Abstract
Organization of presynaptic active zones is essential for development, plasticity, and pathology of the nervous system. Recent studies indicate a trans-synaptic molecular mechanism that organizes the active zones by connecting the pre- and the postsynaptic specialization. The presynaptic component of this trans-synaptic mechanism is comprised of cytosolic active zone proteins bound to the cytosolic domains of voltage-dependent calcium channels (P/Q-, N-, and L-type) on the presynaptic membrane. The postsynaptic component of this mechanism is the synapse organizer (laminin β2) that is expressed by the postsynaptic cell and accumulates specifically on top of the postsynaptic specialization. The pre- and the postsynaptic components interact directly between the extracellular domains of calcium channels and laminin β2 to anchor the presynaptic protein complex in front of the postsynaptic specialization. Hence, the presynaptic calcium channel functions as a scaffolding protein for active zone organization and as an ion-conducting channel for synaptic transmission. In contrast to the requirement of calcium influx for synaptic transmission, the formation of the active zone does not require the calcium influx through the calcium channels. Importantly, the active zones of adult synapses are not stable structures and require maintenance for their integrity. Furthermore, aging or diseases of the central and peripheral nervous system impair the active zones. This review will focus on the molecular mechanisms that organize the presynaptic active zones and summarize recent findings at the neuromuscular junctions and other synapses.
Keywords: Bassoon, Calcium channel, Laminin, LEMS, NMJ, Pierson syndrome
1) Introduction
The nervous system transmits information from the presynaptic neurons to the postsynaptic neurons or to target cells primarily at the chemical synapse. These synapses are formed by the differentiation of axons into a functional presynaptic terminal containing synaptic vesicle release sites, the active zone. Thus, the formation and maintenance of active zones are essential for the function of the central and peripheral nervous system. The neuromuscular junctions (NMJs) of the peripheral nervous system are large and isolated synapses. Thus, they are ideal for studying subcellular structures, such as the presynaptic active zones. The goal of this review is to highlight recent findings on the molecular mechanism of presynaptic active zone formation (and maintenance) at vertebrate NMJs. Similar to the vertebrate NMJ, Drosophila and Caenorhabditis elegans NMJs are ideal systems for studying the molecular mechanism of active zone organization, but these synapses have different active zone proteins compared to vertebrate NMJs and have been reviewed in detail elsewhere [1-7]. The molecular constituents of the active zones in vertebrate synapses have also been well studied in the central nervous system and sensory neurons and are reviewed in detail elsewhere [8-11; 6].
2) Location and Shape of Active Zones at NMJs
Active zones are the electron-dense thickening of the presynaptic membrane where synaptic vesicles fuse with the presynaptic membrane for exocytosis, determined based on the analysis of frog NMJs using transmission electron microscopy [12]. In frog NMJs, freeze-fracture electron microscopy detected long, parallel rows of large intramembranous particles on the P-face (interior of the cytosolic half of a plasma membrane) and exocytosis events adjacent to these particles [13]. The number of active zones correlates well with the quantal content at the frog NMJs [14]. However, the active zones in human, mouse, rat, and lizard NMJs have different organization compared to the frog NMJs. By freeze-fracture electron microscopy, these NMJs have exhibited two parallel arrays of 10-12 nm intramembranous particles arranged in two rows, with each active zone containing 20 of these intramembranous particles [15-18]. These intramembranous particles are thought to include presynaptic voltage-dependent calcium channels. Electron microscope tomography analysis revealed extensive level of detail of the presynaptic structures in the frog [19] and mouse NMJs [20]. Based on these tomography analyses, the active zones detected by transmission electron microscopy and the intramembranous particles detected by the freeze-fracture electron microscopy have been linked together and shown to be part of a large presynaptic protein complex. Three-dimensional reconstruction of serially sectioned transmission electron micrographs has shown the discrete locations of active zones scattered in the presynaptic terminal of mouse NMJs [21]. These ultrastructural analysis data suggest that the active zones of mammalian NMJs are discrete, scattered structures in the presynaptic terminal, and the active zones of frog NMJs are an elongated, continuous structure.
3) Active Zone-Specific Proteins at NMJs
The constituents of the active zones in vertebrate synapses are called the cytoskeletal matrix at the active zone (CAZ) [22] and include Bassoon [23], CAST/ELKS/Erc family proteins [24-26], Munc13 [27; 28], Piccolo [29], and RIM1/2 [30] (Table 1). In embryonic mouse NMJs, the active zone proteins Bassoon, Piccolo, and CAST/ELKS family proteins have exhibited a punctate pattern at the presynaptic terminals [31]. Bassoon and Piccolo proteins also exhibit a punctate pattern in adult mouse NMJs [32; 33] (Fig. 1). The active zone proteins ELKS, Munc13-1, and RIMs, but not CAST/ELKS2α, have also been detected in adult NMJs of rodents [34; 35]. The discrete punctate patterns of these active zone proteins in rodent NMJs are also supported by the following studies. Freeze-fracture electron microscopy and three-dimensional reconstructions of transmission electron micrographs has revealed active zones at discrete locations within the presynaptic terminal of rodent NMJs [15; 18; 21; 20]. The NMJ active zones are not just one large continuum throughout the presynaptic terminal (Fig. 1). The independent probability of release of each active zone within one NMJ can be deduced from the heterogeneity of the synaptic vesicle release within one presynaptic terminal [36; 37]. The CAZ proteins are detected by immunoelectron microscopy and confocal microscopy specifically at the active zones but not diffusely within the presynaptic terminals of different synapses [38-43]. Immunoelectron microscopy of the active zone proteins at the NMJs has not yet been performed, but the various aforementioned analyses suggest that the active zone-specific proteins are distributed primarily at discrete punctate locations in the NMJs. Similar but elongated active zone structures were confirmed in frog NMJs from developmental stages to adult stages by freeze-fracture electron microscopy [44] and in adults by electron microscope tomography [19].
Table 1.
Summary of active zone proteins and active zone organizers of NMJs.
| Active zone proteins |
Localization at embryonic NMJs |
Localization at adult NMJs |
Ultrastructural phenotypes of NMJs in knockout mice |
Physiological phenotypes of NMJs in knockout mice |
Binding partner VDCC subunits |
Binding partner active zone proteins |
|---|---|---|---|---|---|---|
| Bassoon | Yes | Yes | N/A | N/A | β1b, β4 [31] | CAST/ELKS2α [89], ELKS [91], Munc13, Piccolo, Rim [92] |
| CAST/ ELKS2α |
Yesa | No | N/A | N/A | β1b [31] | Bassoon, Rim1 [89], ELKS [26], Piccolo [92] |
| ELKS | Yesa | Yes | Knockout mice not reported | Knockout mice not reported | Not reported | Bassoon [91], CAST/ELKS2α, Rim [26] |
| Munc13-1,2,3 | Not reported | Yes | Munc13-1/2 double KO mice exhibit no qualitative defects [58] |
Munc13-1/2 double KO mice exhibit an increase of mEPP frequency, a decrease of EPP amplitude, and a decrease of quantal content [58] |
Not reported | Bassoon, Piccolo [92], Rim2 [90] |
| Piccolo | Yes | Yes | N/A | N/A | Cav1.2 [84] | Bassoon, CAST/ELKS2α, Munc13, Rim [92] |
| Rim1, 2 | Not reported | Yes | RIM1/2 double KO mice exhibit no qualitative defects [59] |
RIM1/2 double KO mouse exhibit decreases of mEPP frequency and EPP amplitude [59] |
Cav1.2, 2.1, 2.2 [83, 87], β1 - 4 [85] |
Bassoon, Piccolo [92], CAST/ELKS2α [89], ELKS [26], Munc13-1 [90] |
| Active zone organizers |
Localization at embryonic NMJs |
Localization at adult NMJs |
Ultrastructural phenotypes of NMJs in knockout mice |
Physiological phenotypes of NMJs in knockout mice |
Binding partner VDCC subunits |
Binding partner active zone proteins |
|---|---|---|---|---|---|---|
| P/Q-type VDCC (Cav2.1 + β subunit) |
Not reported | Yes | Cav2.1 knockout mice exhibit a decrease of active zone number [32] |
Cav2.1 knockout mice exhibit decreases of mEPP frequency and quantal content [94] |
Cav2.1 subunit: RIM [87], β subunits: Bassoon, CAST/ELKS2α [31], Rim [85, 86] |
|
| Laminin β2 | Yes | Yes | Laminin β2 knockout mice exhibit a decrease of active zone number [32] |
Laminin β2 knockout mice exhibit decreases of mEPP frequency and quantal content [102, 103] |
Cav2.1, 2.2 [32] |
|
| Laminin α4 | Yes | Yes | Laminin α4 knockout mice exhibit miss localized active zones [115] |
N/A | Not reported |
N/A: Not analyzed in knockout mice. [number]: reference number.
Anti-Pan-CAST/ELKS family.
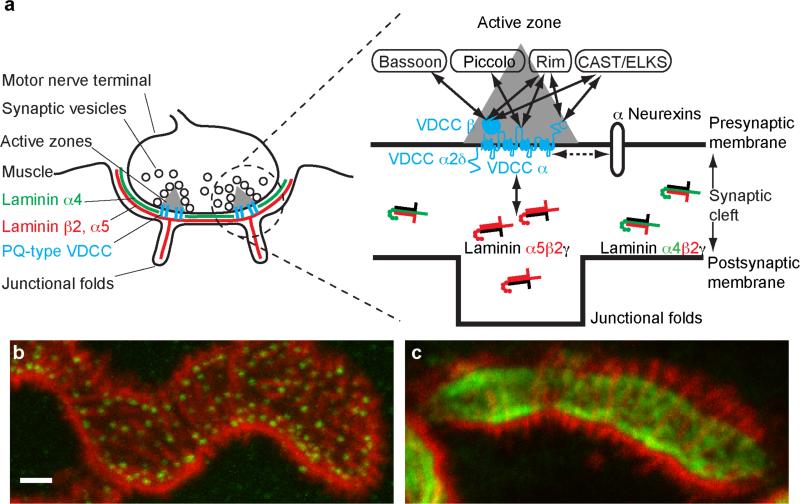
Fig. 1.
(a) A schematic diagram of the vertebrate neuromuscular junction (left) and a close-up of one active zone area (right). Solid arrows represent interactions, and the dotted arrow indicates a functional link. Horizontal lines show pre- and post-synaptic membranes. A junctional fold is indicated as a trough on the postsynaptic side. The space between these lines represents the synaptic cleft. The gray triangle depicts the electron dense material of presynaptic active zones detected by electron microscopy. The size of the proteins and the synaptic cleft are not in scale. VDCC voltage-dependent calcium channels. (b) The active zone protein Bassoon forms puncta and labels the active zones in NMJs of adult mice. (c) The synaptic vesicle associated protein synapsin1 shows a diffuse distribution pattern that visualizes the nerve morphology. Note the difference in the distribution patterns of these two presynaptic proteins. Bassoon, synapsin1, and acetylcholine receptors (AChRs) were visualized at the NMJs of sternomastoid muscles of postnatal day 36 mice by fluorescent immunohistochemistry using anti-Bassoon antibodies (green in b), anti-synapsin1 antibodies (green in c), and Alexa Fluor 594-labeled α-bungarotoxin (red). Many Bassoon puncta are localized above the postsynaptic junctional folds, which were visualized as bright lines of α-bungarotoxin staining. Scale bar 1βm.
These active zone proteins are suggested to function in active zone formation and maintenance at NMJs, in neurotransmitter release, or in both. In the following paragraph, anatomical and functional consequences of gene deletions will be discussed (Table 1). The key protein-protein interactions of these proteins for active zone formation will be discussed in “Tethering Active Zone Proteins by Presynaptic Calcium Channels.”
Bassoon is a large cytosolic scaffolding protein that localizes at the active zones of ribbon synapses and some brain synapses [23; 45]. Piccolo is also a large cytosolic scaffolding protein that is structurally related to Bassoon [46]. Interestingly, Bassoon mutant mice exhibit free-floating ribbons (active zone structures) at photoreceptor synapses [47; 48] and have a reduced level of Ca2+ influx at ribbon synapses of hair cells of the auditory system [49]. The similarities between the ribbon synapses and the NMJs for active zone organization will be described in “Active Zone Organization by a Synapse Organizer Laminin β2 and Its Receptor P/Q-Type VDCC”. The roles of Bassoon and Piccolo in the synaptic vesicle clustering at the active zones have been demonstrated in the analyses of the central nervous system synapses in single knockout mice for Bassoon or Piccolo, as well as in the Piccolo knockout/Bassoon knockdown mice [50; 47; 48; 51-56]. However, NMJs have not been analyzed in these mice, and the consequence of the deletion of these proteins on NMJ active zones remains unknown. An experimental manipulation to decrease the level of endogenous Bassoon protein in mouse NMJs has caused a decrease in the number of active zones [32] (described in “Active Zone Organization by a Synapse Organizer Laminin β2 and Its Receptor P/Q-Type VDCC”). These results suggest that Bassoon may play an important role in the organization of active zones at NMJs.
