Abstract
Objective
The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) reported two equations in 2012: one based on cystatin C concentration (CKD-EPI2012cys) and the other using both serum creatinine and cystatin C concentrations (CKD-EPI2012Scr-cys). We compared the adaptability of new formulae with other four equations.
Methods
Participants (n = 788; median age, 54 [range, 19–94] years) were recruited from the First Affiliated Hospital of Nanjing Medical University. The reference glomerular filtration rate (rGFR) was measured by a 99mTc-DTPA renal dynamic imaging method, and the estimated glomerular filtration rate (eGFR) was calculated separately by the Chinese adapted Modification of Diet in Renal Disease equation (C-MDRD), MacIsaac, Ma, serum creatinine-based CKD-EPI equation (CKD-EPI2009Scr), CKD-EPI2012cys and CKD-EPI2012Scr-cys equations. We compared the performance of six equations with rGFR.
Results
Median rGFR was 76.35 (interquartile range, 59.03–92.50) mL/min/1.73 m2. Compared with CKD-EPI2009Scr, CKD-EPI2012Scr-cys formula had better diagnostic value with larger area under the receiver operating characteristic curve (ROCAUC, 0.879, p = 0.006), especially in young participants (ROCAUC, 0.883, p = 0.005). CKD-EPI2012cys equation did not perform better than other available equations. Accuracy (the proportion of eGFR within 30% of rGFR [P30]) of the CKD-EPI2012Scr-cys equation (77.03%) was inferior only to MacIsaac equation (80.2%) in the entire participants, but performed best in young participants with normal or mildly-injured GFR. Neither of the two new CKD-EPI equations achieved any ideal P30 in the elderly participants with moderately-severely injured GFR. Linear regression analysis demonstrated a consistent result. In this study, CKD-EPI2012Scr-cys had a relatively better diagnosis consistency of GFR stage between the eGFR and rGFR in the whole cohort.
Conclusion
CKD-EPI2012Scr-cys appeared less biased and more accurate in overall participants. Neither of the new CKD-EPI equations achieved ideal accuracy in senior participants with moderately-severely injured GFR. A large-scale study with more subjects and cooperating centers to develop new formulae for the elderly is assumed to be necessary.
Introduction
Chronic kidney disease (CKD) has become a serious threat to human health worldwide [1]. Increasing prevalence of diabetes, hypertension, and obesity will result in an even greater burden of CKD in developing countries such as China [2]. In The Lancet, Zhang et al. presented the results of the first comprehensive study exploring the prevalence of CKD in China. The prevalence was 10.8% in 2012, equivalent to 119.5 million CKD patients [3]. Outcomes of CKD include not only progression to kidney failure but also a series of complications [4]. Early impaired kidney function often has no obvious symptoms, which leads to easily missed or delayed diagnosis. Therefore, accurate assessment of kidney function is essential, which needs not only the public awareness of termly medical examination, but also a simple method to assess the kidney function. Due to the invasiveness, inconveniency and high cost, measuring GFR by the clearance of some exogenous markers is unsuitable in routine clinical practice, although they are the gold-standard methods [5]. Under such circumstance, estimating equations of GFR have gained booming development.
Among a large number of variations, the Modification of Diet in Renal Disease (MDRD), serum creatinine-based Chronic Kidney Disease Epidemiology Collaboration (hereafter referred to as CKD-EPI2009Scr) and MacIsaac equations have been publicly approved and applied [6]. Ma et al. developed the Chinese adapted MDRD equation (hereafter referred to as C-MDRD), which was validated to be better than other MDRD equations in Chinese subjects [7]. However, in patients with near-normal kidney function, the MDRD equations underestimate GFR [8]. The CKD-EPI2009Scr equation partly overcomes the major limitation of the MDRD equation [9]. The MacIsaac equation is a typical cystatin C-based equation developed in 2006. The investigators challenged the traditional view that cystatin C level was independent of body composition. They proved that accounting for body composition improved cystatin C-based GFR estimation [10]. Some researchers shifted their focus to equations based on combination of different markers, for example, the combination of serum creatinine and cystatin C [11]. Ma equation is a representative, which is also based on the data from the Chinese population. They found the equation performed better than the C-MDRD equation, especially in early detection of CKD [12]. Our previous work evaluated the performance of existing equations, showing that they have their own applicability in different CKD stages and age groups [13].
Recently, the CKD-EPI working group has reported two new CKD-EPI equations: one using cystatin C concentration (CKD-EPI2012cys) and the other using both cystatin C and serum creatinine concentrations (CKD-EPI2012Scr-cys). They validate the new equations represent an advance over currently available equations across the range of GFR and in relevant subgroups. The advance even holds true among participants with an extreme body-mass index (the weight in kilograms divided by the square of the height in meters) of less than twenty [14]. The two new equations even have been recommended by KDIGO 2012Clinical Practice Guidelines for the Evaluation and Management of CKD [15]. A series of research for validation of the new formulae has appeared. Mindikoglu et al. evaluated the performance of CKD-EPI2012Scr-cys equation in subjects with cirrhosis, claiming that it was superior to conventional equations for estimating GFR; however, the diagnostic performance was not as good as reported in non-cirrhotic subjects [16]. Obiols et al. found CKD-EPI2012Scr-cys equation was more accurate and precise in hypertensive patients with higher GFR [17]. Kilbride et al. tested the accuracy of the new equations in old people in London [18]. Few data were available in China about the comparison of new equations with other traditional formulae. We compared the adaptability of new formulae with other four equations.
Materials and Methods
Participants
Totally 788 Chinese participants older than 18 years, with or without CKD at the First Affiliated Hospital of Nanjing Medical University between December 2009 and March 2012, were consecutively enrolled in the study. All participants in this study signed the informed consent. The ethics committee of Nanjing Medical University approved the study.
The participants with severe heart failure, acute renal failure, pleural or abdominal effusion, serious edema or malnutrition, skeletal muscle atrophy, amputation, ketoacidosis were excluded. Patients who were taking trimethoprim or cimetidine or ACEI/ARB and those who had recently received glucocorticoid and hemodialysis therapy were also excluded.
