Novel, potent, and well-tolerated antiretroviral agents in appropriate formulations are acutely needed for HIV-infected pediatric patients of all ages. This study was designed to assess the safety and pharmacokinetics of raltegravir in HIV-infected children aged 4 weeks to <19 years.
Keywords: pediatric HIV, raltegravir, pharmacokinetics, adverse event
Abstract
Background. IMPAACT P1066 is a phase I/II open-label multicenter trial to evaluate pharmacokinetics, safety, tolerability, and efficacy of multiple raltegravir formulations in human immunodeficiency virus (HIV)–infected youth.
Methods. Dose selection for each cohort (I: 12 to <19 years; II: 6 to <12 years; and III: 2 to <6 years) was based on review of short-term safety (4 weeks) and intensive pharmacokinetic evaluation. Safety data through weeks 24 and 48, and grade ≥3 or serious adverse events (AEs) were assessed. The primary virologic endpoint was achieving HIV RNA <400 copies/mL or ≥1 log10 reduction between baseline and week 24.
Results. The targeted pharmacokinetic parameters (AUC0-12h and C12h) were achieved for each cohort, allowing dose selection for 2 formulations. Of 96 final dose subjects, there were 15 subjects with grade 3 or higher clinical AEs (1 subject with drug-related [DR] psychomotor hyperactivity and insomnia); 16 subjects with grade 3 or higher laboratory AEs (1 with DR transaminase elevation); 14 subjects with serious clinical AEs (1 with DR rash); and 1 subjects with serious laboratory AEs (1 with DR transaminase increased). There were no discontinuations due to AEs and no DR deaths. Favorable virologic responses at week 48 were observed in 79.1% of patients, with a mean CD4 increase of 156 cells/µL (4.6%).
Conclusions. Raltegravir as a film-coated tablet 400 mg twice daily (6 to <19 years, and ≥25 kg) and chewable tablet 6 mg/kg (maximum dose 300 mg) twice daily (2 to <12 years) was well tolerated and showed favorable virologic and immunologic responses.
Clinical Trials Registration NCT00485264.
Novel, potent, and well-tolerated antiretroviral (ARV) medications in appropriate formulations are acutely needed for pediatric patients of all ages infected with human immunodeficiency virus (HIV), especially for those failing ARV therapy, those with ARV resistance, or those suffering from ARV-related toxicities. Perinatally infected youth are often heavily pretreated and have very limited therapeutic options that can lead to sustained benefit.
Raltegravir is the first Food and Drug Administration– and European Medicines Agency–approved HIV integrase strand transfer inhibitor with demonstrated safety and efficacy in HIV type 1 (HIV-1)–infected adults. Raltegravir at a dose of 400 mg twice daily was approved for use in the treatment of HIV infection in adults based on data from 2 phase III studies in treatment-experienced adults, Blocking Integrase in Treatment-Experienced Patients with a Novel Compound Against HIV, Merck (BENCHMRK-1) and 2 [1, 2], and a phase III study in treatment-naive adults (STARTMRK) [3]. Data extending to 240 weeks for these 3 studies are now available and demonstrate both durable efficacy and a favorable long-term safety profile of raltegravir in adults [4, 5].
The International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) P1066 study was designed to assess the safety and pharmacokinetics (PK) of raltegravir in HIV-infected children aged 4 weeks to <19 years using 3 different formulations with the goal of determining the appropriate raltegravir dose in age-based subgroups. After dose selection, the study provided additional data on safety, efficacy, and population PK. This report describes findings from subjects aged 2 to <19 years of age who received either the film-coated or chewable tablet formulations.
METHODS
In this open-label, nonrandomized, multicenter study, subjects were enrolled into 3 age groups in 4 cohorts. Cohort I, ≥12 to <19 years and cohort IIA, ≥6 to <12 years, were assigned to receive film-coated tablets, as used in the adult formulation. After protocol amendment, cohort IIB, ≥6 to <12 years and cohort III, ≥2 to <6 years, were assigned to receive investigational chewable tablets. Entry criteria included plasma HIV RNA >1000 copies/mL, being ARV-experienced but naive to integrase inhibitors, laboratory values below grade 3 toxicity criteria, and absence of active opportunistic infection or current cancer. The study was conducted at both IMPAACT Network sites in the U.S., South Africa, Botswana, and Brazil after approvals were obtained from local institutional review boards and in-country ethics committees responsible for oversight of the study. Forty sites enrolled at least 1 subject.
The study was conducted in 2 stages. Stage I evaluated intensive PK and short-term safety. Raltegravir was added to a stable, failing regimen (defined as unchanged for ≥12 weeks). Immediately after completing the intensive PK sampling on days 7–12, the background ARV regimen was optimized. Alternatively, subjects who were treatment experienced but off treatment for ≥4 weeks prior to entry could be enrolled with a new optimized background regimen added to raltegravir after completion of intensive PK sampling. Stage II, which enrolled a separate group of subjects, assessed longer-term safety and efficacy of the selected dose. For subjects enrolling in stage II, background antiviral therapy was optimized with initiation of raltegravir.
The dose-finding algorithm for each cohort included review of week 4 safety and intensive PK, and required assessment of the first 4 subjects (“mini-cohort”). Once a mini-cohort met both the safety and PK criteria, further accrual to complete the full cohort could occur. Success, for safety, was defined as (1) no life-threatening suspected adverse drug reaction; (2) no grade 4 event considered probably or definitely attributable to raltegravir; and (3) no more than 25% terminating study treatment due to a grade 3 suspected adverse drug reaction. The PK objective was to achieve a PK profile similar to that attained in adults at the approved raltegravir dose of 400 mg twice daily. The specific PK targets included the geometric mean of the area under the curve (AUC0–12 hours) between 14 and 25 µM*hour and a geometric mean 12-hour postdose concentration (C12 hours) exceeding 33 nM, which corresponds to the in vitro concentration at which 95% of virologic replication is inhibited for antiviral activity. Dose selection in each full cohort occurred after all intensive PK data and at least 4 weeks of safety data were available. In addition, sparse PK sampling was done at intervals until week 48.
Long-term safety was assessed at 24 weeks (primary) and at 48 weeks in all treated subjects. Sites were required to report all grade ≥3 toxicity events, International Conference on Harmonization serious adverse events, malignancies, and pregnancies to the P1066 team and study sponsor (Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health). All subjects completed an adherence questionnaire, and site personnel collected and counted the returned raltegravir tablets to determine missed doses. A taste assessment for the chewable tablets was administered to cohorts IIB and III subjects either at week 4 or at an earlier discontinuation visit, if applicable.