CAST/ELKS/Erc family are scaffolding proteins with four coiled-coil domains and include CAST/ELKS2α/Erc2 and ELKS/CAST2/Erc1[57]. ELKS2α/CAST knockout mice exhibit a normal number of docked synaptic vesicles and normal ultrastructure of synapse morphology in the hippocampal CA1 region [58]. However, the deletion of ELKS2α/CAST caused an increase of inhibitory neurotransmitter release and exploratory behaviors. The NMJs were not analyzed in the ELKS2α/CAST knockout mice, but this mutant is less likely to exhibit an active zone phenotype at the NMJs because ELKS2α/CAST expression was not detected in the adult spinal cord by western blot [58] and in adult NMJs by immunohistochemistry [35].
The Munc13 family is comprised of three homologous members (Munc13-1/2/3) with splice variants for Munc 13-2 (b and ub) [59]. These proteins contain C1- and C2-domains and have roles in neurotransmitter release. Munc13-1 and ubMunc13-2, but not bMunc13-2 nor Munc13-3, were detected at NMJs by western blot analysis [60]. Consistently, Munc13-1/2-double-knockout mice and Munc13-1/2/3-triple-knockout mice exhibited identical phenotypes in the NMJ structure and function [60]. Qualitative analyses of electron micrographs have revealed active zones with synaptic vesicles accumulated around the electron-dense materials and docked synaptic vesicles in the NMJs of double-knockout mice. However, the double-knockout mice have shown an increased number of presynaptic boutons at NMJs, number of axons in peripheral nerves, and frequency of miniature end plate potentials (mEPPs). Interestingly, end plate potential (EPP) amplitude and EPP quantal content was significantly decreased [60]. Thus, the active zones may have looked qualitatively normal, but were functionally impaired in synaptic transmission in Munc13-1/2 double-knockout mice.
RIMs are multi-domain proteins formed by four genes, including three isoforms (α, β, γ) [61; 62]. RIM1α and RIM2α double-knockout mice have decreased mEPP frequency and EPP amplitude compared to control NMJs; however, they demonstrate no obvious ultrastructural defects at the NMJs [61]. These results suggest that the active zone protein RIM functions in synaptic transmission at NMJs. These knockout mice analyses suggest that subtle defects in active zones have significant functional effects on synaptic transmission at NMJs, and sensitive morphological analysis, a quantitative approach, for example, may be better suited for an anatomical analysis of NMJ active zones.
4) Tethering Active Zone Proteins by Presynaptic Calcium Channels
Active zone-specific proteins need to be tethered to presynaptic membranes near voltage-dependent calcium channels (VDCCs) to form active zones (Fig. 1). VDCCs [63-67] are thought to localize at or in the vicinity of the active zones because Ca2+ influx causes the fusion of synaptic vesicles to the presynaptic membrane [68-74], and active zones are synaptic vesicle release sites [12; 13]. Alignment between VDCCs and the synaptic vesicle release machinery is suggested by the direct interactions between presynaptic VDCCs (N- or P/Q-type) and the proteins required for synaptic vesicle release (syntaxin, SNAP-25, synaptotagmin) [75-79] and by the functional modification of VDCCs by syntaxin and SNAP-25 [80; 81]. Electrophysiological studies have demonstrated that developing rodent NMJs utilized a combination of P/Q- and N-type VDCCs but, in young adults, switched to utilizing only P/Q-type VDCCs for synaptic transmission [82; 83]. VDCCs have been detected at vertebrate NMJs using fluorescently tagged ω-conotoxin GVIA [84], Ca2+ indicators [85; 36], and immunohistochemical detection [86-89]. Whereas ultrastructural analysis of VDCCs at the vertebrate NMJ has not yet been performed, higher resolution imaging techniques have detected the VDCC accumulation at the active zones of Drosophila NMJs (using STED microscopy [90]) and ribbon synapses of mouse retina (using immunoelectron microscopy [45]). Similar detailed analysis of VDCC distribution pattern at the vertebrate NMJs needs further analysis.
A direct interaction between the active zone-specific proteins and VDCCs is necessary for tethering CAZ to the presynaptic membrane. Active zone proteins Bassoon and CAST/Erc2 interact with VDCC β subunits [31], which form a tight complex with the pore-forming α subunits of P/Q- and N-type VDCCs [91]. These active zone proteins do not interact with the C-terminal domain of P/Q-type VDCC α subunits [31], which is in contrast to the active zone protein RIM1/2 (see next paragraph). Active zone proteins Bassoon and CAST/Erc2 are significantly decreased at the NMJs of P/Q-type or N-type VDCC (α subunit) knockout mice, as well as in P/Q- and N-type VDCC double-knockout mice [31, 32]. In these knockout mice, reduction in active zones and docked synaptic vesicles was confirmed by a quantitative analysis of electron micrographs. These results suggest that VDCCs function as scaffolding proteins to tether active zone proteins to the presynaptic membrane at the NMJs (also see, “Active Zone Organization by a Synapse Organizer Laminin β2 and its Receptor P/Q-Type VDCC).
Whereas these reports have demonstrated a molecular mechanism to organize NMJ active zones, the first direct interaction between active zone proteins and VDCCs has been reported between the active zone protein RIM and the cytoplasmic loop connecting domain II and III of the N- and L-type VDCCs using recombinant proteins of the subdomains [92]. Similarly, the active zone protein Piccolo interacts with L-type VDCC α subunits at the cytoplasmic loop between domains II and III to facilitate stimulus-secretion coupling in pancreatic beta cells [93]. RIM1 also interacts with VDCC β subunits, and this interaction suppresses the inactivation properties of P/Q- and N-type VDCCs and increases neurotransmitter release from cultured cerebellar granule cells [94; 95]. Furthermore, RIM1/2 interacts with P/Q- and N-type VDCCs at the C-terminal domain of VDCC α subunits to tether VDCCs to the presynaptic active zones via a PDZ domain-mediated direct interaction in cultured hippocampal neurons [96]. Electrophysiological analysis of the calyx of Held in the auditory brainstem revealed a role of RIMs in targeting VDCCs to the presynaptic terminals [97]. Together with the NMJ results of double-knockout mice for RIM1α and RIM2α described in “Active Zone-Specific Proteins at the NMJs”, the interaction between RIMs and VDCCs functions in targeting VDCCs to the presynaptic terminals of the NMJs and thus in organizing the active zones.
Multiple active zone proteins interact with VDCC subunits as described above, and the active zone proteins form a multi-protein complex [25; 26; 98-101]. Thus, active zone proteins and VDCC may form a large protein complex, as reported for synapses of the central nervous system [102]. Consistent with this view, electron microscope tomography analyses of NMJs have revealed macromolecular connections of active zone materials at mouse and frog motor nerve terminals [19; 20].
What happens to the active zones in NMJs lacking VDCCs? Decreased numbers of active zones have been observed at NMJs in mice lacking either P/Q- or N-type VDCC and in mice lacking both P/Q- and N-type VDCCs [31; 32]. These defects do not depend on the activity of either calcium channels or synapses (discussed in “Synaptic Activity and Ca2+ Influx Through VDCC for Active Zone Formation”). Electrophysiological analyses of NMJs in P/Q-type VDCC knockout mice suggested that the R-type VDCCs compensated for the loss of the P/Q-type VDCC at the NMJs by filling the “slot” of the major VDCCs within the presynaptic membrane [103]. Despite the compensation by R-type VDCCs, mEPP frequency and EPP quantal content were significantly decreased in the knockout NMJs [103], which is consistent with the decreased number of active zones. The active zones have remained impaired even with the compensatory upregulation of R-type VDCCs because this channel does not bind to the active zone organizer laminin β2 (described in “Active zone organization by a synapse organizer laminin β2 and its receptor P/Q-type VDCC”) [32]. Synaptic ion channels have non-conducting roles for synapse formation [104], and these analyses demonstrated the essential role of P/Q-type VDCCs for active zone formation.
Non-conducting roles for calcium channels in synaptogenesis have also been demonstrated in invertebrates. At the Drosophila neuromuscular junction, VDCC α2δ subunits play a role in presynaptic differentiation [105-107]. Mutant embryos lacking the α2δ-3 subunit have malformed synaptic boutons and altered active zone organization. This role of the α2δ-3 subunit is independent of the ion-conducting function of the calcium channel complex and is separate from its role of correctly localizing VDCC α subunits at the neuromuscular junctions [107]. Mice lacking the VDCC α2δ-2 subunit (a.k.a. ducky) showed a reduction in NMJ size and muscle fiber diameter but had a near-normal EPP amplitude [108]. The active zones and ultrastructure of NMJs were not analyzed in the VDCC α2δ-2 knockout mice, but the normal mEPP frequency suggests normal active zones. In summary, molecular mechanisms of active zone organization involved the tethering of active zone proteins to the presynaptic membrane by a direct interaction with VDCCs concentrated at the motor nerve terminals.
5) Active Zone Organization by a Synapse Organizer Laminin β2 and Its Receptor P/Q-type VDCC
If active zone proteins could be tethered to membranes by VDCCs as described above, how are these protein complexes anchored to the presynaptic terminal to organize active zones facing the postsynaptic specialization? One molecular mechanism that allows for such organization is an interaction between P/Q-type VDCCs and a synapse organizer, laminin β2 [32] (Fig. 1). The synapse organizer laminin β2 is a muscle-derived extracellular matrix protein specifically concentrated in the synaptic cleft of NMJs [109; 110]. The laminin β2 knockout mouse demonstrated a loss of active zones, an impairment of presynaptic differentiation, and an attenuation of mEPP frequency and quantal content at NMJs [111; 112]. Developmentally, laminin β2 protein is concentrated at higher levels over the acetylcholine receptor clusters compared to the extrasynaptic areas, as early as embryonic day 15, when the innervation of NMJs begins [113-115]. The carboxyl-terminal 20-kDa fragment of laminin β2 promotes presynaptic differentiation of motor neurons, and a tripeptide, leucine-arginine-glutamate, within this 20-kDa fragment is necessary to promote neuronal adhesion to this protein [116; 117].
Specific receptors for the synapse organizer laminin β2 have been identified at the NMJs. Laminin β2 binds specifically and directly to P/Q- and N-type VDCCs [32], which are concentrated at the presynaptic terminals of NMJs [82; 83]. These VDCC pore-forming α subunits bind only to synaptic laminins (containing laminin β2) and not non-synaptic laminins (lacking laminin β2) [32]. Furthermore, synaptic laminins bind to the VDCCs concentrated at presynaptic terminals of NMJs only and not to non-NMJ VDCCs (i.e., R-type and L-type VDCCs (Cav1.2)) [32]. These VDCCs are the first receptors that were shown to bind specifically to synaptic laminins but not to non-synaptic laminins. The well-known laminin receptors, integrins and dystroglycans, do not distinguish between laminins with or without laminin β2 [118-120]. This interaction of laminin β2-VDCC leads to the clustering of VDCCs and presynaptic components [32]. These pore-forming α subunits of VDCCs form a tight complex with the VDCC β subunits, which bind to the active zone proteins Bassoon and CAST/Erc2 [31]. Thus, the synapse organizer laminin β2 anchors the protein complex of VDCC (α and β subunits) and active zone proteins (Bassoon, CAST/ELKS/Erc family, and RIM1/2) from the extracellular side to organize the NMJ active zones.
Following in vivo studies in mice provides compelling arguments that aforementioned extracellular interaction plays a role in organizing the NMJ active zone. Perturbation of the interaction between VDCC and laminin β2 in wild-type mice, and the knockout mice of P/Q-type VDCC or laminin β2 resulted in the active zone disassembly [32]. Moreover, P/Q- and N-type VDCC double-knockout mice exhibit a specific defect of active zones within otherwise normal NMJs [31]. The NMJs in the double-knockout mice have exhibited a decreased number of active zones and docked synaptic vesicles. These defects were twice as severe as the defects of the single knockout mice of P/Q- or N-type VDCCs. However, the double-knockout mice had normal synapse size, synaptic vesicle density at the nerve terminal, and accumulation of synaptic vesicle proteins at the NMJs [31]. These results suggest that axonal outgrowth termination and synaptic vesicle accumulation at NMJs are based on cell adhesion or retrograde signaling, and the active zone formation is based on an additional/different signaling mechanism through VDCCs.
