A number of striking illusions show that visual motion influences perceived position [1]; in all of these, the perceived shift is accompanied or preceded by a visible and salient motion signal. Observers can easily scrutinize the motion: they can attentively track, or at least perceive via inference, the moving features [2–4]. With position shifts that accompany the motion aftereffect (MAE) [5–10], for example, observers can attentively track the moving adaptation stimulus [11, 12]. Even if the shifted test pattern does not display any perceived motion [6, 10], the moving adaptation stimulus is clearly visible, and it could be the visibility of the adaptation stimulus that causes the perceived shift in the test stimulus position. If awareness of motion, mediated by high-level or top-down mechanisms, explains all motion-induced position shifts, then there should be no shift in perceived position without the perception of directional motion. Here, we show that perceived position can be shifted even without awareness of motion.
To test whether the awareness of motion is necessary to shift perceived position, we used a crowding technique developed by He and colleagues [13]. Figure 1 shows the basic stimulus: an array of drifting Gabor patterns (moving sine wave gratings with static gaussian envelopes). The number and spacing was such that subjects were not aware of, or able to report, the direction of motion in any of the central Gabors (see Supplemental data available with this article online). Each Gabor contained either leftward or rightward motion, determined randomly on each trial. Two vertically aligned pairs of Gabors, however, had motion that was always in opposite directions (dashed and solid circled regions in Figure 1A), providing a consistent adaptation direction across trials. After each trial, the array of Gabors was removed, and a single pair of static test Gabors was presented (Figure 1B), located in either of the regions that were previously adapted to motion (either the solid or dashed circled region in Figure 1A).
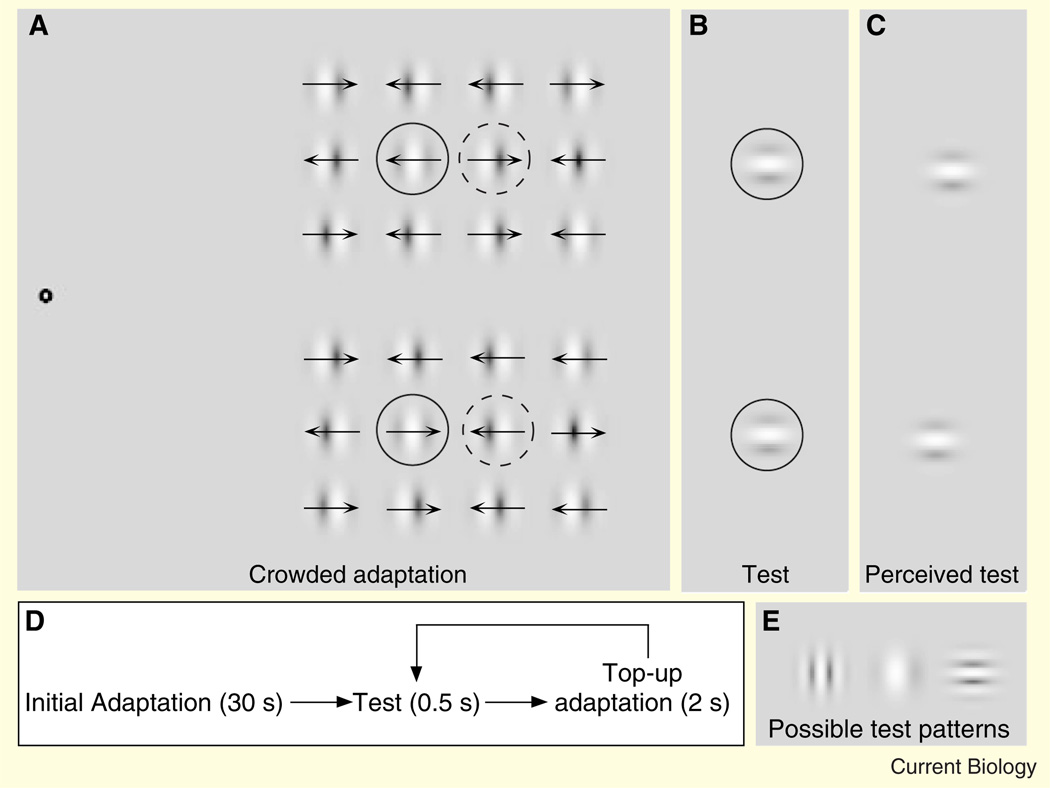
Figure 1. Stimuli used in the first experiment.