Plasma HIV RNA (RNA) concentrations were determined at entry and at regular intervals using the HIV-1 MONITOR Test, version 1.5 (Roche Molecular Diagnostics) or RealTime HIV-1 (Abbott Molecular). Emergence of resistant virus from all subjects displaying virologic failure was monitored by isolating viral RNA from baseline and time of failure, provided plasma RNA was >1000 copies/mL and adequate sample was available, by performing genotypic and phenotypic assays for integrase, protease, and reverse transcriptase resistance. In subjects with virologic failure after 6 months of treatment, plasma specimens with RNA >1000 copies/mL were tested for HIV drug resistance in the polymerase gene encoding protease and reverse transcriptase using the TRUGENE HIV-1 Genotyping Assay (Siemens) and RNA extracted from 140 µL of plasma (QIAamp Viral RNA Mini Kit, Qiagen), following the manufacturer's instructions, and integrase using the PhenoSense and GeneSeq for Integrase (Monogram Biosciences).
Bioanalysis and Pharmacokinetics
Raltegravir plasma concentrations were measured at the University of Alabama at Birmingham using a validated, isocratic, reverse-phase high-performance liquid chromatography/tandem mass spectrometry method consistent with those previously published [6, 7]. The linear calibration range was 10 to 10 000 ng/mL from a 200-µL plasma sample. Whole blood was collected at time 0 (pre-dose) and at 0.5, 1, 2, 3, 4, 6, 8, and 12 hours postdosing. Raltegravir pharmacokinetic parameters were calculated using standard noncompartmental analysis in WinNonlin (Version 5.3, Pharsight Corp, Mountain View, California).
Statistics
The primary analysis group for safety and efficacy was subjects treated with only the final selected dose of raltegravir (FD population), whether enrolled in stage I or II. These results reflect the age-specific doses now approved for commercial use. A secondary set of analyses included the all-treated population (subjects who received any dose of raltegravir during stage I); these analyses are not presented unless specifically noted.
Safety and efficacy results of week 24 and 48 data (primary and key secondary time-points) from cohorts I, IIA, IIB, and III subjects are presented. By the data freeze date (7 February 2013), all subjects enrolled had been on study for >48 weeks. Efficacy responses were secondary objectives. The primary virologic outcome, virologic success, was defined as achieving either plasma RNA <400 copies/mL or ≥1 log10 reduction from baseline at week 24. The secondary efficacy objectives included the proportion achieving RNA <50 copies/mL, RNA <400 copies/mL, and the change from baseline in CD4 cell count and percentage at week 24 and 48 time-points. An observed failure approach was used for handling missing data. For virologic endpoints, missing values were considered to be failures, if missing due to discontinuation of study treatment for lack of efficacy or for non-treatment-related reasons with the last available RNA value not achieving virologic success. For change from baseline in CD4 cell count and percentage, baseline values were carried forward for missing data as described above. Other missing values were excluded. Virologic failure was defined as follows: (1) nonresponder—never achieved either a ≥1 log10 drop from baseline in RNA or RNA <400 copies/mL through week 24; or (2) virologic rebound at week 24 or later—(a) confirmed RNA ≥400 copies/mL after initial response with RNA <400 copies/mL, or (b) confirmed >1.0 log10 increase in RNA above nadir; confirmation required 2 consecutive measurements at least 1 week apart.
RESULTS
Enrollment and Disposition
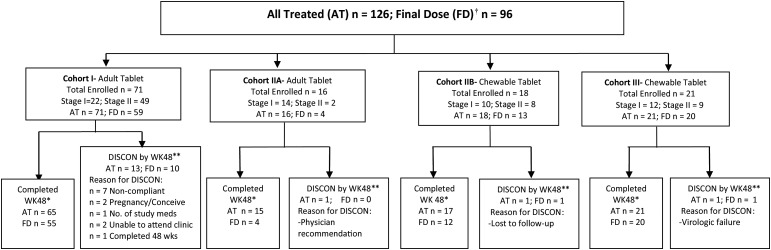
Of the 126 subjects enrolled (Figure 1), 16 subjects discontinued therapy by week 48, 13 of whom were from cohort I. Treatment discontinuation was often associated with poor adherence and virologic failure. Twelve of the 16 subjects (75%) enrolled on cohort IIA received other doses of raltegravir before the final dose was selected.
Figure 1.
Overall disposition of patients. Patients were enrolled in only 1 cohort. †Received only the final recommended dose of raltegravir. *Patient was on study drug to at least Study Day 295. **Disposition is provided for all treated patients based on patient status at the week 48 study visit. In some instances, patients discontinued after Study Day 295 but prior to or at the week 48 study visit and so the number of patients discontinued by week 48 is higher than expected based on the number completed at week 48.
Demographics
In all, 126 subjects were treated with raltegravir (all-treated population); of these, 96 received the final selected dose (FD) (Table 1). The FD population (Table 1) was 49% male; 60% were black and 59% carried a Centers for Disease Control and Prevention (CDC) category B or C disease classification. At baseline, 86% had plasma HIV-1 RNA >4000 copies/mL; mean CD4 count and CD4 percentage were 592 cells/µL and 23%, respectively, and 66.7% had prior experience with at least 3 ARV classes. Adolescents had the greatest prior treatment experience (mean, 9 prior ARVs), and more advanced HIV-1 disease than younger subjects, with >75% having CDC category B or C classification. Fourteen stage I subjects entered the study having failed a prior treatment regimen and on no current ARVs. These subjects received raltegravir monotherapy until after intensive PK sampling, when an optimized background was added. The most common concomitant ARVs were tenofovir (47.9%), ritonavir (as booster, 49%), lamivudine (41.7%), lopinavir/ritonavir (40.6%), and darunavir (39.6%). Nonnucleoside reverse transcriptase inhibitor use was less common (efavirenz, 13.5%; etravirine, 21.9%). There were few differences across cohorts.
Table 1.
