Abstract
Neuromyelitis optica (NMO) is an inflammatory demyelinating disease of the central nervous system that can cause paralysis and blindness. The pathogenesis of NMO involves binding of immunoglobulin G autoantibodies to aquaporin-4 (AQP4) on astrocytes, which is thought to cause complement-dependent cytotoxicity (CDC) and a secondary inflammatory response leading to oligodendrocyte and neuronal damage. Here we investigate in vivo the role of antibody-dependent cell-mediated cytotoxicity (ADCC) triggered by AQP4 autoantibodies (AQP4-IgG) in the development of NMO pathology. A high-affinity, human recombinant monoclonal AQP4-IgG was mutated in its Fc region to produce 'NMO superantibodies' with enhanced CDC and/or ADCC effector functions, without altered AQP4 binding. Pathological effects of these antibodies were studied in a mouse model of NMO produced by intracerebral injection of AQP4-IgG and human complement. The original (non-mutated) antibody produced large NMO lesions in this model, with loss of AQP4 and GFAP immunoreactivity, inflammation and demyelination, as did a mutated antibody with enhanced CDC and ADCC effector functions. As anticipated, a mutated AQP4-IgG lacking CDC but having 10-fold enhanced ADCC produced little pathology, though, unexpectedly, a mutated antibody with 9-fold enhanced CDC but lacking ADCC produced less pathology than the original AQP4-IgG. Also, pathology was greatly reduced following administration of AQP4-IgG and complement to mice lacking the Fc III receptor involved in effector cell activation during ADCC, and to normal mice injected with a Fcγ receptor blocking antibody. Our results provide evidence for the central involvement of ADCC in NMO pathology, and suggest ADCC as a new therapeutic target in NMO.
Keywords: NMO, aquaporin, CDC, ADCC, Fcγ receptor, astrocyte
Introduction
Neuromyelitis optica (NMO) is a severe inflammatory demyelinating disease of the central nervous system (CNS) that often produces paralysis and blindness [42]. A defining feature of NMO is the presence of immunoglobulin G autoantibodies in NMO patient serum directed against aquaporin-4 (AQP4) [14,15], a water channel present on the plasma membrane of astrocyte end-feet [22,26]. It is believed that the anti-AQP4 autoantibody (AQP4-IgG) produces astrocyte damage in NMO by a mechanism involving complement-dependent cytotoxicity (CDC), which induces a secondary inflammatory response leading to oligodendrocyte and neuron death [10]. CDC involves multivalent binding of complement protein C1q to the Fc region of AQP4-IgG bound on AQP4 clusters [27], which leads to formation of a membrane attack complex consisting of complement proteins C5b-C9. The C5 convertase inhibitor eculizumab has demonstrated efficacy in an open-label clinical trial based on the assumed central role of CDC in NMO pathogenesis [28].
In addition to CDC effector function, which involves C1q binding to the antibody Fc region, AQP4-IgG has ADCC (antibody-dependent cell-mediated cytotoxicity) effector function in which the Fc region binds Fc receptors on effector cells and promotes their accumulation, phagocytosis and degranulation. In AQP4-expressing cell cultures, AQP4-IgG can cause cytotoxicity in the presence of natural-killer (NK) cells by an ADCC mechanism [1,40]. We found that AQP4-IgG and NK-cells can produce NMO-like lesions in mouse brain in the absence of complement, with loss of GFAP and AQP4 but not of myelin [30]. Though human NMO lesions contain few NK-cells [33], they show an abundance of other leukocyte cell types, including neutrophils, eosinophils and macrophages, each of which express Fc receptors and can participate in ADCC [17,19,32]. It is unclear whether NMO pathogenesis involves a bona fide ADCC effector mechanism with direct leukocyte interaction with the AQP4-IgG Fc region, or whether the pathogenicity of infiltrating leukocytes is a secondary phenomenon. Elucidation of the role of ADCC in NMO pathogenesis is important, as ADCC may be a potential drug target, and therapeutics targeting components late in the complement cascade may have limited efficacy.
Here, we investigated the involvement of ADCC in NMO using engineered monoclonal recombinant AQP4-IgG antibodies with different effector function profiles, including antibodies with enhanced CDC but no ADCC function, and enhanced ADCC but no CDC effector function. The antibodies were generated by mutation of the Fc region of a recombinant monoclonal AQP4-IgG rAb-53, which was derived from a clonally expanded plasma blast recovered from the cerebrospinal fluid of an NMO patient [1] and characterized extensively for its AQP4 binding and pathogenicity [4,27]. We used a mouse model of NMO involving direct intracerebral injection of AQP4-IgG and complement, which produces human NMO-like pathology with loss of AQP4 and GFAP immunoreactivity, inflammation, perivascular deposition of activated complement, and demyelination [34]. We found here, contrary to initial expectations, that ADCC is a major pathogenic mechanism in NMO.
Materials and Methods
Mice
Experiments were done on wild type mice on CD1 genetic background, generally of age 16–18 weeks. C57BL/6 mice homozygous for the Fc IIItm1Sjv targeted mutation, which eliminates the ligand-binding alpha chain of the Fc III receptor, were purchased from the Jackson Laboratory (Bar Harbor, ME). All procedures were approved by the U.C.S.F Committee on Animal Research.
NMO antibodies, DNA constructs
Purified human monoclonal recombinant AQP4-IgG rAb-53 and control IgG were generated as described [1]. Point mutations were introduced into the IgG1 Fc sequence of the rAb-53 (AQP4-IgGcont) heavy chain to produce antibodies with enhanced CDC and no ADCC (K326W/E333S; AQP4-IgGCDC; [8]), enhanced ADCC and no CDC (S239D/A330L/I332E; AQP4-IgGADCC; [13]), enhanced CDC and ADCC (G236A/S267E/H268F/S324T/I332E; AQP4-IgGCDC/ADCC; [21]), and no CDC or ADCC (L234A/L235A; Aquaporumab, AQmab; [38]). Plasmid pcDNA3.1 encoding the M23 isoform of human AQP4 was generated as described [3].
Cell culture and transfections
Chinese Hamster Ovary (CHO-K1) cells (ATCC CCL-61) were cultured at 37 °C in 5% CO2 / 95% air in F12 Ham’s medium (Sigma-Aldrich, St. Louis, MO) containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. CHO-K1 cells stably expressing human AQP4-M23 were generated previously [3]. Human NK-cells and mouse primary astrocytes were cultured as described [30].
Immunocytochemistry
AQP4-expressing cells were incubated for 20 min in blocking buffer (PBS containing 6 mM glucose, 1 mM pyruvate, 1% bovine serum albumin) and then for 30 min with specified concentrations of AQP4-IgGcont (or mutant antibodies) in blocking buffer. Cells were then rinsed with PBS, fixed in 4% paraformaldehyde (PFA) for 15 min and permeabilized with 0.1% Triton X-100. Cells were blocked again and incubated for 30 min with 0.4 µg/mL polyclonal, C-terminal-specific rabbit anti-AQP4 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), then rinsed with PBS. Cells were then incubated for 30 min with 4 µg/mL goat anti-human IgG-conjugated Alexa Fluor 488 and goat anti-rabbit IgG-conjugated Alexa Fluor 555 (Invitrogen). Quantification of AQP4-IgG binding to AQP4 was performed as described [4].
