We conducted a systematic review and meta-analysis to determine the yield household contact investigation of drug-resistant tuberculosis source cases. The pooled yield was 7.8% (95% CI, 5.6%–10.0%) for active tuberculosis and 47.2% (95 CI%, 30.0%–61.4%) for latent tuberculosis.
Keywords: tuberculosis, contact, drug resistance, transmission
Abstract
Contact investigations among individuals living with drug-susceptible tuberculosis patients (source cases) have shown a high yield of tuberculosis disease and latent tuberculosis, but the yield of such investigations in households of drug-resistant tuberculosis source cases is unknown. In this systematic review and meta-analysis, we found 25 studies that evaluated a median of 111 (interquartile range, 21–302) household contacts of drug-resistant tuberculosis source cases. The pooled yield was 7.8% (95% CI, 5.6%–10.0%) for active tuberculosis and 47.2% (95% CI, 30.0%–61.4%) for latent tuberculosis, although there was significant statistical heterogeneity (P < .0001). More than 50% of secondary cases with drug susceptibility test results were concordant with those of the source case. Among studies that followed household members, the majority of secondary cases were detected within 1 year of the source case's diagnosis. Household contact investigation around drug-resistant tuberculosis patients is a high-yield intervention for detection of drug-resistant tuberculosis and prevention of ongoing transmission.
Drug-resistant tuberculosis is a global epidemic and is a particular threat to persons infected with human immunodeficiency virus (HIV). In 2011, there were an estimated 630 000 cases of multidrug-resistant (MDR) tuberculosis (ie, tuberculosis resistant to at least isoniazid and rifampin) worldwide, representing 5.3% of all tuberculosis cases [1]. The 2-year regimen for MDR tuberculosis is toxic and costly, and average cure rates are only 60%–70% [2–5]. Extensively drug-resistant (XDR) tuberculosis—defined as MDR tuberculosis with additional resistance to a fluoroquinolone and a second-line injectable antituberculosis agent—was first described in 2005 and has since been identified in 77 countries worldwide [6]. The emergence of XDR tuberculosis is of great concern because few treatment options remain against such highly resistant strains, and outcomes for patients with XDR tuberculosis are substantially poorer than outcomes for MDR tuberculosis [7–13]. Thus, prevention of new MDR and XDR tuberculosis cases is paramount to curb these epidemics.
Persons who live with a tuberculosis patient are at high risk for tuberculosis infection and disease due to prolonged, intense exposure to source cases [14, 15]. Thus, a well-established method for preventing tuberculosis cases is the household contact investigation, which seeks to detect and treat active cases earlier and identify latently infected individuals who would benefit from chemoprophylaxis. Contact investigations are a key component of tuberculosis programs [16]. This approach is used widely and effectively in low-burden settings, but rarely in high-incidence settings due in part to financial and human resource constraints [16].
The yield of a household contact investigation can be measured by the proportion of patients with active tuberculosis detected, as well as the proportion of latently infected persons detected. A review of household contact investigations of tuberculosis source cases (both drug-susceptible and drug-resistant) in low- and middle-income countries found that 4.5% of household contacts were diagnosed with active tuberculosis [17]. A more recent review of contact investigations of tuberculosis source cases (not limited to household contacts) found, in low- and middle-income countries, that the prevalence of active tuberculosis among contacts of MDR or XDR tuberculosis source cases (3.4%) was similar to the overall prevalence of active tuberculosis among contacts of all tuberculosis source cases (3.1%) [18].
It is unclear, however, whether the yield of contact investigation specifically in the households of drug-resistant tuberculosis source cases is similar to that of investigations around drug-susceptible source cases. A greater understanding of the anticipated yield from investment in these activities can provide an evidence base to assist tuberculosis programs in incorporating them into routine activities as these expand access to drug-resistant tuberculosis treatment. We conducted a systematic review and meta-analysis of contact investigations among household contacts of drug-resistant tuberculosis source cases, and compared infection and disease rates in children and adults in the household.
METHODS
Search Strategy
We first searched the literature for available systematic and narrative reviews of the yield of contact investigations conducted specifically in the households of drug-resistant tuberculosis patients. None were found.
Our search strategy then aimed to identify all studies that assessed the rate of active or latent tuberculosis among contacts of drug-resistant tuberculosis patients. We reviewed all published articles that discussed results of contact investigations. We did not restrict the language of the publications reviewed.
We searched 7 electronic databases for primary studies published through December 2011: PubMed, Embase, LILACS, IMSEAR, IMEMR, WPRIM, and AIM. The search terms included tuberculosis, resistan*, contact, outbreak, and transmission. The complete search strategy is detailed in Supplementary Appendix 1.
To identify relevant articles not found through our search, we also reviewed the reference lists of primary studies and review articles for additional references.
Initial Review of Studies
We compiled an initial database from the electronic searches and removed duplicate citations. Two reviewers (A. W. T., C. M. Y.) screened these citations by reviewing the title and abstract to capture relevant studies. Studies were eligible for inclusion if they included contact investigation of drug-resistant tuberculosis source cases. Outbreak investigations were included. We also hand-searched the references of reviews of contact investigations to evaluate whether these references met inclusion criteria.
Full text was obtained for relevant citations and reviewed by 2 reviewers (A. W. T., C. M. Y.) to determine eligibility for inclusion. We resolved disagreements between the reviewers by consensus. Studies were excluded for the following reasons: contact investigation was not performed, <5 household contacts were evaluated, contact investigation was restricted to a facility or institution, household contacts were not reported separately from nonhousehold contacts, contacts of drug-resistant source cases were not reported separately from contacts of drug-susceptible source cases, total number of evaluated household contacts was not reported, no original data were reported, either the cohort or dataset was identical to that of another included report, or the full text of the report could not be obtained. For studies in which household contact investigation results were contained within a larger dataset and not presented separately, and which were published after 2000, we contacted the authors requesting the disaggregated data. We also contacted authors of studies in which results were not stratified by adult and pediatric age groups. Articles in German, Japanese, Polish, Romanian, Russian, and Serbian were evaluated with the aid of translators who were trained on inclusion criteria and data extraction.
