiovascular risk, and should be part of routine care (24–29).
There is evidence that patients with RA are less likely to receive both primary and secondary heart disease prevention. Only 55% of RA patients in one study had lipid levels measured; management by rheumatologists was associated with less lipid screening (30). Rheumatologists were less likely to identify and treat cardiac risk factors than primary care physicians (31). Angina may also be under diagnosed, with chest pain attributed to RA instead of CAD, perhaps because the increased risk of CAD is not understood by treating physicians, and referral to a cardiologist is less likely. Patients with RA and an acute MI were less likely to receive reperfusion therapy and secondary prevention medications, such as beta blockers and lipid-lowering agents (32). Patients with RA were also less likely to undergo coronary artery bypass grafting than patients without RA (2).
Effect of RA Therapies on CV Risk in RA
RA therapies target inflammation, a CV risk factor that is also important in patients without rheumatic disease. Understanding how these therapies also modify CV risk in RA may provide insight into the inflammatory component of CV risk for all patients.
Glucocorticoids have been widely used in RA to acutely control pain and inflammation associated with RA flares. However, the beneficial anti-inflammatory effects of glucocorticoids, which can improve mobility, are also accompanied by adverse effects, including increasing CV risk factors and worsening heart disease outcomes. Glucocorticoid use is associated with carotid plaque and arterial stiffness, decreased insulin sensitivity, elevated lipid levels, and hypertension (33,34). Patients treated with high dose steroids (>7.5mg/day prednisone) appear to have twice the risk of heart disease compared with those who do not receive steroids (35). Use of low-dose glucocorticoids to treat active inflammatory disease might have more benefits than harms, but this requires further investigation.
The long standing concern about CV risk with nonsteroidal anti-inflammatory drugs (NSAIDs) use was magnified after studies of the selective cyclo-oxygenase-2 inhibitors (coxibs). The VIGOR (Vioxx Gastrointestinal Outcomes Research) study found an increased risk of heart disease events with rofecoxib use, which led to its removal from the market (36). By contrast, another trial found no differences in heart disease events among subjects randomized to receive celecoxib or ibuprofen (37). Finally, a network meta-analysis of 7 NSAIDs, including 4 coxibs, suggested that naproxen conferred the lowest CV risk, while the remaining 6 NSAIDs appeared to confer similar risk for CVD (38). A large trial currently enrolling patients, including those with RA, will assess differences between NSAIDs and risk of heart disease (39).
Methotrexate, a first-line treatment for RA, has been associated with lower CV risk. A recent meta-analysis found that methotrexate use was associated with 21% fewer CV events (Figure 1)(40). Methotrexate does not appear to alter lipid profiles (41), and there is insufficient evidence about its effects on insulin resistance, hypertension or atherosclerotic plaque burden. Nevertheless, the apparent benefit of methotrexate in reducing heart disease risk in RA, and the strong evidence that CRP is a risk factor for heart disease, led to the development of a large randomized trial (http://ClinicalTrials.gov identifier: NCT01594333) designed to determine whether low dose methotrexate reduces heart disease risk in post-MI patients who have metabolic syndrome or diabetes mellitus (42).
Figure 1.
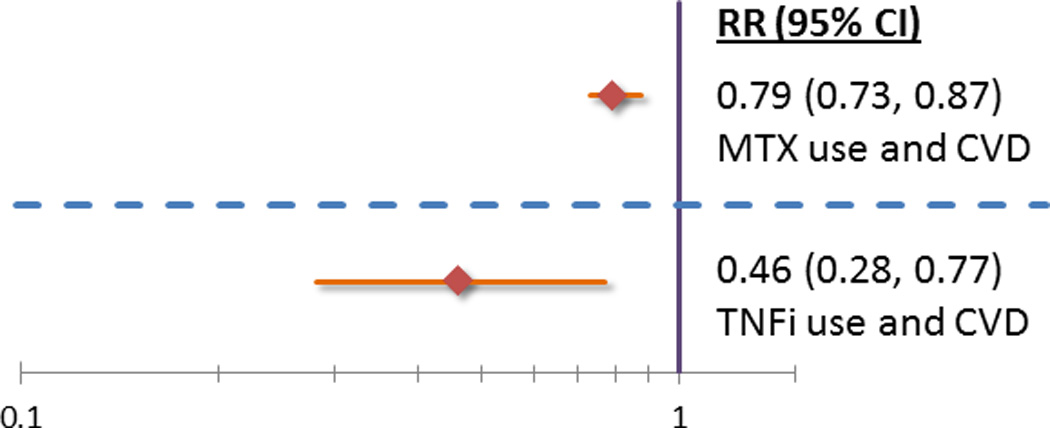
Tumor necrosis factor (TNF) inhibitors are frequently used in RA patients who do not achieve adequate disease control with other therapies. Roughly 30–40% of RA patients in the US received a TNF-inhibitor either as monotherapy or in combination with other medications. TNF-inhibitor therapy in patients with RA appears to be associated with reduced risk of all heart disease events (Figure 1)(43). If this reduced CV risk is true, it may be mediated by effects on controlling inflammation, rather than by favorable modifications of CV risk factors. In general, TNF-inhibitors appear to elevate total and HDL cholesterol, resulting in a stable atherogenic ratio (44). In addition, since RA disease activity appears to be inversely correlated with HDL levels, treatments that control RA activity may favorably affect HDL levels (45). It is unclear, however, whether TNF-inhibitors exert a class effect on lipids, or if specific TNF-inhibitors achieve more favorable lipid profiles. TNF-inhibitors may improve endothelial function and insulin resistance; their effect on arterial stiffness is unclear, with one study showing improvement and another finding no change with therapy (44,46–48).
