Abstract
In the highly social life of humans, rewards that are sought and experienced are intertwined with social relationships and interactions between people. Just as we value non-social rewards such as food or money, we also value social outcomes (e.g., praise from a superior). We use social information to evaluate and form expectations of others and to make decisions involving others. Here we review research demonstrating how the neural circuitry of reward, particularly the striatum, is also involved in processing social information and making decisions in social situations. This research provides an understanding of the neural basis for social behavior from the perspective of how we evaluate social experiences and how our social interactions and decisions are motivated. We review research addressing the common neural systems underlying evaluation of social and non-social rewards. The human striatum, known to play a key role in reward processing, displays signals related to a broad spectrum of social functioning, including evaluating social rewards, making decisions influenced by social factors, learning about social others, cooperating, competing, and following social norms.
Rewards shape our behavior. Out of a vast space of possible actions, the prospect of a reward helps us select those actions that will lead to the most and best rewards, and motivates us to carry out those actions. For example, the prospect of a delicious meal might motivate someone to travel to a distant restaurant. Rewards are not often experienced in isolation in human society, however. We live social lives and the rewards we seek out and experience are intertwined with the social interactions and relationships we have with other people. We value and seek social outcomes, such as praise or approval from others. In addition, our social relationships and social norms determine how we evaluate experiences and how we learn from them. For example, diners might enjoy a meal among friends more than a meal alone and team leaders might prefer to share a prize equally among members rather than keep it for their selves. A full understanding of the neural computations underlying human reward processing, therefore, must include how we recognize and evaluate social rewards, how our social relationships and interactions alter our reward-seeking behavior, and how we learn from other people.
Our understanding of the neural basis of social rewards and behavior builds upon the rich existing studies on basic reward processing – a literature that has provided a perspective on social behavior in terms of how we evaluate social experiences and how our decisions are motivated in a social context. Specifically, recent research efforts highlight commonalities between neural systems underlying social evaluation and decision making and more well-characterized neural systems of reward processing (e.g., 1–3). A common neural structure observed in studies involving social and non-social reinforcers is the human striatum – the input unit of the basal ganglia and a region that, due to its heterogeneity in terms of anatomical connectivity and involvement in distinct, but parallel processes (e.g., affective, cognitive, motor 1, 4), is in a prime position to influence learning and decision-making in a social context. Here we review ongoing research suggesting that signals in the human striatum are relevant to social information processing, including the processing of social factors that influence how we value experiences, learn from them, and make decisions. We focus primarily on knowledge gained from human neuroimaging research due to the complex and somewhat unique social life of humans.
Overview of key neuroanatomical substrates of reward processing
Across species, a broad neural circuit involved in reward processing has been delineated that features, amongst other regions, midbrain dopaminergic areas, the striatum, and ventral and medial prefrontal cortex for review see 4. A key component of the reward circuit, particularly in humans, is the striatum – a likely area of convergence for affective, cognitive, and motor information given its heterogeneity in terms of connectivity and functionality 5, 6. The connectivity between different parts of these structures sets up corticostriatal circuits in partially segregated loops that are posited to have distinct functions (e.g., motor, cognitive, motivational 5) based on specific subsections that are connected. For instance, cells in the ventromedial area of the striatum connect in loops with ventral and medial areas of the prefrontal cortex, while dorsolateral areas of the striatum connect in loops with dorsolateral prefrontal cortex and motor cortex 4, 5, 7, 8. The ventromedial area of the striatum includes the nucleus accumbens and the ventral and medial aspects of the caudate head and putamen, while the dorsolateral area of the striatum includes the dorsal and lateral areas of the caudate head and putamen (see Figure 1). Connections between striatum and dopaminergic midbrain sites (substantia nigra and ventral tegmental area) also tend to differentiate between ventromedial and dorsolateral areas of the striatum. More specifically, midbrain to striatum connectivity can be described as a spiraling pattern in which more medial midbrain sites connect with ventromedial striatum and more lateral midbrain sites connect with dorsolateral areas of the striatum 8. The majority of these connectivity studies utilize neuroanatomical tracing in non-human species, but structural connectivity imaging techniques in humans have begun to find support for the general organization of the corticostriatal circuitry described here 9. Thus, the pattern of connectivity that the striatum exhibits with cortex, midbrain, and other subcortical areas makes it well-positioned to integrate affective, cognitive, and motor information in order to predict, act for, and evaluate rewards.
Figure 1.
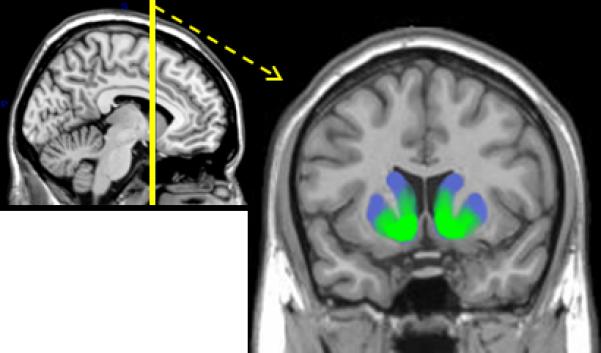
The striatum. Ventromedial areas are colored green and dorsolateral areas are colored blue in the coronal view.
Reward value signals in the striatum
The striatum is a key neural structure involved in reward-related processing, from recognizing and evaluating rewards to learning from them as to predict the best potential reward in the future for review see 1, 10, 11. For primary (e.g., food) and secondary (e.g., money) rewards, striatum activity, as measured by blood oxygen level dependent (BOLD) fMRI signals, has been observed to increase when we encounter cues that predict a likely reward (e.g., a bell rung before mealtime 12, 13) and when we obtain a reward outcome (e.g., receive and consume the meal 14). These changes in striatum BOLD activity coincide with the process of learning how to predict rewards 15, 16 – an observation supported by electrophysiological properties of its dopaminergic afferents.
In a seminal experiment, Schultz, Dayan and Montague 17 recorded from midbrain dopaminergic neurons while non-human primates received liquid rewards. They observed 1) increases in dopamine firing with unexpected deliveries of the reward (a better than expected outcome); 2) increases in dopamine response to cues that predicted the juice reward (the earliest predictor of a reward) rather than to the reward itself over time and; 3) a decrease in firing when a predicted reward was omitted (a worse than expected outcome). This pattern of neural responses is often referred to as a reward prediction error (RPE) signal as it allows an organism to calculate the difference between what was predicted and what is received in order to adjust future expectations and behaviors. The dopamine RPE has provided a potential neurophysiological explanation for computational models describing how individuals learn from reward outcomes 18, 19. Other influential accounts of dopamine function also highlight that dopamine firing to reward cues can reflect the incentive salience of the cue 20 and can in some cases reflect the treatment of the cue as a reward in itself 21. In humans, while the RPE has been seen to activate midbrain structures 22, it has mostly been found to correlate with BOLD signals in the striatum, particularly the ventral striatum during passive learning 23 and dorsal striatum during more instrumental learning 23, 24, with greater levels of correlation between BOLD and RPE indicating better learning and behavioral performance 25.