Data Collection
We designed a data extraction form (Supplementary Appendix 2), which 2 reviewers (N. S. S., C. M. Y.) piloted. These 2 reviewers extracted data from all of the studies included, and disagreements were resolved by consensus. The data elements extracted from each study comprised the yield of each contact investigation, including number of source cases, drug resistance category of source case isolates (eg, isoniazid monoresistant, MDR), number of evaluated contacts, duration of follow-up for contacts, number of active cases detected among contacts (secondary cases), and number of latently infected individuals detected among contacts. Among secondary cases, we also extracted the following data elements: results of tuberculin skin testing (TST), smear, culture, drug susceptibility testing (DST), and genotyping. Where possible, data for contacts were disaggregated by pediatric and adult age groups, and active cases detected at baseline and within 1, 2, and 3 years were indicated.
Definitions
The source case was defined as the tuberculosis case that led to investigation of a household. For data extraction, we used the definition of active tuberculosis reported by each study, including both clinical and bacteriologically confirmed diagnoses. We also used the definition of household contact and of TST positivity reported by each study. A secondary case was defined as a case of active tuberculosis in a household contact. Secondary cases and source cases were classified as having concordant drug resistance patterns if both had isolates in the same resistance category, based on drugs tested in each study (eg, isoniazid monoresistant tuberculosis, MDR tuberculosis, XDR tuberculosis). Secondary cases and source cases were considered genotypically concordant according to the criteria used to compare isolates in each study. We used each study's definition of latent tuberculosis where latent tuberculosis diagnosis was specified; where latent tuberculosis diagnosis was not specified, we deduced that contacts with positive TST results but without active tuberculosis disease had latent tuberculosis. We used the definitions of child and adult in each study if age-stratified data were presented. When contacting authors requesting data disaggregated by age group, we defined the pediatric age range as 0–14 years.
Data Analysis
We computed the proportion of active tuberculosis disease and latent tuberculosis among household contacts for all studies and estimated 95% confidence intervals (CIs) using Wilson's method, which can be applied to studies even with 0% transmission rates [19–21]. We estimated overall proportions by meta-analysis using a random effects model, given the high heterogeneity of the risk of transmission observed across studies. Studies were weighted by the inverse variance of the corresponding transmission risks. Overall yield was also calculated among prespecified subgroups, stratified by drug resistance category of the index case (MDR tuberculosis vs mono- or polyresistant tuberculosis), tuberculosis disease burden (high vs low, based on World Health Organization categories), and age (children aged 0–14 years vs adults). Heterogeneity was measured using the I2 statistic and tested by Q-statistic [22]. Analysis was performed using SAS and S-plus software.
RESULTS
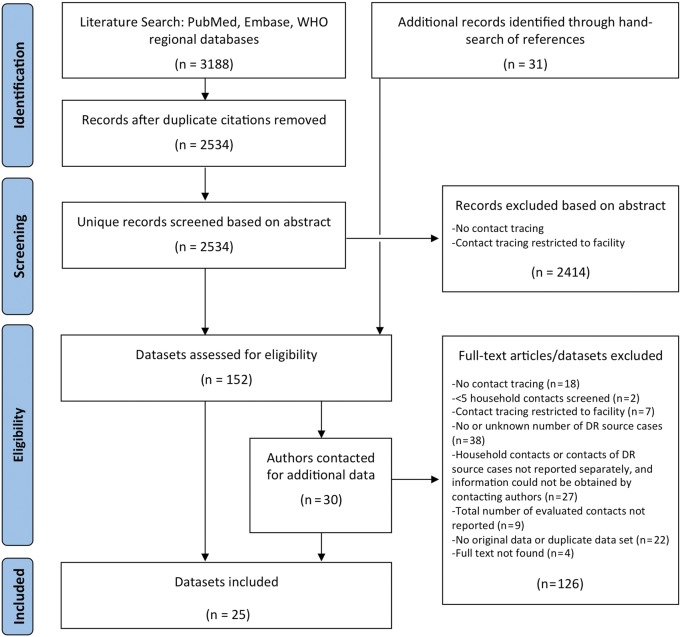
We identified 3188 unique citations through the literature search, of which 25 studies were eligible for inclusion (Figure 1). For 12 of these, data extraction relied on additional unpublished information provided to us by the authors.
Figure 1.
Flow diagram for study selection. Abbreviations: DR, drug-resistant; WHO, World Health Organization.
The included studies evaluated a median of 111 household contacts (interquartile range [IQR], 21–302) and a median of 26 (IQR, 2–87) drug-resistant source cases (Table 1). Eighteen studies included MDR tuberculosis source cases, 2 of which also included XDR tuberculosis source cases. Six studies included monoresistant tuberculosis source cases (mostly to isoniazid, although source cases with isolates with monoresistance to pyrazinamide, rifampicin, and streptomycin were also reported), and 4 studies included source cases with other drug resistance patterns.
Table 1.