CDC and ADCC assays
CHO cells expressing human AQP4-M23 (target cells) were grown in 96-well plates until confluence. For assay of CDC, target cells were incubated for 1 h at 23 °C with specified concentrations of human complement and AQP4-IgGcont or mutant antibodies. For assay of ADCC, target cells were incubated for 1.5 h at 37 °C with NK-cells and AQP4-IgGcont (or mutant antibodies). Target cells were then washed extensively in PBS. In some experiments 1 µM calcein-AM and 2 µM ethidium-homodimer (Invitrogen, Carlsbad, CA) in PBS were added to stain live cells green and dead cells red. In other experiments, target cell viability was measured by addition of 20% AlamarBlue (Invitrogen) for 1 h at 37 °C. Fluorescence was measured with a plate reader at excitation/emission wavelengths of 560/590 nm. Percentage cell viability was computed as: [(sample – 100% lysis)/(no lysis − 100% lysis)] × 100 where ‘100% lysis’ is fluorescence of cells incubated in 1% Triton X-100 and ‘no lysis’ is fluorescence of cells incubated with human complement or NK-cells but no AQP4-IgG.
Intracerebral injection of AQP4-IgG
Intracerebral injection was performed as described [30]. 2 µg AQP4-IgGcont (or mutant antibodies) and 3 µL 20% human complement in 8 µL PBS (~1 µL/min) were infused into the brain. After 24 h or three days mice were anesthetized and brains were processed for immunostaining. For binding experiments 2 µg of antibody was injected without complement and mice were sacrificed 24 h later. In some experiments wild type mice were administered a rat monoclonal antibody that blocks mouse FcγRII/III (clone 2.4G2, 8 µg/g body weight, BD Biosciences, San Jose, CA) or rat purified IgG (as control) by tail vein one hour before intracerebral injection of AQP4-IgGcont and human complement. Mice were sacrificed 24 h later, blood was collected by cardiac puncture, leukocytes were counted using a Hemavet 850 (Drew Scientific, Oxford, CT), and brain tissue was processed.
Immunofluorescence and immunohistochemistry
Paraffin sections were immunostained as described [30] using the following antibodies: rabbit anti-AQP4 (1:200, Santa Cruz Biotechnology), mouse anti-GFAP (1:100, Millipore, Temecula, CA), goat anti-myelin basic protein (MBP) (1:200, Santa Cruz Biotechnology), rabbit anti-Iba1 (1:500, Wako, Richmond, VA), rat anti-CD45 (1:10, BD Biosciences), mouse anti GR-1 (1:10, eBioscience, Hatfield, UK) or mouse anti-C5b-9 (1:50, Santa Cruz Biotechnology). AQP4, GFAP and MBP immunonegative areas were defined by hand and quantified blindly by two observers using ImageJ (http://rsbweb.nih.gov/ij/). Data are presented as percentage of immunonegative area (normalized to total area of hemi-brain slice).
Statistics
Data are presented as mean ± S.E.M. Statistical comparisons were made using the non-parametric Mann-Whitney test when comparing two groups.
Results
‘NMO superantibodies’ with enhanced effector functions
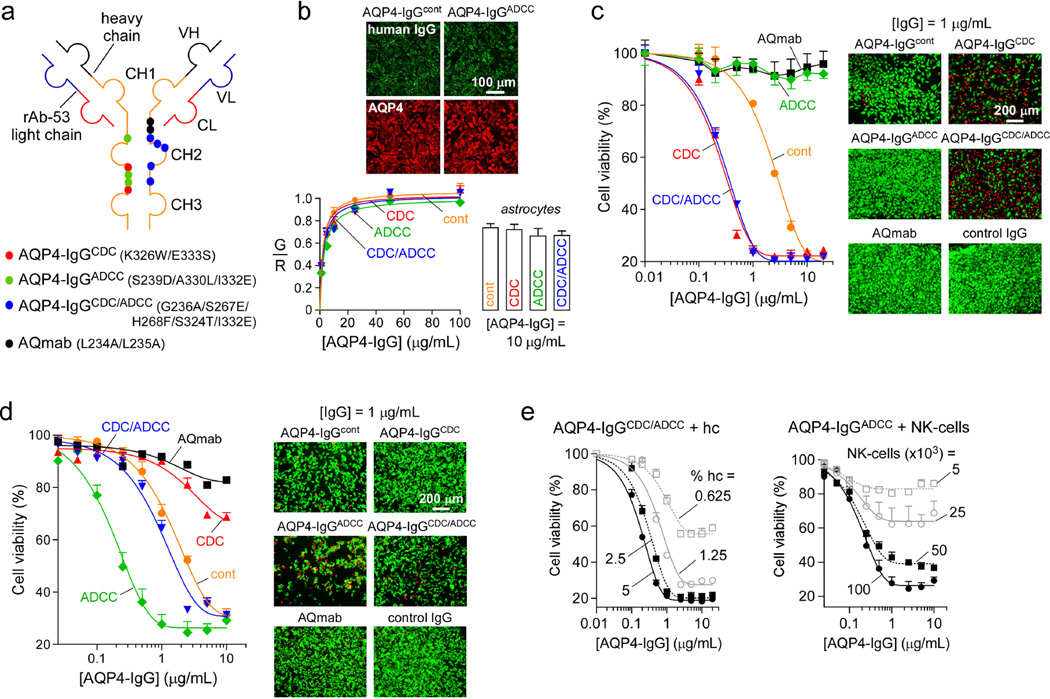
To resolve the roles of CDC and ADCC in NMO, point mutations were introduced in the Fc portion of a monoclonal recombinant human AQP4-IgG (rAb-53, referred as AQP4-IgGcont) that tightly binds to the extracellular surface of both human and mouse AQP4 [1,4,29]. The mutations in the CH2 domain of the heavy chain alter the binding affinity of the Fc fragment to C1q or to Fcγ receptors, thereby altering CDC and/or ADCC effector functions, respectively. Fig. 1a shows locations of the mutations used to generate the four antibodies used in this study: AQP4-IgGCDC (K326W/E333S), with enhanced CDC and no ADCC; AQP4-IgGADCC (S239D/A330L/I332E), with enhanced ADCC and no CDC; AQP4-IgGCDC/ADCC (G236A/S267E/H268F/S324T/I332E), with enhanced CDC and ADCC; and AQmab (L234A/L235A) with no CDC or ADCC.