There is insufficient evidence regarding the effect of other disease modifying antirheumatic drugs and heart disease risk. Hydroxychloroquine is associated with a decreased risk of diabetes mellitus in patients with RA (49), and may also improve lipid profiles, with evidence of decreased LDL and total cholesterol/HDL ratios (50). Tocilizumab, a humanized antibody that targets the IL-6 receptor, increases LDL, HDL and triglyceride levels during clinical trials (51). The long term clinical significance of these perturbations in lipid levels remains unknown.
Effect of Heart Disease Therapies in Patients with RA
There have been relatively few patients with rheumatic diseases enrolled in major clinical trials of primary or secondary prevention. While commonly used CV therapies are presumed to be effective in patients with RA, there is little direct evidence of their efficacy.
Statins (hydroxymethylglutaryl CoA reductase inhibitors) appear to reduce vascular inflammation and, have been tested for efficacy in the treatment of RA. Atorvastatin treatment resulted in a small reduction in RA disease activity compared with placebo, suggesting that statins may reduce inflammation directly (52). Small studies in patients with RA have also shown improvements in arterial stiffness and endothelial dysfunction with atorvastatin (53,54). Statin discontinuation for ≥3 months in patients with RA resulted in a 2% increased risk of acute MI for each month of discontinuation (55). Notably, statin therapy has also been found to impair the effectiveness of rituximab (56), so drug interactions will be important to consider in the clinical management of patients with RA.
Aspirin was the mainstay of RA treatment until the development of prescription NSAIDs in the 1970’s. Aspirin has been highly effective in secondary and primary heart disease prevention, but few studies included patients with rheumatic diseases. Aspirin doses for heart disease prevention are far below those used for RA treatment in the past (ranging from 2600 to 4800mg/day) (57). Moreover, the gastrointestinal bleeding risks of chronic aspirin use in RA patients, who frequently use NSAIDs and glucocorticoids, may shift the balance away from CV benefit.
Mechanisms of RA Pertinent to Cardiovascular Disease
Emerging evidence suggests that T lymphocytes play a crucial pathogenic role in both RA and heart disease (58,59). The major risk gene for RA, HLA-DRB1, predisposes to disease by promoting the selection and survival of autoreactive CD4+ T cells. HLA-DRB1 alleles are also associated with increased risk of MI and various forms of non-RA-associated heart disease(60,61). As in heart disease, T cells isolated from the joints of patients with RA have enhanced production of interferon-γ and interleukin-17, which presumably mediate chronic inflammation (62,63). The proven efficacy of antagonizing T-cell co-stimulation is perhaps the most compelling evidence that T cells are pathogenic in RA (64). Similarly, percutaneous stents that elute T-cell inhibiting drugs (e.g., sirolimus) prevent in-stent restenosis and repeat re-vascularization in CAD(65).
In persons with either RA or heart disease, CD4+ T cells characteristically lose expression of the co-stimulatory molecule, CD28, which ordinarily provides the ‘second signal’ required for T-cell activation. So-called ‘CD28null’ T cells are believed to have undergone reprogramming, leading to premature senescence (66). Expansion of these senescent T cells among persons with RA is associated with extra-articular inflammatory manifestations, including vasculitis and lung disease, as well as CAD (67,68). In the setting of heart disease, CD28null T cells are identified in atherosclerotic plaque, where they are believed to contribute to the inflammatory process by producing cytokines and by killing vascular smooth muscle cells (69). Interestingly, HLA-DRB1, the aforementioned RA-risk gene, also predisposes to expansion of CD28null T cells in RA and in CAD(61,70).