Yield of Secondary Cases of Active Tuberculosis in 25 Included Studies
| Author(s) | Location | Year(s) of Enrollment | Drug-Resistant Tuberculosis Source Cases, No. | Source Case Drug Resistance Category | Household Contacts Evaluated for Active Tuberculosis, No. | Active Secondary Cases, No. (%) | Drug-Resistant Secondary Cases Among Secondary Cases With DST, No. (%) |
|---|---|---|---|---|---|---|---|
| CDC [23] | Federated States of Micronesia | 2007–2009 | 5 | MDR | 163 | 16 (10) | 3/3 (100) |
| Agerton et al [24] | US | 1992–1997 | 12 | MDR | 21 | 3 (14) | 3/3 (100) |
| Bayona et al [25] | Peru | 1997–1999 | 92 | MDR | 464 | 38 (8) | 9/9 (100) |
| Becerra et al [26] | Peru | 1996–2003 | 693 | MDR, XDR | 4503 | 359 (8) | 173/186 (93) |
| Grandjean et al [27] | Peru | 2005–2008 | 358 | MDR | 2112 | 108 (5) | 44/50 (88) |
| Huang et al [28] | Taiwan | 2005–2007 | 19 | MDR | 78 | 0 (0) | NA |
| Johnson et al [29] | US | 1997 | 2 | Mono, MDR | 12 | 0 (0) | NA |
| Kritski et al [30] | Brazil | 1988–1992 | 64 | Poly, MDR | 218 | 17 (8) | 10/13 (77) |
| Mehta et al [31] | US | 1996–2002 | 4 | Poly | 26 | 1 (4) | 1/1 (100) |
| Miramontes et al [32] | US | 2007 | 2 | MDR | 9 | 1 (11) | |
| Mokaddas et al [33] | Kuwait | 2000–2003 | 1 | Mono | 9 | 1 (11) | 1/1 (100) |
| Neely et al [34] | UK | 1995–2000 | 87 | Mono | 129 | 26 (20) | 26/26 (100) |
| Perri et al [35] | US | 2003–2009 | 16 | Mono | 73 | 0 (0) | NA |
| Pineiro Perez et al [36] | Spain | Unknown | 1 | MDR | 9 | 0 (0) | NA |
| Tuberculosis Research Centre [37] | India | 1968–1983 | 209 | Mono | 779 | 188 (24) | 4/22 (18) |
| Reichler et al [38] | US Virgin Islands | 1997–1998 | 1 | MDR | 7 | 0 (0) | NA |
| Salazar-Vergara et al [39] | Philippines | 2001 | 44 | MDR | 111 | 3 (3) | 1/1 (100) |
| Schaaf et al [40] | South Africa | 1994–2000 | 73 | MDR | 125 | 29 (23) | 4/4 (100) |
| Singla et al [41] | India | 2005–2008 | 58 | MDR | 302 | 16 (5) | 2/3 (67) |
| Snider et al [42] | US | Unknown | 180 | Mono, Poly | 601 | 4 (1) | 2/3 (67) |
| Steiner et al [43] | US | 1969 | 1 | Poly | 23 | 6 (26) | 3/3 (100) |
| Teixeira et al [44] | Brazil | 1994–1998 | 26 | MDR | 157 | 9 (6) | 5/6 (83) |
| van Zyl et al [45] | South Africa | 1996–2003 | 55 | MDR | 55 | 16 (29) | |
| Vella et al [46] | South Africa | 2005–2008 | 508 | MDR, XDR | 1766 | 73 (4) | 53/55 (96) |
| Younossian et al [47] | Switzerland | 2003 | 2 | MDR | 13 | 0 (0) | NA |
Abbreviations: CDC, Centers for Disease Control and Prevention; DST, drug susceptibility testing; MDR, multidrug-resistant tuberculosis; Mono, monoresistant tuberculosis (any type); NA, not applicable; Poly, polyresistant tuberculosis; XDR, extensively drug-resistant tuberculosis.
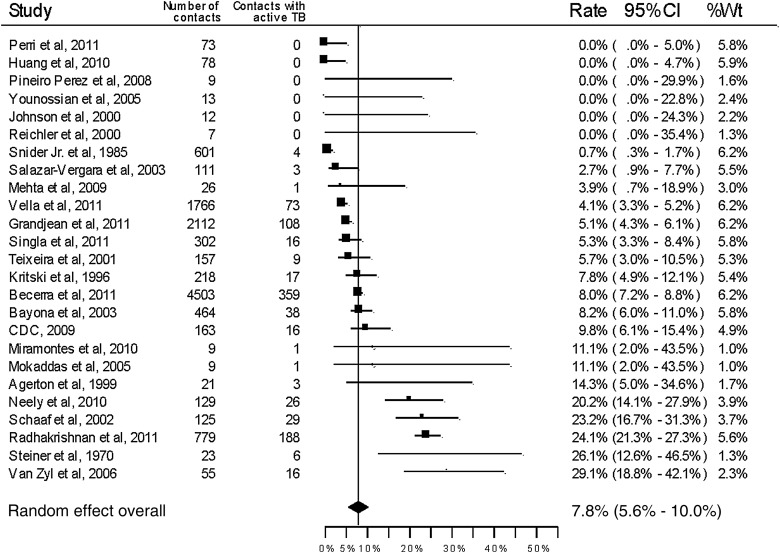
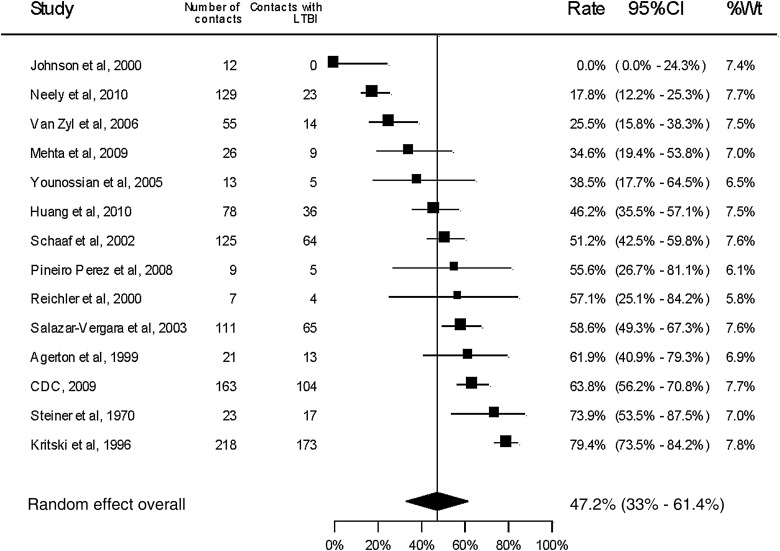
All included studies reported the number of active tuberculosis cases among the household contacts (Table 1 and Figure 2) with an overall pooled yield of 7.8% (95% CI, 5.6%–10.0%). Latent tuberculosis was reported or could be calculated based on TST results in 14 studies (Table 2 and Figure 3). The overall proportion of household contacts with latent tuberculosis was 47.2% (95% CI, 30.0%–61.4%). There was significant statistical heterogeneity in the pooled measures (P < .0001, I2 = 94.9% for active tuberculosis disease; P < .0001, I2 = 96.0% for latent tuberculosis).
Figure 2.
Forest plot for secondary cases of active tuberculosis among household contacts of drug-resistant tuberculosis source cases. Abbreviations: CDC, Centers for Disease Control and Prevention; CI, confidence interval; TB, tuberculosis; Wt, weight.
Table 2.
