Abstract
Secretory diarrheas caused by bacterial and viral enterotoxins remain a significant cause of morbidity and mortality. Enterocyte Cl− channels represent an attractive class of targets for diarrhea therapy, as they are the final, rate-limiting step in enterotoxin-induced fluid secretion in the intestine. Activation of cyclic nucleotide and/or Ca2+ signalling pathways in secretory diarrheas increases the conductance of Cl− channels at the enterocyte luminal membrane, which include the cystic fibrosis transmembrane conductance regulator (CFTR) and Ca2+-activated Cl− channels (CaCCs). High-throughput screens have yielded several chemical classes of small molecule CFTR and CaCC inhibitors that show efficacy in animal models of diarrheas. Natural-product diarrhea remedies with Cl− channel inhibition activity have also been identified, with one product recently receiving FDA approval for HIV-associated diarrhea.
Keywords: diarrhea, cholera, chloride channels, CFTR, CaCC, rotavirus
Introduction
Secretory diarrhea remains a major global health challenge, and represents the second leading cause of mortality globally in children under age 5 [1]. Repeated episodes of dehydration from diarrhea are also associated with impaired physical and mental development [2]. In developing countries major causes of secretory diarrheas include enterotoxin-producing bacteria such as Vibrio cholerae and enterotoxic E coli, viruses such as rotavirus, and enteroinvasive bacteria such as Shigella and Salmonella [1]. In developed countries secretory diarrheas are primarily caused by viruses such as rotavirus, although with the widespread use of rotavirus vaccines other pathogens such as norovirus have become increasingly prevalent [3].
Oral rehydration solution (ORS) to replace fluid losses and promote intestinal fluid absorption has been the primary therapy for secretory diarrhea, reducing mortality four-fold over the last 30 years [4]. However, there remains an unmet need for alternative and adjunctive antidiarrheal therapeutics, as ORS is not always effective, available or administered properly. Antisecretory drug therapy could have broad indications for infectious diarrheas in developing and developed countries, and potentially for diarrheas associated with certain cancer and HIV therapeutics [5]
Antisecretory Targets in the Intestinal Epithelium
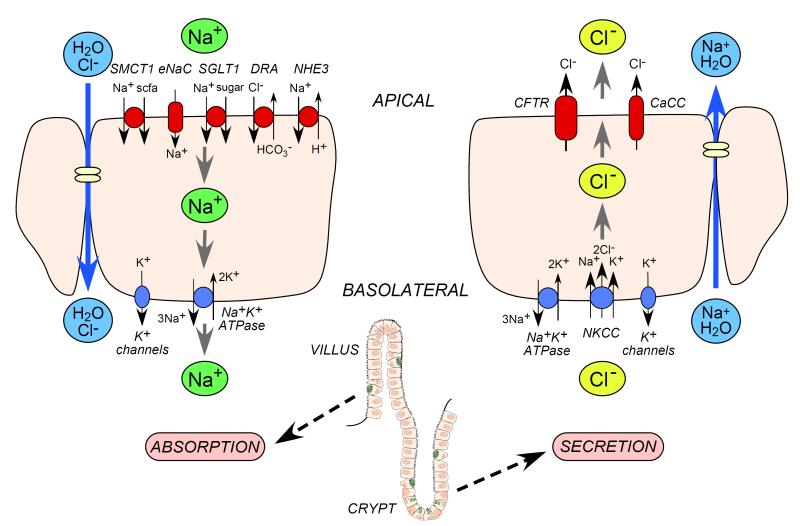
The intestinal epithelium consists of villi and crypts, with absorption occurring mainly in villi and secretion in crypts. Fluid absorption in the small intestine is driven by the luminal Na+/H+ exchanger (NHE3), Na+-glucose cotransporter (SGLT1), and Cl−/HCO −3 exchanger (DRA) [6,7] (Figure 1). As in all epithelia the electrochemical driving force is established by a basolateral Na+K+-ATPase pump. The pro-absorptive solute transporters are constitutively active, though they can be modulated by second-messengers including cAMP and Ca2+ [8, 9]. NHE3, SGLT1 and DRA are thus potential membrane transporter targets to increase intestinal fluid absorption. In the colon, fluid absorption is also facilitated by the epithelial Na+ channel (ENaC) and short-chain fatty acid (scfa) transporters (SMCT1) [10].
Figure 1.
Intestinal transport mechanisms. Left. Fluid absorption, which occurs primarily in villus epithelial cells, involves active transcellular Na+ transport of sodium via apical membrane transporters and channels and the basolateral Na+/K+ ATPase, which drives passive Cl− and water flux. Right. Fluid secretion, which occurs primarily crypt epithelial cells, involves active transcellular Cl− transport from the basolateral side via the NKCC transporter and apical Cl− transport channels, with corresponding passive Na+ and water flux.