Premature senescence of T cells in RA appears to be caused by fundamental defects in the hematopoietic system. CD34+ hematopoietic progenitor cells have accelerated telomere erosion, a sign of senescence (71). Naïve T cells in persons with RA also are prematurely aged, with increased fragility and damage of their DNA due to insufficient activity of basic DNA repair enzymes (72,73). Similarly, telomere shortening in hematopoietic progenitor cells correlates with myocardial dysfunction in patients with CAD (74). The onset of both RA and heart disease coincides with the loss of thymic emigration of naïve T cells in the fifth decade, suggesting that T-cell senescence may underlay the pathogenesis of both of these age-associated conditions. In the foreseeable future, rejuvenation of senescent T cells, using new drugs that restore genomic repair and integrity, could potentially be an effective strategy for the prevention and treatment of cardiovascular disease (75).
CONCLUSION
Heart disease remains a major problem for patients with RA. Systemic inflammation plays a major role, through direct and indirect effects on the vasculature (Figure 2). More research is needed to delineate the disease mechanisms, and to develop and evaluate risk assessment tools, biomarkers, prevention strategies and treatments that are specific to RA. The CV risk in patients with RA is not well recognized by practicing physicians, and better recognition and control of traditional risk factors in patients with RA is important. Coordination of care among rheumatologists, cardiologists, and primary care physicians will be needed for optimal management of CV risk in patients with RA. Tight control of systemic inflammation among patients with RA may also reduce CV risk. Symptoms suggestive of CAD in patients with RA should be evaluated promptly, and early referral to a CV specialist for appropriate evaluation and treatment provides the best chance of optimizing outcomes.
Figure 2.
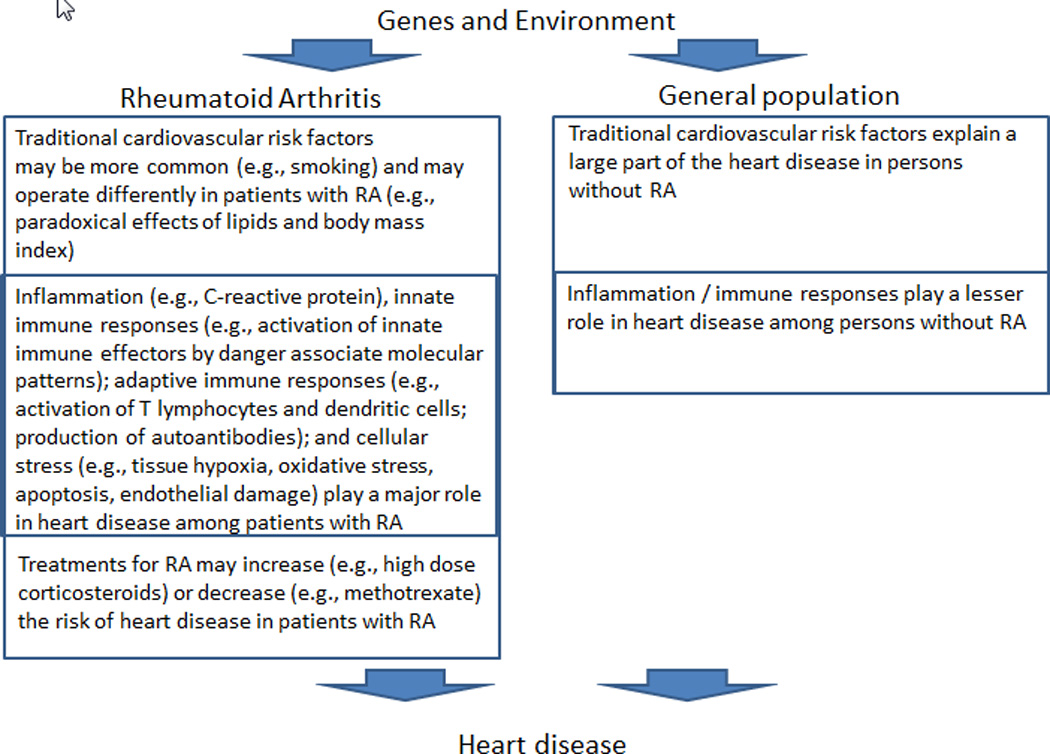
Disentangling the relationship between inflammation, immune modulating treatment and CV risk in RA is difficult. Specific disease modifying drugs (e.g., methotrexate and TNF-inhibitors) effectively control inflammation in RA and also reduce CV risk. In contrast, glucocorticoids increase CV risk because of their adverse metabolic effects, which apparently outweigh their anti-inflammatory benefits. Common treatments to reduce CV risk (e.g., statins) are likely to be effective in patients with RA, but this supposition has little empirical support. Currently enrolling trials such as one administering methotrexate (a first-line treatment for RA) to post-MI patients without RA, should provide insight regarding whether reducing inflammation alone is associated with reduced CV risk (76).