Patient Demographics and Baseline Characteristics, Final Dose Population
| Characteristic | Cohort I (n = 59) | Cohort IIA (n = 4) | Cohort IIB (n = 13) | Cohort III (n = 20) | Total (N = 96) |
|---|---|---|---|---|---|
| Age, y, mean [SD] | 15.2 [1.9] | 10 [1.4] | 8.8 [1.6] | 3.2 [1.2] | 11.6 [5.2] |
| Male sex, n (%) | 30 (50.8) | 3 (75) | 7 (53.8) | 7 (35) | 47 (49) |
| Race, n (%) | |||||
| Black or African American | 35 (59.3) | 3 (75) | 7 (53.8) | 13 (65) | 58 (60.4) |
| White | 21 (35.6) | 1 (25) | 6 (46.2) | 5 (25) | 33 (34.4) |
| Multiracial, American Indian, or unknown | 3 (5.1) | 0 (0) | 0 (0) | 2 (10) | 5 (5.1) |
| Hispanic ethnicity, n (%) | 22 (37.3) | 1 (25) | 7 (53.8) | 8 (40) | 38 (39.6) |
| Plasma HIV RNA, log10 copies/mL | |||||
| Mean [SD] | 4.3 [0.6] | 4.4 [0.6] | 4.2 [0.5] | 4.3 [0.7] | 4.3 [0.6] |
| Median [range] | 4.3 [3.1–6] | 4.6 [3.5–4.9] | 4.2 [3.1–5.2] | 4.5 [2.7–5.3] | 4.3 [2.7–6] |
| CD4 cell count, cells/µL | |||||
| Mean [SD] | 397.5 [229.8] | 850.5 [509.5] | 577.9 [269.8] | 1114.6 [549.7] | 592.2 [441.5] |
| Median [range] | 396.5 [0–872] | 806.5 [274–1515] | 529 [16–1000] | 1086.5 [323–2361] | 481 [0–2361] |
| CD4 percentage | |||||
| Mean [SD] | 19.7 [9.6] | 25.2 [8.7] | 29.1 [10.4] | 28.2 [8.3] | 23 [10.2] |
| Median [range] | 20 [0–44] | 28 [13–31.7] | 33 [2–40] | 28.7 [12.9–41.8] | 23.3 [0–44] |
| CDC HIV clinical classification, n (%) | |||||
| A | 14 (23.7) | 2 (50) | 6 (46.2) | 5 (25) | 27 (28.1) |
| B | 24 (40.7) | 0 (0) | 3 (23.1) | 1 (5) | 28 (29.2) |
| C | 21 (35.6) | 1 (25) | 0 (0) | 7 (35) | 29 (30.2) |
| N | 0 (0) | 1 (25) | 4 (30.8) | 7 (35) | 12 (12.5) |
| Number of ARV classes previously used, n (%) | |||||
| 0 | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 1 (1) |
| 1 | 2 (3.4) | 0 (0) | 0 (0) | 2 (10) | 4 (4.2) |
| 2 | 6 (10.2) | 2 (50) | 7 (53.8) | 12 (60) | 27 (28.1) |
| ≥3 | 51 (86.4) | 2 (50) | 6 (46.2) | 5 (25) | 64 (66.7) |
| Duration of ARVs previously used, y | |||||
| Mean [SD] | 11.9 [4.1] | 10.1 [1.6] | 7.4 [3.1] | 2.4 [1.4] | 9.3 [5.2] |
| Median [range] | 12.8 [1.8–17.9] | 10.7 [7.7–11.4] | 7.7 [1–11.2] | 2.2 [0–5] | 11 [0–17.9] |
| Use of prior NNRTIs, n (%) | 51 (86.4) | 3 (75) | 11 (84.6) | 10 (50) | 75 (78.1) |
| Use of prior PIs, n (%) | 57 (96.6) | 3 (75) | 8 (61.5) | 12 (60) | 80 (83.3) |
| Plasma HIV RNA, copies/mL | |||||
| 1000 to ≤4000 | 9 (15.3) | 1 (25) | 1 (7.7) | 2 (10) | 13 (13.5) |
| >4000 to ≤50 000 | 36 (61) | 2 (50) | 9 (69.2) | 11 (55) | 58 (60.4) |
| >50 000 | 14 (23.7) | 1 (25) | 3 (23) | 7 (35) | 25 (26) |
| Phenotypic sensitivity score, n (%)a | |||||
| 0 | 3 (5.1) | 0 (0) | 1 (7.7) | 0 (0) | 4 (4.2) |
| 1 | 11 (18.6) | 1 (25) | 3 (23.1) | 2 (10) | 17 (17.7) |
| 2 | 22 (37.3) | 0 (0) | 7 (53.8) | 7 (35) | 36 (37.5) |
| ≥3 | 19 (32.2) | 2 (50) | 1 (7.7) | 6 (30) | 28 (29.2) |
| Genotypic sensitivity score, n (%)b | |||||
| 0 | 5 (8.5) | 0 (0) | 1 (7.7) | 0 (0) | 6 (6.3) |
| 1 | 19 (32.2) | 1 (25) | 5 (38.5) | 2 (10) | 27 (28.1) |
| 2 | 18 (30.5) | 2 (50) | 6 (46.2) | 10 (50) | 36 (37.5) |
| ≥3 | 16 (27.1) | 1 (25) | 1 (7.7) | 7 (35) | 25 (26) |
The Genotypic Sensitivity Score and Phenotypic Sensitivity Score were defined as the total number of ARVs in optimized background therapy (not including raltegravir) to which the patient's viral isolate showed genotypic/phenotypic sensitivity, based upon resistance tests performed prestudy (or at screening).
Abbreviations: ARV, antiretroviral; CDC, Centers for Disease Control and Prevention; HIV, human immunodeficiency virus; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; SD, standard deviation.
a Phenotypic sensitivity score was missing for 11.5%.
b Genotypic sensitivity score was missing for 2.1%.
Intensive Pharmacokinetics and Dose Selection
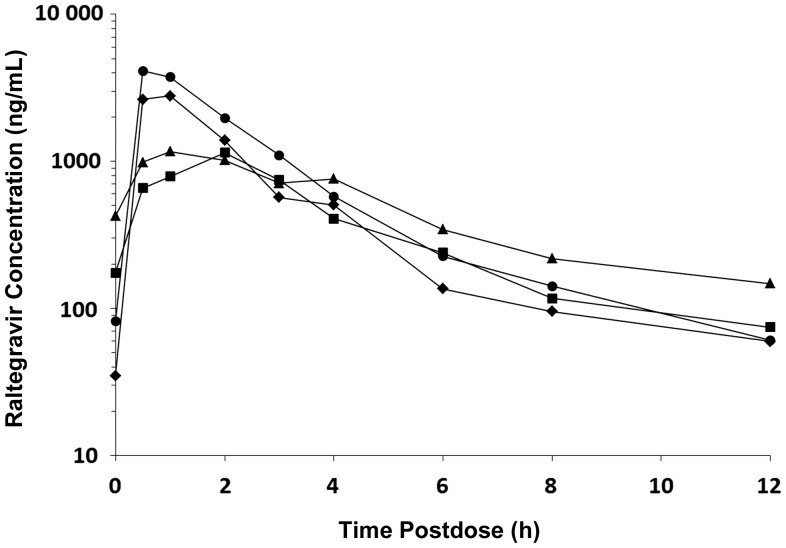
All dose adjustments and decisions were based entirely on PK results; there were no instances in which either the mini-cohort or full cohort dose failed safety criteria. When the geometric mean AUC0–12 hours for a mini-cohort or a full cohort did not meet the PK target, the raltegravir dose was adjusted. A few children (n = 3, cohort 1 only) received individualized dosing higher than the dose selected for the cohort based on protocol-specified PK parameters; no adverse events were associated with this individualized dosing. A dose of 400 mg twice daily of the film-coated tablet was selected for children aged ≥6 years and weighing at least 25 kg. Weight-based dosing of approximately 6 mg/kg twice daily (maximum 300 mg per dose) of the chewable tablet formulation was selected for children 2 to <12 years of age (Tables 2 and 3, Figure 2).