Figure 1. CDC and ADCC effector functions of engineered NMO ‘superantibodies’.
a. Schematic of rAb-53 showing heavy (VH) and light (VL) chain variable regions, light chain constant region (CL), and heavy chain constant regions (CH1–CH3). Locations of amino acid mutations introduced in the heavy chain constant region to generate indicated AQP4-IgG mutant antibodies. b. (top) Representative fluorescence micrographs for binding of 5 µg/mL AQP4-IgGcont and AQP4-IgGADCC (green) to AQP4 in CHO cells stably transfected with human AQP4-M23, together with reference C-terminus AQP4 antibody (red). (bottom left) Binding of NMO antibodies to AQP4-M23 in CHO cells, shown as green-to-red fluorescence ratio (mean ± S.E., n=10). Curves represent fit to a single-site binding model. (bottom right) Binding of 10 µg/mL NMO antibodies to mouse primary astrocytes (mean ± S.E., n=5). c. (left) CDC assay in which CHO cells expressing AQP4-M23 (target cells) were incubated with NMO antibodies and 2.5% human complement for 1 h at 23 °C. Cell viability was measured with AlamarBlue (mean ± S.E., n=3). Data are representative of 3 sets of experiments. (right) Live/dead (green/red) staining of cells incubated with 2.5% complement and 1 µg/mL NMO antibody for 1 h at 23 °C. d. (left) ADCC assay in which target cells are incubated with 105 NK-cells per well and NMO antibodies for 1.5 h at 37 °C. Cell viability was assayed with AlamarBlue (mean ± S.E., n=3). Data are representative of 3 sets of experiments. (right) Live/dead staining of cells incubated with 105 NK-cells per well and 1 µg/mL antibody for 1 h at 37 °C. e. CDC (left) and ADCC (right) assays as in c and d done with specified concentrations of human complement (hc) and NK-cells (mean ± S.E., n=3).
We first verified that mutations introduced in the Fc portion of AQP4-IgG did not alter their binding affinity to AQP4. CHO cells stably expressing human AQP4-M23 were labeled with increasing concentrations of AQP4-IgG (green), and a reference antibody (red) that recognizes the C-terminus of AQP4. Binding was quantified by the green-to-red fluorescence ratio (G/R) as described [4]. Fig. 1b (top) shows representative micrographs. Fig. 1b (bottom left) shows similar AQP4 binding of the various AQP4-IgGs, indicating, as expected, that the Fc mutations did not affect the Fab portion of the antibody. We also verified that the mutant AQP4-IgGs generated for this study had the same binding to native AQP4 in mouse primary astrocytes (Fig. 1b, bottom right).
Fig. 1c shows CDC activity of AQP4-IgGcont and of the four mutant AQP4-IgGs. CHO cells expressing AQP4-M23 were incubated for 1 h with antibody and 2.5 % human complement, and cell viability was measured with AlamarBlue. As expected, AQmab and AQP4-IgGADCC did not produce cytotoxicity, as they lack CDC effector function, whereas AQP4-IgGCDC and AQP4-IgGCDC/ADCC each showed ~ 9-fold enhanced CDC compared to AQP4-IgGcont (as determined by the IC50 ratios). These findings were confirmed by live/dead cell staining (Fig. 1c, right) where killing by AQP4-IgGCDC and AQP4-IgGCDC/ADCC was greatly enhanced compared to AQP4-IgGcont. No killing was seen with AQmab, AQP4-IgGADCC or a control (non-NMO) IgG. Fig. 1e (left) summarizes the dependence of CDC produced by AQP4-IgGCDC/ADCC on complement concentration.
It has been shown previously that human IgGs can bind and activate murine effector cells through all classes of Fcγ receptors [25], and can induce ADCC mediated by mouse neutrophils, eosinophils and macrophages [25,44]. In this study antibody ADCC effector function was assayed in vitro by incubation of AQP4-expressing cells with antibodies and NK-cells, a cell line that has been used extensively in the lab previously [30,38] (Fig. 1d, left). Little ADCC was produced by AQmab or AQP4-IgGCDC compared to AQP4-IgGcont. ADCC produced by AQP4-IgGADCC was enhanced by ~ 10-fold compared to AQP4-IgGcont. These results were confirmed by live/dead cell staining (Fig. 1d, right). Fig. 1e (right) summarizes the dependence of ADCC produced by AQP4-IgGADCC on NK-cell number.
CDC is required for generation of NMO lesions
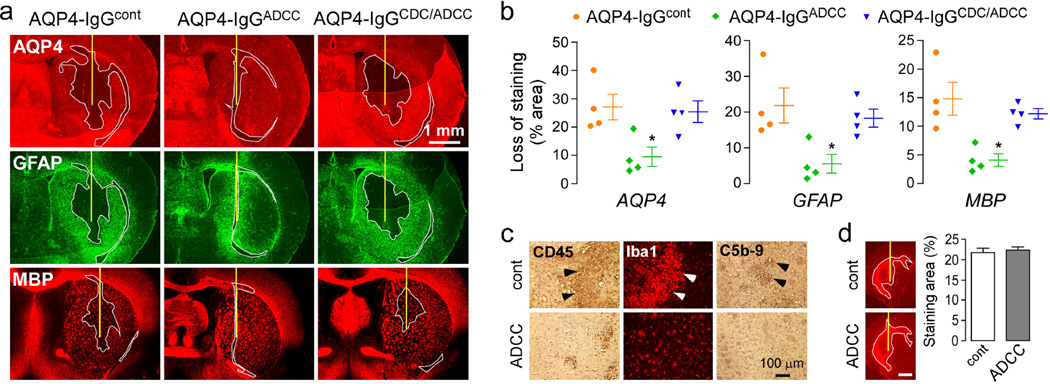
To confirm that complement activation is necessary to produce NMO lesions in our model, AQP4-IgGcont and AQP4-IgGADCC (enhanced ADCC but no CDC function) were injected (separately) with human complement into mouse brains. Three days after injection mice were sacrificed and brain sections were stained for AQP4, GFAP and the myelin marker MBP (Fig. 2a). As previously reported, injection of AQP4-IgGcont induced astrocyte injury as seen by loss of AQP4 and GFAP staining. AQP4-IgGcont also produced myelin damage as shown by reduced MBP immunoreactivity. Injection of AQP4-IgGcont or human complement alone did not produce pathology (data not shown). Administration of AQP4-IgGADCC produced very small lesions compared to AQP4-IgGcont as seen in Fig. 2a and by quantification of lesion size (Fig. 2b). Inflammation, as visualized by CD45 staining, was greatly reduced in mice injected with AQP4-IgGADCC compared to AQP4-IgGcont. The infiltrating cells were mainly macrophages/microglia as shown by Iba1 staining (Fig. 2c). We confirmed by C5b-9 immunofluorescence that complement was not activated in mice injected with AQP4-IgGADCC (Fig. 2c). These results indicate that the CDC effector function of AQP4-IgG is necessary for generation of NMO lesions.
Figure 2. CDC is required for NMO lesions.
a. Brains were injected with 2 g of AQP4-IgGcont, AQP4-IgGADCC or AQP4-IgGCDC/ADCC and 3 µL 20% human complement. Mice were sacrificed 3 days after injection and brain sections were immunostained for AQP4, GFAP and myelin (MBP). Yellow line represents the needle tract. White line shows the area of loss of immunoreactivity. Micrographs are representative of 4 mice per group. b. Areas of loss of AQP4, GFAP and MBP immunoreactivity (mean ± S.E., * P < 0.05 compared with AQP4-IgGcont). c. Immunohistochemistry for CD45, Iba1 and C5b-9. d. Antibody distribution. Brains were injected with 2 µg antibody, mice sacrificed after 24 h, and sections stained with anti-human secondary antibody. (left) Fluorescence micrographs showing areas of diffusion. (right) Areas of diffusion of the NMO antibodies (mean ± S.E., n=3). Scale bar: 1 mm.