Measurement and Estimation of GFR
Patients were informed in advance to avoid any meat consumption on the day of the test. Demographic data and past history were recorded and blood pressure, weight, and height were documented. Serum creatinine (Scr) concentration was assayed by the enzymatic method (Shanghai Kehua Dongling Diagnostic Products Co., Ltd, China), traceable to National Institute of Standards and Technology creatinine standard reference material (SRM 967).Cystatin C concentration was examined by the particle-enhanced immunoturbidimetry method (Beijing Leadman Biomedical Co., Ltd, China),which was calibrated against the international certified reference material ERM-DA471. Both fasting serum samples were assayed on an Olympus AU5400 autoanalyser (Olympus Co., Japan), in strict accordance with the manufacturer's instructions.
All participants had a 99mTc-DTPA renal dynamic imaging measurement as the reference glomerular filtration rate (rGFR), who had been required to have no special change in diet. After measuring height and weight, drinking300 ml water, and emptying the bladder, participants received a bolus injection in the elbow vein of 185 MBq 99mTc-DTPA (purity 95%–99%, Nanjing Senke Co., Ltd, China). The 99mTc-DTPA renal dynamic imaging measurement was carried out and after images acquisition, rGFR was automatically calculated with a computer by the Gates method.
Estimated glomerular filtration rate (eGFR) was calculated separately from six GFR estimating equations including the C-MDRD, MacIsaac equation, Ma equation, CKD-EPI2009Scr, CKD-EPI2012cys and CKD-EPI2012Scr-cys equations. The results are presented in detail in Table 1.
Table 1. Equations to predict glomerular filtration rate.
| Name | Year | Gender | Scr | Scys | Equation |
| C-MDRD | 2006 | 175×Scr−1.234×age−0.179(×0.79,if female) | |||
| MacIsaac | 2006 | (86.7/Scys)-4.2 | |||
| Ma | 2007 | 169×Scr−0.608×Scys−0.63×age−0.157(×0.83,if female) | |||
| CKD-EPI2009Scr | 2009 | female | ≤0.7 | 144× (Scr/0.7)−0.329×0.993age(×1.159,if black) | |
| >0.7 | 144× (Scr/0.7)−1.209×0.993age(×1.159,if black) | ||||
| male | ≤0.9 | 141× (Scr/0.9)−0.411×0.993age(×1.159,if black) | |||
| >0.9 | 141× (Scr/0.9)−1.209×0.993age(×1.159,if black) | ||||
| CKD-EPI2012cys | 2012 | female | ≤0.8 | 133× (Scys/0.8)−0.499×0.996age×0.932 | |
| >0.8 | 133× (Scys/0.8)−1.328×0.996age×0.932 | ||||
| male | ≤0.8 | 133× (Scys/0.8)−0.499×0.996age | |||
| >0.8 | 133× (Scys/0.8)−1.328×0.996age | ||||
| CKD-EPI2012Scr-cys | 2012 | female | ≤0.7 | ≤0.8 | 130× (Scr/0.7)−0.248× (Scys/0.8)−0.375×0.995age(×1.08,if black) |
| >0.8 | 130× (Scr/0.7)−0.248× (Scys/0.8)−0.711×0.995age(×1.08,if black) | ||||
| >0.7 | ≤0.8 | 130× (Scr/0.7)−0.601× (Scys/0.8)−0.375×0.995age(×1.08,if black) | |||
| >0.8 | 130× (Scr/0.7)−0.601× (Scys/0.8)−0.711×0.995age(×1.08,if black) | ||||
| male | ≤0.9 | ≤0.8 | 135× (Scr/0.9)−0.207× (Scys/0.8)−0.375×0.995age(×1.08,if black) | ||
| >0.8 | 135× (Scr/0.9)−0.207× (Scys/0.8)−0.711×0.995age(×1.08,if black) | ||||
| >0.9 | ≤0.8 | 135× (Scr/0.9)−0.601× (Scys/0.8)−0.375×0.995age(×1.08,if black) | |||
| >0.8 | 135× (Scr/0.9)−0.601× (Scys/0.8)−0.711×0.995age(×1.08,if black) |
Note: Scr was shown as mg/dL; Scys was shown as mg/L; age was shown as years.
Abbreviations: Scr: serum creatinine; Scys: serum cystatin C; C-MDRD: the Chinese modified Modification of Diet in Renal Disease equation; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration; CKD-EPI2009Scr: serum creatinine–based CKD-EPI equation which was developed in 2009; CKD-EPI2012cys: cystatin C–based CKD-EPI equation which was newly developed in 2012; CKD-EPI2012Scr-cys: serum creatinine– and cystatin C–based CKD-EPI equation which was newly developed in 2012.
Statistical Analysis
No data sets were normally distributed (P<0.001, Kolmogorov-Smirnov test); thus, nonparametric statistics were used throughout. The receiver operating characteristic (ROC) curve was depicted to analyse the diagnostic value of 6 equations. Abscissa of the curve is the value of (1- specificity) and the vertical is sensitivity. The larger area under the ROC curve (ROCAUC) usually means a better diagnostic value. Bias, precision and accuracy were used to evaluate the performance of each equation. Bias was defined as the median results of differences between eGFR and rGFR (eGFR-rGFR). The interquartile range (IQR) of the differences was a marker of precision. Accuracy was calculated as the proportion of eGFR within 30% of rGFR (P30) and also as root mean square error (RMSE). Wilcoxon matched-pairs signed rank test was used to compare the bias of each eGFR against rGFR. McNemar test was used to compare P30 values of the C-MDRD, MacIsaac, Ma, CKD-EPI2012cys and CKD-EPI2012Scr-cys equations against the P30 value of the CKD-EPI2009Scr equation.