Finally, similar pathways in RA and heart disease might be considered as therapeutic targets, such as T-cell-directed or anti-cytokine therapies (IL-1, IL-6, etc.) (77,78). Indeed, an anti-IL-1β monoclonal antibody (canakinumab) is being studied for heart disease treatment (79). These studies are anticipated to provide valuable new insights into the pathophysiology and treatment of heart disease.
Acknowledgments
Ms. Crowson has research funding from Pfizer and Roche/Genentech. Dr. Liao is supported by NIH K08 AR060257 and Katherine Swan Ginsburg Fund. Dr. Davis is a site primary investigator for industry-sponsored trials with UCB Pharma and Roche. Dr. Solomon is supported by NIH K24 AR 055989, receives research support from Amgen, Lilly, and Abbott, serves as an epidemiologic consultant to CORRONA, and serves in unpaid roles on two Pfizer sponsored trials. Dr. Matteson has research funding from Pfizer and is involved in an industry-sponsored trial with Roche/Genentech. Drs. Knutson and Hlatky reported that they have no relationships relevant to the contents of this paper to disclose. Dr. Hlatky is funded by the American Heart Association. Dr. Gabriel is supported by NIH R01 AR46849, receives research support from Pfizer and serves as a consultant for Roche/Genentech.
Abbreviations
- CAD
coronary artery disease
- CRP
C-reactive protein
- CV
cardiovascular
- HDL
high density lipoprotein
- LDL
low density lipoprotein
- MI
myocardial infarction
- NSAID
non-steroidal anti-inflammatory drug
- RA
rheumatoid arthritis
- TNF
tumor necrosis factor
Appendix
The studies included in this Review were identified by searching PubMed using an extensive list of phrases related to the topic of interest. The searches were restricted to full-text papers in the English language. This manuscript is not intended to be a systematic review or a meta-analysis. Papers cited in this Review were selected based on methodologic strength, whereby evidence from randomized controlled trials and population-based studies were preferred.
No extramural funding was used to support this work. The authors are solely responsible for the design and conduct of this work, the drafting and editing of the paper and its final contents.
References
- 1.Solomon DH, Goodson NJ, Katz JN, et al. Patterns of cardiovascular risk in rheumatoid arthritis. Ann Rheum Dis. 2006;65:1608–1612. doi: 10.1136/ard.2005.050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2005;52:402–411. doi: 10.1002/art.20853. [DOI] [PubMed] [Google Scholar]
- 3.Peters MJ, van Halm VP, Voskuyl AE, et al. Does rheumatoid arthritis equal diabetes mellitus as an independent risk factor for cardiovascular disease? A prospective study. Arthritis Rheum. 2009;61:1571–1579. doi: 10.1002/art.24836. [DOI] [PubMed] [Google Scholar]
- 4.Peters MJ, Symmons DP, McCarey D, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69:325–331. doi: 10.1136/ard.2009.113696. [DOI] [PubMed] [Google Scholar]
- 5.Nicola PJ, Maradit-Kremers H, Roger VL, et al. The risk of congestive heart failure in rheumatoid arthritis: A population-based study over 46 years. Arthritis Rheum. 2005;52:412–420. doi: 10.1002/art.20855. [DOI] [PubMed] [Google Scholar]
- 6.Davis JM, 3rd, Roger VL, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. The presentation and outcome of heart failure in patients with rheumatoid arthritis differs from that in the general population. Arthritis Rheum. 2008;58:2603–2611. doi: 10.1002/art.23798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Francis ML, Varghese JJ, Mathew JM, Koneru S, Scaife SL, Zahnd WE. Outcomes in patients with rheumatoid arthritis and myocardial infarction. Am J Med. 2010;123:922–928. doi: 10.1016/j.amjmed.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 8.Zoller B, Li X, Sundquist J, Sundquist K. Risk of subsequent ischemic and hemorrhagic stroke in patients hospitalized for immune-mediated diseases: a nationwide follow-up study from Sweden. BMC Neurol. 2012;12:41. doi: 10.1186/1471-2377-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bacani AK, Gabriel SE, Crowson CS, Heit JA, Matteson EL. Noncardiac vascular disease in rheumatoid arthritis: increase in venous thromboembolic events? Arthritis Rheum. 