Intestinal fluid secretion is driven by active transepithelial Cl− secretion, which creates the electrochemical force for paracellular Na+ secretion and the osmotic driving force for transcellular water secretion (Figure 1). Cl− is transported into the cell at the basolateral membrane by the Na+/K+/2Cl− cotransporter (NKCC1), which is driven by Na+ and Cl− concentration gradients produced by the Na+K+-ATPase and basolateral K+ channels. The electrochemical gradient drives Cl− secretion across the luminal membrane through CFTR and Ca2+-activated Cl− channels (CaCCs). NKCC1, CFTR, CaCCs and K+ channels (KCNQ1/KNE3, KCNN4) are thus potential membrane transporter targets to reduce intestinal fluid secretion. The intestinal epithelium also expresses other chloride channels including ClC-2 and bestrophins [11, 12].
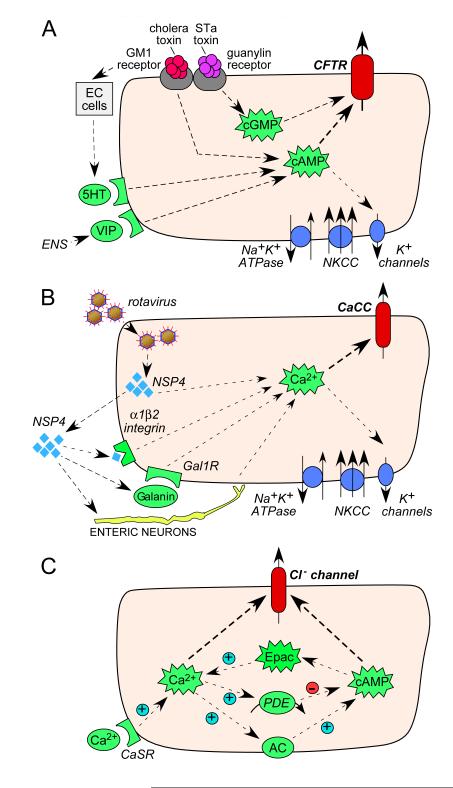
In addition to membrane transporters, a number of the cellular signalling molecules involved in mediating anion secretion represent potential pharmacological targets. Bacterial enterotoxins elevate cyclic nucleotides (cAMP and cGMP) [13, 14] (Figure 2A), and viral enterotoxins and some drugs elevate cytosolic Ca2+ [15, 16] (Figure 2B). There is thought to be significant cross-talk between cyclic nucleotide and Ca2+ signalling, with proposed mechanisms involving cAMP-induced Ca2+ elevation mediated by Epac [17], and compartmentalized Ca2+-induced cAMP elevation mediated by membrane-associated Ca2+-sensitive adenylyl cyclase-1 [18] (Figure 2C). Proof-of concept that these signalling pathways are potential antisecretory targets has been shown by the efficacy of a small molecule phosphodiesterase (PDE) activator, which reduces cAMP and cGMP, in a closed-loop model of intestinal fluid secretion [19]. Other potential targets include modulators of membrane macromolecular complexes such as agonists of lysophosphatidic acid (LPA) receptors, which inhibit CFTR function, or the more recently identified putative modulator MAST205 [20, 21]. Agonists of the Ca2+ sensing receptor CaSR have been shown to inhibit enterotoxin mediated fluid secretion [22] and act through a number of pathways including the enteric nervous system [23].
Figure 2.
Intestinal signal pathways controlling fluid secretion. A. Signaling pathways in CFTR activation by bacterial enterotoxins. Cholera toxin and heat stable enterotoxin (STa) bind to membrane receptors (GM1– ganglioside receptor, guanylin receptor) causing increases in cyclic nucleotides (cAMP, cGMP) and neurotransmitters, resulting in CFTR activation. EC – enterochromaffin cells, 5-HT – 5-hydroxytryptamine, VIP – vasoactive intestinal peptide, ENS – enteric nervous system. B. Signaling pathways in CaCC activation by rotavirus. Rotavirus releases NSP4 (non-structural protein 4), which causes elevation of cytoplasmic Ca2+ either: directly via binding to a membrane receptor (integrin α1β2); via neuropeptide galanin; or through activation of enteric nerves. Gal1-R – galanin 1 receptor. C. Cross-talk between Ca2+ and cAMP pathways in intestinal epithelial cells. Epac – exchange protein directly activated by cAMP, PDE – phosphodiesterase, AC – adenylate cyclase, CaSR – calcium sensing receptor.