Similar studies were done with AQP4-IgGCDC/ADCC, which has both CDC and ADCC effector functions enhanced. Fig. 2a,b shows that AQP4-IgGCDC/ADCC produced similar lesions with reduced AQP4, GFAP and MBP. Lesion size produced by AQP4-IgGcont and AQP4-IgGCDC/ADCC was similar under the conditions used in this study because of the saturating amount of antibody used and its limited volume of deposition, which depends on its diffusion and cellular binding.
The binding and diffusion of the antibodies in the absence of complement were studied by staining of sections with an anti-human secondary antibody at 24 h after antibody injection. Fig. 2d shows similar areas of diffusion of AQP4-IgGcont and AQP4-IgGADCC, as quantified by the area of diffusion of red fluorescence normalized to total area of hemi-brain slice. Therefore, differences in NMO lesion severity are not due to differences in antibody binding or diffusion in brain.
ADCC participates in the generation of NMO lesions
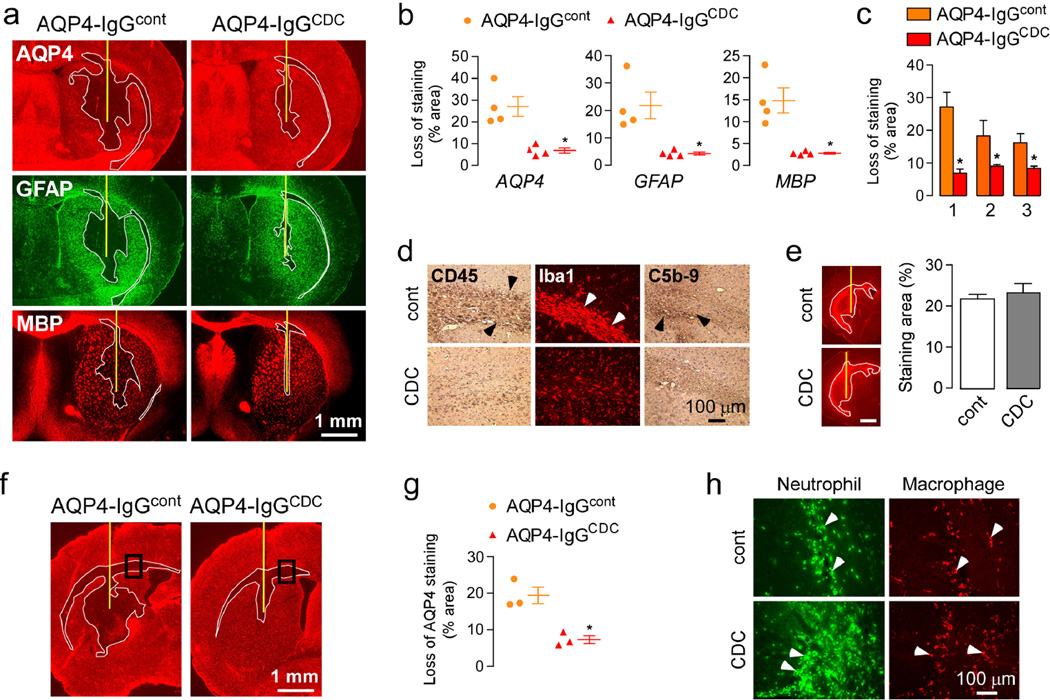
The role of ADCC was studied by comparing data from mice administered AQP4-IgGcont versus AQP4-IgGCDC (enhanced CDC and reduced ADCC), each with human complement. Unexpectedly, AQP4-IgGCDC produced remarkably smaller lesions compared to AQP4-IgGcont, as seen by AQP4, GFAP and MBP immunofluorescence (Fig. 3a) and by quantification of lesion size (Fig. 3b). Similar data were obtained in three separate sets of experiments with four mice in each group (Fig. 3c). Minimal macrophage infiltration was seen in mice injected with AQP4-IgGCDC compared to AQP4-IgGcont (Fig. 3d). These findings indicate that antibody ADCC effector function worsens lesions produced by AQP4-IgG and complement in mouse brain. Fig. 3e shows that differences in antibody binding or diffusion cannot account for the differences in lesion size.
Figure 3. ADCC is required for NMO lesions.
a. Brains were injected with 2 µg of AQP4-IgGcont or AQP4-IgGCDC and 3 µL 20% human complement. Mice were sacrificed after three days and brains were stained as in Fig. 2a. Yellow line represents the needle tract. White line shows the area of loss of immunoreactivity. Data are representative of 4 mice per group. b. Areas of loss of AQP4, GFAP and MBP immunoreactivity (mean ± S.E., * P < 0.05 compared with AQP4-IgGcont). c. Areas of loss of AQP4 staining in 3 independent sets of experiments (mean ± S.E., n=4, * P < 0.05 compared with AQP4-IgGcont). d. Immunohistochemistry for CD45, Iba1 and C5b-9. e. Binding of AQP4-IgGcont and AQP4-IgGCDC as in Fig. 2d. Scale bar: 1 mm. f. AQP4 immunoreactivity 24 h after injection as in a. g. Area of loss of AQP4 immunoreactivity at 24 h (mean ± S.E., n=3, * P < 0.05 compared with AQP4-IgGcont). h. Neutrophil and macrophage infiltration in areas delimited in f.
In this single injection model of NMO, macrophages are seen relatively late in the lesions and their role in the development of the pathology is unclear. Neutrophils infiltrate early in the CNS in response to AQP4-IgG and complement [34]. A previous study reported marked neutrophil infiltration at 24 h after AQP4-IgG administration and showed their crucial role in the pathogenesis of the lesions, as neutropenia reduced severity and neutrophilia increased severity [35]. Because neutrophils are known mediators of ADCC, we investigated the effects of AQP4-IgGcont versus AQP4-IgGCDC at 24 hours after injection. AQP4-IgGcont causes loss of AQP4 (Fig. 3f) and GFAP, but minimal myelin loss at this early time point. At 24 h, loss of AQP4 was reduced with AQP4-IgGCDC (Fig. 3f–g). Neutrophil infiltration was greater in AQP4-IgGCDC than with AQP4-IgGcont (Fig. 3h). These data suggest that the reduced pathology seen with AQP4-IgGCDC is due to reduced ADCC mediated by neutrophils, which are the main inflammatory cells present in the lesion at an early stage.