Table 2.
Raltegravir Pharmacokinetic Parameters by Cohort and Formulation
| Cohort | Descriptor | Dose, mg | Weight, kg | Dose, mg/kg | T1/2, h | Cmax, ng/mL | C12, ng/mL | AUC12, h*mg/L | CL/F, L/h | V/F, L |
|---|---|---|---|---|---|---|---|---|---|---|
| Cohort I (n = 11) | Mean | 390.9 | 43.5 | 9.3 | 4.9 | 2813.1 | 197.0 | 10.2 | 71.7 | 698.6 |
| ≥12 to <19 y | SD | 94.4 | 13.4 | 1.6 | 3.6 | 2673.9 | 154.2 | 10.0 | 56.7 | 1131.5 |
| Film-coated tablet | Median | 440.0 | 39.8 | 9.9 | 3.8 | 1384.1 | 137.6 | 7.4 | 54.1 | 223.6 |
| CV% | 24.1 | 30.8 | 17.3 | 73.9 | 95.1 | 78.3 | 97.6 | 79.1 | 162.0 | |
| GM | 379.6 | 45.1 | 9.1 | 5.0 | 1777.2 | 147.8 | 7.0 | 54.4 | 310.0 | |
| Cohort IIA (n = 11) | Mean | 400.0 | 31.5 | 13.4 | 3.7 | 4055.7 | 399.4 | 13.4 | 112.8 | 475.6 |
| ≥6 to <12 y | SD | 0.0 | 7.3 | 3.7 | 1.4 | 5269.5 | 880.9 | 16.1 | 161.4 | 506.7 |
| Film-coated tablet | Median | 400.0 | 32.4 | 12.3 | 3.4 | 2247.2 | 107.5 | 6.7 | 59.4 | 292.3 |
| CV% | 0.0 | 23.2 | 27.4 | 36.7 | 129.9 | 220.5 | 120.4 | 143.2 | 106.5 | |
| GM | 400.0 | 30.7 | 13.0 | 3.5 | 2135.0 | 109.4 | 7.0 | 56.8 | 287.9 | |
| Cohort IIB (n = 10) | Mean | 230.0 | 36.4 | 6.5 | 4.5 | 5314.2 | 78.7 | 10.5 | 23.6 | 143.2 |
| ≥6 to <12 y | SD | 58.7 | 11.4 | 0.9 | 4.2 | 2842.6 | 68.9 | 3.5 | 8.9 | 131.9 |
| Chewable tablet | Median | 225.0 | 35.1 | 6.2 | 2.9 | 4524.0 | 43.4 | 10.5 | 23.4 | 108.8 |
| CV% | 25.5 | 31.3. | 13.4 | 93.5 | 53.3 | 87.6 | 33.6 | 37.7 | 92.1 | |
| GM | 223.0 | 34.8 | 6.4 | 3.4 | 4660.6 | 57.6 | 10.0 | 22.2 | 110.3 | |
| Cohort III (n = 12) | Mean | 89.6 | 14.2 | 6.2 | 4.1 | 5204.8 | 39.5 | 9.5 | 13.3 | 71.3 |
| 2 to <6 y | SD | 22.5 | 2.2 | 0.7 | 3.2 | 2940.6 | 21.9 | 5.5 | 10.1 | 57.0 |
| Chewable tablet | Median | 75.0 | 13.4 | 6.1 | 3.1 | 5143.5 | 35.8 | 7.3 | 10.3 | 67.8 |
| CV% | 25.1 | 15.7 | 10.7 | 78.5 | 56.5 | 55.5 | 58.6 | 76.1 | 80.0 | |
| GM | 87.5 | 14.1 | 6.2 | 3.3 | 4328.6 | 31.6 | 8.0 | 11.0 | 52.0 |
Abbreviations: AUC12, area under the concentration-time curve from 0 to 12 hours postdose; C12, concentration at 12 hours postdose; CL/F, oral clearance; Cmax, maximum concentration; CV%, percent coefficient of variation; GM, geometric mean; L/h, liters per hour; SD, standard deviation; T1/2, half-life; V/F, oral volume of distribution.
Table 3.
Intensive Pharmacokinetic Parameters at the Final Recommended Raltegravir Dose
| Age | Cohort | Formulation | Final Recommended Dose | No.a | Mean Weight, kg | Mean Dose, mg | Mean Dose, mg/kg | Geometric Mean (CV%) AUC 0–12 h, μM*h | Geometric Mean (CV%) C12 h, nM |
|---|---|---|---|---|---|---|---|---|---|
| 12 to <19 y | I | Film-coated tablet | 400 mg BIDb | 11 | 43.55 | 390.91 | 9.28 | 15.7 (98) | 333 (78) |
| 6 to <12 y | IIA | Film-coated tablet | 400 mg BID, for patients weighing ≥25 kg | 11 | 31.54 | 400.00 | 13.45 | 15.8 (120) | 246 (221) |
| 6 to <12 y | IIB | Chewable tablet | 6 mg/kg BID, maximum of 300 mg BID | 10 | 36.36 | 230.00 | 6.47 | 22.6 (34) | 130 (88) |
| 2 to <6 y | III | Chewable tablet | 6 mg/kg BID, maximum of 300 mg BID | 12 | 14.24 | 89.58 | 6.24 | 18.0 (59) | 71 (55) |
Abbreviations: AUC0–12 h, area under the concentration-time curve from 0 to 12 hours postdose; BID, twice daily; C12 h, concentration at 12 hours postdose; CV%, percent coefficient of variation.
a No. of patients with intensive pharmacokinetic (PK) results at the final recommended dose.
b Cohort I patients received approximately 8 mg/kg dose at time of intensive PK which met PK and safety targets. Based on review of the individual profiles and receipt of a mean dose of 390 mg, the team selected 400 mg BID as the recommended dose for this age group. Patients receiving a dose other than 400 mg BID were switched; no repeat PK was performed.