Interestingly, learning signals in the striatum are present even when feedback is informative but not explicitly rewarding (e.g. feedback that a choice was correct or incorrect) 26, 27. That is, striatum learning signals are not driven exclusively by explicit reward but rather by the informative value of the feedback. This idea is supported by observations in patients with compromised basal ganglia function due to Parkinson's Disease, who show a deficit in learning via trial and error feedback 28. Thus, reward-related signals in the striatum appear to be crucial for alerting and motivating an organism to a reward opportunity, associating predictive cues with reward outcomes, and learning through trial and error in order to adapt behavior to attain rewards in the future1, 10, 11.
A concurrent idea relating to the brain's reward system is that a “value” network is poised to integrate different factors (e.g., magnitude, delay, individual preferences) into a single subjective value signal that allows people to compare different rewards such as money, foods, and consumer products 29, 30. That is, the striatum (along with other components of the reward system such as ventromedial prefrontal cortex discussed elsewhere 31) signals not only the presence or absence of a reward, but also the value of the reward. Higher magnitude rewards elicit greater striatum activity coincident with both reward predictive cues as well as reward outcomes. Furthermore, reward-related signals in the striatum increase with increasing subjective value of the reward (i.e., the value of a reward given a particular individual's personal preferences and motivational states; for review see 32). Collectively, research demonstrates a critical role for the striatum and other regions in providing a signal that integrates all reward information into a subjective value computation that can be used to compare any type of reward to any other type of reward. These signals appear not to be modality specific (i.e., they are evoked by primary food or drug rewards as well as secondary monetary rewards, and by visual as well as auditory reward cues), which allows organisms to pursue a variety of rewards.
Striatal Reward Value Signals in Social Life
Humans use social information to form evaluations and expectations of other people, to modify their evaluations of experienced and potential outcomes, and to make decisions concerning social interactions such as when to trust or cooperate with others. Underlying these functions are calculations of reward value such as the value of a social interaction outcome (e.g., as when a student values praise from a teacher) or the value of an outcome modified by the social context (e.g., as when two teammates value a prize earned by cooperation or when a meal is more enjoyable among friends than alone). Here we review research addressing four broad topics, 1) neural responses to social rewards that mimic neural responses to non-social rewards (e.g., food or money in a non-social context), 2) neural reward-related responses that underlie socially-influenced decision making, 3) neural reward-related responses that underlie learning about other people and interacting with them in cooperative or competitive ways, and 4) neural responses associated with following and enforcing social norms for behavior. We focus predominantly on the striatum due to its key role in reward-related behavior as previously outlined.
Neural responses to social rewards
Social rewards broadly constitute positive experiences (i.e., outcomes or interactions that we seek out) which involve other people. For example, a social reward can be a smile from an attractive person, praise from a teacher, approval from a peer, or a friendly gesture from a new acquaintance. The neural correlates of social rewards have begun to be investigated in humans using fMRI and initial findings suggest that neural responses to social rewards exhibit similarities with neural responses to non-social rewards such as food and money. That is, striatum activity correlates with valued social experiences in a similar manner to non-social rewards. The observation of social stimuli in general (e.g., smiling faces) increases activity in medial prefrontal cortex 33, 34 and striatum in cases where the social stimuli have a high value (e.g., participants will exert effort or pay to view an attractive face) 35–38. Striatum activity also increases when viewing or thinking about people with whom we hold intimate relationships 39, 40, or people that hold high compared to low social rank 41.
The evaluation of social interactions, which may lead to increases in pleasure and impact future decision-making, also seems to involve reward-related processing that occurs in the striatum. For instance, when people learn that they made a positive impression on a new acquaintance, increased subjective feelings of emotion and striatum BOLD signals are observed 2. Moreover, this region of ventral and dorsal striatum responds to both positive social feedback and monetary rewards (scaling accordingly with magnitude for both reinforcers) but not positive social feedback about a third party 2. The ventral striatum also shows evidence of modulation based on receiving positive (i.e., being told another person likes you) compared to neutral or negative social feedback 42, 43. These findings suggest that positive social feedback elicits neural and affective responses similarly to monetary rewards, raising the possibility that neural responses to social rewards in the striatum may underlie socially-influenced behaviors.
Striatal value signals underlie socially-influenced judgments and decisions
Social rewards can influence behaviors just as food and money rewards can influence behavior. People often conform their own judgments to the opinions of other people, act on advice from others, and change their behavior when they know others are watching them. Here, we review research showing that the underlying social context alters the way we evaluate our options in the decision-making process, and this influence is reflected in reward-related processing in the striatum.
Social conformity
We sometimes alter our own evaluations of objects in the environment to conform to the prevailing attitudes of other people, or popular opinion. For example, we might like a song more if other people like it than if other people dislike it. Related to this phenomenon, information about other people's evaluations can influence striatum activity in different ways. In general, striatum activity is greater when we see that the popular opinion agrees rather than disagrees with our own evaluation of an object 44, 45, and is related to our current evaluation of an object after we have seen the popular opinion 46, 47. However, there is mixed evidence concerning whether this activity in the striatum drives conformity to popular opinion. One study observed decreases in ventral striatum activity when participants saw that the popular opinion differed from their own evaluation of a picture 44. In this case, the ventral striatum signal resembled a RPE signal that a) represented the distance between an outcome (i.e., the popular opinion) and a prediction (i.e., the participant's own evaluation) and b) related to a change in the evaluation (i.e., predicted the degree to which participants later changed their evaluations to agree with popular opinion). Also in support of the idea that the striatum influences conformity to popular opinion, ventral striatum activity increases upon seeing a consensus among other people that a financial risk is a good option, and greater ventral striatum activity is related to choices that are more heavily influenced by other people's decisions 48. Other research shows, however, that ventral striatum activity while viewing other people's evaluations is not necessarily related to social conformity 45, or even related to independence from conformity49. That is, ventral striatum activity increased when other people with good reputations agreed with a participants’ evaluation, but other brain regions, including medial prefrontal cortex, predicted changes to participants’ opinions rather than ventral striatum 45. Striatum activity is also increased when participants give an objectively correct answer that disagrees with other people49. Social conformity, as reviewed here, may be regarded as a process involving several steps, including forming an evaluation, interpreting information about others’ evaluations, and updating (or not) an evaluation. The exact neural mechanisms underlying evaluation change due to social conformity are still to be resolved by further research, but the existing research suggests that the striatum plays a role in the process.