Yield of Latent Tuberculosis Detected in 14 Studiesa
| Author(s) | Location | Year(s) of Enrollment | Household Contacts Evaluated for Latent Tuberculosis, No. | Latent Tuberculosis Among Household Contacts, No. (%) |
|---|---|---|---|---|
| CDC [23] | Federated States of Micronesia | 2007–2009 | 163 | 104 (64) |
| Agerton et al [24] | US | 1992–1997 | 21 | 13 (62) |
| Huang et al [28] | Taiwan | 2005–2007 | 78 | 36 (46) |
| Johnson et al [29] | US | 1997 | 12 | 0 (0) |
| Kritski et al [30] | Brazil | 1988–1992 | 218 | 173 (79) |
| Mehta et al [31] | US | 1996–2002 | 26 | 9 (38) |
| Neely et al [34] | UK | 1995–2000 | 129 | 23(18) |
| Pineiro Perez et al [36] | Spain | Unknown | 9 | 5 (56) |
| Reichler et al [38] | US Virgin Islands | 1997–1998 | 7 | 4 (57) |
| Salazar-Vergara et al [39] | Philippines | 2001 | 111 | 65 (59) |
| Schaaf et al [40] | South Africa | 1994–2000 | 125 | 64 (51) |
| Steiner et al [43] | US | 1969 | 23 | 17 (74) |
| van Zyl et al [45] | South Africa | 1996–2003 | 55 | 14 (25) |
| Younossian et al [47] | Switzerland | 2003 | 9 | 4 (44) |
Abbreviation: CDC, Centers for Disease Control and Prevention.
a Studies that reported a diagnosis of latent tuberculosis or where it could be calculated from data on tuberculin skin testing and clinical status of contacts.
Figure 3.
Forest plot for secondary cases of latent tuberculosis infection among household contacts of drug-resistant tuberculosis source cases. Abbreviations: CDC, Centers for Disease Control and Prevention; CI, confidence interval; LTBI, latent tuberculosis; Wt, weight.
Among the 16 studies with only MDR tuberculosis source cases, the overall proportion of household contacts with active tuberculosis disease was 6.5% (95% CI, 4.6%–8.4%, I2 = 87.2%). Among the 7 studies with only mono- or polyresistant tuberculosis source cases, it was 11.6% (95% CI, 2.7%–20.4%, I2 = 97.7%). Latent tuberculosis was reported or calculated in 9 studies that included only MDR tuberculosis source cases, with an overall yield of 50.7% (95% CI, 41.5%–59.9%, I2 = 78.4%). Among 3 studies with mono- or polyresistant tuberculosis source cases, the yield of latent tuberculosis among household contacts was 41.5% (95% CI, 8.19%–74.8%, I2 = 94.7%).
The overall proportion of household contacts with active tuberculosis disease was 8.7% (95% CI, 6.08%–11.2%, I2 = 95.6%) among 12 studies from high-burden tuberculosis settings and 6.3% (95% CI, 2.4%–10.1%, I2 = 79.7%) among 13 studies from low-burden settings (P = .316). Latent tuberculosis among household contacts was reported or could be calculated from 5 studies in high-burden settings and 9 studies in low-burden settings, with overall yields of 52.5% (95% CI, 33.8%–71.2%, I2 = 95.8%) and 44.1% (95% CI, 24.9%–63.4%, I2 = 94.2%), respectively.
Five studies evaluated only pediatric household contacts, 1 study evaluated only adult household contacts, 13 studies evaluated both, and 6 studies did not report age for evaluated household contacts. Of the studies that evaluated both pediatric and adult contacts, it was possible to calculate the yield of active tuberculosis cases for the 2 age groups separately in 11 studies (Table 3). Overall, 4.0% (95% CI, 1.5%–6.5%, I2 = 80.1%) of pediatric contacts and 4.9% (95% CI, 2.7%–7.0%, I2 = 82.3%) of adult contacts had active tuberculosis disease (P = .631). Among 5 studies in which latent tuberculosis was reported or could be calculated for both children and adults, 27.3% (95% CI, 3.9%–50.6%, I2 = 88.5%) of pediatric contacts and 51.9% (95% CI, 25.6%–78.2%, I2 = 93.1%) of adult contacts had latent tuberculosis.
Table 3.
Yield of Active and Latent Tuberculosis Among Household Contacts by Age Group
| Author(s) | Location | Year(s) of Enrollment | Household Contacts Evaluated, No. |
Active Secondary Cases, No. (%) |
Latent Tuberculosis, No. (%) |
|||
|---|---|---|---|---|---|---|---|---|
| Child | Adult | Child | Adult | Child | Adult | |||
| CDC [23] | Federated States of Micronesia | 2007–2009 | 60 | 103 | 9 (15) | 7 (7) | 20 (33) | 84 (82) |
| Bayona et al [25] | Peru | 1997–1999 | 118 | 343 | 3 (3) | 35 (10) | ||
| Becerra et al [26] | Peru | 1996–2003 | 1272 | 3041 | 70 (6) | 237 (8) | ||
| Grandjean et al [27] | Peru | 2005–2008 | 524 | 1567 | 14 (3) | 94 (6) | ||
| Huang et al [28] | Taiwan | 2005–2007 | 16 | 62 | 0 (0) | 0 (0) | 5 (31) | 31 (50) |
| Johnson et al [29] | US | 1997 | 8 | 4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Mehta et al [31] | US | 1996–2002 | 4 | 17 | 1 (25) | 0 (0) | 0 (0) | 7 (41) |
| Miramontes et al [32] | US | 2007 | 2 | 7 | 1 (50) | 0 (0) | ||
| Perri et al [35] | US | 2003–2009 | 10 | 63 | 0 (0) | 0 (0) | ||
| Tuberculosis Research Centre [37] | India | 1968–1983 | 405 | 374 | 4 (1) | 18 (5) | ||
| Steiner et al [43] | US | 1969 | 17 | 6 | 5 (29) | 1 (17) | 12 (71) | 5 (83) |
Abbreviation: CDC, Centers for Disease Control and Prevention.
DST results of secondary cases were reported in 17 studies. In 1 of these studies, only secondary cases with drug resistance patterns and genotypes concordant with their source cases were reported [34]. Of the remaining 16 studies, 15 reported that >50% of secondary cases with DST results were drug-resistant tuberculosis (Table 1), and 14 reported that >50% of secondary cases with DST results had drug resistance categories that were concordant with that of the source case (Table 4). In 7 of the 8 studies that reported genotyping results for secondary cases, at least 75% of secondary cases analyzed had strains whose genotypes were concordant with that of the source case (Table 4).
