Abstract
We estimated the proportion of household contacts whose drug-susceptibility test results matched those of the purported source patient with multidrug-resistant tuberculosis. Ninety-nine (88.4%) contacts had isolates resistant to isoniazid and rifampin, and 41 (36.6%) contacts had isolates with results that also matched the purported source for ethambutol, streptomycin, and pyrazinamide.
Keywords: DST, discordant, presumptive, empirical, contact
The spread of drug-resistant Mycobacterium tuberculosis strains is a looming global health threat that can be contained only by treating those individuals who are sick with drug-resistant tuberculosis disease and by preventing the selection of resistant mutants through better tuberculosis treatment. Despite a limited drug armamentarium, even highly resistant organisms—including those resistant to the 2 most important drugs, isoniazid and rifampin (ie, multidrug-resistant [MDR] tuberculosis)—can be cured in most cases, both in adults and in children [1].
Because tuberculosis is an airborne infectious disease that spreads from person to person via droplet nuclei, close contacts of patients with tuberculosis are more likely than the general population to develop tuberculosis [2]. As a result, tuberculosis contact investigations are key for containing tuberculosis. Close contacts of persons newly diagnosed with tuberculosis disease are investigated for tuberculosis disease and latent infection [2]. Contacts diagnosed with tuberculosis disease, who do not have drug susceptibility testing (DST) results available, often receive initial treatment with a presumptive drug regimen based on the DST of the purported source case, usually the index case that led to evaluation of that household.
When the DST results of the purported source case show resistance to the 2 most effective antituberculous drugs, isoniazid and rifampin (MDR tuberculosis), a rational approach based on studies showing high MDR tuberculosis risks among household contacts with tuberculosis disease involves treating contacts with a presumptive MDR tuberculosis regimen [3–6]. Unfortunately, few reports compare DST results in well-characterized cohorts of close contacts and their index MDR tuberculosis patients. Such data could inform the choice of a drug combination for close contacts when DST results are not available. We compared the DST results of index patients with MDR tuberculosis to DST results from their household contacts for 5 drugs.
PATIENTS AND METHODS
In 1996, Partners In Health, in conjunction with the National Tuberculosis Program of Peru, began providing outpatient, tailored treatment regimens to patients with MDR tuberculosis in Lima [7–9]. We defined the index patient as the first patient in a household to receive an MDR tuberculosis regimen through this program. We previously reported the occurrence of tuberculosis disease in a cohort of household contacts of patients with MDR tuberculosis treated between 1996 and 2003; that analysis was restricted to households where the index MDR tuberculosis patients had isolates tested for extensively drug-resistant (XDR) tuberculosis, which is MDR tuberculosis with resistance to a fluoroquinolone and a second-line injectable agent (capreomycin, amikacin, or kanamycin) [6]. That report did not include an analysis of the concordance of resistance profiles in isolates obtained from each pair (index and contact).
For the present report, the XDR tuberculosis testing criterion did not apply. Rather, we restricted the analysis to those index-contact pairs where both patients were tested for all 5 of the following drugs: isoniazid, rifampin, ethambutol, streptomycin, and pyrazinamide. This study was approved by the research ethics committees of Harvard Medical School and the National Institute of Health of Peru.
DST was performed by Peru's national reference laboratory or the Massachusetts State Laboratory Institute using the proportion method and, for pyrazinamide, the BACTEC method. We constructed an aggregate drug-resistance profile for each index patient, using DST results from all specimens collected on or before the date of the contact's initial specimen collection. We classified the index patient as resistant to a particular drug if resistance was observed in any sample.
For contacts, we used the DST result available for the first specimen collected. A contact was included in the analysis only if that individual had a DST result from a specimen collected within 30 days of beginning any tuberculosis treatment. For each index–contact pair, we compared results for 5 drugs: isoniazid, rifampin, ethambutol, streptomycin, and pyrazinamide. We also assessed whether the resistance profiles in child contacts (age <15 years) were more likely than adult contacts (age ≥15 years) to be concordant with the index for all 5 drugs using the Fisher exact test.