(A) An array of Gabor patches was presented for an adaptation period while subjects fixated on the bull’s-eye. Two pairs of vertically aligned Gabors in the central region of the array had opposing directions of motion, which was fixed throughout an experimental session (adapted regions are circled by dashed and solid lines, respectively; these circles are for illustration and were not presented in the actual stimulus). All other Gabors served as crowding stimuli and had randomly determined directions of motion (leftward or rightward) on each trial. (B) During the test period, two static Gabors were presented in one of the two vertically aligned, adapted regions. (C). After adapting to the stimulus in (A), the test Gabors in (B) appear to be misaligned in a direction opposite that of the prior motion adaptation. (D) Each session began with an initial adaptation period, followed by a repeat test and top-up adaptation periods. (E) Examples of several different kinds of test Gabors that could be presented during the test period.
Because of the crowding, subjects were unable to identify the direction of motion presented within any of the adapted regions (correct direction judgments were not significantly different from chance in the crowded region, provided this was greater than ~20 deg from fixation; see Figure S2 in Supplemental data). Although subjects were unaware of the direction of motion during adaptation, there was a significant shift in the perceived position of the test stimuli (Figure 2).
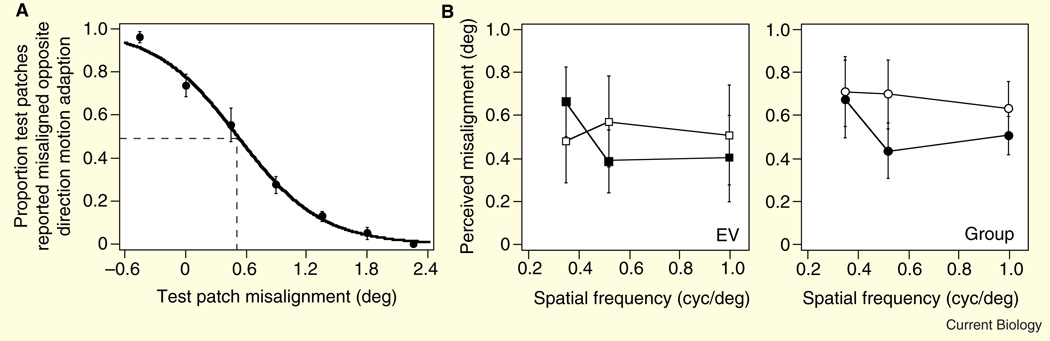
Figure 2. Results of the first experiment.
(A) A representative psychometric function showing a significant shift in the perceived position of the test Gabors as a function of the direction of motion adaptation. The abscissa shows the physical misalignment between the test Gabors: positive values indicate that the Gabors were misaligned in the same direction as the prior motion adaptation, and negative values indicate that they were misaligned in a direction opposite that of the motion adaptation. The ordinate shows the proportion of trials in which the subject perceived the test Gabors to be misaligned opposite the direction of the prior motion adaptation. The point of subjective equality (PSE, the inflexion point) defines the physical misalignment between the Gabors that appeared to be aligned. Because of the motion adaptation, the Gabors had to be presented ~0.6 deg in the same direction as the prior motion adaptation to appear aligned (t(7) = 6.06, P = 0.001). When physically aligned, the Gabors appeared shifted opposite the direction of the motion adaptation. (B) Results for one representative subject (left panel), as well as all four subjects averaged (right panel), as a function of the spatial frequency (abscissa) and orientation of the test Gabors. The orientation of the test Gabors was either the same as (open symbols) or orthogonal to (solid symbols) the orientation of the adaptation Gabors. There was a significant shift in the perceived position of the Gabors with both test orientations (same orientation: t(3) = 4.8, P < 0.01; orthogonal orientation: t(3) = 4.25, P < 0.02). Across all subjects, there was little variation in the illusory position shift as a function of spatial frequency (F(2,6) = 2.6, P = 0.15). There was a larger perceived shift when the test stimuli contained the same orientation as the adaptation stimuli, although this difference did not reach significance (for the group results in the right panel, t(3) = 2.75, P = 0.07). There was a significant overall position shift for each individual subject as well (least significant effect was for subject EV, t(5) = 5.96, P = 0.002; the least significant within-subject condition effect for subject EV was the orthogonally oriented test Gabor with a spatial frequency of 1 cyc/deg: t(30) = 3.3, P < 0.01). Error bars ± s.e.m.