Figure 2.
Raltegravir geometric mean concentration-time results by cohort. Cohort I, triangles; cohort IIA, squares; cohort IIB, circles; cohort III, diamonds.
Safety and Adverse Events
Raltegravir, in either formulation, was well tolerated. The 96 FD subjects included 63 receiving the film-coated tablet and 33 receiving the chewable tablets. Including all adverse events reported up to week 48 in the FD population, there were 84 subjects with clinical events and 85 subjects with laboratory events (Table 4), including 14 subjects with serious clinical events and 1 subject with serious laboratory events. Of the serious adverse events, 2 clinical events in 1 subject (rash [day 17] and drug-induced liver injury [day 141]) were judged by the investigator to be drug related; neither resulted in treatment interruption. Grade 3 or higher clinical adverse events were reported in 15 subjects including 1 subject who on day 41 had the following concurrent 3 drug-related events: psychomotor hyperactivity, abnormal behavior, and insomnia. Grade 3 or higher laboratory adverse events were reported in 16 subjects, including 1 subject with drug-related events of increased alanine aminotransferase and aspartate aminotransferase (multiple reports starting at day 122), which is the same subject noted above with the serious clinical event of drug-induced liver injury. There were no treatment-related discontinuations or deaths in the study through week 48.
Table 4.
Summary of Clinical and Laboratory Adverse Events, Weeks 0–48, Final Dose Population
| Adverse Event | Cohort I (n = 59) | Cohort IIA (n = 4) | Cohort IIB (n = 13) | Cohort III (n = 20) | Total (N = 96) |
|---|---|---|---|---|---|
| Clinical adverse events | |||||
| With ≥1 serious drug-relateda clinical adverse events | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 1 (1) |
| With ≥1 serious clinical adverse events | 10 (16.9) | 0 (0) | 2 (15.4) | 2 (10) | 14 (14.6) |
| With ≥1 grade 3 or higher drug-relateda clinical adverse events | 1 (1.7) | 0 (0) | 0 (0) | 0 (0) | 1 (1) |
| With ≥1 grade 3 or higher clinical adverse events | 10 (16.9) | 0 (0) | 2 (15.4) | 3 (15) | 15 (15.6) |
| With no clinical adverse event | 6 (10.2) | 0 (0) | 2 (15.4) | 4 (20) | 12 (12.5) |
| Discontinued due to an adverse event (clinical or laboratory) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Laboratory adverse events | |||||
| With ≥1 serious drug-relateda laboratory adverse events | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| With ≥1 serious laboratory adverse events | 1 (1.7) | 0 (0) | 0 (0) | 0 (0) | 1 (1) |
| With ≥1 grade 3 or higher drug-relateda laboratory adverse events | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 1 (1) |
| With ≥1 grade 3 or higher laboratory adverse events | 8 (13.6) | 1 (25) | 1 (7.7) | 6 (30) | 16 (16.7) |
| With no laboratory adverse event | 6 (10.2) | 1 (25) | 2 (15.4) | 2 (10) | 11 (11.5) |
Data are presented as No. (%) unless otherwise indicated. Data freeze occurred on 7 February 2013. Events were included if they occurred while on study drug or within 14 days after discontinuation of study drug.
a Drug-related adverse events were determined by the investigator to be possibly, probably, or definitely related to raltegravir.
Efficacy
Virologic success, defined as plasma HIV-1 RNA <400 copies/mL or >1 log10 decline from baseline at week 24, among the 96 FD subjects was 71.6%, with 53.7% having RNA of <50 copies/mL. At week 48, the success rate was 79.1%, with 57.1% having RNA of <50 copies/mL (Table 5). Efficacy results were consistent across the age cohorts, irrespective of the formulation used, although formal comparisons were not performed. Mean increases from baseline in CD4 cells (%) were 119.0 cells/µL (3.8%) at week 24 and 155.7 cells/µL (4.6%) at week 48. Additional subgroup analyses were explored to examine factors predicting virologic success; however, modest sample sizes of subgroups precluded making firm conclusions.
Table 5.
Efficacy Analysis by Cohort, Final Dose Population, Week 24 and Week 48, Observed Failure Approach
| Parameter | Cohort I (n = 59) |
Cohort IIA (n = 4) |
Cohort IIB (n = 13) |
Cohort III (n = 20) |
Total (N = 96) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| no./No. | % (95% CI) | no./No. | % (95% CI) | no./No. | % (95% CI) | no./No. | % (95% CI) | no./No. | % (95% CI) | |
| Proportion with HIV RNA with ≥1 log10 decline or <400 copies/mL | ||||||||||
| Week 24 | 42/58 | 72.4 (59.1, 83.3) | 2/4 | 50 (6.8, 93.2) | 10/13 | 76.9 (46.2, 95) | 14/20 | 70 (45.7, 88.1) | 68/95 | 71.6 (61.4, 80.4) |
| Week 48 | 42/56 | 75 (61.6, 85.6) | 3/4 | 75 (19.4, 99.4) | 10/11 | 90.9 (58.7, 99.8) | 17/20 | 85 (62.1, 96.8) | 72/91 | 79.1 (69.3, 86.9) |
| Proportion with HIV RNA <50 copies/mL | ||||||||||
| Week 24 | 32/58 | 55.2 (41.5, 68.3) | 2/4 | 50 (6.8, 93.2) | 7/13 | 53.8 (25.1, 80.8) | 10/20 | 50 (27.2, 72.8) | 51/95 | 53.7 (43.2, 64) |
| Week 48 | 32/56 | 57.1 (43.2, 70.3) | 2/4 | 50 (6.8, 93.2) | 6/11 | 54.5 (23.4, 83.3) | 12/20 | 60 (36.1, 80.9) | 52/91 | 57.1 (46.3, 67.5) |
| Proportion with HIV RNA <400 copies/mL | ||||||||||
| Week 24 | 40/58 | 69 (55.5, 80.5) | 2/4 | 50 (6.8, 93.2) | 9/13 | 69.2 (38.6, 90.9) | 12/20 | 60 (36.1, 80.9) | 63/95 | 66.3 (55.9, 75.7) |
| Week 48 | 39/56 | 69.6 (55.9, 81.2) | 2/4 | 50 (6.8, 93.2) | 10/11 | 90.9 (58.7, 99.8) | 16/20 | 80 (56.3, 94.3) | 67/91 | 73.6 (63.3, 82.3) |
| Change from baseline in CD4 cell count, cells/µL | Mean | (95% CI) | Mean | (95% CI) | Mean | (95% CI) | Mean | (95% CI) | Mean | (95% CI) |
| Week 24 | 114.4 | (73.7, 155.1) | −35.8 | (−348.8, 277.3) | 143.4 | (−12.9, 299.6) | 147.2 | (−2.7, 297.1) | 119.0 | (74.9, 163.1) |
| Week 48 | 168.2 | (117.5, 218.9) | 189.5 | (−154.2, 533.2) | 76.8 | (−85.3, 238.9) | 158.1 | (11.7, 304.4) | 155.7 | (108.3, 203.1) |
| Change from baseline in CD4 percentage | ||||||||||
| Week 24 | 4.1 | (2.8, 5.3) | 2.2 | (−7.2, 11.5) | 0.8 | (−3.6, 5.2) | 5.3 | (2.9, 7.7) | 3.8 | (2.7, 4.9) |
| Week 48 | 5.2 | (3.9, 6.6) | 6.0 | (−2.6, 14.6) | 1.6 | (−2.7, 5.9) | 4.3 | (1, 7.6) | 4.6 | (3.4, 5.8) |