Table 4.
Frequency of Concordance Between Source and Secondary Case Isolates by Resistance Category and Genotype
| Author(s) | Location | Year(s) of Enrollment | Cases With Concordant Resistance Category Among Cases With DST, No. (%) | Cases With Concordant Genotype Among Cases With Genotype, No. (%) |
|---|---|---|---|---|
| CDC [23] | Federated States of Micronesia | 2007–2009 | 3/3 (100) | 3/3 (100) |
| Agerton et al [24] | US | 1992–1997 | 3/3 (100) | 3/3 (100) |
| Bayona et al [25] | Peru | 1997–1999 | 9/9 (100) | … |
| Becerra et al [26] | Peru | 1996–2003 | 164/186 (88) | … |
| Grandjean et al [27] | Peru | 2005–2008 | 36/50 (72) | … |
| Kritski et al [30] | Brazil | 1988–1992 | 8/13 (62) | … |
| Mehta et al [31] | US | 1996–2002 | 1/1 (100) | 0/1 (0) |
| Mokaddas et al [33] | Kuwait | 2000–2003 | 1/1 (100) | 1/1 (100) |
| Tuberculosis Research Centre [37] | India | 1968–1983 | 4/22 (18) | … |
| Salazar-Vergara et al [39] | Philippines | 2001 | 0/1 (0) | … |
| Schaaf et al [40] | South Africa | 1994–2000 | 4/4 (100) | 3/4 (75) |
| Singla et al [41] | India | 2005–2008 | 2/3 (67) | … |
| Snider et al [42] | US | Unknown | 2/3 (67) | … |
| Steiner et al [43] | US | 1969 | 3/3 (100) | … |
| Teixeira et al [44] | Brazil | 1994–1998 | 5/6 (83) | 6/6 (100) |
| Vella et al [46] | South Africa | 2005–2008 | 33/55 (60) | … |
Cells with missing data indicate that genotyping was not performed or not available for these studies.
Abbreviations: CDC, Centers for Disease Control and Prevention; DST, drug susceptibility testing.
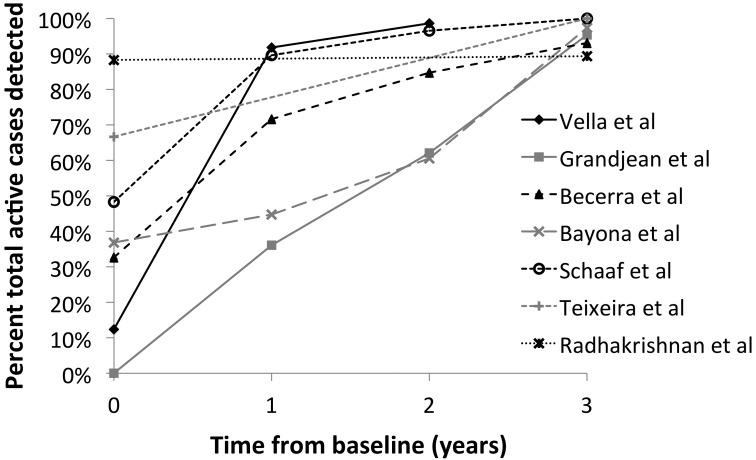
In 7 studies, household contacts were followed up for >1 year, and it was possible to determine the number of secondary cases detected within 1, 2, or 3 years of baseline evaluation (Figure 4). Five studies reported detecting >50% of secondary cases within 1 year of baseline evaluation, and all 6 studies that followed household contacts for >2 years reported detecting >50% of secondary cases within 2 years of baseline evaluation.
Figure 4.
Yield for active tuberculosis disease over time among household contacts of drug-resistant tuberculosis source cases.
DISCUSSION
In this systematic review and meta-analysis, we evaluated the pooled yield of household contact investigation for drug-resistant tuberculosis source cases and found a high overall yield for active tuberculosis cases (7.8%) and latent tuberculosis (47.2%). In almost all of the studies that reported DST and genotyping results, the majority of secondary cases had DST and/or genotyping results concordant with that of the source case. In 5 of the 7 (71%) studies that followed household contacts for >1 year, the majority of secondary cases were detected within 1 year of the source case's diagnosis. Together, these findings support the growing epidemiologic evidence for the high yield of household contact investigation of drug-resistant tuberculosis source cases, particularly within the first year after diagnosis.
Emerging data suggest that drug-resistant tuberculosis cases are primarily occurring as a result of transmission of a tuberculosis strain that is already drug resistant (“primary transmission”) [3, 6]. Although it was initially believed that the mutations that caused drug resistance in tuberculosis would exert a fitness cost, rendering drug-resistant strains less able to cause new cases [48], transmission of drug-resistant tuberculosis strains has been well documented [49–52]. The high concordance between source case and secondary case strain resistance patterns and genotypes observed in this review are further evidence of the transmissibility of drug-resistant tuberculosis strains. Furthermore, the yields of both tuberculosis disease and latent tuberculosis among household contacts in this review were higher than those observed in a systematic review of household contact investigations that was not limited to drug-resistant tuberculosis source cases [17] and another review of all types of contact investigations [18]. This supports the conclusion that the households of both types of source cases merit systematic contact investigation.
Our study found higher yields of household contact investigation for both active tuberculosis disease and latent tuberculosis in high-burden settings as compared to low-burden tuberculosis settings (8.65% vs 6.27% contacts with active tuberculosis disease; 52.5% vs 44.1% contacts with latent tuberculosis), but these differences were not statistically significant. The difference may be attributable in part to community transmission in high-burden settings. Studies from South Africa have shown that although transmission is occurring in households, community transmission may account for up to 50% of drug-resistant tuberculosis cases [46, 53]. Community transmission may also explain the proportion of secondary cases with discordant DST and genotyping results observed in our study (Table 4). The difference between yields of household contact investigations in high- and low-burden settings may also be attributable to resource limitations in high-burden settings that may delay diagnosis and initiation of effective drug-resistant tuberculosis therapy and, therefore, contribute to prolonged infectious periods. For patients with XDR tuberculosis, this is further exacerbated by extremely limited laboratory capacity globally for second-line drug susceptibility testing [54] and the paucity of effective treatment options. However, the increasing availability of rapid phenotypic and genotypic tests for diagnosis of drug-resistant tuberculosis offers great promise for improving case detection, initiating earlier treatment and averting further transmission of drug-resistant tuberculosis in community and congregate settings [55, 56].