The perceived shift in the position of the test Gabors was always in a direction opposite that of the motion adaptation and could be mediated by awareness of motion during the test period. To rule out the possibility that subjects attended to a passively generated motion aftereffect during the test period, we presented orthogonally oriented test Gabors. McGraw and colleagues [6] have shown that this manipulation effectively eliminates the perceived MAE in the test stimuli, but leaves intact the perceived position shift. Figure 2B (solid symbols) shows that there was still a perceived shift in the positions of the test Gabors, even with their orthogonal orientation (t(3) = 4.25, P < 0.02). The local nature of the motion adaptation, and the randomized directions of motion in the array of Gabors, suggest that an ensemble pattern [14] did not contribute to the results.
If subjects became aware of the direction of motion in the adaptation Gabors, this could have influenced judgments on subsequent trials. Two additional experiments were conducted to address this potential problem. In one, each pair of adaptation Gabors contained a randomly determined direction of motion on each trial (leftward or rightward, in contrast to the first experiment where the direction of motion in the adaptation Gabors was fixed across trials). So, the motion adaptation was only guaranteed to accumulate over a single 8 second trial. Although less than ideal for generating directional motion adaptation, this ensures that even if subjects become aware of the direction of motion adaptation on one trial, this knowledge or awareness is not informative and cannot bias judgments on subsequent trials. To avoid the saturation that occurs when both directions of motion are sequentially adapted, the fixation point was repositioned on each trial, ensuring that adaptation occurred at different retinal locations (see Supplemental data). Despite the brevity of the motion adaptation, there was still a significant shift in the perceived positions of the test Gabors (Figure S1 in the Supplemental data), and subjects were unable to identify the direction of motion in the adaptation Gabors (Figure S2).
A third experiment differed from the second only in that each of the crowding Gabors in the array had a randomly determined orientation spanning the entire 360 deg range. This eliminates (or at least dramatically reduces) the possibility that on any particular trial there might be a net difference in the direction of motion presented in the adaptation and crowding Gabors (which could serve as a perceptual grouping cue that might break crowding). Local motion adaptation still produced a significant shift in the perceived positions of the test Gabors (Figure S1).
The results here show that adaptation to motion, even without awareness of the adaptation or any motion aftereffect, can shift the perceived positions of stationary objects. Therefore, perceived position shifts do not entirely depend on high-level (top-down) mechanisms responsible for attentionally driven or inferred motion. Low-level motion does contribute to perceived position, consistent with mounting physiological evidence that the coded location of an object can be influenced by motion at very early stages of visual processing — even as early as the retina [15] and primary visual cortex [16, 17]. These low-level mechanisms do not preclude high-level processes, such as attentive tracking; indeed, the experiments reported here support the idea that there are both types of mechanism: a passive, bottom-up motion detector that influences coded location as well as another mechanism mediated by high-level processes associated with awareness of motion.