For binary endpoints: no./No. with % (95% CI) was reported for each cohort, where no./No. = number of responders/number of patients. For continuous endpoints: mean change with (95% CI) was reported. Normal distributions were assumed for continuous endpoints. Observed failure approach for handling missing data: for binary endpoints, missing values were considered as failures for patients missing data due to discontinuation of study treatment for lack of efficacy or for non-treatment-related reasons with last available HIV RNA value <1 log10 drop from baseline and ≥400 copies/mL; otherwise patients with missing values were excluded. For continuous endpoints (eg, change from baseline in CD4 cell counts and percent), baseline values were carried forward for patients missing data due to discontinuation of study treatment for lack of efficacy or for non-treatment-related reasons with last available HIV RNA value <1 log10 drop from baseline and ≥400 copies/mL; otherwise patients with missing values were excluded.
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus.
Antiretroviral Drug Resistance
Of 36 subjects with virologic failure (either as rebound or nonresponder) by week 48, 34 had on-treatment genotypic data: 12 (35.3%) displayed primary raltegravir signature resistance mutations (amino acid numbers 143, 148, and 155), 2 (5.9%) had other mutations known to confer raltegravir resistance, and 20 (58.8%) did not have genotypes associated with reported raltegravir resistance (ie, wild-type codons). At baseline, among the 36 viral failure subjects, 25 had no raltegravir resistance noted, 3 had either T97A or L74I mutations noted, and data on 8 are lacking. Of those 8 subjects lacking baseline data, by week 48, 3 developed signature raltegravir resistance mutations, whereas 5 continued to have wild-type virus. Among the 2 subjects with other secondary raltegravir resistance mutations, 1 subject had L74I mutation at baseline and at failure and 1 subject had T97A mutation at baseline and L74L/M and T97A mutations at failure. Although these secondary mutations were reported as resistant to raltegravir, these mutations alone are not known to confer clinically significant resistance to raltegravir.
Palatability
At least 1 taste assessment was collected for 41 patients who received the chewable tablet formulation, with 54% of the assessments provided by the participant and 39% provided by the primary caregiver. In 73% of evaluations no problems were reported taking the chewable tablet; 61% reported average or better overall taste.
Adherence
Adherence to ARV treatment was calculated based on data from all treated subjects, but data were not available for each patient or time-point on treatment. Overall, 19% of the patients reported 100% adherence, and 52% were >90% adherent. Across cohorts, 5.6% of cohort I, none of cohort IIA, 5.6% of cohort IIB, and none of cohort III had adherence <70%. Of note, among the 36 patients with virologic failure, 19 (53%) had at least 1 population (sparse) PK sample below the assay limit of quantitation (BLOQ), and 10 (28%) had ≥2 BLOQ PK samples, suggesting likely nonadherence. Furthermore, of the 20 virologic failure patients who had available genotyping data but no raltegravir resistance mutations, 6 (30%) had 1 BLOQ PK sample and 9 others (45%) had ≥2 BLOQ PK samples.
DISCUSSION
Since the earliest stages of the HIV epidemic, drug development for HIV-infected pediatric populations has lagged behind approvals for adult populations. This has been due, in part, to many challenges inherent in pediatric drug development. These include the need for palatable formulations across different age groups, investigations for formulation-dependent toxicities, definition of PK parameters that may be affected by age-related metabolism, and development of simple weight-band dosing schedules. In this prospective, dose-finding PK, safety, and efficacy study across a defined pediatric age range, we report data in HIV-infected treatment-experienced children 2 to <19 years of age. For the pediatric development of raltegravir, IMPAACT P1066 employed a novel study design where PK and safety information from a small number of participants in each age cohort were rapidly evaluated and allowed decisions about expanding the cohort and opening the subsequent younger cohort. This adaptive design allowed the efficient development of raltegravir, sequentially transitioning to younger cohorts and complete parallel analysis of multiple cohorts, resulting in regulatory approval of this ARV for pediatric populations ≥2 years of age, while this study was ongoing in infants and toddlers 4 weeks to <2 years of age. Although a limitation of this study was the small sample size of each cohort, decisions made by the mini-cohort PK and safety assessment were confirmed by analysis of the full stage 1 cohort data and supported by the additional long-term safety, tolerance, and efficacy data collected after dose selection in stages I and II. Note that the study was not designed to compare results between cohorts. A final dose of 400 mg twice daily of the film-coated tablet for children aged ≥6 years and at least 25 kg was chosen, along with a weight-based dose of 6 mg/kg twice daily (maximum of 300 mg per dose) of the chewable tablet formulation for children 2 to <12 years of age.
This study establishes that raltegravir, in combination with an optimized background regimen in these ARV-experienced children, has both an excellent safety and efficacy profile. Consistent with observations in adults, raltegravir demonstrated a rapid onset of ARV effect, with more than half of treated subjects achieving an RNA level of <50 copies/mL by 24 weeks. Raltegravir demonstrated consistent efficacy across age cohorts and for both formulations studied, with 79% of all subjects reaching either a 1 log10 drop from baseline or <400 copies/mL at 48 weeks. Overall, raltegravir was well tolerated in these children and adolescents. Although adverse events overall were common, very few grade 3/4 or serious adverse events were considered drug-related, and none led to raltegravir discontinuation.