2012;64:53–61. doi: 10.1002/art.33322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang KP, Liang KV, Matteson EL, McClelland RL, Christianson TJ, Turesson C. Incidence of noncardiac vascular disease in rheumatoid arthritis and relationship to extraarticular disease manifestations. Arthritis & Rheumatism. 2006;54:642–648. doi: 10.1002/art.21628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stamatelopoulos KS, Kitas GD, Papamichael CM, et al. Subclinical peripheral arterial disease in rheumatoid arthritis. Atherosclerosis. 2010;212:305–309. doi: 10.1016/j.atherosclerosis.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Alkaabi JK, Ho M, Levison R, Pullar T, Belch JJ. Rheumatoid arthritis and macrovascular disease. Rheumatology. 2003;42:292–297. doi: 10.1093/rheumatology/keg083. [DOI] [PubMed] [Google Scholar]
- 13.Holmqvist ME, Neovius M, Eriksson J, et al. Risk of venous thromboembolism in patients with rheumatoid arthritis and association with disease duration and hospitalization. JAMA. 2012;308:1350–1356. doi: 10.1001/2012.jama.11741. [DOI] [PubMed] [Google Scholar]
- 14.Myasoedova E, Crowson CS, Maradit Kremers H, et al. Lipid Paradox in Rheumatoid Arthritis: The Impact of Serum Lipid Measures and Systemic Inflammation on the Risk of Cardiovascular Disease. Ann Rheum Dis. 2010;69:495. doi: 10.1136/ard.2010.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toms TE, Symmons DP, Kitas GD. Dyslipidaemia in rheumatoid arthritis: the role of inflammation, drugs, lifestyle and genetic factors. Curr Vasc Pharmacol. 2010;8:301–326. doi: 10.2174/157016110791112269. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe J, Charles-Schoeman C, Miao Y, et al. Proteomic profiling following immunoaffinity capture of high-density lipoprotein: association of acute-phase proteins and complement factors with proinflammatory high-density lipoprotein in rheumatoid arthritis. Arthritis Rheum. 2012;64:1828–1837. doi: 10.1002/art.34363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maradit Kremers HM, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Prognostic importance of low body mass index in relation to cardiovascular mortality in rheumatoid arthritis. Arthritis Rheum. 2004;50:3450–3457. doi: 10.1002/art.20612. [DOI] [PubMed] [Google Scholar]
- 18.Giles JT, Allison M, Blumenthal RS, et al. Abdominal adiposity in rheumatoid arthritis: Association with cardiometabolic risk factors and disease characteristics. Arthritis Rheum. 2010;62:3173–3182. doi: 10.1002/art.27629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panoulas VF, Douglas KM, Milionis HJ, et al. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford) 2007;46:1477–1482. doi: 10.1093/rheumatology/kem169. [DOI] [PubMed] [Google Scholar]
- 20.Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2005;52:722–732. doi: 10.1002/art.20878. [DOI] [PubMed] [Google Scholar]
- 21.Liang KP, Kremers HM, Crowson CS, et al. Autoantibodies and the risk of cardiovascular events. J Rheumatol. 2009;36:2462–2469. doi: 10.3899/jrheum.090188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sokka T, Hakkinen A. Poor physical fitness and performance as predictors of mortality in normal populations and patients with rheumatic and other diseases. Clin Exp Rheumatol. 2008;26:S14–S20. [PubMed] [Google Scholar]
- 23.Lavie CJ, Thomas RJ, Squires RW, Allison TG, Milani RV. Exercise training and cardiac rehabilitation in primary and secondary prevention of coronary heart disease. Mayo Clin Proc. 2009;84:373–383. doi: 10.1016/S0025-6196(11)60548-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooney JK, Law RJ, Matschke V, et al. Benefits of exercise in rheumatoid arthritis. J Aging Res. 2011;2011:681640. doi: 10.4061/2011/681640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemmey AB, Marcora SM, Chester K, Wilson S, Casanova F, Maddison PJ. Effects of high-intensity resistance training in patients with rheumatoid arthritis: a randomized controlled trial. Arthritis Rheum. 2009;61:1726–1734. doi: 10.1002/art.24891. [DOI] [PubMed] [Google Scholar]