Bias plots were used to compare eGFR with rGFR intuitively. The difference between eGFR and rGFR was regressed against the mean of rGFR and eGFR. The greater slope of regression line against the x-axis means the larger bias. The larger intercept of the regression line against the y-axis indicates poorer accuracy. Kappa test was used to compare the diagnosis consistency of GFR stage between the eGFR and mGFR: kappa value 0.21–0.40 is considered mild agreement, 0.41–0.60 moderate agreement, 0.61–0.80 substantial agreement and 0.81–1.00 near-perfect agreement. Data was considered statistically significant at p<0.05. All statistical analyses were performed using SPSS software (version 17.0; SPSS, Chicago, IL, USA), Epical software (version 1.01; EpiCalc 2000 Application, Brixton Books, USA) and Medcalc for Windows (version 11.6.1.0; Medcalc Software, Mariekerke, Belgium).
Results
Participant characteristics
A total of 788 participants (478 male and 310 female), with or without CKD were enrolled. Median rGFR, cystatin C and Scr were 76.35 (interquartile range, 59.03–92.50) mL/min/1.73 m2, 1.09 (interquartile range, 0.92–1.43) mg/L and 0.94(interquartile range, 0.74–1.22) mg/dL, respectively. Participants were divided into two groups with rGFR<60 and ≥60 mL/min/1.73 m2 and also divided into two groups with age <60 and ≥60 years old. The detailed laboratory and anthropometric measurements are shown in Table 2.
Table 2. Characteristics of the Study Population.
| All subjects | Age<60 | Age≥60 | |
| Age(years) | 54(41–65) | 45(34–53) | 69(64–75) |
| Gender | |||
| Male | 478(60.70) | 298(59.60) | 180(62.5) |
| Female | 310(39.30) | 202(40.40) | 108(37.5) |
| Weight(kg) | 65(55–69) | 65(55–68) | 65(56–70) |
| Height(m) | 1.69(1.60–1.70) | 1.70(1.60–1.70) | 1.68(1.60–1.70) |
| BSA(m2) | 1.74(1.57–1.79) | 1.73(1.56–1.78) | 1.75(1.60–1.79) |
| BMI(kg/m2) | 22.49(21.48–24.49) | 22.49(21.48–24.22) | 22.49(21.48–24.89) |
| BUN(mmol/L) | 5.75(4.52–7.54) | 5.33(4.17–6.78) | 6.74(5.29–9.22) |
| Scr(mg/L) | 0.94(0.74–1.22) | 0.85(0.68–1.06) | 1.09(0.88–1.51) |
| Scys(mg/L) | 1.09(0.92–1.43) | 0.98(0.84–1.20) | 1.34(1.10–1.93) |
| rGFR(mL/min/1.73 m2) | 76.35(59.03–92.50) | 85.35(70.83–100.45) | 62.85(46.35–74.85) |
| GFR category | |||
| ≥60 mL/min/1.73 m2 | 584(74.11) | 425(85.00) | 159(55.21) |
| <60 mL/min/1.73 m2 | 204(28.59) | 75(15.00) | 129(44.79) |
| eGFR(mL/min/1.73 m2) | |||
| C-MDRD | 87.27(62.91–111.25) | 99.39(75.78–123.62) | 66.35(45.24–85.99) |
| MacIsaac | 73.99(54.88–89.50) | 82.58(66.33–96.75) | 58.30(40.50–73.80) |
| Ma | 84.82(58.92–107.25) | 96.61(76.16–116.41) | 63.31(41.15–80.31) |
| CKD-EPI2009Scr | 83.57(58.90–102.16) | 95.82(76.18–110.28) | 61.22(41.19–78.68) |
| CKD-EPI2012cys | 67.75(45.62–90.63) | 81.71(61.19–99.22) | 47.57(30.45–64.65) |
| CKD-EPI2012Scr-cys | 75.80(51.41–94.67) | 88.00(69.42–103.28) | 54.06(34.45–70.38) |
| Comorbid conditions | |||
| Nephritis | 46(5.84) | 28(5.60) | 18(6.25) |
| Kidney neoplasm | 221(28.04) | 132(26.40) | 89(30.90) |
| Hematological disease | 111(14.09) | 101(20.20) | 10(3.47) |
| Hypertension | 162(20.56) | 70(14.00) | 92(31.94) |
| Coronary heart disease | 35(4.44) | 7(1.40) | 28(9.72) |
| Diabetic mellitus | 84(10.66) | 26(5.20) | 58(20.14) |
Note: Values for continuous variables expressed as median (inter-quartile range); values for categorical values expressed as number (percentage). Conversion factors for units: serum creatinine in mg/dL to µmol/L, ×88.4.
Abbreviations: BSA: body surface aera; BMI: body mass index; Scr: serum creatinine; Scys: serum cystatin C; rGFR: reference glomerular filtration rate (using the 99mTc-DTPA renal dynamic imaging method); eGFR: estimated glomerular filtration rate; C-MDRD: the Chinese modified Modification of Diet in Renal Disease equation; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration; CKD-EPI2009Scr: serum creatinine–based CKD-EPI equation which was developed in 2009; CKD-EPI2012cys: cystatin C–based CKD-EPI equation which was newly developed in 2012; CKD-EPI2012Scr-cys: serum creatinine– and cystatin C–based CKD-EPI equation which was newly developed in 2012.
Performance of six equations in all participants
Compared with CKD-EPI2009Scr, CKD-EPI2012Scr-cys equation had a larger ROCAUC (0.879 vs. 0.845) and higher sensitivity (88.7% vs.77.0%) but lower specificity (87.2% vs.92.1%) to diagnose CKD. CKD-EPI2012cys equation did not perform much better than other available equations, except for its sensitivity (Table 3).
Table 3. Diagnostic value of six estimating equations compared with rGFR.
| All subjects | R | ROCAUC | sensitivity | specificity |
| C-MDRD | 0.795(p1 = 0.9) | 0.853(p2 = 0.4) | 75.5 | 95.0 |
| MacIsaac | 0.789(p1 = 0.6) | 0.866(p2 = 0.1) | 84.8 | 88.4 |
| Ma | 0.828(p1 = 0.08) | 0.860(p2 = 0.1) | 78.9 | 93.0 |
| CKD-EPI2009Scr | 0.798 | 0.845 | 77.0 | 92.1 |
| CKD-EPI2012cys | 0.802(p1 = 0.8) | 0.852(p2 = 0.7) | 92.2 | 78.3 |
| CKD-EPI2012Scr-cys | 0.829(p1 = 0.07) | 0.879(p2 = 0.006) | 88.7 | 87.2 |
Note: R = coefficient of relationship between eGFR and rGFR; ROCAUC = aera under receiver operating characteristic curve.