A similar active zone defect is found in retina photoreceptor synapses. Retina specific L-type VDCCs (Cav1.4) are concentrated at the photoreceptor synapse, and Cav1.4 knockout mice exhibit the dissociation of the ribbon structure (active zones) from the presynaptic membranes [121]. Laminin β2 accumulates in the synaptic cleft of photoreceptor synapses [122], and laminin β2 knockout mice have active zone defects at the ribbon synapses [123]. These phenotypes are similar to the NMJs, where the lack of presynaptic VDCCs or laminin β2 exhibited the dissociation of active zone structure from the presynaptic membrane. Thus, it would be interesting to examine if L-type VDCCs (Cav1.4) could bind to laminin β2.
Whereas the aforementioned reports demonstrate that a trans-synaptic molecular mechanism organizes the active zones, the active zone organizer laminin β2 is known to form a trimer with other laminin subunits, and presynaptic VDCCs have been suggested to interact with another synapse organizer, neurexins. Do these molecules contribute to the formation of active zones? The laminin β2 subunit forms trimers with one of the two laminin α subunits (α4 or α5) specifically concentrated at the synaptic cleft and a γ subunit. The concentration of laminin α4 in the synaptic cleft is lower near the active zones and higher between the active zones [124] (Fig. 1). Interestingly, laminin α4 knockout mice have improperly localized active zones at the NMJs but no changes in the total number of active zones, suggesting that laminin α4 controls the location of active zones at mouse NMJs [124]. In contrast to laminin α4, laminin α5 shows a similar distribution pattern as laminin β2 in the synaptic cleft of adult NMJs [124; 125], and laminin α5 knockout mice and laminin α4/α5 double-knockout mice both show presynaptic maturation defects in the postnatal stages, causing partial innervation of the endplate [126; 125]. However, analysis of active zone organization in laminin α5 single or double-knockout mice awaits further study.
Another potential mechanism to anchor VDCCs at presynaptic terminals for active zone organization is the suggested functional link between the N-type VDCCs and presynaptic synapse organizer neurexins [127]. The accumulation of VDCCs at brainstem synapses was impaired in α-neurexins1/2/3 triple-knockout mice [127]. Interestingly, this phenotype is specific to N-type VDCCs, and P/Q-type VDCCs are not affected. However, a direct physical interaction between N-type VDCCs and neurexins awaits further confirmation (thus described here as a functional link). The NMJs of α-neurexin double-knockout mice (neurexin 1/2 or 2/3) have a reduced mEPP frequency and EPP quantal content in the slow twitch muscles, suggesting an impairment of active zones [128]. However, these reductions were not observed in the diaphragm, and the reason for the difference between muscle types is currently unknown. The NMJ ultrastructure has not been analyzed, and the consequence of the deletion of neurexins on NMJ active zones remains unclear. The presynaptic proteins CASK and Mint/X11 could link the VDCCs and α-neurexins [129; 130]. However, the possibility of this interaction functioning in vivo is decreased because CASK knockout mice demonstrated normal active zones and functional presynaptic VDCCs. Similarly, cultured Mint1/2/3 triple-knockout neurons showed normal synaptic ultrastructure and a defect in presynaptic function that could be attributed to the upregulated Munc18-1 [131]. Together, the role of neurexins in organizing NMJ active zones via VDCCs is not clear.
In summary, the active zone organization at NMJs involves a trans-synaptic molecular mechanism. Active zone proteins are tethered to the membrane by binding to P/Q-type VDCCs on the cytosolic side. This protein complex is anchored to the presynaptic membrane of NMJ by the extracellular interaction between P/Q-type VDCCs and muscle-derived synapse organizer laminin β2, which is specifically concentrated in the synaptic cleft above the postsynaptic specialization of NMJs.
6) Synaptic Activity and Ca2+ Influx Through VDCC for Active Zone Formation
The molecular mechanism of active zone formation described in “Active Zone Organization by a Synapse Organizer Laminin β2 and Its Receptor P/Q-Type VDCC” is based on a structural interaction of VDCCs where the channels function as scaffolding proteins. However, does the active zone formation require the Ca2+ influx through VDCCs and/or the synaptic activity at NMJs? Several observations suggest that Ca2+ influx through presynaptic VDCCs and synaptic activities are not required for active zone formation at NMJs. Presynaptic differentiation occurred normally in motor neurons cultured with specific irreversible blockers of P/Q-type VDCCs (ω-agatoxin IVA [132; 133]) and N- type VDCCs (ω-conotoxin GVIA [134; 135]) [32]. These results clearly demonstrated that Ca2+ influx through these VDCCs is not required for presynaptic differentiation in cultured mouse motor neurons.
Previous studies in mutant mice with either reduced or no neuromuscular activity have made compelling arguments that synaptic activity is not a prerequisite for active zone assembly. The lack of synaptic transmission at NMJs did not affect the formation of active zones in choline acetyltransferase knockout mice, which develop a normal density of active zones in embryonic NMJs [136; 137]. Similarly, the lack of synaptic transmission did not affect the formation of active zones at NMJs and synapses of the central nervous system in Munc13-1/2 double-knockout mice [60, 138]. Munc18-1 knockout mice have shown almost complete degeneration of spinal cord neurons, resulting in degeneration of the NMJs, which made the evaluation of NMJ active zone unclear [139]. However, the lack of synaptic transmission did not affect the formation of active zones at synapses of the central nervous system in Munc18-1 knockout mice [140].
The synaptic activity is not required for active zone assembly, and the expression levels of active zone components or active zone organizing molecules are also not dependent on the synaptic activity. The expression levels of active zone protein Bassoon and its mRNA in spinal cord motor neurons were not changed in P/Q- and N-type VDCC double-knockout mice, which lack synaptic transmission at NMJs [32]. Mice lacking choline acetyltransferase (Chat−/−) display no synaptic transmission at NMJs but show a concentration of laminin β2 protein at NMJs [136]. Similarly, the mRNA or protein levels of laminin β2 at NMJs do not appear to change in response to denervation or reinnervation [141-146]. These results suggest that the expression level of active zone proteins and the active zone organizer is not dependent on synaptic activity.
Just as reduced (or no) synaptic activity has little affect on active zone assembly, enhanced synaptic transmission does not affect active zone formation. Knockout mice for collagenous subunit of acetylcholinesterase ColQ exhibited no acetylcholinesterase activity at NMJs and showed elongated mEPPs amplitude [147]. Although quantitative data were not described, the knockout mice showed normal active zones with synaptic vesicles in electron micrographs. Acetylcholinesterase knockout mice exhibited ultrastructurally normal NMJs [148]. These results demonstrated that synaptic activity does not affect active zone formation at NMJs. In summary, NMJ active zone formation does not require synaptic activity or Ca2+ influx, which is in contrast to the postsynaptic differentiation and synapse elimination at NMJs that depend on release of neurotransmitter [149; 150] and synaptic activity [151; 152].
7) Other Synaptic Organizers, Intracellular Pathways, and Junctional Folds.
At the NMJs, multiple synaptic organizers (including laminin β2) orchestrate synaptic differentiation [114; 115; 153-155]. For example, agrin is essential for the postsynaptic differentiation of NMJs [156], and roles of amyloid precursor protein [157] and collagens [154; 158] in NMJ differentiation in vivo have recently been shown. Are these synaptic organizers involved in active zone formation and maintenance?
Agrin, MuSK, and Lrp4
The requirement of agrin and MuSK signaling in NMJ formation is well known, but do they also instruct active zone formation? Nerve-derived agrin containing the Z exon plays an essential role in the organization of NMJs [156] by binding to the postsynaptic receptor Lrp4 and the co-receptor MuSK, and clustering of AChR at the endplate [159-163]. MuSK and Lrp4 knockout mice show aberrant motor axon branching and significantly impaired AChR clustering [159; 160; 164]. Synaptic vesicle-related proteins, SV2 and synaptophysin, accumulated at the intramuscular nerve branches, but the clustering of AChRs and postsynaptic proteins at the opposing sites were hardly detected [159; 161; 164]. Due to the failure of normal NMJ formation, quantitative analysis of active zones was not reported. Similarly, in agrin null mice at embryonic day 18, overall presynaptic differentiation was impaired; specifically, AChR clusters are smaller, less dense, and less numerous [165]. However, active zones were detected in nascent NMJs of the agrin knockout mice by electron microscopy [165]. Based on these results, the chimeric analysis by transplantation, and the analysis of agrin splice variant [156], it has been suggested that agrin may play a role in presynaptic differentiation through yet to be identified retrograde signals. Thus, agrin, MuSK, and Lrp4 do not appear to organize the presynaptic active zones directly by the interactions of these three proteins.
Collagens
The major structural components of the synaptic cleft basement membrane are non-fibrillar collagens, which also have roles as synapse organizers. Enzymatically released bio-active domains of collagen α2, α3, and α6 (IV) chains exhibit synaptogenic activity in vitro and are present at mouse NMJs. Mice with reduced levels of collagen α2 show an impairment of presynaptic differentiation, and mice lacking α3 and α6 collagens show defects of postnatal maintenance of NMJs [154]. The integrity of NMJ active zones in these collagen IV mutants have not been analyzed and await further study. Collagen XIII is expressed by muscles after synapse formation and to accumulate around the end plate region [158]. Collagen XIII knockout mice exhibit poorly matched presynaptic terminals and endplates, less active zones, decreased frequency of mEPPs, and decreased EPP amplitude [158]. However, collagen XIII knockout mice have shown defects in topological maturation of the AChR cluster [158], which is similar to laminin α4/α5 double-knockout mice [125]. Thus, it is not clear whether the active zone phenotype is the primary phenotype or the secondary phenotype caused by postsynaptic defects.
Amyloid Precursor Protein
Amyloid precursor protein (APP) plays a pivotal role in the pathogenesis of Alzheimer’s disease, but APP also accumulates at NMJs [166; 167]. Double-knockout mice for APP and its homolog, APP-like protein 2, exhibit decreased active zone densities, decreased synaptic vesicle density, but a normal number of docked synaptic vesicle in NMJ profiles [157]. Compatible to these defects, mEPP frequency was decreased in the double-knockout mice. The conditional deletion in muscle suggests a postsynaptic requirement of APP and/or APP-like protein 2 for the presynaptic differentiation of the NMJs [168]. The loss of APP and APP-like protein 2 exhibits widespread defects in presynaptic differentiation, including aberrant sprouting of nerve terminals, suggesting that APP has a synaptogenic or synapse maintenance role at the NMJ. However, due to the widespread defects, it is not clear whether the active-zone defect is a primary or secondary phenotype.
At present, few studies have discovered signaling pathways necessary for active zone formation and maintenance at vertebrate NMJs. However, several intracellular mechanisms have been shown to organize the active zones at Drosophila NMJs. The number and spacing of active zones are controlled by spectrin skeleton β-spectrin, spectrin-binding/actin-capping protein adducin, GTPase Rab3, threonine kinase Unc-51, or inositol phosphatase synaptojanin at Drosophila NMJs [169-173]. However, Rab3abcd quadruple knockout mice exhibit no active zone defects, suggesting that the intracellular signaling for active zone organization is different between Drosophila and mice [174]. βIII spectrin knockout mice show impaired synaptogenesis in the cerebellum, but the NMJ active zone awaits further study [175]. Thus, the roles of Rab3s and spectrins in mammalian active zone organization are currently unclear.
In adult NMJs, the active zones align with the postsynaptic specialization called junctional folds, but do the junctional folds have any role in organizing the active zones? The number of junctional folds is significantly lower in the knockout mice for acetylcholine receptor epsilon subunit [176] or double-knockout mice for utrophin and α-dystrobrevin [177] compared to wild-type mice. However, a normal level of active zones was formed at the presynaptic terminal in these mutants [176; 124]. These studies suggest that active zones could form without junctional folds, and the molecular mechanism for active zone formation could be independent from the mechanism of junctional fold formation.
8) Impairment of Active Zones in Neurological Disease
The active zones at NMJs are not stable structures and require mechanisms to maintain their structural integrity. Following experimental manipulations in laboratory animals demonstrate that NMJ active zones are unstable structures. The inhibition of the interaction between presynaptic VDCCs and the synapse organizer laminin β2 have decreased the number of active zones in mouse NMJs after just two days of inhibitor injection, suggesting that mouse active zones are not stable structures [32]. In the adult frog NMJs, decreasing the external calcium concentration leads to the disruption of the relative location between active zones and postsynaptic junctional folds [178]. This disruption of active zones has caused the facilitation of synaptic transmission in frog NMJs during paired pulses and high-frequency stimulations, suggesting that the maintenance of active zones is essential for effective synaptic transmission. These examples suggest that the NMJ active zones may be vulnerable targets in neurological diseases.