The yields of active and latent tuberculosis among pediatric household contacts of drug-resistant tuberculosis in our study were comparable to those observed for drug-susceptible tuberculosis [17]. Although this population is known to be at high risk for disease progression, little is known about the disease burden among children, who likely represent a large pool of exposed, undiagnosed, and untreated latent infections. In one of the largest studies to date of pediatric MDR tuberculosis contacts, tuberculosis prevalence among children who were MDR tuberculosis household contacts was nearly 30 times higher than among children in the general population [57]. The majority of secondary cases had MDR tuberculosis, suggesting transmission in the home. Greater efforts to strengthen contact investigation for children, together with studies on safe and effective chemoprophylaxis, are urgently needed.
There are limitations to this study. First, we were unable to systematically assess the impact of HIV on risk of active tuberculosis disease and latent tuberculosis in household contacts given the limited data reported on this important variable in most studies. HIV is known to increase the risk of progression to active tuberculosis disease [58, 59], so it is likely to have an important effect on secondary case rates observed in high- vs low-HIV prevalence settings. Second, direct comparisons between yields of household contact investigations for drug-susceptible and drug-resistant source cases must be interpreted with caution as testing practices for drug resistance are likely to vary in each country, subjecting drug-resistant source cases to ascertainment bias. Source cases who are sicker or who are infectious for longer periods may have exposed household contacts for a longer time, thus increasing the potential for transmission. With scale-up of simpler, rapid diagnostic tests for drug-resistant tuberculosis, exposure periods and secondary case rates would be expected to decline. Although high-quality treatment for drug-resistant tuberculosis is not yet widely accessible, in some programs where it is available, staff may make greater efforts to identify and screen contacts of drug-resistant tuberculosis source cases, resulting in ascertainment bias among contacts. Third, genotyping of source and secondary cases was not consistently available in all studies included in our analysis, especially those from high-burden tuberculosis settings. Our ability to determine whether transmission occurred in the household or community was thus limited.
All systematic reviews are subject to the possibility of publication bias. A strength of our study is the inclusion of non-English-language papers that expanded the number of included studies and increased the robustness and generalizability of our findings. Likewise, in any meta-analysis, heterogeneity of the included studies is inevitable, and the heterogeneity across studies included in our meta-analysis was very large even in the subgroups. Although heterogeneity was accounted for using a random-effects model, we were unable to determine factors that might have resulted in such a large heterogeneity.
Despite these limitations, our study provides a systematic review and pooled estimates of yield from household contact investigation of drug-resistant tuberculosis source cases. Individuals who live with patients with any form of tuberculosis are at high risk for developing disease or latent infection. Globally, household contact investigation is an underutilized strategy against tuberculosis. As programs are expanding access to drug-resistant tuberculosis treatment and early diagnosis, there is strong evidence to support the prompt implementation of systematic contact investigation in the households of drug-resistant tuberculosis patients.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the following authors who provided us with additional information not included in their published reports: Sapna Bamrah, Louis Grandjean, Maryam Haddad, Yi-Wen Huang, Jean-Paul Janssens, Kammy Johnson, Christoph Lange, Richard Long, Jay Mehta, Roque Miramontes, John Oeltmann, Bianca Perri, H. Simon Schaaf, R. Subramani, Angela Tsai, and Venazio Vella. We also thank the following individuals who read articles in foreign languages and helped to extract data from these articles: Nadza Durakovic, Mayo Hotta, Julia Jezmir, Cristian Jitianu, Maureen Miller, and Aleks Olszewski.
Author contributions. N. S. S., M. C. B., and C. M. Y. conceived of and designed the study. C. M. Y. and A. W. T. conducted the literature search and review of articles. N. S. S., C. M. Y., and A. W. T. extracted and cleaned data. M. H. conducted data analysis and data interpretation. All authors contributed to drafting and critical review of the final version of the manuscript. N. S. S. had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Financial support. There was no specific funding for this project. The study was supported by the National Institute of Allergy and Infectious Diseases (5R01AI089349) and the Einstein-Montefiore Center for AIDS Research (P30 AI 051519).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Geneva, Switzerland: WHO; 2013. Global tuberculosis report 2012. [Google Scholar]
- 2.Kim DH, Kim HJ, Park SK, et al. Treatment outcomes and survival based on drug resistance patterns in multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2010;182:113–19. doi: 10.1164/rccm.200911-1656OC. [DOI] [PubMed] [Google Scholar]
- 3.Gandhi NR, Nunn P, Dheda K, et al. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet. 2010;375:1830–43. doi: 10.1016/S0140-6736(10)60410-2. [DOI] [PubMed] [Google Scholar]