Abbreviations: C-MDRD: the Chinese modified Modification of Diet in Renal Disease equation; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration; CKD-EPI2009Scr: serum creatinine–based CKD-EPI equation which was developed in 2009; CKD-EPI2012cys: cystatin C–based CKD-EPI equation which was newly developed in 2012; CKD-EPI2012Scr-cys: serum creatinine– and cystatin C–based CKD-EPI equation which was newly developed in 2012.
Correlation coefficients of the C-MDRD equation, MacIsaac equation, Ma equation, CKD-EPI2012cys equation and CKD-EPI2012Scr-cys equation were compared against that of CKD-EPI2009Scr equation (p1).
ROCAUC of the C-MDRD equation, MacIsaac equation, Ma equation, CKD-EPI2012cys equation and CKD-EPI2012Scr-cys equation were compared against that of CKD-EPI2009Scr equation (p2).
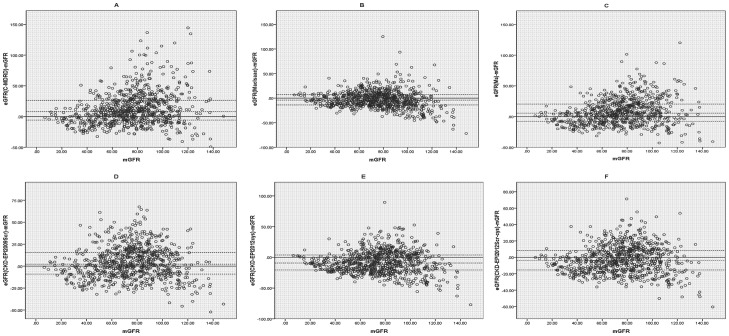
Performance of the equations is summarized in Table 4, and bias plots of the 6 equations against rGFR are shown in Figure 1. Both CKD-EPI2012Scr-cys and CKD-EPI2012cys underestimated GFR [(bias, −4.11; 95%CI, −5.17to−2.29 mL/min/1.73 m2; P<0.001) and (bias, −9.23; 95%CI, −10.60 to−7.40 mL/min/1.73 m2; P<0.001,) respectively] in the whole cohort. CKD-EPI2009Scr, Ma and C-MDRD equations overestimated GFR. Of all the 6 equations, CKD-EPI2009Scr possessed the smallest difference (bias, 2.21; 95%CI, 0.73to4.58 mL/min/1.73 m2; P<0.001). MacIsaac, CKD-EPI2012Scr-cys and CKD-EPI2009Scr equation appeared to be more accurate with higher P30 value (80.20%, 77.03% and 75.76%, respectively).CKD-EPI2012cys equation performed not as well as previously expected (P30 value, 68.40%). Concurrently, CKD-EPI2012Scr-cys equation had the lowest RMSE and relative lower IQR.
Table 4. Performance of six estimating equations compared with rGFR.
| All subjects | Bias (median difference) (95%CI) | Precision (IQR of the difference) | Accuracy P30 (95%CI) | Accuracy (RMSE) |
| C-MDRD | 8.17(6.59,10.01)(p3<0.001) | 32.11 | 64.21(60.74,67.55)(p4<0.001) | 29.74 |
| MacIsaac | −4.08(−5.58, −2.77)(p3<0.001) | 20.88 | 80.20(77.21,82.90) (p4 = 0.02) | 18.40 |
| Ma | 5.52(3.51,6.88)(p3<0.001) | 28.01 | 71.19(67.87,74.31) (p4 = 0.003) | 22.81 |
| CKD-EPI2009Scr | 2.21(0.73,4.58)(p3<0.001) | 24.49 | 75.76(72.58,78.68) | 18.94 |
| CKD-EPI2012cys | −9.23(−10.60, −7.40)(p3<0.001) | 24.39 | 68.40(65.01,71.61)(p4<0.001) | 20.10 |
| CKD-EPI2012Scr-cys | −4.11(−5.17, −2.29)(p3<0.001) | 23.84 | 77.03(73.90,79.89)(p4 = 0.5) | 17.29 |
Note: Bias = median difference between eGFR and rGFR; P30 = the proportion of eGFR within 30% of rGFR; RMSE = root mean square error.
Abbreviations: rGFR: reference glomerular filtration rate; CI: confidence interval; IQR = the inter-quartile range of difference; C-MDRD: the Chinese modified Modification of Diet in Renal Disease equation; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration; CKD-EPI2009Scr: serum creatinine–based CKD-EPI equation which was developed in 2009; CKD-EPI2012cys: cystatin C–based CKD-EPI equation which was newly developed in 2012; CKD-EPI2012Scr-cys: serum creatinine– and cystatin C–based CKD-EPI equation which was newly developed in 2012.
Wilcoxon matched-pairs signed rank test was used to compare the difference between the eGFR and rGFR (p3); McNemar test was used to compare the P30 of the C-MDRD equation, MacIsaac equation, Ma equation, CKD-EPI2012cys equation and CKD-EPI2012Scr-cys equation against the P30 of CKD-EPI2009Scr equation (p4).
Figure 1. Bias plots intuitively compare estimated glomerular filtration rate (eGFR) with reference glomerular filtration rate (rGFR).
The difference between eGFR and rGFR was regressed against the mean of rGFR and eGFR. The eGFRs were calculated separately from six estimating equations. (A) C-MDRD: the Chinese modified Modification of Diet in Renal Disease equation; (B) MacIsaac equation; (C) Ma equation; (D) CKD-EPI2009Scr: serum creatinine–based CKD-EPI equation which was developed in 2009; (E) CKD-EPI2012cys: cystatin C–based CKD-EPI equation which was newly developed in 2012; (F) CKD-EPI2012Scr-cys: serum creatinine– and cystatin C–based CKD-EPI equation which was newly developed in 2012. GFR were measured in mL/min/1.73 m2. Horizontal solid lines represent zero bias. Horizontal dashed lines represent 25th percentiles bias, median bias and 75th percentiles of the bias.