Following advice
Social influence need not always come from a motivation to conform to other people's opinions. Sometimes, other people may have advice that can help us make better decisions. Whereas social conformity involves altering our evaluations to accord with popular opinion, following advice involves altering our evaluations based on information from someone who is perceived to have valuable information. The influence of advice may come from definite endorsement of an option by an expert, such as a music reviewer giving a positive review for a song50, or a more subtle association between an expert and an option, such as a celebrity athlete appearing to be associated with a sports product51. Value signals in the striatum appear to reflect a) how we evaluate the advice itself (e.g., valuing an expert's opinion) 52, b) how much the advice changes our opinion (e.g., valuing an option more highly after it is endorsed by an expert) 50, 51, and c) how we evaluate new information that agrees with or conflicts with advice we have received (e.g., valuing positive information about an expertly endorsed stock but discounting negative information) 53. Specifically, knowing that advice is coming from an expert rather than a novice increases ventral striatum activity prior to actually receiving advice 52. Once we receive expert advice, increased activity in ventromedial and dorsomedial striatum might either reflect the social reward experienced by agreeing with an expert 50, 52, or the higher value placed on a decision option 50, 51 . For example, ventromedial striatum activity increases when experts agree with our own preference for a song 50. Findings from the same study suggest that ventromedial striatum activity when we receive an outcome (i.e., a token to purchase our preferred song) is related to whether we change our own evaluation after learning the experts’ evaluation50. Advice also influences striatal signals related to learning about our decisions. Ventral striatum activity shows a weaker distinction between positive and negative outcomes of advice-based choices compared to outcomes of choices without advice 53. This finding suggests that positive outcomes may be treated as more positive (i.e., better indicators of a good choice) and negative outcomes may be treated as less negative (i.e., more easily dismissed as an anomalous outcome of a good choice) when the outcome results from a choice that was advised by another person. Much like people will stick to a decision when they believe the odds are higher 25, people are more likely to stick with an advised choice in the face of negative outcomes 53. While striatal responses appear to reflect the value of advice as well as its influence on decisions, other research suggests that dorsomedial prefrontal cortex activity is involved in determining the validity of advice from a questionable source 3. Finally, ventromedial prefrontal cortex appears to integrate information concerning advice from others, the validity of the advice, and the value of decision options 3, 52.
Altering behavior when others are watching
Even when other people provide no feedback or interaction, the mere presence of another person can affect how reward information is perceived and processed in the brain, leading to influences on reward-related behavior (e.g., peer pressure to take risks or make a charitable donation). For example, the experience and evaluation of an objectively quantifiable reward such as a $2 gain on a gamble is modified by the presence of another person 54. Specifically, when a monetary gain is shared with a friend (compared to a stranger or a computer) people rate the experience as more exciting, and striatum activity is increased (see Figure 2). The modulation of striatal responses by the presence of others can also result in changes in behavior. Adolescents tend to make more risky decisions when in the presence of their peers and this phenomenon is associated with increased ventral striatum responses to the potential rewards of risky decisions such as speeding through a yellow traffic light 55. It remains unclear whether increased striatal responses are related to increased value of the potential positive outcomes (e.g., experiencing greater thrills from positive outcomes in the presence of peers) or increased expectation of a positive outcome (e.g., greater optimism in the presence of peers). With monetary rewards, research shows that ventral striatum activity can signal both anticipated reward magnitude and probability 56, 57. Interestingly, other research shows that adolescents, like adults, display striatum responses to positive social feedback 42, 43 (i.e., being liked), but also that adolescents higher in social anxiety display an increased striatum response to positive relative to negative social feedback 42. It may be that adolescents alter their behavior (i.e., take more risks) in an effort to seek out positive social feedback. Indeed, positive social feedback and general social support may play an important role in regulating behavior, and recent research suggests that striatal responses can underlie affect-regulating effects of social support 58. One direction for research in this area might be to incorporate research on the cognitive regulation of reward-related responses (see sidebar 2) with manipulations of social encouragement to understand how social support can influence neural, affective, and behavioral responses in reward-related behavior.
Figure 2.
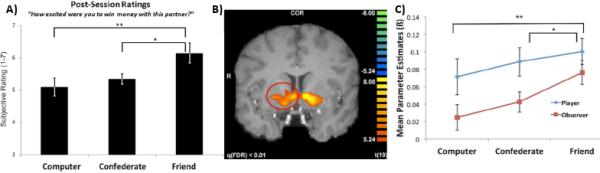
Striatum response to monetary rewards is modulated by the social context. A) In a card guessing task14, participants are more excited to receive monetary rewards when they are shared with a friend compared to a stranger or computer. B) Striatum activity increases for monetary gains compared to losses. C) Striatum activity (from region circled in part B) to monetary gains is greater when sharing the rewards with a friend. Figure adapted from Fareri and colleagues54.
Giving to others
The knowledge that we are being watched can also motivate behaviors that benefit other people even at a cost to oneself (e.g., donate money to a charity). In such cases, social reward (e.g., gaining a good reputation as a philanthropist) has its own value that offsets the loss of other valued resources (e.g., money). Several studies link activity in the striatum with giving behavior, suggesting that striatum activity signals the benefits of giving 59–63. For example, people make more charitable donations when they believe their decisions are being watched, and ventral striatum activity is greater when making a donation that is watched by others compared to an anonymous donation 59. Other research shows that striatum activity is influenced not only by the enhanced reputation when others witness a donation, but other benefits of giving as well. That is, ventral and dorsal striatum appear to signal a vicarious reward when other parties (i.e., a charity or another person) receive rewards, regardless of whether any enhanced reputation can be gained 60–63. In particular, people value seeing charities they think are important 59 or people similar to themselves receive rewards 62. Interestingly, people value vicarious rewards and exhibit increased ventral striatum activity even when they are not responsible for the reward 61–63. However, people are more satisfied when their own free choice (i.e., donation) benefits a charity and they exhibit greater ventral striatum activity compared to when the charity benefits the same amount without the donator's choice 61, 64. Moreover, striatal activity may underlie difficult giving decisions, such as how to efficiently and equitably allocate limited resources to others 65. On the whole this area of research shows that acts of giving can be valuable for many reasons (e.g., reputation benefit, vicarious reward, satisfaction of giving, efficiency, equity) and that striatum activity integrates these sources of value in a manner resembling the representation of subjective value for non-social rewards 32. These striatal responses provide a possible mechanism for humans to value actions and outcomes that do not directly benefit themselves but that help maintain social relationships, which are integral to individual survival.