Involvement of CFTR and CaCC in Secretory Diarrhea
The involvement of the major apical Cl− channels, CFTR and the intestinal CaCC, in secretory diarrheas is supported by numerous studies. There is strong evidence that CFTR is the Cl− pathway in secretory diarrheas caused by the bacterial enterotoxins released in cholera and Traveler’s diarrhea in both the small intestine and colon [24] (Figure 2A). Intestinal Cl− and fluid secretion are absent in mice lacking CFTR and in CF patients [25], and colonic Cl− transport in human tissue is effectively blocked by CFTR inhibitors [24]. The alternative, CaCC-mediated pathway, may be involved as well in these diarrheas, but likely represents the primary pathway for apical membrane Cl− secretion in rotavirus (Figure 2B) and possibly in drug-induced secretory diarrheas [26].
Small-Molecule CFTR and CaCC Inhibitors
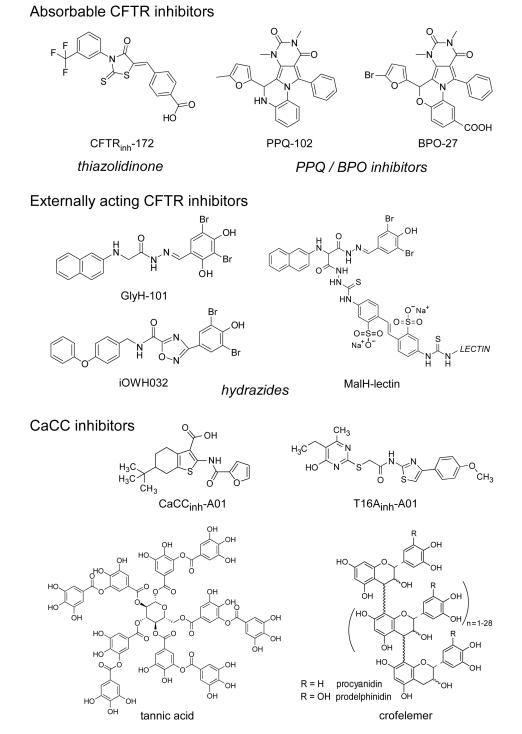
Three chemical classes of small-molecule CFTR inhibitors have emerged from high-throughput screening. The thiazolidinone CFTRinh-172 [27] (Figure 3), which has been used widely in cystic fibrosis research, inhibits CFTR by binding at or near arginine-347 and stabilizing the channel closed-state [28]. The IC50 for inhibition of CFTR Cl− current by CFTRinh-172 ranges from ~300 nM to several μM depending on cell type and membrane potential. CFTRinh-172 has low toxicity and is excreted with minimal metabolism [29]. Studies in mouse models of cholera and STa toxin-induced intestinal fluid secretion have demonstrated CFTRinh-172 efficacy [24]. Structure–activity studies have identified thiazolidinones with greater water solubility than CFTRinh-172 [30], including an analogue containing a 4-tetrazolophenyl in place of the 4-carboxyphenyl in CFTRinh- 172 that has shown efficacy in mouse models of polycystic kidney disease (PKD) [31].
Figure 3.
Chemical structures of CFTR and CaCC inhibitors. Absorbable CFTR inhibitors include thiazolidiones and PPQ/BPO inhibitors. Externally acting CFTR inhibitors include hydrazide derivatives. Small-molecule and macromolecular CaCC inhibitors shown.
PPQ/BPO compounds (Figure 3) are a second class of absorbable CFTR inhibitors with cytoplasmic site-of-action [32]. The IC50 is ~90 nM for PPQ-102 inhibition of CFTR Cl− conductance. Structure-activity studies yielded BPO-27, which contains structural changes that greatly increase its metabolic stability, inhibition potency and aqueous solubility [33]. The IC50 for CFTR inhibition by (racemic) BPO-27 is ~8 nM. Chiral separation yielded an active R-enantiomer of BPO-27 with IC50 ~ 4 nM, with the S-enantiomer being inactive [32]. PPQ-102 and BPO-27 have shown efficacy in models of PKD, but have not been tested in diarrhea models.
Glycine hydrazides such as GlyH-101 (Figure 3) are a third class of CFTR inhibitors that target the CFTR pore on its extracellular surface [34]. Patch-clamp analysis showed a characteristic signature of an extracellular pore blocking inhibitor, including a linear current–voltage relationship that becomes inwardly rectifying following GlyH-101, with rapid single-channel flicker. CFTR inhibition by a membrane-impermeant PEG-hydrazide conjugate [35], and molecular modelling [36], further supported an extracellular site-of-action, which provides a unique opportunity to develop non-absorbable compounds for antisecretory therapy. The GlyH-101 analog iOWH032 (Figure 3), which weakly inhibits CFTR (IC50 ~ 8 μM), is in clinical trials [37]. However, it is theoretically unlikely that a low-affinity small-molecule glycine hydrazide will have antisecretory efficacy because of predicted rapid washout (by convection) of an externally targeted inhibitor (see below), and the poor inhibition potency of glycine hydrazides at interior-negative membrane potentials.