Linear regression analysis also demonstrated a consistent result (Table 5). The MacIsaac, CKD-EPI2012Scr-cys and CKD-EPI2009Scr equations showed better correlation, with lower slope and smaller intercept.
Table 5. Regression analysis of the difference between eGFR and rGFR against the average of eGFR and rGFR.
| All subjects | Slope of regression line with the X-axias(95% CI) | Intercept of regression line with the Y-axis(95%CI) |
| C-MDRD | 0.53(0.49,0.58)(p5<0.001) | −30.91(−35.06, −26.76)(p6<0.001) |
| MacIsaac | 0.13(0.08,0.18)(p5 = 0.001) | −12.73(−16.61, −8.85) (p6 = 0.4) |
| Ma | 0.39(0.35,0.44)(p5<0.001) | −23.28(−26.91, −19.66)(p6 = 0.002) |
| CKD-EPI2009Scr | 0.25(0.20,0.29) | −15.07(−18.77, −11.38) |
| CKD-EPI2012cys | 0.21(0.16,0.26)(p5 = 0.3) | −22.97(−26.55, −19.40)(p6 = 0.003) |
| CKD-EPI2012Scr-cys | 0.22(0.17,0.26)(p5 = 0.4) | −18.73(−22.05, −15.41)(p6 = 0.1) |
Note: The slope of the regression line against the X axis stands for the bias for eGFR; the trend of accuracy for eGFR was expressed as the intercept of the regression line against the Y-axis. The difference between eGFR and rGFR was regressed against the average of eGFR and rGFR. X-axis represented the average of eGFR and rGFR. Y-axis represented the difference between eGFR and rGFR.
Abbreviations: eGFR: estimated glomerular filtration rate; rGFR: reference glomerular filtration rate; CI: confidence interval; C-MDRD: the Chinese modified Modification of Diet in Renal Disease equation; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration; CKD-EPI2009Scr: serum creatinine–based CKD-EPI equation which was developed in 2009; CKD-EPI2012cys: cystatin C–based CKD-EPI equation which was newly developed in 2012; CKD-EPI2012Scr-cys: serum creatinine– and cystatin C–based CKD-EPI equation which was newly developed in 2012.
ANCOVA test was used to compare the slopes (p5) and intercepts (p6) of the regression line of the C-MDRD equation, MacIsaac equation, Ma equation, CKD-EPI2012cys equation and CKD-EPI2012Scr-cys equation against the slope and intercept of CKD-EPI2009Scr equation.
In the comparison of the diagnosis consistency of GFR stage between the eGFR and mGFR, no equation achieved substantial agreement in this study. The CKD-EPI2012Scr-cys had a relatively better diagnosis consistency with a kappa value of 0.513.However, CKD-EPI2012cys equation did not perform well in the whole cohort (Table 6).
Table 6. Comparison of the diagnosis consistency of GFR stage between the eGFR and rGFR.
| rGFR≥90 | rGFR 60–89 | rGFR<60 | sum | Kappa | |
| All subjects | |||||
| C-MDRD | |||||
| eGFR≥90 | 197(87.9) | 169(46.9) | 7(3.4) | 373 | |
| eGFR60–89 | 27(12.1) | 162(45.0) | 43(21.1) | 232 | 0.480 |
| eGFR<60 | 0 | 29(8.1) | 154(75.5) | 183 | |
| MacIsaac | |||||
| eGFR≥90 | 131(58.5) | 63(17.5) | 0 | 194 | |
| eGFR60–89 | 90(40.2) | 232(64.4) | 31(15.2) | 353 | 0.505 |
| eGFR<60 | 3(1.3) | 65(18.1) | 173(84.8) | 241 | |
| Ma | |||||
| eGFR≥90 | 192(85.7) | 151(41.9) | 3(1.5) | 346 | |
| eGFR60–89 | 32(14.3) | 168(46.7) | 40(19.6) | 240 | 0.494 |
| eGFR<60 | 0 | 41(11.4) | 161(78.9) | 202 | |
| CKD-EPI2009Scr | |||||
| eGFR≥90 | 188(83.90) | 143(39.7) | 6(2.9) | 337 | |
| eGFR60–89 | 36(16.1) | 171(47.5) | 41(20.1) | 248 | 0.483 |
| eGFR<60 | 0 | 46(12.8) | 157(77.0) | 203 | |
| CKD-EPI2012cys | |||||
| eGFR≥90 | 135(60.3) | 66(18.3) | 0 | 201 | |
| eGFR60–89 | 79(35.3) | 177(49.2) | 16(7.8) | 272 | 0.451 |
| eGFR<60 | 10(4.5) | 117(32.5) | 188(92.2) | 315 | |
| CKD-EPI2012Scr-cys | |||||
| eGFR≥90 | 157(70.1) | 91(25.3) | 0 | 248 | |
| eGFR60–89 | 65(29.0) | 196(54.4) | 23(11.3) | 284 | 0.513 |
| eGFR<60 | 2(0.9) | 73(20.3) | 181(88.7) | 256 | |
| Sum | 224 | 360 | 204 | 788 | |
| Age<60 y | |||||
| C-MDRD | |||||
| eGFR≥90 | 180(88.2) | 125(58.6) | 4(5.3) | 309 | |
| eGFR60–89 | 24(11.8) | 83(37.6) | 16(21.3) | 123 | 0.412 |
| eGFR<60 | 0 | 13(5.9) | 55(73.3) | 68 | |
| MacIsaac | |||||
| eGFR≥90 | 126(61.8) | 50(22.6) | 0 | 176 | |
| eGFR60–89 | 75(36.8) | 147(66.5) | 16(21.3) | 238 | 0.458 |
| eGFR<60 | 3(1.5) | 24(10.9) | 59(78.7) | 86 | |
| Ma | |||||
| eGFR≥90 | 178(87.3) | 120(54.3) | 1(1.3) | 299 | |
| eGFR60–89 | 26(12.7) | 88(39.8) | 19(25.3) | 133 | 0.421 |
| eGFR<60 | 0 | 13(5.9) | 55(73.3) | 68 | |
| CKD-EPI2009Scr | |||||
| eGFR≥90 | 176(86.3) | 122(55.2) | 4(5.3) | 302 | |
| eGFR60–89 | 28(13.7) | 84(38.0) | 19(25.3) | 131 | 0.391 |