- 26.de Jong Z, Munneke M, Zwinderman AH, et al. Is a long-term high-intensity exercise program effective and safe in patients with rheumatoid arthritis? Results of a randomized controlled trial. Arthritis Rheum. 2003;48:2415–2424. doi: 10.1002/art.11216. [DOI] [PubMed] [Google Scholar]
- 27.van den Ende CH, Breedveld FC, le Cessie S, Dijkmans BA, de Mug AW, Hazes JM. Effect of intensive exercise on patients with active rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2000;59:615–621. doi: 10.1136/ard.59.8.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neuberger GB, Aaronson LS, Gajewski B, et al. Predictors of exercise and effects of exercise on symptoms, function, aerobic fitness, and disease outcomes of rheumatoid arthritis. Arthritis Rheum. 2007;57:943–952. doi: 10.1002/art.22903. [DOI] [PubMed] [Google Scholar]
- 29.Noreau L, Martineau H, Roy L, Belzile M. Effects of a modified dance-based exercise on cardiorespiratory fitness, psychological state and health status of persons with rheumatoid arthritis. Am J Phys Med Rehabil. 1995;74:19–27. doi: 10.1097/00002060-199501000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Maradit Kremers HM, Bidaut-Russell M, Scott CG, Reinalda MS, Zinsmeister AR, Gabriel SE. Preventive medical services among patients with rheumatoid arthritis. J Rheumatol. 2003;30:1940–1947. [PubMed] [Google Scholar]
- 31.Desai SS, Myles JD, Kaplan MJ. Suboptimal cardiovascular risk factor identification and management in patients with rheumatoid arthritis: a cohort analysis. Arthritis Res Ther. 2012;14:R270. doi: 10.1186/ar4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Doornum S, Brand C, Sundararajan V, Ajani AE, Wicks IP. Rheumatoid arthritis patients receive less frequent acute reperfusion and secondary prevention therapy after myocardial infarction compared with the general population. Arthritis Res Ther. 2010;12:R183. doi: 10.1186/ar3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dessein PH, Joffe BI, Stanwix AE, Christian BF, Veller M. Glucocorticoids and insulin sensitivity in rheumatoid arthritis. J Rheumatol. 2004;31:867–874. [PubMed] [Google Scholar]
- 34.Hafstrom I, Rohani M, Deneberg S, Wornert M, Jogestrand T, Frostegard J. Effects of low-dose prednisolone on endothelial function, atherosclerosis, and traditional risk factors for atherosclerosis in patients with rheumatoid arthritis--a randomized study. J Rheumatol. 2007;34:1810–1816. [PubMed] [Google Scholar]
- 35.Davis JM, 3rd, Maradit Kremers H, Crowson CS, et al. Glucocorticoids and cardiovascular events in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2007;56:820–830. doi: 10.1002/art.22418. [DOI] [PubMed] [Google Scholar]
- 36.Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med. 2000;343:1520–1528. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]
- 37.Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA. 2000;284:1247–1255. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- 38.Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342:c7086. doi: 10.1136/bmj.c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Becker MC, Wang TH, Wisniewski L, et al. Rationale, design, and governance of Prospective Randomized Evaluation of Celecoxib Integrated Safety versus Ibuprofen Or Naproxen (PRECISION), a cardiovascular end point trial of nonsteroidal antiinflammatory agents in patients with arthritis. Am Heart J. 2009;157:606–612. doi: 10.1016/j.ahj.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 40.Micha R, Imamura F, Wyler von Ballmoos M, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol. 2011;108:1362–1370. doi: 10.1016/j.amjcard.2011.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Georgiadis AN, Papavasiliou EC, Lourida ES, et al. Atherogenic lipid profile is a feature characteristic of patients with early rheumatoid arthritis: effect of early treatment--a prospective, controlled study. Arthritis Res Ther. 2006;8:R82. doi: 10.1186/ar1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ridker PM. Testing the inflammatory hypothesis of atherothrombosis: scientific rationale for the cardiovascular inflammation reduction trial (CIRT) J Thromb Haemost. 2009;7Suppl1:332–339. doi: 10.1111/j.1538-7836.2009.03404.x. [DOI] [PubMed] [Google Scholar]