- 4.Seung KJ, Omatayo DB, Keshavjee S, et al. Early outcomes of MDR TB treatment in a high HIV-prevalence setting in southern Africa. PLoS One. 2009;4:e7186. doi: 10.1371/journal.pone.0007186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orenstein EW, Basu S, Shah NS, et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis. 2009;9:153–61. doi: 10.1016/S1473-3099(09)70041-6. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Geneva, Switzerland: WHO; 2010. Multidrug and extensively drug-resistant TB (M/XDR TB): 2010 global report on surveillance and response. [Google Scholar]
- 7.Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs—worldwide, 2000–2004. MMWR Morb Mortal Wkly Rep. 2006;55:301–5. [PubMed] [Google Scholar]
- 8.Gandhi NR, Shah NS, Andrews JR, et al. HIV coinfection in multidrug- and extensively drug-resistant tuberculosis results in high early mortality. Am J Respir Crit Care Med. 2010;181:80–6. doi: 10.1164/rccm.200907-0989OC. [DOI] [PubMed] [Google Scholar]
- 9.Keshavjee S, Gelmanova IY, Farmer PE, et al. Treatment of extensively drug-resistant tuberculosis in Tomsk, Russia: a retrospective cohort study. Lancet. 2008;372:1403–9. doi: 10.1016/S0140-6736(08)61204-0. [DOI] [PubMed] [Google Scholar]
- 10.Migliori GB, Besozzi G, Girardi E, et al. Clinical and operational value of the extensively drug-resistant tuberculosis definition. Eur Respir J. 2007;30:623–6. doi: 10.1183/09031936.00077307. [DOI] [PubMed] [Google Scholar]
- 11.Mitnick CD, Shin SS, Seung KJ, et al. Comprehensive treatment of extensively drug-resistant tuberculosis. N Engl J Med. 2008;359:563–74. doi: 10.1056/NEJMoa0800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satti H, Seung K, Keshavjee S, et al. Extensively drug-resistant tuberculosis, Lesotho. Emerg Infect Dis. 2008;14:992–3. doi: 10.3201/eid1406.071654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah NS, Pratt R, Armstrong L, et al. Extensively drug-resistant tuberculosis in the United States, 1993–2007. JAMA. 2008;300:2153–60. doi: 10.1001/jama.300.18.2153. [DOI] [PubMed] [Google Scholar]
- 14.Andrews RH, Evadatta S, Ox W, et al. Prevalence of tuberculosis among close family contacts of tuberculous patients in south India, and influence of segregation of the patient on early attack rate. Bull World Health Organ. 1960;23:463–510. [PMC free article] [PubMed] [Google Scholar]
- 15.Sepkowitz KA. How contagious is tuberculosis? Clin Infect Dis. 1996;23:954–62. doi: 10.1093/clinids/23.5.954. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Geneva, Switzerland: WHO, 2013; Recommendations for investigating contacts of persons with infectious tuberculosis in low- and middle-income countries. [PubMed] [Google Scholar]
- 17.Morrison J, Pai M, Hopewell PC. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8:359–68. doi: 10.1016/S1473-3099(08)70071-9. [DOI] [PubMed] [Google Scholar]
- 18.Fox GJ, Barry SE, Britton WJ, et al. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2013;41:140–56. doi: 10.1183/09031936.00070812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agresti A, Coull BA. Approximate is better than “exact” for interval estimation of binomial proportions. Am Stat. 1998;52:119–26. [Google Scholar]
- 20.Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22:209–12. [Google Scholar]
- 21.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–72. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. Two simultaneous outbreaks of multidrug-resistant tuberculosis—Federated States of Micronesia, 2007–2009. MMWR Morb Mortal Wkly Rep. 2009;58:253–6. [PubMed] [Google Scholar]
- 24.Agerton TB, Valway SE, Blinkhorn RJ, et al. Spread of strain W, a highly drug-resistant strain of Mycobacterium tuberculosis, across the United States. Clin Infect Dis. 1999;29:85–92. doi: 10.1086/520187. [DOI] [PubMed] [Google Scholar]
- 25.Bayona J, Chavez-Pachas AM, Palacios E, et al. Contact investigations as a means of detection and timely treatment of persons with infectious multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2003;7(12 suppl 3):S501–9. [PubMed] [Google Scholar]
- 26.Becerra MC, Appleton SC, Franke MF, et al. Tuberculosis burden in households of patients with multidrug-resistant and extensively drug-resistant tuberculosis: a retrospective cohort study. Lancet. 2011;377:147–52. doi: 10.1016/S0140-6736(10)61972-1. [DOI] [PubMed] [Google Scholar]
- 27.Grandjean L, Crossa A, Gilman RH, et al. Tuberculosis in household contacts of multidrug-resistant tuberculosis patients. Int J Tuberc Lung Dis. 2011;15:1164–9. doi: 10.5588/ijtld.11.0030. i. [DOI] [PubMed] [Google Scholar]
- 28.Huang YW, Shen GH, Lee JJ, et al. Latent tuberculosis infection among close contacts of multidrug-resistant tuberculosis patients in central Taiwan. Int J Tuberc Lung Dis. 2010;14:1430–5. [PubMed] [Google Scholar]
- 29.Johnson KR, Braden CR, Cairns KL, et al. Transmission of Mycobacterium tuberculosis from medical waste. JAMA. 2000;284:1683–8. doi: 10.1001/jama.284.13.1683. [DOI] [PubMed] [Google Scholar]
- 30.Kritski AL, Marques MJ, Rabahi MF, et al. Transmission of tuberculosis to close contacts of patients with multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 1996;153:331–5. doi: 10.1164/ajrccm.153.1.8542139. [DOI] [PubMed] [Google Scholar]
- 31.Mehta J, Keith R, Al HM, et al. Mini epidemic of isoniazide resistant TB in rural TN: a need for supervised preventive therapy. Tenn Med. 2009;102:41–4. [PubMed] [Google Scholar]
- 32.Miramontes R, Lambert L, Haddad MB, et al. Public health response to a multidrug-resistant tuberculosis outbreak among Guatemalans in Tennessee, 2007. South Med J. 2010;103:882–6. doi: 10.1097/SMJ.0b013e3181eba488. [DOI] [PubMed] [Google Scholar]
- 33.Mokaddas E, Ahmad S, Abal AT, et al. Molecular fingerprinting reveals familial transmission of rifampin-resistant tuberculosis in Kuwait. Ann Saudi Med. 2005;25:150–3. doi: 10.5144/0256-4947.2005.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neely F, Maguire H, Le BF, et al. High rate of transmission among contacts in large London outbreak of isoniazid mono-resistant tuberculosis. J Public Health (Oxf) 2010;32:44–51. doi: 10.1093/pubmed/fdp056. [DOI] [PubMed] [Google Scholar]