Regulatory approval for children of novel ARV agents with demonstrated safety and efficacy in adult populations is supported by open-label noncomparative studies designed to determine the dose and safety across the spectrum of pediatric developmental stages. Many of these trials have a design similar to our study reported here: test drug plus optimized background, and inclusion criteria for ARV experienced subjects failing their current regimen. Similar to our study, a recent study of darunavir/ritonavir in treatment-experienced HIV-infected children aged ≥6 years showed 59% of subjects with HIV-1 RNA <400 copies/mL at 48 weeks [8]. In a study comparing low-dose to high-dose tipranavir (plus ritonavir) at 48 weeks, 39.7% low-dose and 45.6% high-dose tipranavir/ritonavir recipients had a viral load <400 copies/mL [9]. Overall, efficacy in our study (at 48 weeks) was higher with 74% of subjects achieving viral loads <400 copies/mL, and 79% reaching protocol-defined success.
Of the 34 subjects who experienced virologic failure and for whom resistance data are available, resistance due to raltegravir was noted in 35%, and raltegravir was frequently undetectable in sparse PK samples. Given deficiencies in adherence, it is possible that in this highly ARV-experienced group, incomplete adherence leading to suboptimal drug plasma concentrations and not treatment potency was responsible for the poor response and selection of resistance. Because of the substantial amount of missing adherence data, these results should be interpreted with caution, but suggest that attention to adherence may result in viral suppression even if ARVs are recycled. Two polymorphisms, L74I/L/M and T97A, when detected alone do not confer phenotypic resistance to raltegravir. However, L74I can enhance resistance conferred by signature resistance mutations, and viruses bearing both L74M and T97A have reduced susceptibility to raltegravir.
These results establish that raltegravir given in age-appropriate formulations is safe, well tolerated, and has favorable antiretroviral and immunological effects in HIV-1–infected ARV-experienced children aged 2 to <19 years. As the first approved HIV integrase inhibitor, raltegravir is an important addition to the HIV treatment armamentarium for children and adolescents.
Notes
Acknowledgments. Members of the P1066 Protocol Team include: Sharon Nachman, MD, Department of Pediatrics, State University of New York at Stony Brook; Nan Zheng, MA, Terence Fenton, EdD, and Carmelito Alvero, MS, Statistical and Data Analysis Center, Harvard School of Public Health, Boston, MA; Edward P. Acosta, PharmD, Division of Clinical Pharmacology, University of Alabama at Birmingham; Hedy Teppler, MD, Brenda Homony, MS, Xia Xu, PhD, Larissa Wenning, PhD, Merck & Co, West Point, PA; Bobbie Graham, BS, Frontier Science and Technology Research Foundation, Amherst, NY; Stephen A. Spector, MD, Department of Pediatrics, University of California, San Diego and Rady Children's Hospital San Diego, La Jolla, CA; Lisa M. Frenkel, MD, Departments of Pediatrics, Laboratory Medicine, and Global Health, University of Washington and Seattle Children's Hospital and Research Institute, Seattle, Washington; Maripat Toye, RN, MS, CCRP, Baystate Medical Center, Springfield, MA; Carol Worrell, MD, Maternal and Pediatric Infectious Disease Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD; Ed Handelsman, MD, Paul Sato, MD, Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID), Bethesda, MD; Elizabeth Petzold, PhD, Social and Scientific Systems, Silver Spring, MD; Andrew Wiznia, MD, Jacobi Medical Center, Bronx, NY, and the IMPAACT P1066 Study Team.
Participating sites and site personnel include: Shandukani Research (Harry Moultrie, MD MSc; Angela Oosthuizen, BPharm; Gurpreet Kindra, MD PhD); Chicago Children's (Margaret Ann Sanders, MPH; Ruth Williams, RN; Jennifer Jensen, PNP); San Juan City Hospital PR NICHD (Midnela Acevedo, MD; Lizbeth Fabregas, MS); Columbia IMPAACT (Andrea Jurgrau, PNP; Marc Foca, MD; Alice Higgins, RN); UCLA–Los Angeles/Brazil AIDS Consortium (Jaime G. Deville, MD; Karin Nielsen-Saines, MD; Michele F. Carter, RN); DUMC Pediatric (John Swetnam, MD; Joan Wilson, RN, BSN; Margaret Donnelly, PA-C); NYU NY NICHD, supported in part by grant UL1 TR000038 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) (Siham Akleh, RN; Mona Rigaud, MD; Aditya Kaul, MD); St Jude/UTHSC (Nehali Patel, MD; Aditya Gaur, MD; L. Jill Utech, RN, MSN); Hospital Nossa Senhora da Conceicao (Edmundo Cardoso, MD; Ana Maria Moreira, MD; Breno Santos, MD); Durban Pediatric HIV (Raziya Bobat, MD; Rosie Mngqibisa, MS); Jacobi Medical Center Bronx NICHD (Marlene Burey, PNP; Jacob Abadi, MD; Michael Rosenberg, MD); WNE Maternal Pediatric Adolescent AIDS (Katherine Luzuriaga, MD; Donna Picard, RN; Jessica Pagano-Therrien, RN, PNP; CTSI: UL1TR000161); Soweto IMPAACT (Sylvia Dittmer, MBBCH, DCH; Hilda Ntatule Ndiweni, BC, Ed ET Admin; Amisha Patel, BPharm); Texas Children's Hospital (Michelle DelRey, RN; Chivon McMullen-Jackson, RN, BSN, CCRP; Mary E. Paul, MD); Seattle Children's Hospital (Ann Melvin, MD, MPH; Corry Venema-Weiss, ARNP; Jenna Lane, ARNP). This publication was supported by the NCATS of the NIH under Award Number UL1TR000423); SUNY Stony Brook NICHD (Christy Beneri, DO; Denise Ferraro, FNP; Erin Infanzon); Rush University Cook County Hospital Chicago NICHD (James B McAuley, MD, MPH; Mariam Aziz, MD; Maureen McNichols, RN, MSN, CCRC); Boston Medical Center Pediatric HIV Program NICHD (Stephen Pelton, MD; Deb McLaud, RN; Diana Clarke, PharmD); Children's National Medical Center Washington DC NICHD (Steven Zeichner, MD, PhD; Arezou Akar, MPH; Deidre Thompson, RN); The Children's Hospital of Philadelphia IMPAACT (Steven D. Douglas, MD, Richard M. Rutstein, MD, Carol A. Vincent, PhD, CRNP); Bronx-Lebanon Hospital IMPAACT (Mary Elizabeth Vachon, MPH; Martha Cavallo, NP; Murli Udharam