Striatum value signals relate to learning about, cooperating with, and competing with other people
We learn about other people by interacting with them directly as well as by observing others acting without our involvement. These experiences influence the impressions that we form of other people and inform our decisions about how to interact with others. We learn to appreciate others and how to effectively cooperate and compete with others. The neural signals underlying these types of social learning display similarities to neural signals underlying learning from non-social rewards. Here we review research demonstrating that signals in the striatum change as we learn about other people in a manner that allows us to form predictions about the outcomes of our interactions with other people.
Learning about others
One simple way that we learn about other people is by experiencing positive and negative interactions with them. When we have a positive interaction with a new acquaintance, such as receiving a compliment or a friendly gesture, activity in ventral striatum increases 2, 66. Moreover, striatal responses transform in a manner that reflects what we have learned to expect from a person after repeated interaction with them – that is, the acquisition of a reputation 67. This pattern of striatal activity while learning from social information resembles the reward prediction error learning signal exhibited in the striatum while learning from monetary or food rewards 38, 66, 67. For example, ventral striatum responses to social experiences (e.g., receiving a friendly gesture from a person) index the degree to which a social experience differs from what was expected 66. That is, a friendly gesture elicits a larger increase in ventral striatum activity when it comes from someone who has rarely been friendly in the past compared to someone who is usually friendly. This signal may play a role in changing our evaluations of others (e.g., how much we like them, how competent we think they are) 66, 68, 69 and even in changing evaluations of ourselves 70. Moreover, striatum activity increases in anticipation of a positive social interaction after repeated interactions with one person, suggesting that this neural signal is important for learning to predict others’ behavior 67. The ability to learn from and predict others’ behavior becomes very important in two-way interactions, where we are not just receiving information about others but also making decisions that affect others, such as a deciding to cooperate with another person.
Cooperating with others
Activity in the striatum displays properties consistent with representing the value of cooperation. People can sometimes gain larger rewards by cooperating with others than by acting alone. Socially-interactive economic games have provided a useful way of operationalizing cooperation in research. In such games, participants might choose to cooperate or not cooperate with a partner (who is often described as another participant in the study) and then receive outcomes (e.g. monetary rewards) depending on whether their partner chose to reciprocate their cooperation or not. As with rewards such as money or positive social feedback, ventral and dorsal striatum activity increases when receiving rewards as a result of reciprocated cooperation and when making a decision to cooperate 67, 71, 72. Evidence suggests that striatum activity represents more than just the outcome gained by cooperation. That is, earning a reward by cooperation with a partner elicits greater ventral striatum activity compared to earning an equivalent reward in a non-social context and even compared to earning a larger reward by not cooperating at the expense of one's partner 71. Along with other research on shared rewards 54 (see Figure 2), this research suggests that humans may value the experience of cooperation over and above the objective value of rewards yielded by cooperation 73, 74. This increased value of cooperation can be beneficial because consistent cooperative behavior may often result in greater long-term gains 71.
Decisions to cooperate with another person are colored by the information we have learnt about the person thus far. Further research utilizing cooperative economic games has shown that the prior reputation of a partner alters striatum responses to reciprocated or non-reciprocated cooperation and influences decisions to cooperate or not 75–79. For example, a first impression of a partner can bias the way we process interactions with them 80. Once we initially make a positive impression of someone, we are more likely to continue cooperating with that person even when they fail to consistently reciprocate our cooperation. Furthermore, ventral striatum activity does not distinguish between reciprocated versus non-reciprocated cooperation to the same degree when we have a prior (good or bad) first impression of the partner compared to when we have a neutral first impression of the partner 78. This finding parallels the effect of advice on outcomes of risk decisions 53 and suggests that biases introduced by social information can affect our experience of outcomes and the way we learn from them. Further research has specifically examined how prior social information (i.e., a good or bad first impression) influences neural activity related to learning about others through positive and negative interactions 77. This research suggests that, whereas striatal responses correlate with learning to trust a new partner with no prior knowledge, lateral prefrontal cortex may bring declarative knowledge to bear on further decisions to cooperate with the partner, similarly to the manner in which declarative knowledge is brought to bear on non-social reward learning 81. Future research on this topic promises further insight into how we evaluate other people and shape our interactions with them.
Competing with others
Whereas cooperation can often yield increased rewards, humans still value the experience of outperforming others and having higher status than others. Research has suggested that reward signals in the striatum are sensitive to comparisons of our own reward outcomes and status with those of other people. Even when our own outcomes do not depend on other people, we prefer when our own outcomes are better than someone else's and we make decisions to try to gain better outcomes than someone else 82, 83. Ventral striatum activity is greater when we gain a larger reward than someone else or even lose less than someone else 82–84. These socially comparative reward responses influence our behavior, even when our own outcomes do not depend on others’ actions. For example, people who show a larger ventral striatal increase for outperforming another person will take more risks to outperform someone else 83. Evidence suggests that ventral striatal responses to reward outcomes are comparable regardless of whether the competitor is a close friend or a stranger, and indeed we are as competitive (or more) with friend as with a stranger 85. Sensitivity of striatal activity to social comparison may serve a purpose to increase or maintain social status. Consistent with a role in tracking social status, ventral striatal activity is modulated by the relative ranking of social others, especially in an unstable hierarchy 41, 86. In addition to comparing our own outcomes with others’ outcomes, we also prefer for our groups to do better than other groups. That is, sports fans experience greater positive emotions and increased ventral striatum activity when seeing their favorite team do well or their rival team do poorly in reference to a neutral team 87. Ventral striatum responses are also involved in decisions where we are in direct competition with someone else, as in the case of bidding for an item in an auction. That is, the social competition inherent in an auction drives overbidding and is related to striatal value computations for winning and losing an auction 88, 89. Additionally, ventral striatum and medial prefrontal cortex signals encode information learned about strategic actions of competitors 72, 88, 90. These findings suggest an integral role for the striatum in engaging in and learning from competitive interactions.