Two examples of human neurological diseases exhibit a reduced number of NMJ active zones. In Lambert-Eaton Myasthenia Syndrome (LEMS), patients have a reduced number of NMJ active zones, reduced synaptic transmission, and weakened muscles [179; 16]. Majority of the patients (75-85%) possess autoantibodies against P/Q-type VDCC, which is speculated to internalize the VDCCs into presynaptic terminals and to decrease the active zones [16; 180]. Importantly, LEMS patients have autoantibodies against the laminin-binding domain of the P/Q-type VDCC [181]. Thus, the decrease of active zones may be caused, in part, by autoantibodies that block the signaling from the synapse organizer laminin β2 to the presynaptic VDCCs required for the active zone organization [32]. A LEMS animal model further supports that the loss of active zones is a part of the etiological mechanism. Mice injected with the LEMS patient IgGs exhibit a reduced number of NMJ active zones [182]. Together with the previous paragraph, these results suggest that mature NMJs require presynaptic P/Q-type VDCCs to maintain the active zones. In Pierson syndrome, patients exhibited a loss of NMJ active zones, an impairment of synaptic transmission, and denervation of NMJs because they lack laminin β2 due to gene mutations [183; 184]. These phenotypes are identical to laminin β2 knockout mice [111], suggesting that the synapse organizer laminin β2 is also required for active zone organization in human.
Other human neurological diseases also exhibit impairment of active zones in the peripheral and central nervous systems. In amyotrophic lateral sclerosis, the active zone length is significantly reduced in synapses contacting the chromatolytic neurons in the ventral horn of the spinal cord [185]. A genome-wide association study for major depressive disorder has suggested implication of active zone protein Piccolo [186]. Human prion diseases, including Creutzfeldt–Jakob disease and Gerstmann–Sträussler–Scheinker (GSS) syndrome, are fatal neurodegenerative diseases, and animal models exhibit impairment of active zones. A Drosophila model of GSS syndrome, expressing mutated mouse prion protein, exhibits a decreased protein level of active zone protein Bruchpilot at NMJs [187]. Scrapie-infected mice exhibit a decreased protein level of active zone protein ELKS in the hippocampus [187]. These results suggest that impairment of active zones may be part of the etiological mechanisms of these neurological diseases.
Are active zones impaired in neurodegenerative diseases characterized by the detection of proteinaceous inclusions called Lewy body? Lewy bodies are found in Parkinson’s disease, dementia with Lewy bodies, and a Lewy body variant of Alzheimer’s disease, and contain α-synuclein as a major constituent [188]. Recent studies support a role of α-synuclein overexpression in pathogenesis of Parkinson’s disease [189; 190]. An increased expression level of α-synuclein in transgenic mice does not alter the total number of synaptic vesicles associated with the active zones, but reduces the number of synaptic vesicles adjacent to the active zones and reduces the density of synaptic vesicles within the synaptic boutons in the hippocampal CA1 region [191]. These defects reduce the synaptic transmission and suggest that the overexpression of α-synuclein inhibits the reclustering of synaptic vesicles after endocytosis. This result is consistent with the immunoelectron microscopy that detected synuclein within the presynaptic terminal but not at the active zones of cultured rat hippocampal neurons [192]. Synuclein co-localizes with synapsin I in the presynaptic terminal but not with the active zone proteins at the presynaptic membranes. Thus, the role of active zones in the etiological mechanisms of synucleinopathies is not clear.
Interestingly, α-synuclein is also detected at NMJs and in muscles and has been suggested to play a role in the pathogenesis of inclusion-body myositis [193]. α-Synuclein knockout mice exhibit repetitive compound muscle action potentials in response to a single stimulation, suggesting that α-synuclein may play a role in acetylcholine compartmentalization at the NMJs [194]. Importantly, neuronal overexpression of human or mouse α-synuclein in transgenic mice causes axonal damage and denervation of the NMJs [195; 196]. However, NMJ active zones have not been analyzed in these mice, and the role of active zones in the pathogenesis of motor nerve neuropathy is unknown.
In summary, active zones of adult synapses are not stable structures and may play a role in the pathogenesis of some neurological diseases.
9) Perspectives
The NMJ serves as an attractive model to study presynaptic differentiation due to its size and isolation from neighboring synapses compared to synapses in the central nervous system. These advantages may aid in solving important questions that still remain to elucidate the molecular mechanism of active zone organization. First, what is the essential interaction for the maintenance of NMJ active zones, which might be affected in some neuromuscular diseases? The knowledge of molecular identity of active zone-specific proteins and the interactions between these proteins were increased, but the hierarchy of these molecular interactions at the NMJs is not known. The study of ribbon synapses in Bassoon knockout mice suggests that there is an essential interaction of active zone proteins that could cause the dissociation of active zone structure [45]. Second, how common are the molecular mechanisms that organize the active zones between central and peripheral synapses or between motor and sensory nervous systems? Is the same set of CAZ proteins found in all the synapses? The scaffolding protein P/Q-type or N-type VDCCs are concentrated at many presynaptic terminals of the central and peripheral synapses. However, differences among synapses are suggested by the fact that the synapse organizer laminin β2 is specific to the NMJs and ribbon synapses of the retina. Third, what neurological diseases have impairments in the active zones? Considering the interaction of active zone proteins with VDCCs, channelopathies (diseases with mutations in ion channels) affecting central synapses and NMJs [197] may also exhibit impairments in the active zones. These are just a few questions that await further investigation to elucidate the mechanism of active zone formation and maintenance.
Acknowledgments
I thank Nicolas G. Bazan for the invitation for this review and Michael A. Fox and Beth Levant for comments on the manuscript. The work in my laboratory is supported by grants from the Whitehall Foundation and NIH-National Center for Research Resources (RR024214), National Institute of Child Health and Human Development (HD002528).
Footnotes
The author declares that he has no conflict of interest.
References
- 1.Broadie KS, Richmond JE. Establishing and sculpting the synapse in Drosophila and C. elegans. Current Opinion in Neurobiology. 2002;12(5):491–498. doi: 10.1016/s0959-4388(02)00359-8. [DOI] [PubMed] [Google Scholar]
- 2.Jin Y. Synaptogenesis. WormBook; 2005. p. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins CA, DiAntonio A. Synaptic development: insights from Drosophila. Current Opinion in Neurobiology. 2007;17(1):35–42. doi: 10.1016/j.conb.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Stryker E, Johnson KG. LAR, liprin alpha and the regulation of active zone morphogenesis. J Cell Sci. 2007;120(21):3723–3728. doi: 10.1242/jcs.03491. [DOI] [PubMed] [Google Scholar]
- 5.Owald D, Sigrist SJ. Assembling the presynaptic active zone. Curr Opin Neurobiol. 2009;19(3):311–318. doi: 10.1016/j.conb.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Sigrist SJ, Schmitz D. Structural and functional plasticity of the cytoplasmic active zone. Current Opinion in Neurobiology. 2010;21:1–7. doi: 10.1016/j.conb.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Wichmann C, Sigrist SJ. The active zone T-bar--a plasticity module? J Neurogenet. 2010;24(3):133–145. doi: 10.3109/01677063.2010.489626. [DOI] [PubMed] [Google Scholar]
- 8.Rosenmund C, Rettig J, Brose N. Molecular mechanisms of active zone function. Current Opinion in Neurobiology. 2003;13(5):509–519. doi: 10.1016/j.conb.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Prescott ED, Zenisek D. Recent progress towards understanding the synaptic ribbon. Current Opinion in Neurobiology. 2005;15(4):431–436. doi: 10.1016/j.conb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Fejtova A, Gundelfinger ED. Molecular organization and assembly of the presynaptic active zone of neurotransmitter release. Results Probl Cell Differ. 2006;43:49–68. doi: 10.1007/400_012. [DOI] [PubMed] [Google Scholar]
- 11.Jin Y, Garner CC. Molecular mechanisms of presynaptic differentiation. Annual review of cell and developmental biology. 2008;24:237–262. doi: 10.1146/annurev.cellbio.23.090506.123417. [DOI] [PubMed] [Google Scholar]
- 12.Couteaux R, Pecot-Dechavassine M. Synaptic vesicles and pouches at the level of "active zones" of the neuromuscular junction. C R Acad Sci Hebd Seances Acad Sci D. 1970;271(25):2346–2349. [PubMed] [Google Scholar]
- 13.Heuser JE, Reese TS, Dennis MJ, Jan Y, Jan L, Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. The Journal of cell biology. 1979;81(2):275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Propst J, Ko C. Correlations between active zone ultrastructure and synaptic function studied with freeze-fracture of physiologically identified neuromuscular junctions. The Journal of Neuroscience. 1987;7(11):3654–3664. doi: 10.1523/JNEUROSCI.07-11-03654.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellisman MH, Rash JE, Staehelin LA, Porter KR. Studies of excitable membranes. II. A comparison of specializations at neuromuscular junctions and nonjunctional sarcolemmas of mammalian fast and slow twitch muscle fibers. The Journal of cell biology. 1976;68(3):752–774. doi: 10.1083/jcb.68.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukunaga H, Engel AG, Osame M, Lambert EH. Paucity and disorganization of presynaptic membrane active zones in the lambert-eaton myasthenic syndrome. Muscle & Nerve. 1982;5(9):686–697. [Google Scholar]
- 17.Walrond J, Reese T. Structure of axon terminals and active zones at synapses on lizard twitch and tonic muscle fibers. The Journal of Neuroscience. 1985;5(5):1118–1131. doi: 10.1523/JNEUROSCI.05-05-01118.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukuoka T, Engel AG, Lang B, Newsom-Davis J, Prior C, Wray DW. Lambert-Eaton myasthenic syndrome: I. Early morphological effects of IgG on the presynaptic membrane active zones. Ann Neurol. 1987;22(2):193–199. doi: 10.1002/ana.410220203. [DOI] [PubMed] [Google Scholar]
- 19.Harlow ML, Ress D, Stoschek A, Marshall RM, McMahan UJ. The architecture of active zone material at the frog's neuromuscular junction. Nature. 2001;409(6819):479–484. doi: 10.1038/35054000. [DOI] [PubMed] [Google Scholar]
- 20.Nagwaney S, Harlow ML, Jung JH, Szule JA, Ress D, Xu J, Marshall RM, McMahan UJ. Macromolecular connections of active zone material to docked synaptic vesicles and presynaptic membrane at neuromuscular junctions of mouse. The Journal of Comparative Neurology. 2009;513(5):457–468. doi: 10.1002/cne.21975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rowley KL, Mantilla CB, Ermilov LG, Sieck GC. Synaptic vesicle distribution and release at rat diaphragm neuromuscular junctions. Journal of neurophysiology. 2007;98(1):478–487. doi: 10.1152/jn.00251.2006. [DOI] [PubMed] [Google Scholar]