- 43.Barnabe C, Martin BJ, Ghali WA. Systematic review and meta-analysis: anti-tumor necrosis factor alpha therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2011;63:522–529. doi: 10.1002/acr.20371. [DOI] [PubMed] [Google Scholar]
- 44.Tam LS, Tomlinson B, Chu TT, Li TK, Li EK. Impact of TNF inhibition on insulin resistance and lipids levels in patients with rheumatoid arthritis. Clin Rheumatol. 2007;26:1495–1498. doi: 10.1007/s10067-007-0539-8. [DOI] [PubMed] [Google Scholar]
- 45.Jamnitski A, Visman IM, Peters MJ, Dijkmans BA, Voskuyl AE, Nurmohamed MT. Beneficial effect of 1-year etanercept treatment on the lipid profile in responding patients with rheumatoid arthritis: the ETRA study. Ann Rheum Dis. 2010;69:1929–1933. doi: 10.1136/ard.2009.127597. [DOI] [PubMed] [Google Scholar]
- 46.Hurlimann D, Forster A, Noll G, et al. Anti-tumor necrosis factor-alpha treatment improves endothelial function in patients with rheumatoid arthritis. Circulation. 2002;106:2184–2187. doi: 10.1161/01.cir.0000037521.71373.44. [DOI] [PubMed] [Google Scholar]
- 47.Angel K, Provan SA, Gulseth HL, Mowinckel P, Kvien TK, Atar D. Tumor necrosis factor-alpha antagonists improve aortic stiffness in patients with inflammatory arthropathies: a controlled study. Hypertension. 2010;55:333–338. doi: 10.1161/HYPERTENSIONAHA.109.143982. [DOI] [PubMed] [Google Scholar]
- 48.Van Doornum S, McColl G, Wicks IP. Tumour necrosis factor antagonists improve disease activity but not arterial stiffness in rheumatoid arthritis. Rheumatology (Oxford) 2005;44:1428–1432. doi: 10.1093/rheumatology/kei033. [DOI] [PubMed] [Google Scholar]
- 49.Wasko MC, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298:187–193. doi: 10.1001/jama.298.2.187. [DOI] [PubMed] [Google Scholar]
- 50.Morris SJ, Wasko MC, Antohe JL, et al. Hydroxychloroquine use associated with improvement in lipid profiles in rheumatoid arthritis patients. Arthritis Care Res (Hoboken) 2011;63:530–534. doi: 10.1002/acr.20393. [DOI] [PubMed] [Google Scholar]
- 51.Singh JA, Beg S, Lopez-Olivo MA. Tocilizumab for rheumatoid arthritis: a Cochrane systematic review. J Rheumatol. 2011;38:10–20. doi: 10.3899/jrheum.100717. [DOI] [PubMed] [Google Scholar]
- 52.McCarey DW, McInnes IB, Madhok R, et al. Trial of Atorvastatin in Rheumatoid Arthritis (TARA): double-blind, randomised placebo-controlled trial. Lancet. 2004;363:2015–2021. doi: 10.1016/S0140-6736(04)16449-0. [DOI] [PubMed] [Google Scholar]
- 53.Van Doornum S, McColl G, Wicks IP. Atorvastatin reduces arterial stiffness in patients with rheumatoid arthritis. Ann Rheum Dis. 2004;63:1571–1575. doi: 10.1136/ard.2003.018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hermann F, Forster A, Chenevard R, et al. Simvastatin improves endothelial function in patients with rheumatoid arthritis. J Am Coll Cardiol. 2005;45:461–464. doi: 10.1016/j.jacc.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 55.De Vera MA, Choi H, Abrahamowicz M, Kopec J, Goycochea-Robles MV, Lacaille D. Statin discontinuation and risk of acute myocardial infarction in patients with rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2011;70:1020–1024. doi: 10.1136/ard.2010.142455. [DOI] [PubMed] [Google Scholar]
- 56.Arts EE, Jansen TL, Den Broeder A, et al. Statins inhibit the antirheumatic effects of rituximab in rheumatoid arthritis: results from the Dutch Rheumatoid Arthritis Monitoring (DREAM) registry. Ann Rheum Dis. 2011;70:877–878. doi: 10.1136/ard.2010.136093. [DOI] [PubMed] [Google Scholar]
- 57.Fries JF, Ramey DR, Singh G, Morfeld D, Bloch DA, Raynauld JP. A reevaluation of aspirin therapy in rheumatoid arthritis. Arch Intern Med. 1993;153:2465–2471. [PubMed] [Google Scholar]
- 58.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 59.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. The New England journal of medicine. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 60.Paakkanen R, Lokki ML, Seppanen M, Tierala I, Nieminen MS, Sinisalo J. Proinflammatory HLA-DRB1*01-haplotype predisposes to ST-elevation myocardial infarction. Atherosclerosis. 2012;221:461–466. doi: 10.1016/j.atherosclerosis.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 61.Sun W, Cui Y, Zhen L, Huang L. Association between HLA-DRB1, HLA-DRQB1 alleles, and CD4(+)CD28(null) T cells in a Chinese population with coronary heart disease. Mol Biol Rep. 2011;38:1675–1679. doi: 10.1007/s11033-010-0279-8. [DOI] [PubMed] [Google Scholar]