| eGFR<60 | 0 | 15(6.8) | 52(69.3) | 67 | |
| CKD-EPI2012cys | |||||
| eGFR≥90 | 132(64.7) | 60(27.1) | 0 | 192 | |
| eGFR60–89 | 65(31.9) | 119(53.8) | 10(13.3) | 194 | 0.423 |
| eGFR<60 | 7(3.4) | 42(19.0) | 65(86.7) | 114 | |
| CKD-EPI2012Scr-cys | |||||
| eGFR≥90 | 153(75.0) | 79(35.7) | 0 | 232 | |
| eGFR60–89 | 49(24.0) | 124(56.1) | 14(18.7) | 187 | 0.478 |
| eGFR<60 | 2(1.0) | 18(8.1) | 61(81.3) | 81 | |
| Sum | 204 | 221 | 75 | 500 | |
| Age≥60 y | |||||
| C-MDRD | |||||
| eGFR≥90 | 17(85.0) | 44(31.7) | 3(2.3) | 64 | |
| eGFR60–89 | 3(15.0) | 79(56.8) | 27(20.9) | 109 | 0.482 |
| eGFR<60 | 0 | 16(11.5) | 99(76.7) | 115 | |
| MacIsaac | |||||
| eGFR≥90 | 5(25.0) | 13(9.4) | 0 | 18 | |
| eGFR60–89 | 15(75.0) | 85(61.2) | 15(11.6) | 115 | 0.481 |
| eGFR<60 | 0 | 41(29.5) | 114(88.4) | 155 | |
| Ma | |||||
| eGFR≥90 | 14(70.0) | 31(22.3) | 2(1.6) | 47 | |
| eGFR60–89 | 6(30.0) | 80(57.6) | 21(16.3) | 107 | 0.492 |
| eGFR<60 | 0 | 28(20.1) | 106(82.2) | 134 | |
| CKD-EPI2009Scr | |||||
| eGFR≥90 | 12(60.0) | 21(15.1) | 2(1.6) | 35 | |
| eGFR60–89 | 8(40.0) | 87(62.6) | 22(17.1) | 117 | 0.501 |
| eGFR<60 | 0 | 31(22.3) | 105(81.4) | 136 | |
| CKD-EPI2012cys | |||||
| eGFR≥90 | 3(15.0) | 6(4.3) | 0 | 9 | |
| eGFR60–89 | 14(70.0) | 58(41.7) | 6(4.7) | 78 | 0.349 |
| eGFR<60 | 3(15.0) | 75(54.0) | 123(95.3) | 201 | |
| CKD-EPI2012Scr-cys | |||||
| eGFR≥90 | 4(20.0) | 12(8.6) | 0 | 16 | |
| eGFR60–89 | 16(80.0) | 72(51.8) | 9(7.0) | 97 | 0.431 |
| eGFR<60 | 0 | 55(39.6) | 120(93.0) | 175 | |
| Sum | 20 | 139 | 129 | 288 |
Note: eGFR and rGFR were given in mL/min/1.73 m2; bold font cells represent agreement; data were expressed as n (percentage).
Abbreviations: rGFR: reference glomerular filtration rate; eGFR: estimated glomerular filtration rate; C-MDRD: the Chinese modified Modification of Diet in Renal Disease equation; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration; CKD-EPI2009Scr: serum creatinine–based CKD-EPI equation which was developed in 2009; CKD-EPI2012cys: cystatin C–based CKD-EPI equation which was newly developed in 2012; CKD-EPI2012Scr-cys: serum creatinine– and cystatin C–based CKD-EPI equation which was newly developed in 2012.
Performance of six equations in subgroups
In young participants, CKD-EPI2012Scr-cys equation had a larger ROCAUC thanCKD-EPI2009Scr equation (0.883 vs. 0.829, p = 0.005) to diagnose CKD. CKD-EPI2012cys equation did not perform much better than other available equations, especially in old participants. In subgroups with rGFR≥60 mL/min/1.73 m2 or the group with age<60 years old, CKD-EPI2009Scr, Ma and C-MDRD equations continued to overestimate GFR and CKD-EPI2012Scr-cys equation was unbiased whereas all equations underestimated GFR when rGFR<60 mL/min/1.73 m2.Accuracy of the CKD-EPI2012Scr-cys equation was superior (higher P30) to that of the CKD-EPI2009Scr and MacIsaac equations at rGFR≥60 mL/min/1.73 m2 or age<60 years old. Neither of the two new CKD-EPI equation achieved an ideal P30 value under the condition of rGFR<60 mL/min/1.73 m2 or age ≥60 years old (Table 4).
Linear regression analysis demonstrated a similar result that MacIsaac, CKD-EPI2012Scr-cys and CKD-EPI2009Scr formulae performed better than other 3 equations in young participants with normal or mildly- injured GFR.
As for the diagnosis consistency, neither of the two new equations performed well in old participants and the CKD-EPI2012cys equation in particular. The CKD-EPI2009Scr equation had a comparatively better diagnosis consistency with a kappa value of 0.501under the condition of age ≥60 years old (Table 6).
Discussion
This study compared the adaptability of new formulae with other four equations in788 participants. The principal finding of the present study was that CKD-EPI2012Scr-cys formula had better diagnostic value and accuracy in the entire participants, particularly in young participants with normally or mildly- injured GFR. CKD-EPI2012cys equation did not perform much better than other available equations. Concurrently, CKD-EPI2012Scr-cys had a better diagnosis consistency of GFR stage between the eGFR and rGFR, especially in young participants. An important issue has to be explained. In the present study, the unbalanced subgroups (the number of participants in rGFR ≥60 ml/min/1.73 m2 and the number of participants in age <60 years old were much greater than the other two subgroups) might result in selective bias.