- 22.Dresbach T, Qualmann B, Kessels MM, Garner CC, Gundelfinger ED. The presynaptic cytomatrix of brain synapses. Cell Mol Life Sci. 2001;58(1):94–116. doi: 10.1007/PL00000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.tom Dieck S, Sanmarti-Vila L, Langnaese K, Richter K, Kindler S, Soyke A, Wex H, Smalla KH, Kampf U, Franzer JT, Stumm M, Garner CC, Gundelfinger ED. Bassoon, a novel zinc-finger CAG/glutamine-repeat protein selectively localized at the active zone of presynaptic nerve terminals. The Journal of cell biology. 1998;142(2):499–509. doi: 10.1083/jcb.142.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohtsuka T, Takao-Rikitsu E, Inoue E, Inoue M, Takeuchi M, Matsubara K, Deguchi-Tawarada M, Satoh K, Morimoto K, Nakanishi H, Takai Y. Cast: a novel protein of the cytomatrix at the active zone of synapses that forms a ternary complex with RIM1 and munc13-1. The Journal of cell biology. 2002;158(3):577–590. doi: 10.1083/jcb.200202083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Liu X, Biederer T, Südhof TC. A family of RIM-binding proteins regulated by alternative splicing: implications for the genesis of synaptic active zones. Proceedings of the National Academy of Sciences. 2002;99(22):14464–14469. doi: 10.1073/pnas.182532999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deguchi-Tawarada M, Inoue E, Takao-Rikitsu E, Inoue M, Ohtsuka T, Takai Y. CAST2: identification and characterization of a protein structurally related to the presynaptic cytomatrix protein CAST. Genes to Cells. 2004;9(1):15–23. doi: 10.1111/j.1356-9597.2004.00697.x. [DOI] [PubMed] [Google Scholar]
- 27.Brose N, Hofmann K, Hata Y, Südhof TC. Mammalian homologues of Caenorhabditis elegans unc-13 gene define novel family of C2-domain proteins. The Journal of biological chemistry. 1995;270(42):25273–25280. doi: 10.1074/jbc.270.42.25273. [DOI] [PubMed] [Google Scholar]
- 28.Betz A, Ashery U, Rickmann M, Augustin I, Neher E, Südhof TC, Rettig J, Brose N. Munc13-1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron. 1998;21(1):123–136. doi: 10.1016/s0896-6273(00)80520-6. [DOI] [PubMed] [Google Scholar]
- 29.Cases-Langhoff C, Voss B, Garner AM, Appeltauer U, Takei K, Kindler S, Veh RW, De Camilli P, Gundelfinger ED, Garner CC. Piccolo, a novel 420 kDa protein associated with the presynaptic cytomatrix. Eur J Cell Biol. 1996;69(3):214–223. [PubMed] [Google Scholar]
- 30.Wang Y, Okamoto M, Schmitz F, Hofmann K, Südhof TC. Rim is a putative Rab3 effector in regulating synaptic-vesicle fusion. Nature. 1997;388(6642):593–598. doi: 10.1038/41580. [DOI] [PubMed] [Google Scholar]
- 31.Chen J, Billings SE, Nishimune H. Calcium channels link the muscle-derived synapse organizer laminin β2 to bassoon and CAST/Erc2 to organize presynaptic active zones. The Journal of Neuroscience. 2011;31(2):512–525. doi: 10.1523/JNEUROSCI.3771-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishimune H, Sanes JR, Carlson SS. A synaptic laminin-calcium channel interaction organizes active zones in motor nerve terminals. Nature. 2004;432(7017):580–587. doi: 10.1038/nature03112. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Mizushige T, Nishimune H. Active zone density is conserved during synaptic growth but impaired in aged mice. J Comp Neurol. 2012;520(2):434–452. doi: 10.1002/cne.22764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Juranek J, Mukherjee K, Rickmann M, Martens H, Calka J, Südhof TC, Jahn R. Differential expression of active zone proteins in neuromuscular junctions suggests functional diversification. European Journal of Neuroscience. 2006;24(11):3043–3052. doi: 10.1111/j.1460-9568.2006.05183.x. [DOI] [PubMed] [Google Scholar]
- 35.Tokoro T, Higa S, Deguchi-Tawarada M, Inoue E, Kitajima I, Ohtsuka T. Localization of the active zone proteins CAST, ELKS, and Piccolo at neuromuscular junctions. Neuroreport. 2007;18(4):313–316. doi: 10.1097/WNR.0b013e3280287abe. [DOI] [PubMed] [Google Scholar]
- 36.Wyatt RM, Balice-Gordon RJ. Heterogeneity in synaptic vesicle release at neuromuscular synapses of mice expressing synaptophluorin. The Journal of Neuroscience. 2008;28(1):325–335. doi: 10.1523/JNEUROSCI.3544-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo F, Dittrich M, Stiles JR, Meriney SD. Single-pixel optical fluctuation analysis of calcium channel function in active zones of motor nerve terminals. The Journal of Neuroscience. 2011;31(31):11268–11281. doi: 10.1523/JNEUROSCI.1394-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Landis DM, Hall AK, Weinstein LA, Reese TS. The organization of cytoplasm at the presynaptic active zone of a central nervous system synapse. Neuron. 1988;1(3):201–209. doi: 10.1016/0896-6273(88)90140-7. [DOI] [PubMed] [Google Scholar]
- 39.Satzler K, Sohl LF, Bollmann JH, Borst JG, Frotscher M, Sakmann B, Lubke JH. Three-dimensional reconstruction of a calyx of Held and its postsynaptic principal neuron in the medial nucleus of the trapezoid body. The Journal of Neuroscience. 2002;22(24):10567–10579. doi: 10.1523/JNEUROSCI.22-24-10567.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siksou L, Rostaing P, Lechaire J-P, Boudier T, Ohtsuka T, Fejtova A, Kao H-T, Greengard P, Gundelfinger ED, Triller A, Marty S. Three-dimensional architecture of presynaptic terminal cytomatrix. The Journal of Neuroscience. 2007;27(26):6868–6877. doi: 10.1523/JNEUROSCI.1773-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tao-Cheng JH. Ultrastructural localization of active zone and synaptic vesicle proteins in a preassembled multi-vesicle transport aggregate. Neuroscience. 2007;150(3):575–584. doi: 10.1016/j.neuroscience.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Regus-Leidig H, tom Dieck S, Specht D, Meyer L, Brandstätter JH. Early steps in the assembly of photoreceptor ribbon synapses in the mouse retina: the involvement of precursor spheres. The Journal of Comparative Neurology. 2009;512(6):814–824. doi: 10.1002/cne.21915. [DOI] [PubMed] [Google Scholar]
- 43.Dondzillo A, Sätzler K, Horstmann H, Altrock WD, Gundelfinger ED, Kuner T. Targeted three-dimensional immunohistochemistry reveals localization of presynaptic proteins Bassoon and Piccolo in the rat calyx of Held before and after the onset of hearing. The Journal of Comparative Neurology. 2010;518(7):1008–1029. doi: 10.1002/cne.22260. [DOI] [PubMed] [Google Scholar]
- 44.Ko CP. Formation of the active zone at developing neuromuscular junctions in larval and adult bullfrogs. J Neurocytol. 1985;14(3):487–512. doi: 10.1007/BF01217757. [DOI] [PubMed] [Google Scholar]
- 45.tom Dieck S, Altrock WD, Kessels MM, Qualmann B, Regus H, Brauner D, Fejtova A, Bracko O, Gundelfinger ED, Brandstatter JH. Molecular dissection of the photoreceptor ribbon synapse: physical interaction of Bassoon and RIBEYE is essential for the assembly of the ribbon complex. The Journal of cell biology. 2005;168(5):825–836. doi: 10.1083/jcb.200408157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fenster SD, Chung WJ, Zhai R, Cases-Langhoff C, Voss B, Garner AM, Kaempf U, Kindler S, Gundelfinger ED, Garner CC. Piccolo, a presynaptic zinc finger protein structurally related to bassoon. Neuron. 2000;25(1):203–214. doi: 10.1016/s0896-6273(00)80883-1. [DOI] [PubMed] [Google Scholar]
- 47.Dick O, tom Dieck S, Altrock WD, Ammermuller J, Weiler R, Garner CC, Gundelfinger ED, Brandstatter JH. The presynaptic active zone protein bassoon is essential for photoreceptor ribbon synapse formation in the retina. Neuron. 2003;37(5):775–786. doi: 10.1016/s0896-6273(03)00086-2. [DOI] [PubMed] [Google Scholar]
- 48.Specht D, tom Dieck S, Ammermuller J, Regus-Leidig H, Gundelfinger ED, Brandstatter JH. Structural and functional remodeling in the retina of a mouse with a photoreceptor synaptopathy: plasticity in the rod and degeneration in the cone system. European Journal of Neuroscience. 2007;26(9):2506–2515. doi: 10.1111/j.1460-9568.2007.05886.x. [DOI] [PubMed] [Google Scholar]
- 49.Frank T, Rutherford MA, Strenzke N, Neef A, Pangrsic T, Khimich D, Fetjova A, Gundelfinger ED, Liberman MC, Harke B, Bryan KE, Lee A, Egner A, Riedel D, Moser T. Bassoon and the synaptic ribbon organize Ca2+ channels and vesicles to add release sites and promote refilling. Neuron. 2010;68(4):724–738. doi: 10.1016/j.neuron.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Altrock WD, tom Dieck S, Sokolov M, Meyer AC, Sigler A, Brakebusch C, Fassler R, Richter K, Boeckers TM, Potschka H, Brandt C, Loscher W, Grimberg D, Dresbach T, Hempelmann A, Hassan H, Balschun D, Frey JU, Brandstatter JH, Garner CC, Rosenmund C, Gundelfinger ED. Functional inactivation of a fraction of excitatory synapses in mice deficient for the active zone protein bassoon. Neuron. 2003;37(5):787–800. doi: 10.1016/s0896-6273(03)00088-6. [DOI] [PubMed] [Google Scholar]
- 51.Angenstein F, Hilfert L, Zuschratter W, Altrock WD, Niessen HG, Gundelfinger ED. Morphological and metabolic changes in the cortex of mice lacking the functional presynaptic active zone protein Bassoon: a combined 1H-NMR spectroscopy and histochemical study. Cereb Cortex. 2008;18(4):890–897. doi: 10.1093/cercor/bhm122. [DOI] [PubMed] [Google Scholar]
- 52.Buran BN, Strenzke N, Neef A, Gundelfinger ED, Moser T, Liberman MC. Onset coding is degraded in auditory nerve fibers from mutant mice lacking synaptic ribbons. The Journal of Neuroscience. 2010;30(22):7587–7597. doi: 10.1523/JNEUROSCI.0389-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goetze B, Schmidt K-F, Lehmann K, Altrock WD, Gundelfinger ED, Lowel S. Vision and visual cortical maps in mice with a photoreceptor synaptopathy: reduced but robust visual capabilities in the absence of synaptic ribbons. NeuroImage. 2010;49(2):1622–1631. doi: 10.1016/j.neuroimage.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 54.Hallermann S, Fejtova A, Schmidt H, Weyhersmuller A, Silver RA, Gundelfinger ED, Eilers J. Bassoon speeds vesicle reloading at a central excitatory synapse. Neuron. 2010;68(4):710–723. doi: 10.1016/j.neuron.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lanore F, Blanchet C, Fejtova A, Pinheiro P, Richter K, Balschun D, Gundelfinger ED, Mulle C. Impaired development of hippocampal mossy fibre synapses in mouse mutants for the presynaptic scaffold protein Bassoon. The Journal of physiology. 2010;588(12):2133–2145. doi: 10.1113/jphysiol.2009.184929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mukherjee K, Yang X, Gerber SH, Kwon H-B, Ho A, Castillo PE, Liu X, Südhof TC. Piccolo and bassoon maintain synaptic vesicle clustering without directly participating in vesicle exocytosis. Proceedings of the National Academy of Sciences. 2010;107(14):6504–6509. doi: 10.1073/pnas.1002307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hida Y, Ohtsuka T. CAST and ELKS proteins: structural and functional determinants of the presynaptic active zone. Journal of Biochemistry. 2010;148(2):131–137. doi: 10.1093/jb/mvq065. [DOI] [PubMed] [Google Scholar]
- 58.Kaeser PS, Deng L, Chavez AE, Liu X, Castillo PE, Südhof TC. ELKS2alpha/CAST deletion selectively increases neurotransmitter release at inhibitory synapses. Neuron. 2009;64(2):227–239. doi: 10.1016/j.neuron.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brose N, Rosenmund C, Rettig J. Regulation of transmitter release by Unc-13 and its homologues. Current Opinion in Neurobiology. 2000;10(3):303–311. doi: 10.1016/s0959-4388(00)00105-7. [DOI] [PubMed] [Google Scholar]
- 60.Varoqueaux F, Sons MS, Plomp JJ, Brose N. Aberrant morphology and residual transmitter release at the Munc13-deficient mouse neuromuscular synapse. Mol Cell Biol. 2005;25(14):5973–5984. doi: 10.1128/MCB.25.14.5973-5984.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schoch S, Mittelstaedt T, Kaeser PS, Padgett D, Feldmann N, Chevaleyre V, Castillo PE, Hammer RE, Han W, Schmitz F, Lin W, Südhof TC. Redundant functions of RIM1alpha and RIM2alpha in Ca2+-triggered neurotransmitter release. The EMBO journal. 2006;25(24):5852–5863. doi: 10.1038/sj.emboj.7601425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mittelstaedt T, Alvarez-Baron E, Schoch S. RIM proteins and their role in synapse function. Biol Chem. 2010;391(6):599–606. doi: 10.1515/BC.2010.064. [DOI] [PubMed] [Google Scholar]
- 63.Mori Y, Friedrich T, Kim M-S, Mikami A, Nakai J, Ruth P, Bosse E, Hofmann F, Flockerzi V, Furuichi T, Mikoshiba K, Imoto K, Tanabe T, Numa S. Primary structure and functional expression from complementary DNA of a brain calcium channel. Nature. 1991;350(6317):398–402. doi: 10.1038/350398a0. [DOI] [PubMed] [Google Scholar]