Striatum value signals associated with following and enforcing social norms
Social norms specify what patterns of behavior are expected or acceptable in social interactions. Increasing evidence demonstrates that striatal signals underlying reward valuation and decision making incorporate commonly accepted social norms, in a manner consistent with motivating behavior in ourselves as well as others that abides by social norms. One commonly accepted social norm is that people should act in ways that promote fairness. In research, fairness has been operationalized as the relatively equal distribution of monetary rewards. Whereas increased responses in insular cortex have been associated with perceived unfairness of monetary outcomes 91, a growing literature suggests that striatum responses reflect the positive value of fair outcomes 73, 92, 93. For example, in an economic exchange game termed the ultimatum game, a player decides whether to accept a monetary amount from a second player who has been given a conditional monetary endowment. If the first player accepts the offer, both players receive money according to the second player's offer. If the first player rejects the offer then neither player gets any money. Fair offers are close to a 50/50 split of the endowment between the two players; unfair offers occur when the second player attempts to keep most of the endowment and offers little to the first player. Research shows that the first player experiences increased positive emotion and ventral striatum and medial prefrontal cortex activity when receiving fair offers from the second player, independently of the material outcome 93. Ventral striatum and medial prefrontal cortex show a similar response to outcomes that restore equality amongst partners even when they are not directly interacting 92.
One perspective on social norms is that they provide expectations about what should happen. Consistent with the human ventral striatum association with RPE, ventral striatum and medial prefrontal cortex responses to ultimatum game offers correlate with the difference between social expectations and actual outcomes, even when expectations of fairness are altered by repeated experiences 94. Striatum responses appear more closely related to the fairness of others’ actions than the fairness of our own actions towards others. Ventral striatum activity, as well as medial prefrontal cortex, increases when people decide to unfairly keep a monetary endowment instead of share it with a partner (i.e., keep more than the partner expects according to social norms) 95. Furthermore, people who are less troubled by the guilt of acting unfairly towards others exhibit greater ventral striatum activity, suggesting that ventral striatum activity during one's own unfair actions is more tightly linked to the material reward than the social consequences.
One way that social norms are enforced is through punishment to norm violators. The striatum displays signals that are related to making a decision to punish someone for acting unfairly 96, 97, as well as related to observing a punishment meted out to someone who has acted unfairly 98. Increased dorsal striatum activity is associated with a decision to punish a partner who has acted unfairly, even when we must incur a cost to punish the partner 96, 97. One idea is that people experience a positive feeling, or schadenfreude, upon seeing a norm violator receive a deserved misfortune. This feeling can occur even when the misfortune is not a direct punishment for the norm violation. For example, when someone acts uncooperatively with us we might later feel joy if that person bumps their head in a doorway. In certain conditions, ventral striatum activity appears related to seeing others receive a deserved misfortune. That is, in male participants, ventral striatum activity increases when passively seeing partner, who has previously acted unfairly, incur pain 98. Furthermore, the level of ventral striatum activation is related to male participants’ desire for revenge against the unfair partner98. Interestingly, decisions to punish a partner's unfairness (e.g., refuse an unfair ultimatum game offer) can be considered disadvantageous in cases where there is no further interaction with the partner. Indeed, decisions to punish unfairness at a personal cost are associated with impulsivity 99, and serotonin appears to play an important role in regulating striatal responses that influence punishment decisions 97. These findings suggest complex relationships between expectations, outcomes, and emotions as well as interactions between striatum, medial prefrontal cortex, and insula, which are explored in ongoing research 100, 101.
Conclusion
Research reviewed here highlights signals in the striatum that appear to play a role in social behavior and display properties that enable humans to evaluate and learn from both non-social and social experiences. Signals in the striatum are related to a broad spectrum of social functions, including the evaluation of social rewards, the modulation of reward experiences and behavior by social relationships and interactions, the process of learning about social others, and following and enforcing social norms. Along with the striatum, other brain sites including the amygdala, ventromedial prefrontal cortex, and lateral prefrontal cortex play roles in reward processing and social behavior. Activity in these brain networks allows for flexible changes in evaluations of stimuli in the social world. Exciting directions for future research include improving our understanding of how social encouragement and support can promote beneficial behavior and better understanding links between social relationships and the brain mechanisms regulating reward-related behavior.
Cognitive regulation of reward-related responses and cortico-striatal interactions.
One mechanism by which social factors may influence reward-seeking behavior is by increasing cognitive regulation of reward-related responses. That is, social support may bolster efforts to focus on healthy eating, smoking cessation, or other forms of self-regulation of reward seeking 102. Neuroimaging research has probed cortico-striatal activity when people use cognitive strategies to regulate their response to a reward. Strategies include mentally distancing oneself from a reward, focusing on future consequences, or focusing on an alternative goal. Lateral prefrontal cortex activity increases when people use cognitive strategies to regulate responses to food cues 103–105, smoking cues 106, or monetary reward cues 107, 108. This increase in lateral prefrontal cortex activity coincides with diminished activity in the striatum (compared to the striatum response when no cognitive strategy is employed) 106–108. Increased lateral prefrontal activity has been linked with healthier food choices 104 and decreased striatum activity following regulation has been associated with a decrease in risk taking in monetary decisions 107. One suggestion is that cognitive strategies recruit lateral prefrontal activity to down-regulate striatum activity, which diminishes appetitive responses 106. Alternatively, lateral prefrontal cortex activity may alter value computations in ventromedial prefrontal cortex, such that alternative goals (e.g., health) are incorporated into the computation of a reward's value 104. Future research might explore how these neural systems underlying self-regulation may be influenced by social factors and whether social support may bolster self-regulatory efforts by influencing these neural processes.
Acknowledgements
The authors were supported by funds from the National Institute of Mental Health (to M.R.D., NIMH 084081) and the National Science Foundation (to J.P.B., NSF SPRF).
Contributor Information
Jamil P. Bhanji, Department of Psychology, Rutgers University, Newark, NJ
Mauricio R. Delgado, Department of Psychology, Rutgers University, Newark, NJ.