- 62.Eid RE, Rao DA, Zhou J, et al. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009;119:1424–1432. doi: 10.1161/CIRCULATIONAHA.108.827618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nistala K, Adams S, Cambrook H, et al. Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proc Natl Acad Sci U S A. 2010;107:14751–14756. doi: 10.1073/pnas.1003852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kremer JM, Genant HK, Moreland LW, et al. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2006;144:865–876. doi: 10.7326/0003-4819-144-12-200606200-00003. [DOI] [PubMed] [Google Scholar]
- 65.Schomig A, Dibra A, Windecker S, et al. A meta-analysis of 16 randomized trials of sirolimus-eluting stents versus paclitaxel-eluting stents in patients with coronary artery disease. Journal of the American College of Cardiology. 2007;50:1373–1380. doi: 10.1016/j.jacc.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 66.Vallejo AN. CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol Rev. 2005;205:158–169. doi: 10.1111/j.0105-2896.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 67.Gerli R, Schillaci G, Giordano A, et al. CD4+CD28− T lymphocytes contribute to early atherosclerotic damage in rheumatoid arthritis patients. Circulation. 2004;109:2744–2748. doi: 10.1161/01.CIR.0000131450.66017.B3. [DOI] [PubMed] [Google Scholar]
- 68.Martens PB, Goronzy JJ, Schaid D, Weyand CM. Expansion of unusual CD4+ T cells in severe rheumatoid arthritis. Arthritis Rheum. 1997;40:1106–1114. doi: 10.1002/art.1780400615. [DOI] [PubMed] [Google Scholar]
- 69.Nakajima T, Schulte S, Warrington KJ, et al. T-cell-mediated lysis of endothelial cells in acute coronary syndromes. Circulation. 2002;105:570–575. doi: 10.1161/hc0502.103348. [DOI] [PubMed] [Google Scholar]
- 70.Schonland SO, Lopez C, Widmann T, et al. Premature telomeric loss in rheumatoid arthritis is genetically determined and involves both myeloid and lymphoid cell lineages. Proc Natl Acad Sci U S A. 2003;100:13471–13476. doi: 10.1073/pnas.2233561100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Colmegna I, Diaz-Borjon A, Fujii H, Schaefer L, Goronzy JJ, Weyand CM. Defective proliferative capacity and accelerated telomeric loss of hematopoietic progenitor cells in rheumatoid arthritis. Arthritis Rheum. 2008;58:990–1000. doi: 10.1002/art.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shao L, Fujii H, Colmegna I, Oishi H, Goronzy JJ, Weyand CM. Deficiency of the DNA repair enzyme ATM in rheumatoid arthritis. J Exp Med. 2009;206:1435–1449. doi: 10.1084/jem.20082251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fujii H, Shao L, Colmegna I, Goronzy JJ, Weyand CM. Telomerase insufficiency in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2009;106:4360–4365. doi: 10.1073/pnas.0811332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spyridopoulos I, Hoffmann J, Aicher A, et al. Accelerated telomere shortening in leukocyte subpopulations of patients with coronary heart disease: role of cytomegalovirus seropositivity. Circulation. 2009;120:1364–1372. doi: 10.1161/CIRCULATIONAHA.109.854299. [DOI] [PubMed] [Google Scholar]
- 75.Weyand CM, Fujii H, Shao L, Goronzy JJ. Rejuvenating the immune system in rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:583–588. doi: 10.1038/nrrheum.2009.180. [DOI] [PubMed] [Google Scholar]
- 76.Ridker PM. Moving beyond JUPITER: will inhibiting inflammation reduce vascular event rates? Curr Atheroscler Rep. 2013;15:295. doi: 10.1007/s11883-012-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Genovese MC, Becker JC, Schiff M, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353:1114–1123. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- 78.Smolen JS, Beaulieu A, Rubbert-Roth A, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371:987–997. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 79.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: Rationale and Design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]