- 64.Starr TV, Prystay W, Snutch TP. Primary structure of a calcium channel that is highly expressed in the rat cerebellum. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(13):5621–5625. doi: 10.1073/pnas.88.13.5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dubel SJ, Starr TV, Hell J, Ahlijanian MK, Enyeart JJ, Catterall WA, Snutch TP. Molecular cloning of the alpha-1 subunit of an omega-conotoxin-sensitive calcium channel. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(11):5058–5062. doi: 10.1073/pnas.89.11.5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Williams ME, Brust PF, Feldman DH, Patthi S, Simerson S, Maroufi A, McCue AF, Velicelebi G, Ellis SB, Harpold MM. Structure and functional expression of an omega-conotoxin-sensitive human N-type calcium channel. Science. 1992;257(5068):389–395. doi: 10.1126/science.1321501. [DOI] [PubMed] [Google Scholar]
- 67.Fujita Y, Mynlieff M, Dirksen RT, Kim M-S, Niidome T, Nakai J, Friedrich T, Iwabe N, Miyata T, Furuichi T, Furutama D, Mikoshiba K, Mori Y, Beam KG. Primary structure and functional expression of the [omega]-conotoxin-sensitive N-type calcium channel from rabbit brain. Neuron. 1993;10(4):585–598. doi: 10.1016/0896-6273(93)90162-k. [DOI] [PubMed] [Google Scholar]
- 68.Katz B, Miledi R. A study of synaptic transmission in the absence of nerve impulses. The Journal of physiology. 1967;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Katz B, Miledi R. The timing of calcium action during neuromuscular transmission. The Journal of physiology. 1967;189(3):535–544. doi: 10.1113/jphysiol.1967.sp008183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pumplin DW, Reese TS, Llinas R. Are the presynaptic membrane particles the calcium channels?. Proceedings of the National Academy of Sciences of the United States of America; 1981. pp. 7210–7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoshikami D, Bagabaldo Z, Olivera BM. The inhibitory effects of omega-conotoxins on Ca channels and synapses. Annals of the New York Academy of Sciences. 1989;560:230–248. doi: 10.1111/j.1749-6632.1989.tb24100.x. [DOI] [PubMed] [Google Scholar]
- 72.Llinas R, Sugimori M, Silver RB. Microdomains of high calcium concentration in a presynaptic terminal. Science. 1992;256(5057):677–679. doi: 10.1126/science.1350109. [DOI] [PubMed] [Google Scholar]
- 73.Stanley EF. The calcium channel and the organization of the presynaptic transmitter release face. Trends in neurosciences. 1997;20(9):404–409. doi: 10.1016/s0166-2236(97)01091-6. [DOI] [PubMed] [Google Scholar]
- 74.Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 1998;20(3):389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 75.Sheng ZH, Rettig J, Takahashi M, Catterall WA. Identification of a syntaxin-binding site on N-type calcium channels. Neuron. 1994;13(6):1303–1313. doi: 10.1016/0896-6273(94)90417-0. [DOI] [PubMed] [Google Scholar]
- 76.Martin-Moutot N, Charvin N, Leveque C, Sato K, Nishiki T, Kozaki S, Takahashi M, Seagar M. Interaction of SNARE complexes with P/Q-type calcium channels in rat cerebellar synaptosomes. The Journal of biological chemistry. 1996;271(12):6567–6570. doi: 10.1074/jbc.271.12.6567. [DOI] [PubMed] [Google Scholar]
- 77.Rettig J, Sheng ZH, Kim DK, Hodson CD, Snutch TP, Catterall WA. Isoform-specific interaction of the alpha1A subunits of brain Ca2+ channels with the presynaptic proteins syntaxin and SNAP-25. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(14):7363–7368. doi: 10.1073/pnas.93.14.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sheng ZH, Rettig J, Cook T, Catterall WA. Calcium-dependent interaction of N-type calcium channels with the synaptic core complex. Nature. 1996;379(6564):451–454. doi: 10.1038/379451a0. [DOI] [PubMed] [Google Scholar]
- 79.Sheng ZH, Yokoyama CT, Catterall WA. Interaction of the synprint site of N-type Ca2+ channels with the C2B domain of synaptotagmin I. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(10):5405–5410. doi: 10.1073/pnas.94.10.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bezprozvanny I, Scheller RH, Tsien RW. Functional impact of syntaxin on gating of N-type and Q-type calcium channels. Nature. 1995;378(6557):623–626. doi: 10.1038/378623a0. [DOI] [PubMed] [Google Scholar]
- 81.Wiser O, Bennett MK, Atlas D. Functional interaction of syntaxin and SNAP-25 with voltage-sensitive L- and N-type Ca2+ channels. The EMBO journal. 1996;15(16):4100–4110. [PMC free article] [PubMed] [Google Scholar]
- 82.Uchitel OD, Protti DA, Sanchez V, Cherksey BD, Sugimori M, Llinas R. P-type voltage-dependent calcium channel mediates presynaptic calcium influx and transmitter release in mammalian synapses. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(8):3330–3333. doi: 10.1073/pnas.89.8.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosato Siri MD, Uchitel OD. Calcium channels coupled to neurotransmitter release at neonatal rat neuromuscular junctions. The Journal of physiology. 1999;514(Pt 2):533–540. doi: 10.1111/j.1469-7793.1999.533ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Robitaille R, Adler EM, Charlton MP. Strategic location of calcium channels at transmitter release sites of frog neuromuscular synapses. Neuron. 1990;5(6):773–779. doi: 10.1016/0896-6273(90)90336-e. [DOI] [PubMed] [Google Scholar]
- 85.Wachman ES, Poage RE, Stiles JR, Farkas DL, Meriney SD. Spatial distribution of calcium entry evoked by single action potentials within the presynaptic active zone. The Journal of Neuroscience. 2004;24(12):2877–2885. doi: 10.1523/JNEUROSCI.1660-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Day NC, Wood SJ, Ince PG, Volsen SG, Smith W, Slater CR, Shaw PJ. Differential localization of voltage-dependent calcium channel alpha1 subunits at the human and rat neuromuscular junction. The Journal of Neuroscience. 1997;17(16):6226–6235. doi: 10.1523/JNEUROSCI.17-16-06226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Westenbroek RE, Hoskins L, Catterall WA. Localization of Ca2+ channel subtypes on rat spinal motor neurons, interneurons, and nerve terminals. The Journal of Neuroscience. 1998;18(16):6319–6330. doi: 10.1523/JNEUROSCI.18-16-06319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De Laet A, Adriaensen D, Van Bogaert PP, Scheuermann DW, Timmermans JP. Immunohistochemical localization of voltage-activated calcium channels in the rat oesophagus. Neurogastroenterology and motility. 2002;14(2):173–181. doi: 10.1046/j.1365-2982.2002.00320.x. [DOI] [PubMed] [Google Scholar]
- 89.Santafé MM, Sabaté MM, Garcia N, Ortiz N, Lanuza MA, Tomas J. Changes in the neuromuscular synapse induced by an antibody against gangliosides. Annals of Neurology. 2005;57(3):396–407. doi: 10.1002/ana.20403. [DOI] [PubMed] [Google Scholar]
- 90.Fouquet W, Owald D, Wichmann C, Mertel S, Depner H, Dyba M, Hallermann S, Kittel RJ, Eimer S, Sigrist SJ. Maturation of active zone assembly by Drosophila Bruchpilot. The Journal of cell biology. 2009;186(1):129–145. doi: 10.1083/jcb.200812150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annual review of cell and developmental biology. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 92.Coppola T, Magnin-Luthi S, Perret-Menoud V, Gattesco S, Schiavo G, Regazzi R. Direct interaction of the Rab3 effector RIM with Ca2+ channels, SNAP-25, and synaptotagmin. Journal of Biological Chemistry. 2001;276(35):32756–32762. doi: 10.1074/jbc.M100929200. [DOI] [PubMed] [Google Scholar]
- 93.Shibasaki T, Sunaga Y, Fujimoto K, Kashima Y, Seino S. Interaction of ATP sensor, cAMP sensor, Ca2+ sensor, and voltage-dependent Ca2+ channel in insulin granule exocytosis. Journal of Biological Chemistry. 2004;279(9):7956–7961. doi: 10.1074/jbc.M309068200. [DOI] [PubMed] [Google Scholar]
- 94.Kiyonaka S, Wakamori M, Miki T, Uriu Y, Nonaka M, Bito H, Beedle AM, Mori E, Hara Y, De Waard M, Kanagawa M, Itakura M, Takahashi M, Campbell KP, Mori Y. RIM1 confers sustained activity and neurotransmitter vesicle anchoring to presynaptic Ca2+ channels. Nature neuroscience. 2007;10(6):691–701. doi: 10.1038/nn1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Uriu Y, Kiyonaka S, Miki T, Yagi M, Akiyama S, Mori E, Nakao A, Beedle AM, Campbell KP, Wakamori M, Mori Y. Rab3-interacting molecule g isoforms lacking the Rab3-binding domain induce long-lasting currents but block neurotransmitter vesicle-anchoring in voltage-dependent P/Q-type Ca2+ channels. Journal of Biological Chemistry. 2010;285(28):21750–21767. doi: 10.1074/jbc.M110.101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kaeser PS, Deng L, Wang Y, Dulubova I, Liu X, Rizo J, Südhof TC. RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell. 2011;144(2):282–295. doi: 10.1016/j.cell.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Han Y, Kaeser PS, Südhof TC, Schneggenburger R. RIM determines Ca2+ channel density and vesicle docking at the presynaptic active zone. Neuron. 2011;69(2):304–316. doi: 10.1016/j.neuron.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takao-Rikitsu E, Mochida S, Inoue E, Deguchi-Tawarada M, Inoue M, Ohtsuka T, Takai Y. Physical and functional interaction of the active zone proteins, CAST, RIM1, and Bassoon, in neurotransmitter release. The Journal of cell biology. 2004;164(2):301–311. doi: 10.1083/jcb.200307101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dulubova I, Lou X, Lu J, Huryeva I, Alam A, Schneggenburger R, Südhof TC, Rizo J. A Munc13/RIM/Rab3 tripartite complex: from priming to plasticity? The EMBO journal. 2005;24(16):2839–2850. doi: 10.1038/sj.emboj.7600753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ohara-Imaizumi M, Ohtsuka T, Matsushima S, Akimoto Y, Nishiwaki C, Nakamichi Y, Kikuta T, Nagai S, Kawakami H, Watanabe T, Nagamatsu S. ELKS, a protein structurally related to the active zone-associated protein CAST, is expressed in pancreatic {beta} cells and functions in insulin exocytosis: interaction of ELKS with exocytotic machinery analyzed by total internal reflection fluorescence microscopy. Mol Biol Cell. 2005;16(7):3289–3300. doi: 10.1091/mbc.E04-09-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang X, Hu B, Zieba A, Neumann NG, Kasper-Sonnenberg M, Honsbein A, Hultqvist G, Conze T, Witt W, Limbach C, Geitmann M, Danielson H, Kolarow R, Niemann G, Lessmann V, Kilimann MW. A protein interaction node at the neurotransmitter release site: domains of Aczonin/Piccolo, Bassoon, CAST, and Rim converge on the N-terminal domain of Munc13-1. The Journal of Neuroscience. 2009;29(40):12584–12596. doi: 10.1523/JNEUROSCI.1255-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Muller C, Haupt A, Bildl W, Schindler J, Knaus H, Meissner M, Rammner B, Striessnig J, Flockerzi V, Fakler B, Schulte U. Quantitative proteomics of the Cav2 channel nano-environments in the mammalian brain. Proceedings of the National Academy of Sciences. 2010;107(34):14950–14957. doi: 10.1073/pnas.1005940107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Urbano FJ, Piedras-Renteria ES, Jun K, Shin HS, Uchitel OD, Tsien RW. Altered properties of quantal neurotransmitter release at endplates of mice lacking P/Q-type Ca2+ channels. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(6):3491–3496. doi: 10.1073/pnas.0437991100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nishimune H. Transsynaptic channelosomes: non-conducting roles of ion channels in synapse formation. Channels. 2011;5(5):432, 439. doi: 10.4161/chan.5.5.16472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dickman DK, Kurshan PT, Schwarz TL. Mutations in a Drosophila {alpha}2{delta} voltage-gated calcium channel subunit reveal a crucial synaptic function. The Journal of Neuroscience. 2008;28(1):31–38. doi: 10.1523/JNEUROSCI.4498-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ly CV, Yao C-K, Verstreken P, Ohyama T, Bellen HJ. Straightjacket is required for the synaptic stabilization of cacophony, a voltage-gated calcium channel [alpha]1 subunit. The Journal of cell biology. 2008;181(1):157–170. doi: 10.1083/jcb.200712152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kurshan PT, Oztan A, Schwarz TL. Presynaptic [alpha]2[delta]-3 is required for synaptic morphogenesis independent of its Ca2+-channel functions. Nat Neurosci. 2009;12(11):1415–1423. doi: 10.1038/nn.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kaja S, Todorov B, van de Ven RCG, Ferrari MD, Frants RR, van den Maagdenberg AMJM, Plomp JJ. Redundancy of Cav2.1 channel accessory subunits in transmitter release at the mouse neuromuscular junction. Brain Research. 2007;1143:92–101. doi: 10.1016/j.brainres.2007.01.063. [DOI] [PubMed] [Google Scholar]