In an attempt to address the washout/potency liabilities, several non-absorbable macromolecular conjugates were synthesized containing a malonic acid hydrazide (MalH) CFTR-inhibiting moiety, including a MalH-lectin conjugate [38] (Figure 3). MalH-lectin conjugates had IC50 down to 50 nM and remained bound to CFTR for many hours, as compared to seconds for GlyH-101 or iOWH032. The improved potency of the MalH-lectin conjugate and its resistance to washout is likely due to trapping in the enterocyte glycocalyx. Multivalent MalH-PEG conjugates were also synthesized with nanomolar CFTR inhibition potency [35]. The development potential of these lectin and PEG conjugates is unclear.
Motivated by the potential efficacy of CaCC inhibitors for some secretory diarrheas, a phenotype-based small molecule screen was done using the human colonic cell line HT-29 [39]. Several classes of CaCC inhibitors were identified, the most potent being the 3-acyl-2-aminothiophene CaCCinh-A01 (Figure 3). CaCCinh-A01 fully inhibited CaCC-dependent halide flux in different intestinal cell lines and in response to different agonists, with IC50 down to 1 μM, and was shown recently to prevent watery diarrhea in a neonatal mouse model of secretory diarrhea (unpublished observations). Subsequent target-based screening yielded TMEM16A-selective inhibitors [40], the most potent being T16Ainh-A01 (Figure 3). Though TMEM16A is not a major enterocyte CaCC, it is the principal CaCC in interstitial cells of Cajal and required for intestinal motility [41].
Convective Washout Reduces Efficacy of Surface-Targeted Cl− Channel Inhibitors
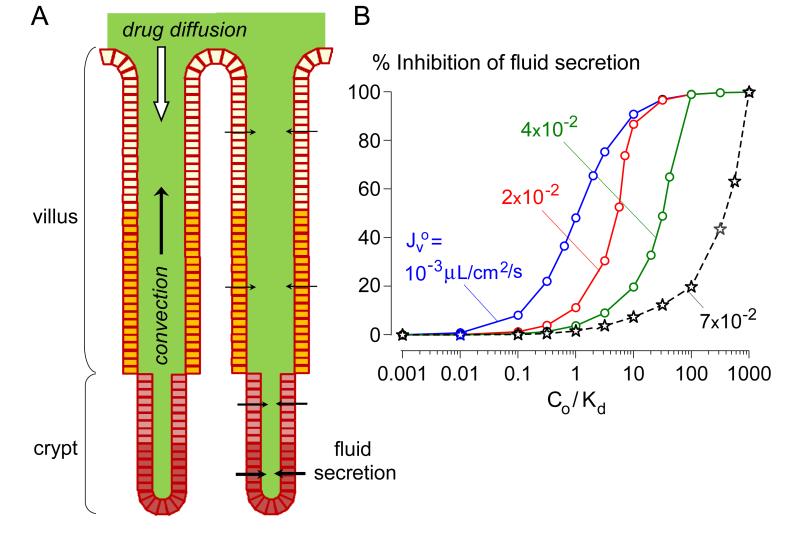
As mentioned above, a concern for drugs with an extracellular target in intestinal crypts is convective drug washout, which reduces drug efficacy (Figure 4A). A convection-diffusion model was developed recently of drug washout in an anatomically accurate 3-dimensional model of the human intestine [42]. The model predicted greatly reduced inhibitor efficacy for rapid crypt fluid secretion as occurs in cholera. Figure 4B shows a single-crypt computation in which inhibitor efficacy in reducing fluid secretion is plotted as a function of inhibitor concentration (relative to its binding dissociation constant, Kd). Whereas 50% inhibition of fluid secretion occurs for inhibitor concentration ~ Kd when secretion rate is low, orders of magnitude greater inhibitor concentration is needed to prevent fluid secretion at high secretion rates as in cholera. It was concluded that the antisecretory efficacy of an oral, membrane-impermeant, surface-targeted inhibitor requires high inhibitor affinity (low nanomolar Kd) in order to obtain sufficiently high luminal inhibitor concentration (> 100-fold Kd), and sustained high luminal inhibitor concentration or slow inhibitor dissociation. Convective washout considerations are relevant to glycine hydrazide- and some natural-product-based Cl− channel-targeted therapies.
Figure 4.