ADCC in NMO lesions is mediated by Fcγ receptor III
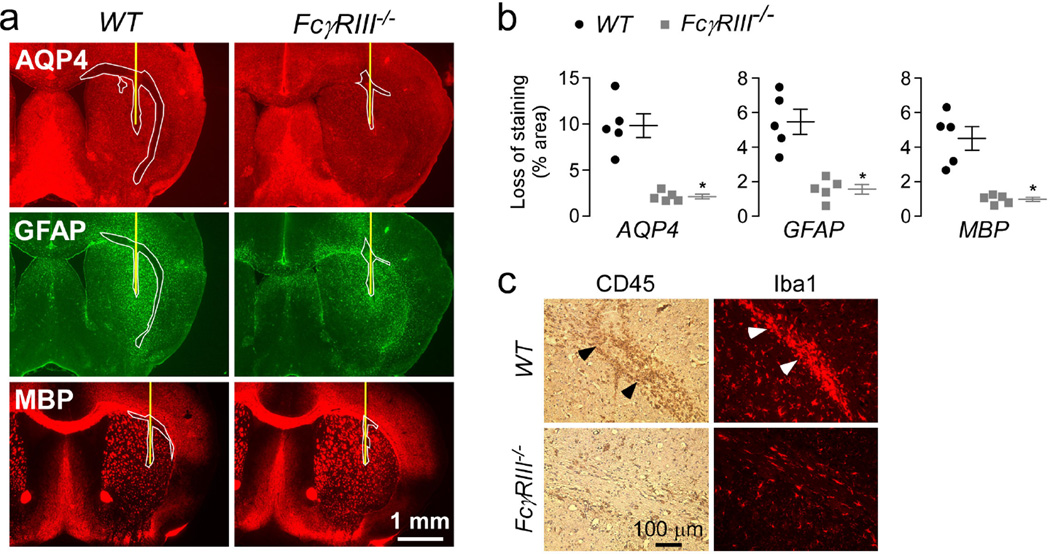
The ADCC data above suggest the involvement of Fcγ receptors in NMO pathogenesis. Murine immune cells express three activating Fcγ receptors: FcγRI, FcγRIII and FcγRIV. The IgG1 response in mice is mainly mediated by FcγRIII [6,24]. FcγRIII is expressed in macrophages, neutrophils and eosinophils [2], which are seen abundantly in human NMO lesions and in the NMO intracerebral model in mice [17,34]. Inactivation of FcγRIII in mice prevents ADCC produced by NK-cells and phagocytosis of IgG1-opsonized cells by macrophages [7]. As AQP4-IgG is an IgG1-subclass antibody, we investigated the role of FcγRIII by intracerebral injection of AQP4-IgGcont and human complement in wild type and FcγRIII C57BL/6 knockout mice. Submaximal AQP4-IgGcont was used in this study to generate small (non-saturating) lesions. Fig. 4a shows significantly reduced pathology in the FcγRIII knock-out mice, as confirmed by quantification of lesion size in Fig. 4b. Inflammation was reduced in FcγRIII knock-out mice as seen by CD45 and Iba1 staining (Fig. 4c). ADCC in this NMO model is therefore mediated mainly by FcγRIII.
Figure 4. Inactivation of FcγRIII in mice protects against NMO lesions.
a. Brains of C57BL/6 wild type (WT) and FcγRIII knock-out (FcγRIII−/−) mice were injected with 0.5 g of AQP4-IgGcont and 3 µL of 20% human complement and stained 3 days later as in Fig. 2a. Yellow line represents the needle tract. White line shows the area of loss of immunoreactivity. Micrographs are representative of 5 mice per group. b. Areas of loss of AQP4, GFAP and MBP immunoreactivity (mean ± S.E., * P < 0.05 compared with WT). c. CD45 and Iba1 immunohistochemistry.
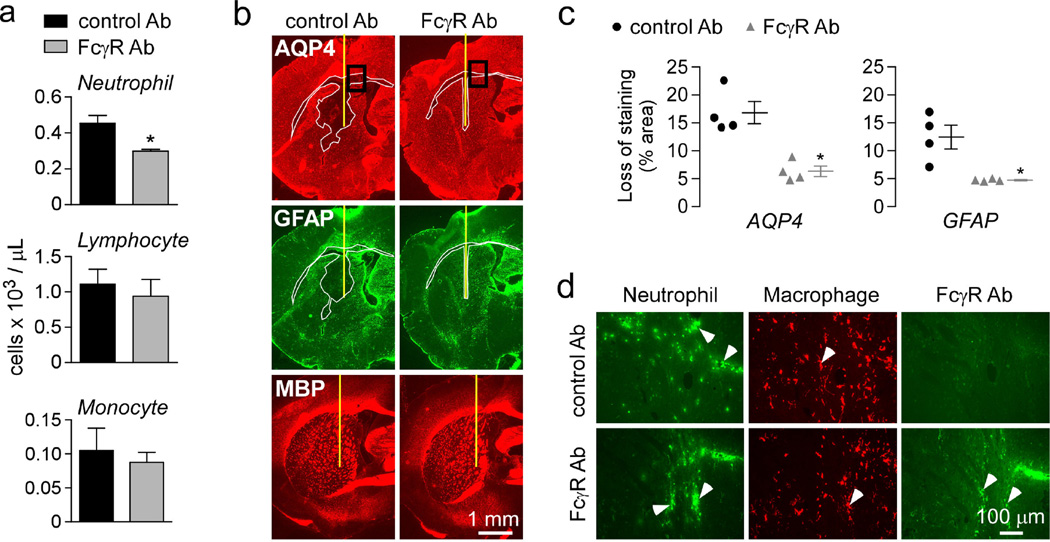
To explore inhibition of ADCC as a potential new therapeutic option for NMO, we treated mice with a monoclonal antibody that blocks mouse FcγRII and III function by inhibition of IgG binding to the receptors [39]. The FcγRII/III antibody and control IgG were deglycosylated using EndoS enzyme, as done previously [37], to inhibit their effector functions. Antibody was administered intravenously one hour prior intracerebral injection of AQP4-IgGcont and complement and the mice were sacrificed 24 h later at which time blood was collected and the brain was harvested. The FcγRII/III antibody reduced by ~30 % the peripheral neutrophil count, with other leukocytes unaffected (Fig. 5a). Treatment with FcγRII/III antibody significantly reduced lesion size in brain as seen by AQP4 and GFAP immunoreactivity (Fig. 5b–c). Inflammation in the treated mice was comparable to control mice (Fig. 5d). We confirmed the binding of the FcγRII/III antibody to neutrophils in the treated mice by staining with a fluorescent secondary antibody (Fig. 5d).
Figure 5. Administration of a FcγR blocking antibody reduces the severity of NMO lesions.
Mice were administered with FcγRII/III blocking antibody or control antibody (Ab) one hour prior intracerebral injection of 2 µg AQP4-IgGcont and 3 µL 20% complement. Mice were sacrificed at 24 h. a. Leukocyte count in mice injected with FcγRII/III blocking or control antibody at the time of sacrifice. b. AQP4, GFAP and myelin immunoreactivity. Yellow line represents the needle tract. White line shows the area of loss of immunoreactivity. Data are representative of 4 mice per group. c. Areas of loss of AQP4 and GFAP immunoreactivity (mean ± S.E., * P < 0.05 compared with control Ab). d. Neutrophil, macrophage and FcγR blocking antibody staining in areas delimited in b.
Discussion
We used engineered monoclonal AQP4-IgGs to investigate the importance of antibody CDC vs. ADCC effector functions in the generation of NMO lesions in mice. Addition of complement in the absence of effector leukocytes can produce CDC efficiently in various in vitro AQP4-transfected target cells and ex vivo spinal cord explants [27,43]. We found here that AQP4-IgG lacking ADCC effector function, even with enhanced CDC effector function, produced reduced NMO pathology in vivo. Together with data showing reduced pathology in Fc III receptor knockout mice and mice treated with a Fcγ receptor blocking antibody, we conclude that ADCC plays a central pathogenic role in NMO lesions produced by AQP4-IgG. We also confirmed CDC as a primary pathogenic process in NMO, as seen by the absence of NMO lesions with an AQP4-IgG lacking CDC effector function but with enhanced ADCC effector function. Though a limited number of mice were used in the studies here, the findings were robust and clear-cut.