There were some other findings in this study. The MacIsaac equation, another well-behaved formula, also possessed a good diagnostic value and an impressive accuracy, even in old participants with moderately-severely injured GFR. Meanwhile, it had a fine diagnosis consistency of GFR stage between the eGFR and rGFR. It is a typical cystatin C-based equation.
It is well-known that serum creatinine is a classic kidney function indicator; however, it is easily influenced by many factors, such as body mass, dietary intake, aging, and analytic problems with assay methods [19]. Consequently the serum creatinine-based equations have these inherent limitations. Cystatin C is an endogenous 13 kDa protein that is freely filtered at the glomerulus, and then almost completely reabsorbed and catabolized by proximal tubular epithelial cells with only small amounts excreted in the urine. Cystatin C generation was felt to be constant, which resulted in cystatin C-based equations having a tendency to replace creatinine-based equations for a time [20]–[25]. However, previous studies have found non-GFR determinants of cystatin C, including non-renal elimination, differences in generation among individuals, relation to such factors as inflammation, steroid use and thyroid disease [26]–[28]. Thus, we cannot simply say cystatin C and cystatin C-based equations are better than serum creatinine and its formulae. Recently, Rule et al. reported serum creatinine-based equations are better than cystatin C-based ones for evaluating risk factors associated with CKD [29]. Therefore, there is no perfect formula and clinicians should choose an appropriate one depending on different study objectives.
The CKD-EPI2012Scr-cysequation, using the combination of serum creatinine and cystatin C, provides more precise GFR estimates. The CKD-EPI working group explained that errors due to the non-GFR determinants of serum creatinine and cystatin C are independent and smaller in an equation that uses both markers than in an equation that uses only one marker. They also claimed the addition of race as a variable had improved the performance of the CKD-EPI2012Scr-cysequation [14]. Bouvet et al. revealed that estimation of GFR using the four covariates (cystatin C, serum creatinine, body weight, and age) was less biased and more precise [11]. Thus, equations based on combination of different markers might become the final recommendation.
There are two strengths in this study. First, most of the studies, especially the two Chinese equations (the C-MDRD equation and Ma equation), were based on the patients with CKD [7], [10], [12].But eGFR always plays a role of screening the patients who might contract CKD, suggesting that those who use the equation may be healthy or with other disorders. Our study covered a proportion of subjects who might not suffer from CKD, which means we have evaluated the six famous equations in a more general population. Second, in our previous work, we investigated whether formulae possessed different diagnostic values between non-elderly and elderly subjects. Kilbride et al. tested the accuracy of the new equations in 74-year-olds or older in London [18]. We attempted to identify the value of the new equations in both non-elderly and elderly subjects.
There are also some limitations of this study. First of all, sample size, especially the number of participants with rGFR <30 ml/min/1.73 m2, is limited, and this is a single center study. Additionally, participants were all recruited from different departments of the hospital, which means rarely there was absolutely healthy population, and none of the data sets came from the population of patients with markedly reduced muscle mass or malnutrition. Thirdly, rGFR measurement by 99mTc-DTPA renal dynamic imaging method was still used in this study. It is, however, different from the renal clearance of Inulin used in the Mac study and 125I-iothalamate clearance in CKD-EPI equations. Therefore, the inconsistent rGFR may partially affect the true values. A nephrourology committee recommended double plasma clearance as the rGFR; however, before a unified rGFR can be carried out globally, this study may provide some exploratory information for clinicians and researchers. Moreover, the spectrum of disease is not uniform and the disease effect cannot be eliminated.
In summary, CKD-EPI2012Scr-cys formula had better diagnostic value and accuracy in the whole cohort; however, its performance was substantially worse in old subjects with moderately-severely injured GFR. CKD-EPI2012cys equation did not perform much better than other available equations. No magic formula has existed and every equation has its own characteristics. A large-scale study with many subjects and cooperating centers to develop a new formula for the general Chinese is necessary and urgent. Combination of different indicators should be recommended or more ideal endogenous indicators remain to be identified. Additionally, with the progress of medicine and the extension of human life, many countries have stepped into an aging society where elderly CKD population is rapidly expanding. We need to develop special formulae for this special population.
Acknowledgments
We thank the staff of Division of Nephrology, Department of Geriatrics of The First Affiliated Hospital of Nanjing Medical University for their cooperation and help. The study received statistical advice at the proposal stage from Professor Rongbin Yu of the Department of Epidemiology and Biostatistics, School of Public Health, Nanjing Medical University.