References
- 1.Delgado MR. Reward-related responses in the human striatum. Ann NY Acad Sci. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- 2.Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008:58:284–294. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 3.Behrens TEJ, Hunt LT, Woolrich MW, Rushworth MFS. Associative learning of social value. Nature. 2008;456:245–249. doi: 10.1038/nature07538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 6.Cardinal RN, Parkinson J a, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–52. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 7.Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- 8.Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehéricy S, Ducros M, Van de Moortele P-F, Francois C, Thivard L, Poupon C, Swindale N, Ugurbil K, Kim D-S. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann Neurol. 2004;55:522–9. doi: 10.1002/ana.20030. [DOI] [PubMed] [Google Scholar]
- 10.Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr Opin Neurobiol. 2005;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- 11.Liljeholm M, O'Doherty JP. Contributions of the striatum to learning, motivation, and performance: an associative account. Trends Cogn Sci. 2012;16:467–75. doi: 10.1016/j.tics.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- 14.Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- 15.O'Doherty J, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38:329–337. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- 16.McClure SM, Berns GS, Montague PR. Temporal prediction errors in a passive learning task activate human striatum. Neuron. 2003;38:339–346. doi: 10.1016/s0896-6273(03)00154-5. [DOI] [PubMed] [Google Scholar]
- 17.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 18.Rescorla RA, Wagner AR. A theory of Pavolovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical Conditioning II. Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- 19.Sutton RS, Barto AG. Reinforcement Learning. MIT Press; Cambridge, MA: 1998. [Google Scholar]
- 20.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 21.Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry. 2009;65:869–73. doi: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D'Ardenne K, McClure SM, Nystrom LE, Cohen JD. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science. 2008;319:1264–1267. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- 23.O'Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- 24.Tricomi EM, Delgado MR, Fiez J a. Modulation of caudate activity by action contingency. Neuron. 2004;41:281–92. doi: 10.1016/s0896-6273(03)00848-1. [DOI] [PubMed] [Google Scholar]
- 25.Schonberg T, Daw ND, Joel D, O'Doherty JP. Reinforcement learning signals in the human striatum distinguish learners from nonlearners during reward-based decision making. J Neurosci. 2007;27:12860–12867. doi: 10.1523/JNEUROSCI.2496-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tricomi E, Fiez JA. Information content and reward processing in the human striatum during performance of a declarative memory task. Cogn Affect Behav Neurosci. 2012;12:361–372. doi: 10.3758/s13415-011-0077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez PF, Aron AR, Poldrack RA. Ventral-striatal/nucleus-accumbens sensitivity to prediction errors during classification learning. Hum Brain Mapp. 2006;27:306–13. doi: 10.1002/hbm.20186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shohamy D, Myers CE, Onlaor S, Gluck M a. Role of the basal ganglia in category learning: how do patients with Parkinson's disease learn? Behav Neurosci. 2004;118:676–86. doi: 10.1037/0735-7044.118.4.676. [DOI] [PubMed] [Google Scholar]
- 29.Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chib VS, Rangel A, Shimojo S, O'Doherty JP. Evidence for a common representation of decision values for dissimilar goods in human ventromedial prefrontal cortex. J Neurosci. 2009;29:12315–20. doi: 10.1523/JNEUROSCI.2575-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rangel A, Hare T. Neural computations associated with goal-directed choice. Curr Opin Neurobiol. 2010;20:262–70. doi: 10.1016/j.conb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Peters J, Büchel C. Neural representations of subjective reward value. Behav Brain Res. 2010;213:135–141. doi: 10.1016/j.bbr.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 33.O'Doherty J, Winston J, Critchley H, Perrett D, Burt DM, Dolan RJ. Beauty in a smile: the role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia. 2003;41:147–155. doi: 10.1016/s0028-3932(02)00145-8. [DOI] [PubMed] [Google Scholar]
- 34.Cunningham WA, Johnson MK, Gatenby JC, Gore JC, Banaji MR. Neural components of social evaluation. J Pers Soc Psychol. 2003;85:639–49. doi: 10.1037/0022-3514.85.4.639. [DOI] [PubMed] [Google Scholar]
- 35.Aharon I, Etcoff N, Ariely D, Chabris CF, O'Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32:537–551. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- 36.Smith D V, Hayden BY, Truong T-K, Song AW, Platt ML, Huettel SA. Distinct value signals in anterior and posterior ventromedial prefrontal cortex. J Neurosci. 2010;30:2490–2495. doi: 10.1523/JNEUROSCI.3319-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spreckelmeyer KN, Krach S, Kohls G, Rademacher L, Irmak A, Konrad K, Kircher T, Gründer G. Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Soc Cogn Affect Neurosci. 2009;4:158–165. doi: 10.1093/scan/nsn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin A, Adolphs R, Rangel A. Social and monetary reward learning engage overlapping neural substrates. Soc Cogn Affect Neurosci. 2012;7:274–281. doi: 10.1093/scan/nsr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fisher HE, Aron A, Brown LL. Romantic love: a mammalian brain system for mate choice. Philos Trans R Soc Lond B Biol Sci. 2006;361:2173–2186. doi: 10.1098/rstb.2006.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hughes BL, Beer JS. Orbitofrontal cortex and anterior cingulate cortex are modulated by motivated social cognition. Cerebral Cortex. 2012;22:1372–1381. doi: 10.1093/cercor/bhr213. [DOI] [PubMed] [Google Scholar]
- 41.Zink CF, Tong Y, Chen Q, Bassett DS, Stein JL, Meyer-Lindenberg A. Know your place: neural processing of social hierarchy in humans. Neuron. 2008;58:273–283. doi: 10.1016/j.neuron.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gunther Moor B, van Leijenhorst L, Rombouts SARB, Crone EA, Van der Molen MW. Do you like me? Neural correlates of social evaluation and developmental trajectories. Soc Neurosci. 2010;5:461–482. doi: 10.1080/17470910903526155. [DOI] [PubMed] [Google Scholar]
- 43.Davey CG, Allen NB, Harrison BJ, Dwyer DB, Yücel M. Being liked activates primary reward and midline self-related brain regions. Hum Brain Mapp. 2010;31:660–668. doi: 10.1002/hbm.20895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klucharev V, Hytönen K, Rijpkema M, Smidts A, Fernández G. Reinforcement learning signal predicts social conformity. Neuron. 2009;61:140–151. doi: 10.1016/j.neuron.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 45.Izuma K, Adolphs R. Social manipulation of preference in the human brain. Neuron. 2013;78:563–73. doi: 10.1016/j.neuron.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mason MF, Dyer R, Norton MI. Neural mechanisms of social influence. Organ Behav Hum Decis Process. 2009;110:152–159. [Google Scholar]