NMO lesion pathology and experimental models have suggested a key role for granulocytes and macrophages in NMO pathogenesis [9]. Neutrophils, eosinophils and macrophages are prominently seen in human NMO lesions [17,19,32]. Studies using the mouse intracerebral injection model show reduced lesion size in mice made neutropenic or hypoeosinophilic, and increased lesion size in mice made neutrophilic or hypereosinophilic [35,44]. Also, addition of purified neutrophil elastase or eosinophil granule toxins worsened astrocyte loss and demyelination in an ex vivo spinal cord slice culture model of NMO. Finally, the neutrophil elastase inhibitor Sivelestat and the second-generation antihistamine Cetirizine (which has eosinophil stabilizing actions) reduced NMO pathology in in vivo and ex vivo mouse models [35,44], suggesting the potential utility of neutrophil- and eosinophil-targeted therapies in NMO. The addition of macrophages to spinal cord cultures also exacerbated AQP4-IgG tissue injury [43], though their role in CNS injury in vivo has not been studied.
Mice express three different activating Fcγ receptors: neutrophils and eosinophils express FcγRIII and FcγRIV, and macrophages express FcγRI, FcγRIII and FcγRIV [41]. Binding of Fcγ receptors to the Fc portion of IgG mediates effector cell activation and promotes ADCC, resulting in effector cell degranulation and target cell lysis or phagocytosis. Since AQP4-IgG lacking ADCC effector function has decreased affinity for Fcγ receptors in general, the reduced pathology could also be partly due to reduced phagocytic activity from infiltrating cells. We found an important role of FcγRIII in AQP4-IgG-mediated ADCC in the intracerebral injection model of NMO. FcγRIII deficient mice showed little cell infiltration and astrocyte damage when administered AQP4-IgG and human complement. Previous data from FcγRIII−/− mice have demonstrated that FcγRIII triggers Arthus reaction and passive cutaneous anaphylaxis [7], IgG1-induced passive systemic anaphylaxis [20], and collagen-induced arthritis [5]. A major role of FcγRIII in NMO lesion formation is consistent with observations that FcγRI is expressed mainly on macrophages and not in neutrophils or eosinophils, which are the immune cells present early in NMO lesions [35] and probably the major mediators of astrocyte damage, as well as the observation that FcγRIV binds mainly mouse IgG2a and IgG2b [23]. Administration of a FcγRII/III blocking antibody reduced pathology, probably by a combination of direct ADCC inhibition and reduced peripheral neutrophil count.
Our findings have implications for new NMO therapeutics. As ADCC aggravates NMO pathology, therapeutics targeting ADCC are predicted to have potential benefit in NMO when used alone or together with therapies targeting CDC, AQP4-IgG binding to CNS astrocytes, or neutrophil/eosinophil degranulation. However, translation of our findings concerning the specific role of FcγRIII in NMO lesions to the human pathology are limited by dissimilarities in the binding specificity and expression pattern of human versus murine Fcγ receptors. For example, in humans FcγRIIIA is not expressed in neutrophils, but only in NK-cells and macrophages [2]. Identification of the Fcγ receptors involved in human NMO pathology is therefore required before development of therapeutics targeting ADCC in NMO.
The data presented here suggest an interplay between CDC and ADCC in the development of NMO pathology. CDC is the principle mechanism causing pathology in many diseases such as paroxysmal nocturnal hemoglobulinuria, hereditary angioedema, age-related macular degeneration, and atypical hemolytic-uremic syndrome [31]. ADCC is the principle effector mechanism employed by antibodies in tumor surveillance and viral clearance. Polymorphisms in FcγRs that affect their binding to IgGs have been associated with altered clinical efficacy of rituximab, an anti-CD20 monoclonal antibody used in the treatment of B-cell lymphomas [41]. It was shown previously that both complement and FcγRs are necessary for pathology in several autoimmune diseases, including rheumatoid arthritis, anti-GBM glomerulonephritis and blistering diseases [18]. Our data support the addition of NMO to this list.
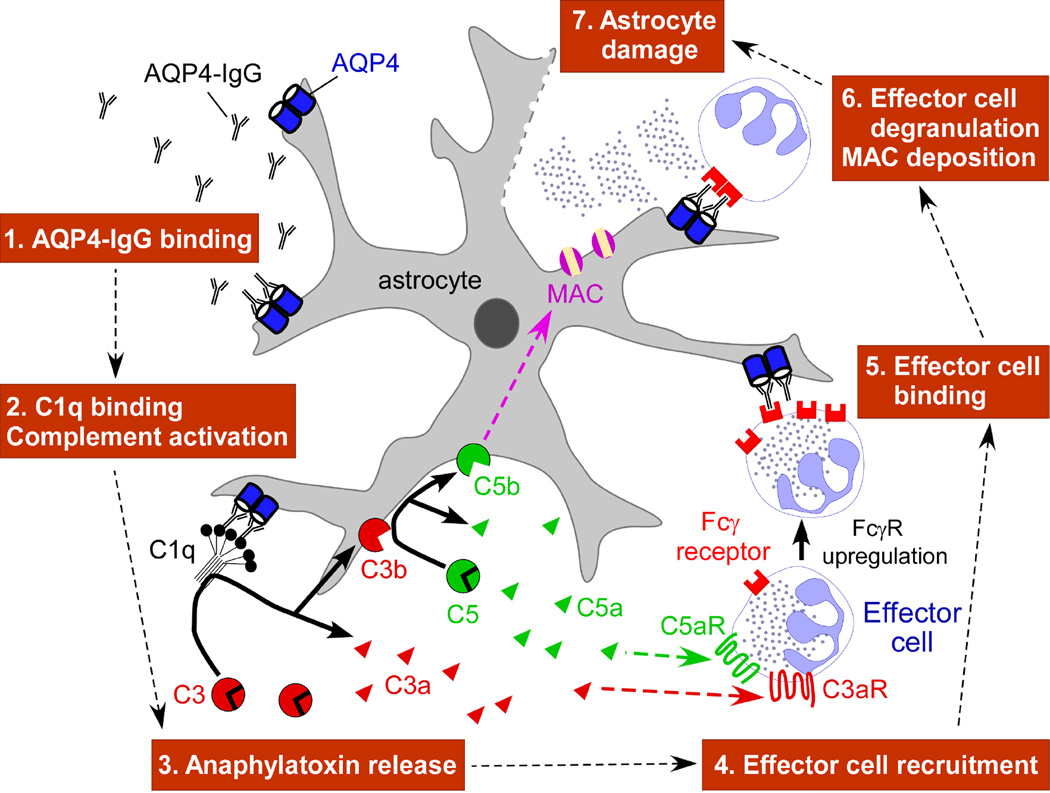
Fig. 6 diagrams a proposed mechanism for AQP4-IgG pathogenesis based on our data. AQP4-IgG enters the CNS, by an unknown mechanism, and binds AQP4 on astrocyte end-feet. This is followed by C1q binding and activation of the complement cascade. Cleavage of complement proteins C3 and C5 produces anaphylatoxins C3a and C5a, which are potent chemoattractants of neutrophils, eosinophils, monocytes and macrophages into the CNS [11]. It has been shown that anaphylatoxins participate in CNS inflammation in several neurodegenerative diseases [11] and increase microvascular permeability [11], which may augment the CNS entry of AQP4-IgG. Importantly, binding of C5a to its receptor on inflammatory cells increases expression of activating FcγRs [36]. Thus, C5a has a dual role in the cross-talk between CDC and ADCC: recruitment of inflammatory cells towards sites of complement activation, and enhancement of ADCC mediated by these cells. The involvement of C5a in NMO is supported by a recent study showing elevation of C5a levels in cerebrospinal fluid of NMO patients [12]. Inflammatory cells that enter the CNS bind the Fc portion of AQP4-IgG through FcγRs, which lead to their degranulation. Secondary oligodendrocyte death, demyelination, and neuronal injury may result from the direct effects of activated complement products such as anaphylatoxins and toxic granules, or indirectly through astrocyte injury.