Purswani, MD); Gaborone Prevention/Treatment Trials (Gaerolwe Masheto, MD; Anthony Ogwu, MD, MPH; Tebogo Kakhu, BS, RN); University of California San Diego Maternal, Child, and Adolescent HIV (Rolando M. Viani, MD, MTP; Anita, Darcey, RN; Kimberly Norris, RN, BSN); Children's Hospital of Boston NICHD (Sandra K. Burchett, MD, MS; Catherine Kneut, RN, MS, CPNP; Nancy Karthas, RN, MS, CPNP); University of South Florida–Tampa NICHD (Denise Casey, RN; Patricia Emmanuel, MD; Jorge Lujan-Zilbermann, MD); Howard University, Washington DC, NICHD (Sohail Rana, MD; Patricia Houston, MS; Mulu Mengistab, Pharm D); University of Florida College of Medicine, Jacksonville NICHD (Mobeen Rathore, MD; Ayesha Mirza, MD; Tabetha Gayton, MS, RN, FNP; The UF CTSI is supported in part by the NIH/National Center for Research Resources (NCRR) Clinical and Translational Science Award UL1 RR029890); University of Colorado Denver NICHD (Emily Barr, CPNP, CNM, MSN; Jennifer Dunn, FNP-C; Kerry Hahn, BS, CCRP); South Florida CDC Fort Lauderdale NICHD (Zulma Eysallenne, RN; F. Sholar Howard, ARNP; Kathleen Graham, Pharm D); Instituto de Infectologia Emílio Ribas Sao Paulo Brazil NICHD (Marinella Della Negra, MD, PhD; Wladimir Queiroz, MD, MSc; Yu Ching Lian, MD, MSc); University of California, San Francisco NICHD (Diane Wara, MD; Ted Ruel, MD; This publication was supported by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131); Tulane University New Orleans NICHD (Russell VanDyke, MD; Patricia Reilly, RN; Sheila Bradford, RN); Stellenbosch University (Anita Janse van Rensburg, RN; Els Dobbels, MD; Marietjie Bester, RN); Metropolitan Hospital NICHD (Mahrukh Bamji, MD; Santa Paul, MD; Mirala Sarza, MS); USC LA NICHD (Andrea Kovacs, MD; James Homans, MD; LaShonda Spencer, MD); Inst of Pediatrics Fed Univ Rio de Janeiro NICHD (Cristna Hofer, MD, PhD; Thalita Abreu, MD; Ricardo Oliveira, MD); Hospital Federal dos Servidores do Estado Rio de Janeiro NICHD (Esau C. Joao, MD, PhD); SOM Federal University Minas Gerais Brazil NICHD (Jorge Pinto, MD; Flavia Ferreira, MD; Fabiana Kakehasi, MD); University of Sao Paulo Brazil NICHD (Maria Celia Cervi; Marcia De Lima Isaac); Hospital General de Agudos Buenos Aires Argentina NICHD (Marcelo H. Losso, MD; Erica Stankievich, MD; Irene Foradori, MD); Children's Hospital of Los Angeles NICHD (Diane Tucker, MSN; Joseph Church, MD; Marvin Belzer, MD, FACP, FSAM); and Johns Hopkins University Baltimore NICHD (Jonathan Ellen MD; Allison Agwu MD; Laurel Borkovic MS ED).
Financial support. Overall support for the IMPAACT Group was provided by the NIAID (grant number U01 AI068632), the NICHD, and the National Institute of Mental Health (grant number AI068632). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work was supported by the Statistical and Data Analysis Center at Harvard School of Public Health, under the NIAID cooperative agreement 5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group and 1 U01 AI068616 with the IMPAACT Group. Support of the sites was provided by the NIAID and the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD (contract number N01-DK-9-001/HHSN267200800001C), and by Merck & Co Inc.
Potential conflicts of interest. H. T., B. H., L. W., and X. X. are employees of Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, and may own stock and/or stock options in the company. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Steigbigel RT, Cooper DA, Kumar PN, et al. BENCHMRK Study Teams. Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med. 2008;359:339–54. doi: 10.1056/NEJMoa0708975. [DOI] [PubMed] [Google Scholar]
- 2.Steigbigel RT, Cooper DA, Teppler H, et al. BENCHMRK Study Teams. Long-term efficacy and safety of raltegravir combined with optimized background therapy in treatment-experienced patients with drug-resistant HIV infection: week 96 results of the BENCHMRK 1 and 2 phase III trials. Clin Infect Dis. 2010;50:605–12. doi: 10.1086/650002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lennox JL, DeJesus E, Lazzarin A, et al. STARTMRK investigators. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet. 2009;374:796–806. doi: 10.1016/S0140-6736(09)60918-1. [DOI] [PubMed] [Google Scholar]
- 4.De Castro N, Braun J, Charreau I, et al. EASIER ANRS 138 study group. Switch from enfuvirtide to raltegravir in virologically suppressed multidrug-resistant HIV-1-infected patients: a randomized open-label trial. Clin Infect Dis. 2009;49:1259–67. doi: 10.1086/605674. [DOI] [PubMed] [Google Scholar]
- 5.Rockstroh JK, Lennox JL, Dejesus E, et al. STARTMRK Investigators. Long-term treatment with raltegravir or efavirenz combined with tenofovir/emtricitabine for treatment-naive human immunodeficiency virus-1-infected patients: 156-week results from STARTMRK. Clin Infect Dis. 2011;53:807–16. doi: 10.1093/cid/cir510. [DOI] [PubMed] [Google Scholar]
- 6.Long MC, Bennetto-Hood C, Acosta EP. A sensitive HPLC-MS-MS method for the determination of raltegravir in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;867:165–71. doi: 10.1016/j.jchromb.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 7.Merschman SA, Vallano PT, Wenning LA, Matuszewski BK, Woolf EJ. Determination of the HIV integrase inhibitor, MK-0518 (raltegravir), in human plasma using 96-well liquid-liquid extraction and HPLC MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;857:15–24. doi: 10.1016/j.jchromb.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 8.Blanche S, Bologna R, Cahn P, et al. Pharmacokinetics, safety and efficacy of darunavir/ritonavir in treatment-experienced children and adolescents. AIDS. 2009;23:2005–13. doi: 10.1097/QAD.0b013e328330abaa. [DOI] [PubMed] [Google Scholar]
- 9.Salazar JC, Cahn P, Yogev R, et al. Efficacy, safety and tolerability of tipranavir coadministered with ritonavir in HIV-1 infected children and adolescents. AIDS. 2008;22:1789–98. doi: 10.1097/QAD.0b013e32830c481b. [DOI] [PMC free article] [PubMed] [Google Scholar]