- 47.Zaki J, Schirmer J, Mitchell JP. Social influence modulates the neural computation of value. Psychol Sci. 2011;22:894–900. doi: 10.1177/0956797611411057. [DOI] [PubMed] [Google Scholar]
- 48.Burke CJ, Tobler PN, Schultz W, Baddeley M. Striatal BOLD Response Reflects the Impact of Herd Information on Financial Decisions. Front Hum Neurosci. 2010;4:48. doi: 10.3389/fnhum.2010.00048. June. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berns GS, Chappelow J, Zink CF, Pagnoni G, Martin-Skurski ME, Richards J. Neurobiological correlates of social conformity and independence during mental rotation. Biol Psychiatry. 2005;58:245–53. doi: 10.1016/j.biopsych.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 50.Campbell-Meiklejohn DK, Bach DR, Roepstorff A, Dolan RJ, Frith CD. How the opinion of others affects our valuation of objects. Curr Biol. 2010;20:1165–1170. doi: 10.1016/j.cub.2010.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klucharev V, Smidts A, Fernández G. Brain mechanisms of persuasion: how “expert power” modulates memory and attitudes. Soc Cogn Affect Neurosci. 2008;3:353–66. doi: 10.1093/scan/nsn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meshi D, Biele G, Korn CW, Heekeren HR. How expert advice influences decision making. PLoS One. 2012;7:1–12. doi: 10.1371/journal.pone.0049748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Biele G, Rieskamp J, Krugel LK, Heekeren HR. The neural basis of following advice. PLoS Biol. 2011;9:1–11. doi: 10.1371/journal.pbio.1001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fareri DS, Niznikiewicz MA, Lee VK, Delgado MR. Social network modulation of reward-related signals. J Neurosci. 2012;32:9045–9052. doi: 10.1523/JNEUROSCI.0610-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chein J, Albert D, O'Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain's reward circuitry. Dev Sci. 2011;14:F1–10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. J Neurosci. 2005;25:4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Preuschoff K, Bossaerts P, Quartz SR. Neural differentiation of expected reward and risk in human subcortical structures. Neuron. 2006;51:381–390. doi: 10.1016/j.neuron.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 58.Onoda K, Okamoto Y, Nakashima K, Nittono H, Ura M, Yamawaki S. Decreased ventral anterior cingulate cortex activity is associated with reduced social pain during emotional support. Soc Neurosci. 2009;4:443–454. doi: 10.1080/17470910902955884. [DOI] [PubMed] [Google Scholar]
- 59.Izuma K, Saito DN, Sadato N. Processing of the incentive for social approval in the ventral striatum during charitable donation. J Cogn Neurosci. 2010;22:621–631. doi: 10.1162/jocn.2009.21228. [DOI] [PubMed] [Google Scholar]
- 60.Moll J, Krueger F, Zahn R, Pardini M, de Oliveira-Souza R, Grafman J. Human fronto-mesolimbic networks guide decisions about charitable donation. Proc Natl Acad Sci U S A. 2006;103:15623–15628. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harbaugh WT, Mayr U, Burghart DR. Neural responses to taxation and voluntary giving reveal motives for charitable donations. Science. 2007;316:1622–1625. doi: 10.1126/science.1140738. [DOI] [PubMed] [Google Scholar]
- 62.Mobbs D, Yu R, Meyer M, Passamonti L, Seymour B, Calder AJ, Schweizer S, Frith CD, Dalgleish T. A key role for similarity in vicarious reward. Science. 2009;324:900. doi: 10.1126/science.1170539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuss K, Falk A, Trautner P, Elger CE, Weber B, Fliessbach K. A reward prediction error for charitable donations reveals outcome orientation of donators. Soc Cogn Affect Neurosci. 2013;8:216–223. doi: 10.1093/scan/nsr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hare TA, Camerer CF, Knoepfle DT, Rangel A. Value computations in ventral medial prefrontal cortex during charitable decision making incorporate input from regions involved in social cognition. J Neurosci. 2010;30:583–590. doi: 10.1523/JNEUROSCI.4089-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hsu M, Anen C, Quartz SR. The right and the good: distributive justice and neural encoding of equity and efficiency. Science. 2008;320:1092–1095. doi: 10.1126/science.1153651. [DOI] [PubMed] [Google Scholar]
- 66.Jones RM, Somerville LH, Li J, Ruberry EJ, Libby V, Glover G, Voss HU, Ballon DJ, Casey BJ. Behavioral and neural properties of social reinforcement learning. J Neurosci. 2011;31:13039–13045. doi: 10.1523/JNEUROSCI.2972-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.King-Casas B, Tomlin D, Anen C, Camerer CF, Quartz SR, Montague PR. Getting to know you: reputation and trust in a two-person economic exchange. Science. 2005;308:78–83. doi: 10.1126/science.1108062. [DOI] [PubMed] [Google Scholar]
- 68.Bhanji JP, Beer JS. Dissociable neural modulation underlying lasting first impressions, changing your mind for the better, and changing it for the worse. J Neurosci. 2013;33:9337–9344. doi: 10.1523/JNEUROSCI.5634-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harris LT, Fiske ST. Neural regions that underlie reinforcement learning are also active for social expectancy violations. Soc Neurosci. 2010;5:76–91. doi: 10.1080/17470910903135825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Korn CW, Prehn K, Park SQ, Walter H, Heekeren HR. Positively biased processing of self-relevant social feedback. J Neurosci. 2012;32:16832–16844. doi: 10.1523/JNEUROSCI.3016-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rilling J, Gutman D, Zeh T, Pagnoni G, Berns G, Kilts C. A neural basis for social cooperation. Neuron. 2002;35:395–405. doi: 10.1016/s0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- 72.Yoshida W, Seymour B, Friston KJ, Dolan RJ. Neural mechanisms of belief inference during cooperative games. J Neurosci. 2010;30:10744–10751. doi: 10.1523/JNEUROSCI.5895-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tabibnia G, Lieberman MD. Fairness and Cooperation Are Rewarding Evidence from Social Cognitive. Neuroscience. 2007;9747:90–101. doi: 10.1196/annals.1412.001. [DOI] [PubMed] [Google Scholar]
- 74.Decety J, Jackson PL, Sommerville JA, Chaminade T, Meltzoff AN. The neural bases of cooperation and competition: an fMRI investigation. Neuroimage. 2004;23:744–751. doi: 10.1016/j.neuroimage.2004.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Phan KL, Sripada CS, Angstadt M, McCabe K. Reputation for reciprocity engages the brain reward center. Proc Natl Acad Sci U S A. 2010;107:13099–13104. doi: 10.1073/pnas.1008137107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fareri DS, Chang LJ, Delgado MR. Effects of direct social experience on trust decisions and neural reward circuitry. Front Neurosci. 2012;6:148. doi: 10.3389/fnins.2012.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fouragnan E, Chierchia G, Greiner S, Neveu R, Avesani P, Coricelli G. Reputational priors magnify striatal responses to violations of trust. J Neurosci. 2013;33:3602–11. doi: 10.1523/JNEUROSCI.3086-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Delgado MR, Frank RH, Phelps EA. Perceptions of moral character modulate the neural systems of reward during the trust game. Nat Neurosci. 2005;8:1611–1618. doi: 10.1038/nn1575. [DOI] [PubMed] [Google Scholar]