Figure 6. Proposed roles of CDC and ADCC in NMO pathogenesis.
MAC: membrane attack complex. FcγR: Fcγ receptor.
Engineered monoclonal AQP4-IgGs with altered effector function profiles offer unique opportunities to understand NMO pathogenesis and develop novel therapies. A variety of approaches to modulate IgG effector functions have been developed, involving mutagenesis of the IgG Fc region or alteration in IgG glycosylation [16]. We previously introduced the concept of aquaporumab anti-AQP4 antibodies as a therapeutic strategy in NMO [38]. As a non-pathogenic, high-affinity antibody lacking CDC and ADCC effector functions, aquaporumab competes with pathogenic AQP4-IgG for binding to AQP4, preventing downstream astrocyte cytotoxicity, inflammatory cell recruitment and demyelination. An alternative strategy involving enzymatic deglycosylation of AQP4-IgG was also developed [37]. In addition to the application here to resolve CDC vs. ADCC effector functions, AQP4-IgGs with enhanced effectors functions, or ‘NMO superantibodies’, may be useful in creating more robust animal models of NMO than are currently available, such as passive-transfer models based on peripheral AQP4- IgG administration.
In conclusion, our results indicate the central involvement of both CDC and ADCC in NMO pathogenesis. As such, therapies targeting ADCC are predicted to be effective in NMO.
Acknowledgments
Fundings: This work was supported by grants from the Guthy-Jackson Charitable Foundation (ASV, JLB), grants EY13574, EB00415, DK35124, HL73856, DK86125 and DK72517 (ASV) and EY022936 (JLB) from the National Institutes of Health.
References
- 1.Bennett JL, Lam C, Kalluri SR, Saikali P, Bautista K, Dupree C, Glogowska M, Case D, Antel JP, Owens GP, Gilden D, Nessler S, Stadelmann C, Hemmer B. Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann Neurol. 2009;66(5):617–629. doi: 10.1002/ana.21802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruhns P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood. 2012;119(24):5640–5649. doi: 10.1182/blood-2012-01-380121. [DOI] [PubMed] [Google Scholar]
- 3.Crane JM, Bennett JL, Verkman AS. Live cell analysis of aquaporin-4 m1/m23 interactions and regulated orthogonal array assembly in glial cells. J Biol Chem. 2009;284(51):35850–35860. doi: 10.1074/jbc.M109.071670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crane JM, Lam C, Rossi A, Gupta T, Bennett JL, Verkman AS. Binding affinity and specificity of neuromyelitis optica autoantibodies to aquaporin-4 m1/m23 isoforms and orthogonal arrays. J Biol Chem. 2011;286(18):16516–16524. doi: 10.1074/jbc.M111.227298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diaz de Stahl T, Andren M, Martinsson P, Verbeek JS, Kleinau S. Expression of FcgammaRIII is required for development of collagen-induced arthritis. Eur J Immunol. 2002;32(10):2915–2922. doi: 10.1002/1521-4141(2002010)32:10<2915::AID-IMMU2915>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 6.Gessner JE, Heiken H, Tamm A, Schmidt RE. The IgG Fc receptor family. Ann Hematol. 1998;76(6):231–248. doi: 10.1007/s002770050396. [DOI] [PubMed] [Google Scholar]
- 7.Hazenbos WL, Gessner JE, Hofhuis FM, Kuipers H, Meyer D, Heijnen IA, Schmidt RE, Sandor M, Capel PJ, Daeron M, van de Winkel JG, Verbeek JS. Impaired IgG-dependent anaphylaxis and Arthus reaction in Fc gamma RIII (CD16) deficient mice. Immunity. 1996;5(2):181–188. doi: 10.1016/s1074-7613(00)80494-x. [DOI] [PubMed] [Google Scholar]
- 8.Idusogie EE, Wong PY, Presta LG, Gazzano-Santoro H, Totpal K, Ultsch M, Mulkerrin MG. Engineered antibodies with increased activity to recruit complement. J Immunol. 2001;166(4):2571–2575. doi: 10.4049/jimmunol.166.4.2571. [DOI] [PubMed] [Google Scholar]
- 9.Jacob A, Saadoun S, Kitley J, Leite M, Palace J, Schon F, Papadopoulos MC. Detrimental role of granulocyte-colony stimulating factor in neuromyelitis optica: clinical case and histological evidence. Mult Scler. 2012;18(12):1801–1803. doi: 10.1177/1352458512443994. [DOI] [PubMed] [Google Scholar]
- 10.Jarius S, Wildemann B. AQP4 antibodies in neuromyelitis optica: diagnostic and pathogenetic relevance. Nat Rev Neurol. 2010;6(7):383–392. doi: 10.1038/nrneurol.2010.72. [DOI] [PubMed] [Google Scholar]
- 11.Klos A, Tenner AJ, Johswich KO, Ager RR, Reis ES, Kohl J. The role of the anaphylatoxins in health and disease. Mol Immunol. 2009;46(14):2753–2766. doi: 10.1016/j.molimm.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuroda H, Fujihara K, Takano R, Takai Y, Takahashi T, Misu T, Nakashima I, Sato S, Itoyama Y, Aoki M. Increase of complement fragment C5a in cerebrospinal fluid during exacerbation of neuromyelitis optica. J Neuroimmunol. 2013;254(1–2):178–182. doi: 10.1016/j.jneuroim.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Lazar GA, Dang W, Karki S, Vafa O, Peng JS, Hyun L, Chan C, Chung HS, Eivazi A, Yoder SC, Vielmetter J, Carmichael DF, Hayes RJ, Dahiyat BI. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci U S A. 2006;103(11):4005–4010. doi: 10.1073/pnas.0508123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202(4):473–477. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, Nakashima I, Weinshenker BG. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 16.Liu XY, Pop LM, Vitetta ES. Engineering therapeutic monoclonal antibodies. Immunol Rev. 2008;222:9–27. doi: 10.1111/j.1600-065X.2008.00601.x. [DOI] [PubMed] [Google Scholar]
- 17.Lucchinetti CF, Mandler RN, McGavern D, Bruck W, Gleich G, Ransohoff RM, Trebst C, Weinshenker B, Wingerchuk D, Parisi JE, Lassmann H. A role for humoral mechanisms in the pathogenesis of Devic's neuromyelitis optica. Brain. 2002;125(Pt 7):1450–1461. doi: 10.1093/brain/awf151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mihai S, Nimmerjahn F. The role of Fc receptors and complement in autoimmunity. Autoimmun Rev. 2012 doi: 10.1016/j.autrev.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Misu T, Fujihara K, Kakita A, Konno H, Nakamura M, Watanabe S, Takahashi T, Nakashima I, Takahashi H, Itoyama Y. Loss of aquaporin 4 in lesions of neuromyelitis optica: distinction from multiple sclerosis. Brain. 2007;130(Pt 5):1224–1234. doi: 10.1093/brain/awm047. [DOI] [PubMed] [Google Scholar]