- 109.Sanes JR, Hall ZW. Antibodies that bind specifically to synaptic sites on muscle fiber basal lamina. The Journal of cell biology. 1979;83(2 Pt 1):357–370. doi: 10.1083/jcb.83.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hunter DD, Shah V, Merlie JP, Sanes JR. A laminin-like adhesive protein concentrated in the synaptic cleft of the neuromuscular junction. Nature. 1989;338(6212):229–234. doi: 10.1038/338229a0. [DOI] [PubMed] [Google Scholar]
- 111.Noakes PG, Gautam M, Mudd J, Sanes JR, Merlie JP. Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin beta 2. Nature. 1995;374(6519):258–262. doi: 10.1038/374258a0. [DOI] [PubMed] [Google Scholar]
- 112.Knight D, Tolley LK, Kim DK, Lavidis NA, Noakes PG. Functional analysis of neurotransmission at beta2-laminin deficient terminals. The Journal of physiology. 2003;546(Pt 3):789–800. doi: 10.1113/jphysiol.2002.030924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Patton BL, Miner JH, Chiu AY, Sanes JR. Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice. The Journal of cell biology. 1997;139(6):1507–1521. doi: 10.1083/jcb.139.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annual review of neuroscience. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- 115.Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2(11):791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- 116.Hunter DD, Cashman N, Morris-Valero R, Bulock JW, Adams SP, Sanes JR. An LRE (leucine-arginine-glutamate)-dependent mechanism for adhesion of neurons to S-laminin. The Journal of Neuroscience. 1991;11(12):3960–3971. doi: 10.1523/JNEUROSCI.11-12-03960.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Porter BE, Weis J, Sanes JR. A motoneuron-selective stop signal in the synaptic protein S-laminin. Neuron. 1995;14(3):549–559. doi: 10.1016/0896-6273(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 118.Belkin AM, Stepp MA. Integrins as receptors for laminins. Microsc Res Tech. 2000;51(3):280–301. doi: 10.1002/1097-0029(20001101)51:3<280::AID-JEMT7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 119.Suzuki N, Yokoyama F, Nomizu M. Functional sites in the laminin alpha chains. Connective tissue research. 2005;46(3):142–152. doi: 10.1080/03008200591008527. [DOI] [PubMed] [Google Scholar]
- 120.Barresi R, Campbell KP. Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci. 2006;119(2):199–207. doi: 10.1242/jcs.02814. [DOI] [PubMed] [Google Scholar]
- 121.Mansergh F, Orton NC, Vessey JP, Lalonde MR, Stell WK, Tremblay F, Barnes S, Rancourt DE, Bech-Hansen NT. Mutation of the calcium channel gene Cacna1f disrupts calcium signaling, synaptic transmission and cellular organization in mouse retina. Human molecular genetics. 2005;14(20):3035–3046. doi: 10.1093/hmg/ddi336. [DOI] [PubMed] [Google Scholar]
- 122.Hunter DD, Murphy MD, Olsson CV, Brunken WJ. S-Laminin expression in adult and developing retinae: a potential cue for photoreceptor morphogenesis. Neuron. 1992;8(3):399–413. doi: 10.1016/0896-6273(92)90269-j. [DOI] [PubMed] [Google Scholar]
- 123.Libby RT, Lavallee CR, Balkema GW, Brunken WJ, Hunter DD. Disruption of laminin beta2 chain production causes alterations in morphology and function in the CNS. The Journal of Neuroscience. 1999;19(21):9399–9411. doi: 10.1523/JNEUROSCI.19-21-09399.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Patton BL, Cunningham JM, Thyboll J, Kortesmaa J, Westerblad H, Edstrom L, Tryggvason K, Sanes JR. Properly formed but improperly localized synaptic specializations in the absence of laminin alpha4. Nat Neurosci. 2001;4(6):597–604. doi: 10.1038/88414. [DOI] [PubMed] [Google Scholar]
- 125.Nishimune H, Valdez G, Jarad G, Moulson CL, Muller U, Miner JH, Sanes JR. Laminins promote postsynaptic maturation by an autocrine mechanism at the neuromuscular junction. The Journal of cell biology. 2008;182(6):1201–1215. doi: 10.1083/jcb.200805095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bierman J, Knittel L, Yang D, Tarumi YS, Miner JH, Sanes JR, Patton BL. Defects in developing neuromuscular junctions of mice lacking laminin 5; Society for Neuroscience, 33rd annual meeting; New Orleans, LA. 2003. 2003. pp. 898–897. Society for Neuroscience. [Google Scholar]
- 127.Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, Südhof TC. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 2003;423(6943):939–948. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- 128.Sons MS, Busche N, Strenzke N, Moser T, Ernsberger U, Mooren FC, Zhang W, Ahmad M, Steffens H, Schomburg ED, Plomp JJ, Missler M. Alpha-neurexins are required for efficient transmitter release and synaptic homeostasis at the mouse neuromuscular junction. Neuroscience. 2006;138(2):433–446. doi: 10.1016/j.neuroscience.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 129.Hata Y, Butz S, Südhof T. CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. The Journal of Neuroscience. 1996;16(8):2488–2494. doi: 10.1523/JNEUROSCI.16-08-02488.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Maximov A, Südhof TC, Bezprozvanny I. Association of neuronal calcium channels with modular adaptor proteins. The Journal of biological chemistry. 1999;274(35):24453–24456. doi: 10.1074/jbc.274.35.24453. [DOI] [PubMed] [Google Scholar]
- 131.Atasoy D, Schoch S, Ho A, Nadasy KA, Liu X, Zhang W, Mukherjee K, Nosyreva ED, Fernandez-Chacon R, Missler M, Kavalali ET, Südhof TC. Deletion of CASK in mice is lethal and impairs synaptic function. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(7):2525–2530. doi: 10.1073/pnas.0611003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mintz IM, Venema VJ, Swiderek KM, Lee TD, Bean BP, Adams ME. P-type calcium channels blocked by the spider toxin [omega]-Aga-IVA. Nature. 1992;355(6363):827–829. doi: 10.1038/355827a0. [DOI] [PubMed] [Google Scholar]
- 133.Protti DA, Uchitel OD. Transmitter release and presynaptic Ca2+ currents blocked by the spider toxin omega-Aga-IVA. Neuroreport. 1993;5(3):333–336. doi: 10.1097/00001756-199312000-00039. [DOI] [PubMed] [Google Scholar]
- 134.Kasai H, Aosaki T, Fukuda J. Presynaptic Ca-antagonist [omega]-conotoxin irreversibly blocks N-type Ca-channels in chick sensory neurons. Neuroscience Research. 1987;4(3):228–235. doi: 10.1016/0168-0102(87)90014-9. [DOI] [PubMed] [Google Scholar]
- 135.Plummer MR, Logothetis DE, Hess P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron. 1989;2(5):1453–1463. doi: 10.1016/0896-6273(89)90191-8. [DOI] [PubMed] [Google Scholar]
- 136.Misgeld T, Burgess RW, Lewis RM, Cunningham JM, Lichtman JW, Sanes JR. Roles of neurotransmitter in synapse formation: development of neuromuscular junctions lacking choline acetyltransferase. Neuron. 2002;36(4):635–648. doi: 10.1016/s0896-6273(02)01020-6. [DOI] [PubMed] [Google Scholar]
- 137.Brandon EP, Lin W, D'Amour KA, Pizzo DP, Dominguez B, Sugiura Y, Thode S, Ko CP, Thal LJ, Gage FH, Lee KF. Aberrant patterning of neuromuscular synapses in choline acetyltransferase-deficient mice. The Journal of Neuroscience. 2003;23(2):539–549. doi: 10.1523/JNEUROSCI.23-02-00539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Varoqueaux F, Sigler A, Rhee JS, Brose N, Enk C, Reim K, Rosenmund C. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(13):9037–9042. doi: 10.1073/pnas.122623799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Heeroma JH, Plomp JJ, Roubos EW, Verhage M. Development of the mouse neuromuscular junction in the absence of regulated secretion. Neuroscience. 2003;120(3):733–744. doi: 10.1016/s0306-4522(03)00258-6. [DOI] [PubMed] [Google Scholar]
- 140.Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, Toonen RF, Hammer RE, van den Berg TK, Missler M, Geuze HJ, Südhof TC. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287(5454):864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- 141.Sanes JR, Schachner M, Covault J. Expression of several adhesive macromolecules (N-CAM, L1, J1, NILE, uvomorulin, laminin, fibronectin, and a heparan sulfate proteoglycan) in embryonic, adult, and denervated adult skeletal muscle. The Journal of cell biology. 1986;102(2):420–431. doi: 10.1083/jcb.102.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sanes JR, Engvall E, Butkowski R, Hunter DD. Molecular heterogeneity of basal laminae: isoforms of laminin and collagen IV at the neuromuscular junction and elsewhere. The Journal of cell biology. 1990;111(4):1685–1699. doi: 10.1083/jcb.111.4.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Patton BL, Connoll AM, Martin PT, Cunningham JM, Mehta S, Pestronk A, Miner JH, Sanes JR. Distribution of ten laminin chains in dystrophic and regenerating muscles. Neuromuscul Disord. 1999;9(6-7):423–433. doi: 10.1016/s0960-8966(99)00033-4. [DOI] [PubMed] [Google Scholar]
- 144.Batt J, Bain J, Goncalves J, Michalski B, Plant P, Fahnestock M, Woodgett J. Differential gene expression profiling of short and long term denervated muscle. FASEB J. 2005;20:115–117. doi: 10.1096/fj.04-3640fje. [DOI] [PubMed] [Google Scholar]
- 145.Kostrominova TY, Dow DE, Dennis RG, Miller RA, Faulkner JA. Comparison of gene expression of 2-mo denervated, 2-mo stimulated-denervated, and control rat skeletal muscles. Physiol Genomics. 2005;22(2):227–243. doi: 10.1152/physiolgenomics.00210.2004. [DOI] [PubMed] [Google Scholar]
- 146.Zhou Z, Cornelius CP, Eichner M, Bornemann A. Reinnervation-induced alterations in rat skeletal muscle. Neurobiology of Disease. 2006;23(3):595–602. doi: 10.1016/j.nbd.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 147.Feng G, Krejci E, Molgo J, Cunningham JM, Massoulie J, Sanes JR. Genetic analysis of collagen Q: roles in acetylcholinesterase and butyrylcholinesterase assembly and in synaptic structure and function. The Journal of cell biology. 1999;144(6):1349–1360. doi: 10.1083/jcb.144.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xie W, Stribley JA, Chatonnet A, Wilder PJ, Rizzino A, McComb RD, Taylor P, Hinrichs SH, Lockridge O. Postnatal developmental delay and supersensitivity to organophosphate in gene-targeted mice lacking acetylcholinesterase. Journal of Pharmacology and Experimental Therapeutics. 2000;293(3):896–902. [PubMed] [Google Scholar]
- 149.Lin W, Dominguez B, Yang J, Aryal P, Brandon EP, Gage FH, Lee K-F. Neurotransmitter acetylcholine negatively regulates neuromuscular synapse formation by a Cdk5-dependent mechanism. Neuron. 2005;46(4):569–579. doi: 10.1016/j.neuron.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 150.Misgeld T, Kummer TT, Lichtman JW, Sanes JR. Agrin promotes synaptic differentiation by counteracting an inhibitory effect of neurotransmitter. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(31):11088–11093. doi: 10.1073/pnas.0504806102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lichtman JW, Wilkinson RS, Rich MM. Multiple innervation of tonic endplates revealed by activity-dependent uptake of fluorescent probes. Nature. 1985;314(6009):357–359. doi: 10.1038/314357a0. [DOI] [PubMed] [Google Scholar]
- 152.Buffelli M, Burgess RW, Feng G, Lobe CG, Lichtman JW, Sanes JR. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature. 2003;424(6947):430–434. doi: 10.1038/nature01844. [DOI] [PubMed] [Google Scholar]
- 153.Lai K-O, Ip NY. Central synapse and neuromuscular junction: same players, different roles. Trends in Genetics. 2003;19(7):395–402. doi: 10.1016/S0168-9525(03)00147-1. [DOI] [PubMed] [Google Scholar]
- 154.Fox MA, Sanes JR, Borza D-B, Eswarakumar VP, Fassler R, Hudson BG, John SWM, Ninomiya Y, Pedchenko V, Pfaff SL, Rheault MN, Sado Y, Segal Y, Werle MJ, Umemori H. Distinct target-derived signals organize formation, maturation, and maintenance of motor nerve terminals. Cell. 2007;129(1):179–193. doi: 10.1016/j.cell.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 155.Song Y, Balice-Gordon R. New dogs in the dogma: Lrp4 and Tid1 in neuromuscular synapse formation. Neuron. 2008;60(4):526–528. doi: 10.1016/j.neuron.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 156.Burgess RW, Nguyen QT, Son Y-J, Lichtman JW, Sanes JR. Alternatively spliced isoforms of nerve- and muscle-derived agrin: their roles at the neuromuscular junction. Neuron. 1999;23(1):33–44. doi: 10.1016/s0896-6273(00)80751-5. [DOI] [PubMed] [Google Scholar]