Convective washout reduces the efficacy of enterocyte surface-targeted Cl− channel inhibitors. A. Schematic of epithelial cell-lined crypt-villus units. Fluid secretion into the lumen produces convective (upward) solute transport opposing drug diffusion. B. Convective inhibitor washout requires a high concentration of a membrane-impermeant inhibitor in the intestinal lumen for antisecretory efficacy. Computations were done for a single crypt with human mid-jejunal anatomy. Percentage inhibition of net secreted fluid as a function of Co/Kd (lumen inhibitor concentration / inhibitor dissociation constant) for indicated J ov (single-crypt fluid secretion in the absence of inhibitor). J o ~7 × 10−2 v μL/cm2/s is typical in cholera. Adapted from ref. 43.
Natural-Product Cl− Channel Inhibitors
Natural products have been identified with antidiarrheal efficacy in humans and a putative mechanism of action involving Cl− channel inhibition. Crofelemer, a heterogeneous proanthocyanidin oligomer extracted from the bark latex of South American tree Croton lechleri, was approved recently for HIV-associated diarrhea following clinical trials showing efficacy in reducing the number and severity of diarrhea episodes [43]. Investigation of the antisecretory mechanism of crofelemer revealed weak and partial (maximum ~60 %) inhibition of CFTR, though complete inhibition of CaCC with IC50 < 10 μM [44]. Whether CaCC inhibition by crofelemer can explain its efficacy in HIV-associated diarrhea is unclear. Crofelemer has not been tested in animal models having defined diarrheas.
Following a natural product screen that identified tannic acid as a general CaCC inhibitor, we found that red wines containing polyphenolic gallotannins fully inhibited intestinal CaCC without effect on CFTR [45]. In recent follow-up work, we generated an alcohol-free red wine extract with potent CaCC inhibition activity, and showed its efficacy in a neonatal mouse model of rotaviral diarrhea (unpublished data). The wine extract inhibited intestinal Ca2+-activated Cl− current and fluid secretion without affecting rotaviral infection of intestinal epithelial cells. CaCC inhibition may account for anecdotal reports of antidiarrheal action of red wines. Motivated by the possibility that known herbal antidiarrheal remedies might act by Cl− channel inhibition, we recently screened a selection of diarrhea remedies from sources worldwide and identified a commonly used Thai herbal remedy that fully inhibited both CFTR and CaCC (unpublished observations). The herbal remedy showed efficacy in mouse models of cholera and rotaviral diarrhea. Chemical analysis of active ingredient(s) is in progress. Natural products thus represent a potentially inexpensive and immediately available therapy for secretory diarrheas with a defined mechanism of action.
Conclusions
Antisecretory drug therapy has considerable potential in reducing morbidity and mortality associated with infectious and some drug-induced and other diarrheas. Because severe secretory diarrhea is largely a concern in developing countries, challenges in drug development include the need for very low cost and high stability in a hot /humid environment, as well as obtaining funding to support commercial development of a new chemical entity with relatively low profit potential. The development or repurposing of existing natural products, such as wine or herbal extracts, is of particular interest based on their low cost and immediate availability for clinical testing against a variety of pathogens. The overall human and economic cost of diarrheal disease globally justifies a multi-factorial approach that includes pharmacological therapies as well as improvements in access to ORS, education, vaccination and sanitation.
Highlights.
cAMP (CFTR) and Ca2+-activated (CaCC) Cl− channels are expressed on enterocytes.
Enterocyte Cl− channels are activated in major infectious secretory diarrheas.
Cl− channel-targeted therapeutics for secretory diarrheas are emerging.
Acknowledgments
This work was supported by grants DK72517, DK35124, EY13574 and EB00415 from the National Institutes of Health, and a Research Development Program grant from the Cystic Fibrosis Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Annotated Reference List
- 1.Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O’Brien KL, Campbell H, Black RE. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381:1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2 *.Moore SR, Lima NL, Soares AM, Oriá RB, Pinkerton RC, Barrett LJ, Guerrant RL, Lima AA. Prolonged episodes of acute diarrhea reduce growth and increase risk of persistent diarrhea in children. Gastroenterology. 2010;139:1156–1164. doi: 10.1053/j.gastro.2010.05.076. One of an important group of cohort studies showing morbidity outcomes resulting from prolonged acute diarrheal episodes.
- 3 *.Payne DC, Vinjé J, Szilagyi PG, Edwards KM, Staat MA, Weinberg GA, Hall CB, Chappell J, Bernstein DI, Curns AT, et al. Norovirus and medically attended gastroenteritis in U. S. children. N Engl J Med. 2013;368:1121–1130. doi: 10.1056/NEJMsa1206589. Shows that norovirus diarrhea is replacing rotavirus as the most frequent cause of acute diarrhea in the U.S.