- 79.Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58:639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 80.Reeder GD, Brewer MB. A Schematic Model of Dispositional Attribution in Interpersonal Perception. Psychological Review. 1979;86:61–79. [Google Scholar]
- 81.Li J, Delgado MR, Phelps EA. How instructed knowledge modulates the neural systems of reward learning. Proc Natl Acad Sci U S A. 2011;108:55–60. doi: 10.1073/pnas.1014938108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dvash J, Gilam G, Ben-Ze'ev A, Hendler T, Shamay-Tsoory SG. The envious brain: the neural basis of social comparison. Hum Brain Mapp. 2010;31:1741–50. doi: 10.1002/hbm.20972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bault N, Joffily M, Rustichini A, Coricelli G. Medial prefrontal cortex and striatum mediate the influence of social comparison on the decision process. Proc Natl Acad Sci U S A. 2011;108:16044–16049. doi: 10.1073/pnas.1100892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fliessbach K, Weber B, Trautner P, Dohmen T, Sunde U, Elger CE, Falk A. Social comparison affects reward-related brain activity in the human ventral striatum. Science. 2007;318:1305–1308. doi: 10.1126/science.1145876. [DOI] [PubMed] [Google Scholar]
- 85.Fareri DS, Delgado MR. Differential reward responses during competition against in- and out-of-network others. Soc Cogn Affect Neurosci. 2013 doi: 10.1093/scan/nst006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ly M, Haynes MR, Barter JW, Weinberger DR, Zink CF. Subjective socioeconomic status predicts human ventral striatal responses to social status information. Curr Biol. 2011;21:794–797. doi: 10.1016/j.cub.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 87.Cikara M, Botvinick MM, Fiske ST. Us versus them: social identity shapes neural responses to intergroup competition and harm. Psychol Sci. 2011;22:306–313. doi: 10.1177/0956797610397667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Van den Bos W, Talwar A, McClure SM. Neural correlates of reinforcement learning and social preferences in competitive bidding. J Neurosci. 2013;33:2137–2146. doi: 10.1523/JNEUROSCI.3095-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Delgado MR, Schotter A, Ozbay EY, Phelps EA. Understanding overbidding: using the neural circuitry of reward to design economic auctions. Science. 2008;321:1849–1852. doi: 10.1126/science.1158860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhu L, Mathewson KE, Hsu M. Dissociable neural representations of reinforcement and belief prediction errors underlie strategic learning. Proc Natl Acad Sci U S A. 2012;109:1419–1424. doi: 10.1073/pnas.1116783109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the Ultimatum Game. Science. 2003;300:1755–1758. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- 92.Tricomi E, Rangel A, Camerer CF, O'Doherty JP. Neural evidence for inequality-averse social preferences. Nature. 2010;463:1089–1091. doi: 10.1038/nature08785. [DOI] [PubMed] [Google Scholar]
- 93.Tabibnia G, Satpute AB, Lieberman MD. The sunny side of fairness: preference for fairness activates reward circuitry (and disregarding unfairness activates self-control circuitry). Psychol Sci. 2008;19:339–347. doi: 10.1111/j.1467-9280.2008.02091.x. [DOI] [PubMed] [Google Scholar]
- 94.Xiang T, Lohrenz T, Montague PR. Computational substrates of norms and their violations during social exchange. J Neurosci. 2013;33:1099–1108. doi: 10.1523/JNEUROSCI.1642-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chang LJ, Smith A, Dufwenberg M, Sanfey AG. Triangulating the neural, psychological, and economic bases of guilt aversion. Neuron. 2011;70:560–572. doi: 10.1016/j.neuron.2011.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.De Quervain DJ-F, Fischbacher U, Treyer V, Schellhammer M, Schnyder U, Buck A, Fehr E. The neural basis of altruistic punishment. Science. 2004;305:1254–1258. doi: 10.1126/science.1100735. [DOI] [PubMed] [Google Scholar]
- 97.Crockett MJ, Apergis-Schoute A, Herrmann B, Lieberman M, Müller U, Robbins TW, Clark L. Serotonin Modulates Striatal Responses to Fairness and Retaliation in Humans. J Neurosci. 2013;33:3505–3513. doi: 10.1523/JNEUROSCI.2761-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Singer T, Seymour B, O'Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439:466–469. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Crockett MJ, Clark L, Lieberman MD, Tabibnia G, Robbins TW. Emotion. 2010;Impulsive choice and altruistic punishment are correlated and increase in tandem with serotonin depletion.10:855–862. doi: 10.1037/a0019861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vostroknutov A, Tobler PN, Rustichini A. Causes of social reward differences encoded in human brain. J Neurophysiol. 2012;107:1403–1412. doi: 10.1152/jn.00298.2011. [DOI] [PubMed] [Google Scholar]
- 101.Van den Bos W, van Dijk E, Westenberg M, Rombouts SA, Crone EA. What motivates repayment? Neural correlates of reciprocity in the Trust Game. Soc Cogn Affect Neurosci. 2009;4:294–304. doi: 10.1093/scan/nsp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bandura A. Social cognitive theory of self-regulation. Organ Behav Hum Decis Process. 1991;50:248–287. 50(2), 248-287. [Google Scholar]
- 103.Bhanji JP, Beer JS. Taking a different perspective: Mindset influences neural regions that represent value and choice. Soc Cogn Affect Neurosci. 2012;7:782–793. doi: 10.1093/scan/nsr062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hare TA, Malmaud J, Rangel A. Focusing attention on the health aspects of foods changes value signals in vmPFC and improves dietary choice. J Neurosci. 2011;31:11077–11087. doi: 10.1523/JNEUROSCI.6383-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hutcherson CA, Plassmann H, Gross JJ, Rangel A. Cognitive Regulation during Decision Making Shifts Behavioral Control between Ventromedial and Dorsolateral Prefrontal Value Systems. J Neurosci. 2012;32:13543–13554. doi: 10.1523/JNEUROSCI.6387-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci U S A. 2010;107:14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Martin L, Delgado M. The influence of emotion regulation on decision-making under risk. J Cogn Neurosci. 2011;23:2569–2581. doi: 10.1162/jocn.2011.21618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Delgado MR, Gillis MM, Phelps EA. Regulating the expectation of reward via cognitive strategies. Nat Neurosci. 2008;11:880–881. doi: 10.1038/nn.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]