- 157.Wang P, Yang G, Mosier DR, Chang P, Zaidi T, Gong YD, Zhao NM, Dominguez B, Lee KF, Gan WB, Zheng H. Defective neuromuscular synapses in mice lacking amyloid precursor protein (APP) and APP-Like protein 2. The Journal of Neuroscience. 2005;25(5):1219–1225. doi: 10.1523/JNEUROSCI.4660-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Latvanlehto A, Fox MA, Sormunen R, Tu H, Oikarainen T, Koski A, Naumenko N, Shakirzyanova A, Kallio M, Ilves M, Giniatullin R, Sanes JR, Pihlajaniemi T. Muscle-derived collagen XIII regulates maturation of the skeletal neuromuscular junction. The Journal of Neuroscience. 2010;30(37):12230–12241. doi: 10.1523/JNEUROSCI.5518-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.DeChiara TM, Bowen DC, Valenzuela DM, Simmons MV, Poueymirou WT, Thomas S, Kinetz E, Compton DL, Rojas E, Park JS, Smith C, DiStefano PS, Glass DJ, Burden SJ, Yancopoulos GD. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell. 1996;85(4):501–512. doi: 10.1016/s0092-8674(00)81251-9. [DOI] [PubMed] [Google Scholar]
- 160.Glass DJ, Bowen DC, Stitt TN, Radziejewski C, Bruno J, Ryan TE, Gies DR, Shah S, Mattsson K, Burden SJ, DiStefano PS, Valenzuela DM, DeChiara TM, Yancopoulos GD. Agrin acts via a MuSK receptor complex. Cell. 1996;85(4):513–523. doi: 10.1016/s0092-8674(00)81252-0. [DOI] [PubMed] [Google Scholar]
- 161.Lin W, Burgess RW, Dominguez B, Pfaff SL, Sanes JR, Lee K-F. Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature. 2001;410(6832):1057–1064. doi: 10.1038/35074025. [DOI] [PubMed] [Google Scholar]
- 162.Kim N, Stiegler AL, Cameron TO, Hallock PT, Gomez AM, Huang JH, Hubbard SR, Dustin ML, Burden SJ. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell. 2008;135(2):334–342. doi: 10.1016/j.cell.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang B, Luo S, Wang Q, Suzuki T, Xiong WC, Mei L. LRP4 serves as a coreceptor of agrin. Neuron. 2008;60(2):285–297. doi: 10.1016/j.neuron.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Weatherbee SD, Anderson KV, Niswander LA. LDL-receptor-related protein 4 is crucial for formation of the neuromuscular junction. Development. 2006;133(24):4993–5000. doi: 10.1242/dev.02696. [DOI] [PubMed] [Google Scholar]
- 165.Gautam M, Noakes PG, Moscoso L, Rupp F, Scheller RH, Merlie JP, Sanes JR. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell. 1996;85(4):525–535. doi: 10.1016/s0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- 166.Schubert W, Prior R, Weidemann A, Dircksen H, Multhaup G, Masters CL, Beyreuther K. Localization of Alzheimer beta A4 amyloid precursor protein at central and peripheral synaptic sites. Brain Research. 1991;563(1-2):184–194. doi: 10.1016/0006-8993(91)91532-6. [DOI] [PubMed] [Google Scholar]
- 167.Akaaboune M, Allinquant B, Farza H, Roy K, Magoul R, Fiszman M, Festoff BW, Hantai D. Developmental regulation of amyloid precursor protein at the neuromuscular junction in mouse skeletal muscle. Molecular and cellular neurosciences. 2000;15(4):355–367. doi: 10.1006/mcne.2000.0834. [DOI] [PubMed] [Google Scholar]
- 168.Wang Z, Wang B, Yang L, Guo Q, Aithmitti N, Songyang Z, Zheng H. Presynaptic and postsynaptic interaction of the amyloid precursor protein promotes peripheral and central synaptogenesis. The Journal of Neuroscience. 2009;29(35):10788–10801. doi: 10.1523/JNEUROSCI.2132-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dickman DK, Lu Z, Meinertzhagen IA, Schwarz TL. Altered synaptic development and active zone spacing in endocytosis mutants. Curr Biol. 2006;16(6):591–598. doi: 10.1016/j.cub.2006.02.058. [DOI] [PubMed] [Google Scholar]
- 170.Pielage J, Fetter RD, Davis GW. A postsynaptic Spectrin scaffold defines active zone size, spacing, and efficacy at the Drosophila neuromuscular junction. J Cell Biol. 2006;175(3):491–503. doi: 10.1083/jcb.200607036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Graf ER, Daniels RW, Burgess RW, Schwarz TL, DiAntonio A. Rab3 dynamically controls protein composition at active zones. Neuron. 2009;64(5):663–677. doi: 10.1016/j.neuron.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wairkar YP, Toda H, Mochizuki H, Furukubo-Tokunaga K, Tomoda T, Diantonio A. Unc-51 controls active zone density and protein composition by downregulating ERK signaling. The Journal of Neuroscience. 2009;29(2):517–528. doi: 10.1523/JNEUROSCI.3848-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pielage J, Bulat V, Zuchero JB, Fetter RD, Davis GW. Hts/adducin controls synaptic elaboration and Elimination. Neuron. 2011;69(6):1114–1131. doi: 10.1016/j.neuron.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Schluter OM, Schmitz F, Jahn R, Rosenmund C, Südhof TC. A complete genetic analysis of neuronal Rab3 function. The Journal of Neuroscience. 2004;24(29):6629–6637. doi: 10.1523/JNEUROSCI.1610-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Stankewich MC, Gwynn B, Ardito T, Ji L, Kim J, Robledo RF, Lux SE, Peters LL, Morrow JS. Targeted deletion of betaIII spectrin impairs synaptogenesis and generates ataxic and seizure phenotypes. Proceedings of the National Academy of Sciences. 2010;107(13):6022–6027. doi: 10.1073/pnas.1001522107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Missias AC, Mudd J, Cunningham JM, Steinbach JH, Merlie JP, Sanes JR. Deficient development and maintenance of postsynaptic specializations in mutant mice lacking an 'adult' acetylcholine receptor subunit. Development. 1997;124(24):5075–5086. doi: 10.1242/dev.124.24.5075. [DOI] [PubMed] [Google Scholar]
- 177.Grady RM, Zhou H, Cunningham JM, Henry MD, Campbell KP, Sanes JR. Maturation and maintenance of the neuromuscular synapse: genetic evidence for roles of the dystrophin-glycoprotein complex. Neuron. 2000;25(2):279–293. doi: 10.1016/s0896-6273(00)80894-6. [DOI] [PubMed] [Google Scholar]
- 178.Meriney SD, Wolowske B, Ezzati E, Grinnell AD. Low calcium-induced disruption of active zone structure and function at the frog neuromuscular junction. Synapse. 1996;24(1):1–11. doi: 10.1002/(SICI)1098-2396(199609)24:1<1::AID-SYN1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 179.Lambert EH, Elmqvist D. Quantal components of end-plate potentials in the myasthenic syndrome. Ann N Y Acad Sci. 1971;183:183–199. doi: 10.1111/j.1749-6632.1971.tb30750.x. [DOI] [PubMed] [Google Scholar]
- 180.Flink MT, Atchison WD. Ca2+ channels as targets of neurological disease: Lambert-Eaton Syndrome and other Ca2+ channelopathies. J Bioenerg Biomembr. 2003;35(6):697–718. doi: 10.1023/b:jobb.0000008033.02320.10. [DOI] [PubMed] [Google Scholar]
- 181.Takamori M, Komai K, Iwasa K. Antibodies to calcium channel and synaptotagmin in Lambert-Eaton myasthenic syndrome. Am J Med Sci. 2000;319(4):204–208. doi: 10.1097/00000441-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 182.Fukunaga H, Engel AG, Lang B, Newsom-Davis J, Vincent A. Passive transfer of Lambert-Eaton myasthenic syndrome with IgG from man to mouse depletes the presynaptic membrane active zones. Proceedings of the National Academy of Sciences of the United States of America. 1983;80(24):7636–7640. doi: 10.1073/pnas.80.24.7636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zenker M, Aigner T, Wendler O, Tralau T, Muntefering H, Fenski R, Pitz S, Schumacher V, Royer-Pokora B, Wuhl E, Cochat P, Bouvier R, Kraus C, Mark K, Madlon H, Dotsch J, Rascher W, Maruniak-Chudek I, Lennert T, Neumann LM, Reis A. Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Human molecular genetics. 2004;13(21):2625–2632. doi: 10.1093/hmg/ddh284. [DOI] [PubMed] [Google Scholar]
- 184.Maselli RA, Ng JJ, Anderson JA, Cagney O, Arredondo J, Williams C, Wessel HB, Abdel-Hamid H, Wollmann RL. Mutations in LAMB2 causing a severe form of synaptic congenital myasthenic syndrome. Journal of Medical Genetics. 2009;46(3):203–208. doi: 10.1136/jmg.2008.063693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sasaki S, Iwata M. Ultrastructural study of the synapses of central chromatolytic anterior horn cells in motor neuron disease. Journal of neuropathology and experimental neurology. 1996;55(8):932–939. doi: 10.1097/00005072-199608000-00009. [DOI] [PubMed] [Google Scholar]
- 186.Sullivan PF, de Geus EJC, Willemsen G, James MR, Smit JH, Zandbelt T, Arolt V, Baune BT, Blackwood D, Cichon S, Coventry WL, Domschke K, Farmer A, Fava M, Gordon SD, He Q, Heath AC, Heutink P, Holsboer F, Hoogendijk WJ, Hottenga JJ, Hu Y, Kohli M, Lin D, Lucae S, MacIntyre DJ, Maier W, McGhee KA, McGuffin P, Montgomery GW, Muir WJ, Nolen WA, Nothen MM, Perlis RH, Pirlo K, Posthuma D, Rietschel M, Rizzu P, Schosser A, Smit AB, Smoller JW, Tzeng JY, van Dyck R, Verhage M, Zitman FG, Martin NG, Wray NR, Boomsma DI, Penninx BWJH. Genome-wide association for major depressive disorder: a possible role for the presynaptic protein piccolo. Mol Psychiatry. 2008;14(4):359–375. doi: 10.1038/mp.2008.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Choi JK, Jeon YC, Lee DW, Oh JM, Lee HP, Jeong BH, Carp RI, Koh YH, Kim YS. A Drosophila model of GSS syndrome suggests defects in active zones are responsible for pathogenesis of GSS syndrome. Human molecular genetics. 2010;19(22):4474–4489. doi: 10.1093/hmg/ddq379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bennett MC. The role of alpha-synuclein in neurodegenerative diseases. Pharmacol Ther. 2005;105(3):311–331. doi: 10.1016/j.pharmthera.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 189.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. Alpha-synuclein locus triplication causes Parkinson's disease. Science. 2003;302(5646):841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 190.Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destee A. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004;364(9440):1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 191.Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, Lee MK, Chaudhry FA, Nicoll RA, Edwards RH. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. 2010;65(1):66–79. doi: 10.1016/j.neuron.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tao-Cheng JH. Activity-related redistribution of presynaptic proteins at the active zone. Neuroscience. 2006;141(3):1217–1224. doi: 10.1016/j.neuroscience.2006.04.061. [DOI] [PubMed] [Google Scholar]
- 193.Askanas V, Engel WK, Alvarez RB, McFerrin J, Broccolini A. Novel immunolocalization of alpha-synuclein in human muscle of inclusion-body myositis, regenerating and necrotic muscle fibers, and at neuromuscular junctions. Journal of neuropathology and experimental neurology. 2000;59(7):592–598. doi: 10.1093/jnen/59.7.592. [DOI] [PubMed] [Google Scholar]
- 194.Pelkonen A, Yavich L. Neuromuscular pathology in mice lacking alpha-synuclein. Neuroscience Letters. 2011;487(3):350–353. doi: 10.1016/j.neulet.2010.10.054. [DOI] [PubMed] [Google Scholar]
- 195.van der Putten H, Wiederhold KH, Probst A, Barbieri S, Mistl C, Danner S, Kauffmann S, Hofele K, Spooren WP, Ruegg MA, Lin S, Caroni P, Sommer B, Tolnay M, Bilbe G. Neuropathology in mice expressing human alpha-synuclein. The Journal of neuroscience. 2000;20(16):6021–6029. doi: 10.1523/JNEUROSCI.20-16-06021.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rieker C, Dev KK, Lehnhoff K, Barbieri S, Ksiazek I, Kauffmann S, Danner S, Schell H, Boden C, Ruegg MA, Kahle PJ, van der Putten H, Shimshek DR. Neuropathology in mice expressing mouse alpha-synuclein. PLoS ONE. 2011;6(9):e24834. doi: 10.1371/journal.pone.0024834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Jen J, Wan J, Graves M, Yu H, Mock AF, Coulin CJ, Kim G, Yue Q, Papazian DM, Baloh RW. Loss-of-function EA2 mutations are associated with impaired neuromuscular transmission. Neurology. 2001;57(10):1843–1848. doi: 10.1212/wnl.57.10.1843. [DOI] [PubMed] [Google Scholar]