- 4.Munos MK, Walker CL, Black RE, Lipecka J, Bali M, Thomas A, Fanen P, Edelman A. The effect of oral rehydration solution and recommended home fluids on diarrhoea mortality. Int J Epidemiol. 2010;39:i75–87. doi: 10.1093/ije/dyq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacArthur RD, DuPont HL. Etiology and pharmacologic management of noninfectious diarrhea in HIV-infected individuals in the highly active antiretroviral therapy era. Clin Infect Dis. 2012;55:860–867. doi: 10.1093/cid/cis544. [DOI] [PubMed] [Google Scholar]
- 6 *.Lin R, Murtazina R, Cha B, Chakraborty M, Sarker R, Chen TE, Lin Z, Hogema BM, de Jonge HR, Seidler U, et al. D-Glucose acts via sodium/glucose cotransporter 1 to increase NHE3 in mouse jejunal brush border by a Na+/H+ exchange regulatory factor 2-dependent process. Gastroenterology. 2011;140:560–571. doi: 10.1053/j.gastro.2010.10.042. Shows that Na+/H+ and SGLT1 transporters are regulated by a common signaling complex, which may be targeted to reduce fluid losses in diarrhea.
- 7.Walker NM, Simpson JE, Brazill JM, Gill RK, Dudeja PK, Schweinfest CW, Clarke LL. Role of down-regulated in adenoma anion exchanger in HCO −3 secretion across murine duodenum. Gastroenterology. 2009;136:893–901. doi: 10.1053/j.gastro.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gawenis LR, Franklin CL, Simpson JE, Palmer BA, Walker NM, Wiggins TM, Clarke LL. cAMP inhibition of murine intestinal Na/H exchange requires CFTR-mediated cell shrinkage of villus epithelium. Gastroenterology. 2003;125:1148–1163. doi: 10.1016/s0016-5085(03)01212-5. [DOI] [PubMed] [Google Scholar]
- 9.Murtazina R, Kovbasnjuk O, Chen TE, Zachos NC, Chen Y, Kocinsky HS, Hogema BM, Seidler U, de Jonge HR, Donowitz M. NHERF2 is necessary for basal activity, second messenger inhibition, and LPA stimulation of NHE3 in mouse distal ileum. Am J Physiol Cell Physiol. 2011;301:C126–136. doi: 10.1152/ajpcell.00311.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teramae H, Yoshikawa T, Inoue R, Ushida K, Takebe K, Nio-Kobayashi J, Iwanaga T. The cellular expression of SMCT2 and its comparison with other transporters for monocarboxylates in the mouse digestive tract. Biomed Res. 2010;31:239–249. doi: 10.2220/biomedres.31.239. [DOI] [PubMed] [Google Scholar]
- 11.Fritsch J. Distribution of ClC-2 chloride channel in rat and human epithelial tissues. Am J Physiol Cell Physiol. 2002;282:C805–816. doi: 10.1152/ajpcell.00291.2001. [DOI] [PubMed] [Google Scholar]
- 12.Yu K, Lujan R, Marmorstein A, Gabriel S, Hartzell HC. Bestrophin-2 mediates bicarbonate transport by goblet cells in mouse colon. J Clin Invest. 2010;120:1722–1735. doi: 10.1172/JCI41129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Field M, Fromm D, al-Awqati Q, Greenough WB., 3rd Effect of cholera enterotoxin on ion transport across isolated ileal mucosa. J Clin Invest. 1972;51:796–804. doi: 10.1172/JCI106874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao MC, Guandalini S, Smith PL, Field M. Mode of action of heat-stable Escherichia coli enterotoxin. Tissue and subcellular specificities and role of cyclic GMP. Biochim Biophys Acta. 1980;632:35–46. doi: 10.1016/0304-4165(80)90247-0. [DOI] [PubMed] [Google Scholar]
- 15.Ball JM, Tian P, Zeng CQ, Morris AP, Estes MK. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science. 1996;272:101–104. doi: 10.1126/science.272.5258.101. [DOI] [PubMed] [Google Scholar]
- 16.Morris AP, Scott JK, Ball JM, Zeng CQ, O’Neal WK, Estes MK. NSP4 elicits age-dependent diarrhea and Ca2+ mediated I− influx into intestinal crypts of CF mice. Am J Physiol. 1999;277:G431–44. doi: 10.1152/ajpgi.1999.277.2.G431. [DOI] [PubMed] [Google Scholar]
- 17.Hoque KM, Woodward OM, van Rossum DB, Zachos NC, Chen L, Leung GP, Guggino WB, Guggino SE, Tse CM. Epac1 mediates protein kinase A-independent mechanism of forskolin-activated intestinal chloride secretion. J Gen Physiol. 2010;135:43–58. doi: 10.1085/jgp.200910339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Namkung W, Finkbeiner WE, Verkman AS. CFTR-adenylyl cyclase I association responsible for UTP activation of CFTR in well-differentiated primary human bronchial cell cultures. Mol Biol Cell. 2010;21:2639–2648. doi: 10.1091/mbc.E09-12-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tradtrantip L, Yangthara B, Padmawar P, Morrison C, Verkman AS. Thiophenecarboxylate suppressor of cyclic nucleotides discovered in a small-molecule screen blocks toxin-induced intestinal fluid secretion. Mol Pharmacol. 