Supplementary Material
Footnotes
Supplemental data
Supplemental data containing additional references and experimental procedures are available at http://www.currentbiology.com/cgi/content/full/15/9/R324/DC1/
References
- 1.Whitney D. The influence of visual motion on perceived position. Trends Cogn. Sci. 2002;6:211–216. doi: 10.1016/s1364-6613(02)01887-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shim WM, Cavanagh P. The motion-induced position shift depends on the perceived direction of bistable quartet motion. Vision Res. 2004;44:2393–2401. doi: 10.1016/j.visres.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe K, Nijhawan R, Shimojo S. Shifts in perceived position of flashed stimuli by illusory object motion. Vision Res. 2002;42:2645–2650. doi: 10.1016/s0042-6989(02)00296-1. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe K, Sato TR, Shimojo S. Perceived shifts of flashed stimuli by visible and invisible object motion. Perception. 2003;32:545–559. doi: 10.1068/p5047. [DOI] [PubMed] [Google Scholar]
- 5.Fang F, He S. Strong influence of test patterns on the perception of motion aftereffect and position. J. Vis. 2004;4:637–642. doi: 10.1167/4.7.9. [DOI] [PubMed] [Google Scholar]
- 6.McGraw PV, Whitaker D, Skillen J, Chung ST. Motion adaptation distorts perceived visual position. Curr. Biol. 2002;12:2042–2047. doi: 10.1016/s0960-9822(02)01354-4. [DOI] [PubMed] [Google Scholar]
- 7.Nishida S, Johnston A. Influence of motion signals on the perceived position of spatial pattern. Nature. 1999;397:610–612. doi: 10.1038/17600. [DOI] [PubMed] [Google Scholar]
- 8.Snowden RJ. Shifts in perceived position following adaptation to visual motion. Curr. Biol. 1998;8:1343–1345. doi: 10.1016/s0960-9822(07)00567-2. [DOI] [PubMed] [Google Scholar]
- 9.Whitaker D, McGraw PV, Pearson S. Non-veridical size perception of expanding and contracting objects. Vision Res. 1999;39:2999–3009. doi: 10.1016/s0042-6989(99)00010-3. [DOI] [PubMed] [Google Scholar]
- 10.Whitney D, Cavanagh P. Motion adaptation shifts apparent position without the motion aftereffect. Percept. Psychophys. 2003;65:1011–1018. doi: 10.3758/bf03194830. [DOI] [PubMed] [Google Scholar]
- 11.Cavanagh P. Attention-based motion perception. Science. 1992;257:1563–1565. doi: 10.1126/science.1523411. [DOI] [PubMed] [Google Scholar]
- 12.Culham JC, Verstraten FA, Ashida H, Cavanagh P. Independent aftereffects of attention and motion. Neuron. 2000;28:607–615. doi: 10.1016/s0896-6273(00)00137-9. [DOI] [PubMed] [Google Scholar]
- 13.He S, Cavanagh P, Intriligator J. Attentional resolution and the locus of visual awareness. Nature. 1996;383:334–337. doi: 10.1038/383334a0. [DOI] [PubMed] [Google Scholar]
- 14.Parkes L, Lund J, Angelucci A, Solomon JA, Morgan M. Compulsory averaging of crowded orientation signals in human vision. Nat. Neurosci. 2001;4:739–744. doi: 10.1038/89532. [DOI] [PubMed] [Google Scholar]
- 15.Berry MJ, 2nd, Brivanlou IH, Jordan TA, Meister M. Anticipation of moving stimuli by the retina. Nature. 1999;398:334–338. doi: 10.1038/18678. [DOI] [PubMed] [Google Scholar]
- 16.Fu YX, Shen Y, Gao H, Dan Y. Asymmetry in visual cortical circuits underlying motion-induced perceptual mislocalization. J. Neurosci. 2004;24:2165–2171. doi: 10.1523/JNEUROSCI.5145-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitney D, Goltz HC, Thomas CG, Gati JS, Menon RS, Goodale MA. Flexible retinotopy: motion-dependent position coding in the visual cortex. Science. 2003;302:878–881. doi: 10.1126/science.1087839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.