- 35.Perri BR, Proops D, Moonan PK, et al. Mycobacterium tuberculosis cluster with developing drug resistance, New York, New York, USA, 2003–2009. Emerg Infect Dis. 2011;17:372–8. doi: 10.3201/eid1703.101002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pineiro Perez R, Mellado Pena MJ, Mendez Echevarria A, et al. Exposicion a tuberculosis multirresistente: estudio y seguimiento de nueve ninos [in Spanish] An Pediatr (Barc) 2008;68:490–5. doi: 10.1157/13120048. [DOI] [PubMed] [Google Scholar]
- 37.Tuberculosis Research Centre, Indian Council of Medical Research (ICMR), Chennai, India. Risk of tuberculosis among contacts of isoniazid-resistant and isoniazid-susceptible cases. Int J Tuberc Lung Dis. 2011;15:782–8. doi: 10.5588/ijtld.09.0327. [DOI] [PubMed] [Google Scholar]
- 38.Reichler MR, Valway SE, Onorato IM. Transmission in the United States Virgin Islands and Florida of a multidrug-resistant Mycobacterium tuberculosis strain acquired in Puerto Rico. Clin Infect Dis. 2000;30:617–8. doi: 10.1086/313698. [DOI] [PubMed] [Google Scholar]
- 39.Salazar-Vergara RM, Sia IG, Tupasi TE, et al. Tuberculosis infection and disease in children living in households of Filipino patients with tuberculosis: a preliminary report. Int J Tuberc Lung Dis. 2003;7(12 suppl 3):S494–500. [PubMed] [Google Scholar]
- 40.Schaaf HS, Gie RP, Kennedy M, et al. Evaluation of young children in contact with adult multidrug-resistant pulmonary tuberculosis: a 30-month follow-up. Pediatrics. 2002;109:765–71. doi: 10.1542/peds.109.5.765. [DOI] [PubMed] [Google Scholar]
- 41.Singla N, Singla R, Jain G, et al. Tuberculosis among household contacts of multidrug-resistant tuberculosis patients in Delhi, India. Int J Tuberc Lung Dis. 2011;15:1326–30. doi: 10.5588/ijtld.10.0564. [DOI] [PubMed] [Google Scholar]
- 42.Snider DE, Jr, Kelly GD, Cauthen GM, et al. Infection and disease among contacts of tuberculosis cases with drug-resistant and drug-susceptible bacilli. Am Rev Respir Dis. 1985;132:125–32. doi: 10.1164/arrd.1985.132.1.125. [DOI] [PubMed] [Google Scholar]
- 43.Steiner M, Chaves AD, Lyons HA, et al. Primary drug-resistant tuberculosis. Report of an outbreak. N Engl J Med. 1970;283:1353–8. doi: 10.1056/NEJM197012172832501. [DOI] [PubMed] [Google Scholar]
- 44.Teixeira L, Perkins MD, Johnson JL, et al. Infection and disease among household contacts of patients with multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2001;5:321–8. [PubMed] [Google Scholar]
- 45.van Zyl S, Marais BJ, Hesseling AC, et al. Adherence to anti-tuberculosis chemoprophylaxis and treatment in children. Int J Tuberc Lung Dis. 2006;10:13–8. [PubMed] [Google Scholar]
- 46.Vella V, Racalbuto V, Guerra R, et al. Household contact investigation of multidrug-resistant and extensively drug-resistant tuberculosis in a high HIV prevalence setting. Int J Tuberc Lung Dis. 2011;15:1170–5. doi: 10.5588/ijtld.10.0781. [DOI] [PubMed] [Google Scholar]
- 47.Younossian AB, Rochat T, Ketterer JP, et al. High hepatotoxicity of pyrazinamide and ethambutol for treatment of latent tuberculosis. Eur Respir J. 2005;26:462–4. doi: 10.1183/09031936.05.00006205. [DOI] [PubMed] [Google Scholar]
- 48.Cohen T, Sommers B, Murray M. The effect of drug resistance on the fitness of Mycobacterium tuberculosis. Lancet Infect Dis. 2003;3:13–21. doi: 10.1016/s1473-3099(03)00483-3. [DOI] [PubMed] [Google Scholar]
- 49.Gagneux S. Fitness cost of drug resistance in Mycobacterium tuberculosis. Clin Microbiol Infect. 2009;15(suppl 1):66–8. doi: 10.1111/j.1469-0691.2008.02685.x. [DOI] [PubMed] [Google Scholar]
- 50.Gagneux S, Long CD, Small PM, et al. The competitive cost of antibiotic resistance in Mycobacterium tuberculosis. Science. 2006;312:1944–6. doi: 10.1126/science.1124410. [DOI] [PubMed] [Google Scholar]
- 51.Gagneux S, Burgos MV, DeRiemer K, et al. Impact of bacterial genetics on the transmission of isoniazid-resistant Mycobacterium tuberculosis. PLoS Pathog. 2006;2:e61. doi: 10.1371/journal.ppat.0020061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen T, Murray M. Modeling epidemics of multidrug-resistant M. tuberculosis of heterogeneous fitness. Nat Med. 2004;10:1117–21. doi: 10.1038/nm1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Verver S, Warren RM, Munch Z, et al. Transmission of tuberculosis in a high incidence urban community in South Africa. Int J Epidemiol. 2004;33:351–7. doi: 10.1093/ije/dyh021. [DOI] [PubMed] [Google Scholar]
- 54.World Health Organization. Geneva, Switzerland: WHO; 2008. Anti-tuberculosis drug resistance in the world: fourth global report. [Google Scholar]
- 55.Basu S, Friedland GH, Medlock J, et al. Averting epidemics of extensively drug-resistant tuberculosis. Proc Natl Acad Sci U S A. 2009;106:7672–7. doi: 10.1073/pnas.0812472106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dowdy DW, Chaisson RE, Maartens G, et al. Impact of enhanced tuberculosis diagnosis in South Africa: a mathematical model of expanded culture and drug susceptibility testing. Proc Natl Acad Sci U S A. 2008;105:11293–8. doi: 10.1073/pnas.0800965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Becerra MC, Franke MF, Appleton SC, et al. Tuberculosis in children exposed at home to multidrug-resistant tuberculosis. Pediatr Infect Dis J. 2013;32:115–9. doi: 10.1097/INF.0b013e31826f6063. [DOI] [PubMed] [Google Scholar]
- 58.Selwyn PA, Hartel D, Lewis VA, et al. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med. 1989;320:545–50. doi: 10.1056/NEJM198903023200901. [DOI] [PubMed] [Google Scholar]
- 59.Lawn SD, Bekker LG, Middelkoop K, et al. Impact of HIV infection on the epidemiology of tuberculosis in a peri-urban community in South Africa: the need for age-specific interventions. Clin Infect Dis. 2006;42:1040–7. doi: 10.1086/501018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.