- 20.Miyajima I, Dombrowicz D, Martin TR, Ravetch JV, Kinet JP, Galli SJ. Systemic anaphylaxis in the mouse can be mediated largely through IgG1 and Fc gammaRIII. Assessment of the cardiopulmonary changes, mast cell degranulation, and death associated with active or IgE- or IgG1-dependent passive anaphylaxis. J Clin Invest. 1997;99(5):901–914. doi: 10.1172/JCI119255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore GL, Chen H, Karki S, Lazar GA. Engineered Fc variant antibodies with enhanced ability to recruit complement and mediate effector functions. MAbs. 2010;2(2):181–189. doi: 10.4161/mabs.2.2.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17(1):171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nimmerjahn F, Lux A, Albert H, Woigk M, Lehmann C, Dudziak D, Smith P, Ravetch JV. FcgammaRIV deletion reveals its central role for IgG2a and IgG2b activity in vivo. Proc Natl Acad Sci U S A. 2010;107(45):19396–19401. doi: 10.1073/pnas.1014515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8(1):34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 25.Overdijk MB, Verploegen S, Ortiz Buijsse A, Vink T, Leusen JH, Bleeker WK, Parren PW. Crosstalk between human IgG isotypes and murine effector cells. J Immunol. 2012;189(7):3430–3438. doi: 10.4049/jimmunol.1200356. [DOI] [PubMed] [Google Scholar]
- 26.Papadopoulos MC, Verkman AS. Aquaporin water channels in the nervous system. Nat Rev Neurosci. 2013;14(4):265–277. doi: 10.1038/nrn3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phuan PW, Ratelade J, Rossi A, Tradtrantip L, Verkman AS. Complement-dependent cytotoxicity in neuromyelitis optica requires aquaporin-4 protein assembly in orthogonal arrays. J Biol Chem. 2012;287(17):13829–13839. doi: 10.1074/jbc.M112.344325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pittock SJ, Lennon VA, McKeon A, Mandrekar J, Weinshenker BG, Lucchinetti CF, O'Toole O, Wingerchuk DM. Eculizumab in AQP4-IgG-positive relapsing neuromyelitis optica spectrum disorders: an open-label pilot study. Lancet Neurol. 2013;12(6):554–562. doi: 10.1016/S1474-4422(13)70076-0. [DOI] [PubMed] [Google Scholar]
- 29.Ratelade J, Bennett JL, Verkman AS. Intravenous neuromyelitis optica autoantibody in mice targets aquaporin-4 in peripheral organs and area postrema. PLoS One. 2011;6(11):e27412. doi: 10.1371/journal.pone.0027412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ratelade J, Zhang H, Saadoun S, Bennett JL, Papadopoulos MC, Verkman AS. Neuromyelitis optica IgG and natural killer cells produce NMO lesions in mice without myelin loss. Acta Neuropathol. 2012;123(6):861–872. doi: 10.1007/s00401-012-0986-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roemer SF, Parisi JE, Lennon VA, Benarroch EE, Lassmann H, Bruck W, Mandler RN, Weinshenker BG, Pittock SJ, Wingerchuk DM, Lucchinetti CF. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain. 2007;130(Pt 5):1194–1205. doi: 10.1093/brain/awl371. [DOI] [PubMed] [Google Scholar]
- 33.Saadoun S, Bridges LR, Verkman AS, Papadopoulos MC. Paucity of natural killer and cytotoxic T cells in human neuromyelitis optica lesions. Neuroreport. 2012;23(18):1044–1047. doi: 10.1097/WNR.0b013e32835ab480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saadoun S, Waters P, Bell BA, Vincent A, Verkman AS, Papadopoulos MC. Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain. 2010;133(Pt 2):349–361. doi: 10.1093/brain/awp309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saadoun S, Waters P, Macdonald C, Bell BA, Vincent A, Verkman AS, Papadopoulos MC. Neutrophil protease inhibition reduces neuromyelitis optica-immunoglobulin G-induced damage in mouse brain. Ann Neurol. 2012;71(3):323–333. doi: 10.1002/ana.22686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skokowa J, Ali SR, Felda O, Kumar V, Konrad S, Shushakova N, Schmidt RE, Piekorz RP, Nurnberg B, Spicher K, Birnbaumer L, Zwirner J, Claassens JW, Verbeek JS, van Rooijen N, Kohl J, Gessner JE. Macrophages induce the inflammatory response in the pulmonary Arthus reaction through G alpha i2 activation that controls C5aR and Fc receptor cooperation. J Immunol. 2005;174(5):3041–3050. doi: 10.4049/jimmunol.174.5.3041. [DOI] [PubMed] [Google Scholar]
- 37.Tradtrantip L, Ratelade J, Zhang H, Verkman AS. Enzymatic deglycosylation converts pathogenic neuromyelitis optica anti-aquaporin-4 immunoglobulin G into therapeutic antibody. Ann Neurol. 2013;73(1):77–85. doi: 10.1002/ana.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tradtrantip L, Zhang H, Saadoun S, Phuan PW, Lam C, Papadopoulos MC, Bennett JL, Verkman AS. Anti-Aquaporin-4 monoclonal antibody blocker therapy for neuromyelitis optica. Ann Neurol. 2012;71(3):314–322. doi: 10.1002/ana.22657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Unkeless JC. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979;150(3):580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vincent T, Saikali P, Cayrol R, Roth AD, Bar-Or A, Prat A, Antel JP. Functional consequences of neuromyelitis optica-IgG astrocyte interactions on blood-brain barrier permeability and granulocyte recruitment. J Immunol. 2008;181(8):5730–5737. doi: 10.4049/jimmunol.181.8.5730. [DOI] [PubMed] [Google Scholar]
- 41.Willcocks LC, Smith KG, Clatworthy MR. Low-affinity Fcgamma receptors, autoimmunity and infection. Expert Rev Mol Med. 2009;11:e24. doi: 10.1017/S1462399409001161. [DOI] [PubMed] [Google Scholar]
- 42.Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6(9):805–815. doi: 10.1016/S1474-4422(07)70216-8. [DOI] [PubMed] [Google Scholar]
- 43.Zhang H, Bennett JL, Verkman AS. Ex vivo spinal cord slice model of neuromyelitis optica reveals novel immunopathogenic mechanisms. Ann Neurol. 2011;70(6):943–954. doi: 10.1002/ana.22551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang H, Verkman AS. Eosinophil pathogenicity mechanisms and therapeutics in neuromyelitis optica. J Clin Invest. 2013;123(5):2306–2316. doi: 10.1172/JCI67554. [DOI] [PMC free article] [PubMed] [Google Scholar]