2009;75:134–142. doi: 10.1124/mol.108.050567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C, Dandridge KS, Di A, Marrs KL, Harris EL, Roy K, Jackson JS, Makarova NV, Fujiwara Y, Farrar PL, et al. Lysophosphatidic acid inhibits cholera toxin-induced secretory diarrhea through CFTR-dependent protein interactions. J Exp Med. 2005;202:975–986. doi: 10.1084/jem.20050421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren A, Zhang W, Yarlagadda S, Sinha C, Arora K, Moon CS, Naren AP. MAST205 competes with cystic fibrosis transmembrane conductance regulator (CFTR)-associated ligand for binding to CFTR to regulate CFTR-mediated fluid transport. J Biol Chem. 2013;288:12325–12334. doi: 10.1074/jbc.M112.432724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geibel J, Sritharan K, Geibel R, Geibel P, Persing JS, Seeger A, Roepke TK, Deichstetter M, Prinz C, Cheng SX, et al. Calcium-sensing receptor abrogates secretagogue-induced increases in intestinal net fluid secretion by enhancing cyclic nucleotide destruction. Proc Natl Acad Sci U S A. 2006;103:9390–9397. doi: 10.1073/pnas.0602996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23 *.Cheng SX. Calcium-sensing receptor inhibits secretagogue-induced electrolyte secretion by intestine via the enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2012;303:G60–70. doi: 10.1152/ajpgi.00425.2011. Shows the importance of the enteric nervous system in calcium-sensing receptor-mediated inhibition of intestinal fluid secretion.
- 24.Thiagarajah J, Broadbent T, Hsieh E, Verkman AS. Prevention of toxin-induced intestinal ion and fluid secretion by a small-molecule CFTR inhibitor. Gastroenterology. 2004;126:511–519. doi: 10.1053/j.gastro.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Berschneider HM, Knowles MR, Azizkhan RG, Boucher RC, Tobey NA, Orlando RC, Powell DW. Altered intestinal chloride transport in cystic fibrosis. FASEB J. 1988;2:2625–2629. doi: 10.1096/fasebj.2.10.2838365. [DOI] [PubMed] [Google Scholar]
- 26.Rufo PA, Lin PW, Andrade A, Jiang L, Rameh L, Flexner C, Alper SL, Lencer WI. Diarrhea-associated HIV-1 APIs potentiate muscarinic activation of Cl− secretion by T84 cells via prolongation of cytosolic Ca2+ signaling. Am J Physiol Cell Physiol. 2004;286:C998–C1008. doi: 10.1152/ajpcell.00357.2003. [DOI] [PubMed] [Google Scholar]
- 27.Ma T, Thiagarajah JR, Yang H, Sonawane ND, Folli C, Galietta LJ, Verkman AS. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest. 2002;110:1651–1658. doi: 10.1172/JCI16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caci E, Caputo A, Hinzpeter A, Arous N, Fanen P, Sonawane ND, Verkman AS, Ravazzolo R, Zegarra-Moran O, Galietta LJ. Evidence for direct CFTR inhibition by CFTRinh- 172 based on arginine 347 mutagenesis. Biochem J. 2008;413:135–142. doi: 10.1042/BJ20080029. [DOI] [PubMed] [Google Scholar]
- 29.Sonawane ND, Muanprasat C, Nagatani R, Song Y, Verkman AS. In vivo pharmacology and antidiarrheal efficacy of a thiazolidinone CFTR inhibitor in rodents. J Pharm Sci. 2005;94:134–43. doi: 10.1002/jps.20228. [DOI] [PubMed] [Google Scholar]
- 30.Sonawane ND, Verkman AS. Thiazolidinone CFTR inhibitors with improved water solubility identified by structure-activity analysis. Bioorg Med Chem. 2008;16:8187–8195. doi: 10.1016/j.bmc.2008.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tradtrantip L, Sonawane ND, Namkung W, Verkman AS. Nanomolar potency pyrimido-pyrrolo-quinoxalinedione CFTR inhibitor reduces cyst size in a polycystic kidney disease model. J Med Chem. 2009;52:6447–6455. doi: 10.1021/jm9009873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snyder D, Tradtrantip L, Yao C, Fettinger J, Kurth MJ, Verkman AS. Absolute configuration and biological properties of enantiometers of CFTR inhibitor BPO-27. ACS Med Chem Lett. 2013;4:456–459. doi: 10.1021/ml400069k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33 **.Snyder DS, Tradtrantip L, Yao C, Kurth MJ, Verkman AS. Potent, metabolically stable benzopyrimido-pyrrolo-oxazine-dione (BPO) CFTR inhibitors for polycystic kidney disease. J Med Chem. 2011;54:5468–5477. doi: 10.1021/jm200505e. Synthesis of CFTR inhibitors with low nanomolar potency and improved pharmacological properties that inhibit fluid secretion in polycystic kidney disease.