Funding Statement
This work was supported by the Innovation of Science and Technology Achievement Transformation Fund of Jiangsu Province BL2012066, the National Natural Science Foundation of China H0511-810705, the grants from the Major State Basic Research Development Program of China 2013CB530803, and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions JX10231801. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Levey AS, Eckardt K-U, Tsukamoto Y, Levin A, Coresh J, et al. (2005) Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 67 2089–2100. [DOI] [PubMed] [Google Scholar]
- 2. Nugent RA, Fathima SF, Feigl AB, Chyung D (2011) The Burden of Chronic Kidney Disease on Developing Nations: A 21st Century Challenge in Global Health. Nephron Clin Pract 118: c269–c277. [DOI] [PubMed] [Google Scholar]
- 3. Zhang LX, Wang F, Wang L, Wang WK, Liu BC, et al. (2012) Prevalence of chronic kidney disease in China: a cross-sectional survey. The Lancet 379: 815–822. [DOI] [PubMed] [Google Scholar]
- 4. Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, et al. (2007) Chronic kidney disease as a global public health problem: Approaches and initiatives–a position statement from Kidney Disease Improving Global Outcomes. Kidney Int 72: 247–259. [DOI] [PubMed] [Google Scholar]
- 5. Stevens LA, Coresh J, Greene T, Levey AS (2006) Assessing Kidney Function—Measured and Estimated Glomerular Filtration Rate. N Engl J Med 354 2473–83. [DOI] [PubMed] [Google Scholar]
- 6. Pei XH, Yang WY, Wang SN, Zhu B, Wu JQ, et al. (2013) Using Mathematical Algorithms to Modify Glomerular Filtration Rate Estimation Equations. PLoS One 8 e57852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ma Y-C, Zuo L, Chen J-H, Luo Q, Yu X-Q, et al. (2006) Modified Glomerular Filtration Rate Estimating Equation for Chinese Patients with Chronic Kidney Disease. J Am Soc Nephrol 17: 2937–2944. [DOI] [PubMed] [Google Scholar]
- 8. Zuo L, Ma Y-C, Zhou Y-H, Wang M, G-B Xu, et al. (2005) Application of GFR-Estimating Equations in Chinese Patients with Chronic Kidney Disease. Am J Kidney Dis 45: 463–472. [DOI] [PubMed] [Google Scholar]
- 9. Levey AS, Stevens LA, Schmid CH, Zhang YP, Castro AF, et al. (2009) A New Equation to Estimate Glomerular Filtration Rate. Ann Intern Med 150 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Macdonald J, Marcora S, Jibani M, Roberts G, Kumwenda M, et al. (2006) GFR Estimation Using Cystatin C Is Not Independent of Body Composition. Am J Kidney Dis 48: 712–719. [DOI] [PubMed] [Google Scholar]
- 11. Bouvet Y, Bouissou F, Coulais Y, Seronie-Vivien S, Tafani M, et al. (2006) GFR is better estimated by considering both serum cystatin C and creatinine levels. Pediatr Nephrol 21: 1299–1306. [DOI] [PubMed] [Google Scholar]
- 12. Ma Y-C, Zuo L, Chen J-H, Luo Q, Yu X-Q, et al. (2007) Improved GFR estimation by combined creatinine and cystatin C measurements. Kidney Int 72: 1535–1542. [DOI] [PubMed] [Google Scholar]
- 13. Pei X-H, He J, Liu Q, Zhu B, Bao L-H, et al. (2012) Evaluation of serum creatinine-and cystatin C-based equations for the estimation of glomerular filtration rate in a Chinese population. Scand J Urol Nephrol 46 223–231. [DOI] [PubMed] [Google Scholar]
- 14. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, et al. (2012) Estimating Glomerular Filtration Rate from Serum Creatinine and Cystatin C. N Engl J Med 367: 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int, Suppl 3: 1–150.
- 16.Mindikoglu AL, Dowling TC, Weir MR, Seliger SL, Christenson RH, et al.. (2013) Performance of Chronic Kidney Disease Epidemiology Collaboration Creatinine-Cystatin C Equation for Estimating Kidney Function in Cirrhosis. Hepatology: In press. [DOI] [PMC free article] [PubMed]
- 17.Obiols J, Bargnoux A-S, Kuster N, Fesler P, Pieroni L, et al.. (2013) Validation of a new standardized cystatin C turbidimetric assay: Evaluation of the three novel CKD-EPI equations in hypertensive patients. Clin Biochem: In press. [DOI] [PubMed]
- 18. Kilbride HS, Stevens PE, Eaglestone G, Knight S, Carter JL, et al. (2013) Accuracy of the MDRD (Modification of Diet in Renal Disease) study and CKD-EPI (CKD Epidemiology Collaboration) Equations for Estimation of GFR in the Elderly. Am J Kidney Dis 61: 57–66. [DOI] [PubMed] [Google Scholar]
- 19. Dharnidharka VR, Kwon C, Stevens G (2002) Serum cystatin C Is Superior to Serum Creatinine as a Marker of Kidney Function: A Meta-Analysis. Am J Kidney Dis 40: 221–226. [DOI] [PubMed] [Google Scholar]
- 20. Vinge E, Lindergard B, Nilsson-Ehle P, G.rubb A (1999) Relationships among serum cystatin C, serum creatinine, lean tissue mass and glomerular filtration rate in healthy adults. Scand J Clin Lab Invest 59: 587–592. [DOI] [PubMed] [Google Scholar]
- 21. Grubb A, Nyman U, Björk J, Lindström V, Rippe B, et al. (2005) Simple Cystatin C–Based Prediction Equations for Glomerular Filtration Rate Compared with the Modification of Diet in Renal Disease Prediction Equation for Adults and the Schwartz and the Counahan–Barratt Prediction Equations for Children. Clin Chem 51: 1420–1431. [DOI] [PubMed] [Google Scholar]
- 22. Hojs R, Bevc S, Ekart R, Gorenjak M, Puklavec L (2008) Serum cystatin C-based equation compared to serum creatinine-based equations for estimation of glomerular filtration rate in patients with chronic kidney disease. Clin Nephrol 70 10–17. [DOI] [PubMed] [Google Scholar]
- 23. Stevens LA, Schmid CH, Greene T, Liang L, Beck GJ, et al. (2009) Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int 75: 652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hojs R, Bevc S, Ekart R, Gorenjak M, Puklavec L (2009) Serum cystatin C-based formulas for prediction of glomerular filtration rate in patients with chronic kidney disease. Nephron Clin Pract 114 c118–c126. [DOI] [PubMed] [Google Scholar]
- 25. Tangri N, Stevens LA, Schmid CH, Zhang YP, Beck GJ, et al. (2011) Changes in dietary protein intake has no effect on serum cystatin C levels independent of the glomerular filtration rate. Kidney Int 79 471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wasén E, Isoaho R, Mattila K, Vahlberg T, Kivelä S-L, et al. (2003) Serum Cystatin C in the Aged: Relationships With Health Status. Am J Kidney Dis 42 36–43. [DOI] [PubMed] [Google Scholar]
- 27. Sjöström P, Tidman M, Jones I (2005) Determination of the production rate and non-renal clearance of cystatin C and estimation of the glomerular filtration rate from the serum concentration of cystatin C in humans. Scand J Clin Lab Invest 65: 111–124. [DOI] [PubMed] [Google Scholar]
- 28. Rule AD, Bergstralh EJ, Slezak JM, Bergert J, Larson TS (2006) Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int 69: 399–405. [DOI] [PubMed] [Google Scholar]
- 29. Rule AD, Bailey KR, Lieske JC, Peyser PA, Turner ST (2013) Estimating the glomerular filtration rate from serum creatinine is better than from cystatin C for evaluating risk factors associated with chronic kidney disease. Kidney Int 83: 1169–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]