- 34.Muanprasat C, Sonawane ND, Salinas D, Taddei A, Galietta LJ, Verkman AS. Discovery of glycine hydrazide pore-occluding CFTR inhibitors: mechanism, structure-activity analysis, and in vivo efficacy. J Gen Physiol. 2004;124:125–137. doi: 10.1085/jgp.200409059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sonawane ND, Zhao D, Zegarra-Moran O, Galietta LJ, Verkman AS. Nanomolar CFTR inhibition by pore-occluding divalent polyethylene glycol-malonic acid hydrazides. Chem Biol. 2008;15:718–728. doi: 10.1016/j.chembiol.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norimatsu Y, Ivetac A, Alexander C, O’Donnell N, Frye L, Sansom MS, Dawson DC. Locating a plausible binding site for an open channel blocker, GlyH-101, in the pore of the cystic fibrosis transmembrane conductance regulator. Mol Pharmacol. 2012;82:1042–1055. doi: 10.1124/mol.112.080267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Hostos EL, Choy RK, Nguyen T. Developing novel antisecretory drugs to treat infectious diarrhea. Future Med Chem. 2011;3:1317–1325. doi: 10.4155/fmc.11.87. [DOI] [PubMed] [Google Scholar]
- 38.Sonawane ND, Zhao D, Zegarra-Mora O, Galietta LJV, Verkman AS. Lectin conjugates as potent, nonabsorbable CFTR inhibitors for reducing intestinal fluid secretion in cholera. Gastroenterology. 2007;132:1234–1244. doi: 10.1053/j.gastro.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 39.De La Fuente R, Namkung W, Mills S, Verkman AS. Small-molecule screen identifies inhibitors of a human intestinal calcium-activated chloride channel. Mol Pharmacol. 2008;73:758–768. doi: 10.1124/mol.107.043208. [DOI] [PubMed] [Google Scholar]
- 40 *.Namkung W, Phuan P, Verkman AS. TMEM16A inhibitors reveal TMEM16A as a minor component of CaCC conductance in airway and intestinal epithelial cells. J Biol Chem. 2011;286:2365–2374. doi: 10.1074/jbc.M110.175109. Small molecule inhibitors of TMEM16A were identified, and used to show that the major intestinal calcium activated chloride conductance is not TMEM16A.
- 41.Namkung W, Yao Z, Finkbeiner WE, Verkman AS. Small-molecule activators of TMEM16A, a calcium-activated chloride channel, stimulate epithelial chloride secretion and intestinal contraction. FASEB J. 2011;25:4048–4062. doi: 10.1096/fj.11-191627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42 **.Jin BJ, Thiagarajah JR, Verkman AS. Convective washout reduces the antidiarrheal efficacy of enterocyte surface-targeted antisecretory drugs. J Gen Physiol. 2013;141:261–272. doi: 10.1085/jgp.201210885. Model of intestinal fluid secretion that quantitatively predicts that luminally active chloride channel inhibitors are unlikely to be effective due to convective drug washout.
- 43.Yeo QM, Crutchley R, Cottreau J, Tucker A, Garey KW. Crofelemer, a novel antisecretory agent approved for the treatment of HIV-associated diarrhea. Drugs Today (Barc) 2013;49:239–252. doi: 10.1358/dot.2013.49.4.1947253. [DOI] [PubMed] [Google Scholar]
- 44 *.Tradtrantip L, Namkung W, Verkman AS. Crofelemer, an antidiarrheal proanthocyanidin oligomer extracted from Croton lechleri, targets two distinct intestinal chloride channels. Mol Pharmacol. 2010;77:69–78. doi: 10.1124/mol.109.061051. Mechanism of action studies of the recently approved drug crofelemer showing inhibition of calcium-activated chloride channels and weak CFTR inhibition.
- 45.Namkung W, Thiagarajah JR, Phuan PW, Verkman AS. Inhibition of Ca2+-activated Cl− channels by gallotannins as a possible molecular basis of health benefits of green tea and red wine. FASEB J. 2010;24:4178–4186. doi: 10.1096/fj.10-160648. [DOI] [PMC free article] [PubMed] [Google Scholar]