RESULTS
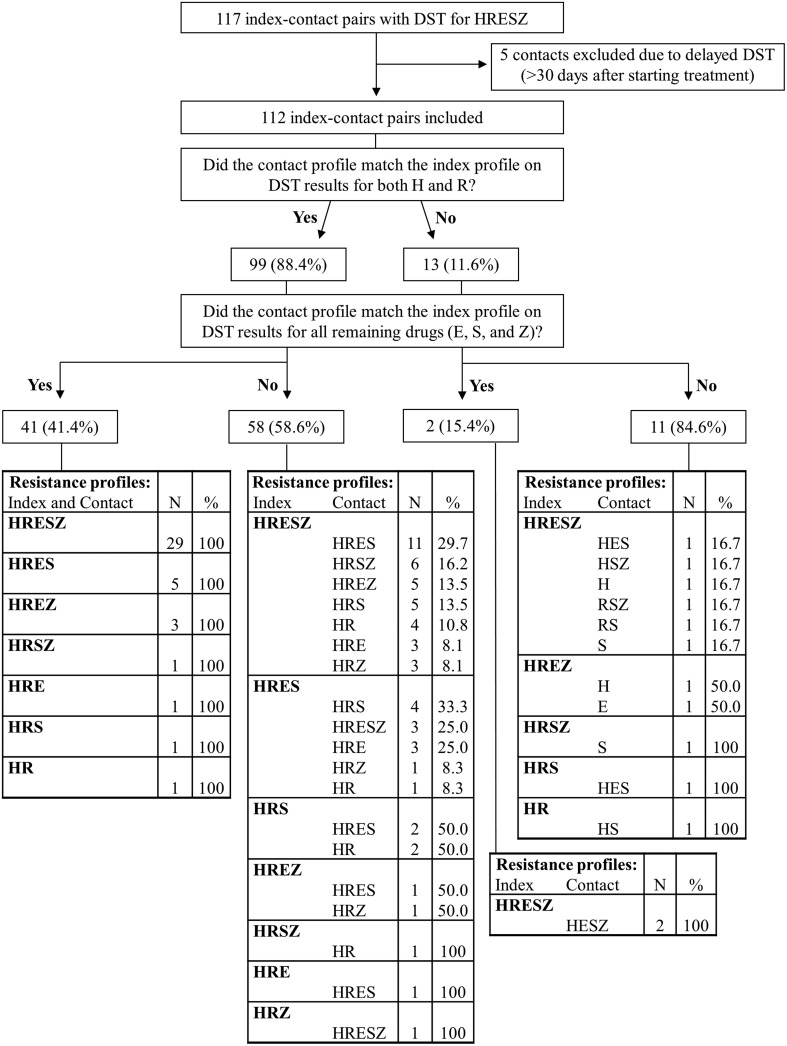
Among 758 households of index patients with confirmed MDR tuberculosis, there were 117 households in which both the index patient and at least 1 other patient had isolates tested for isoniazid, rifampin, ethambutol, streptomycin, and pyrazinamide. Of these 117 index–contact pairs, 5 were excluded from analysis because the contact's DST was performed on a specimen collected after that individual received >30 days of tuberculosis treatment. The remaining 112 index–contact pairs were compared. These included 101 of the contacts with tuberculosis disease that we previously reported [6].
Nearly 90% of contacts had isolates resistant to both isoniazid and rifampin, and 36.6% of contacts had isolates matching the index case's DST for all 5 drugs (Figure 1). Age information was available for 108 contacts. Four of 16 (25.0%) child contacts and 35 of 92 (32.4%) adult contacts had isolates that matched the index's DST for all 5 drugs (P = .4).
Figure 1.
Index–contact pairs with drug-susceptibility testing (DST) results for isoniazid (H), rifampin (R), ethambutol (E), streptomycin (S), and pyrazinamide (Z).
Eleven contacts (9.8%) had isolates resistant to at least 1 more (or different) drug than their purported source case. Of the 74 pairs in which the index isolates were resistant to all 5 drugs, 45 (60.8%) of their contacts had isolates that were susceptible to at least 1 of the 5 drugs.
DISCUSSION
This study provides strong empirical evidence to support the practice of treating a close contact without DST results by using a presumptive MDR tuberculosis regimen tailored to the DST results of the purported source case. In all but 11 index–contact pairs, a regimen devised using the resistance profile of the index patient would have been at least adequate for the contact.
Among household contacts who developed tuberculosis, 88.4% had MDR tuberculosis. Full resistance profiles in index–contact pairs were similar, but they were identical across all 5 drugs in <40% of the pairs. Discordance most frequently took the form of contact susceptibility to drugs to which the index strain was resistant, especially for ethambutol, streptomycin, and pyrazinamide. Among index patients whose isolates were resistant to all 5 drugs tested, nearly two-thirds of their contacts had isolates that were susceptible to 1 or more of those drugs. This finding has 2 important implications.
First, in this setting, a presumptive regimen that is tailored to the DST result of an index patient is likely to provide good coverage for the contact's resistance profile. If this regimen contains at least 5 likely effective drugs, including a fluoroquinolone and an injectable, previous work suggests that it will reduce mortality and recurrence in patients with MDR tuberculosis [8, 9]. Our findings also support the approach used by specialists to design preventive therapy regimens for child contacts based on the index patient's DST result [10].
Second, it remains critical to obtain specimens for DST in close contacts of patients with drug-resistant tuberculosis. With this information, clinicians can adjust the regimen to the household contact's own susceptibility pattern—including prescription of a first-line regimen for fully susceptible strains—assuring effectiveness while minimizing toxicity due to unnecessary drugs [11]. Because a small but important proportion (9.8%) of contact strains had resistance to more of the 5 drugs than did the index strains, DST results from the contact's isolate are also essential to assure a curative regimen.
There are several possible explanations for the differences between index and contact DST results. First, the contact might have been infected with the index patient's strain before the index patient acquired additional drug resistance; we have previously described such a likely case in this setting [12]. Second, the contact might have been infected by a source outside the home. Genotyping studies performed on a subset of this cohort suggested that between 10% and 38% of cases in the household contacts likely resulted from an MDR tuberculosis strain that was different from that infecting the index MDR tuberculosis patient [13]. Third, the index patient might have harbored 2 strains but passed only 1 strain to the household contact. Fourth, reproducibility of DST in some drugs is variable. Finally, the use of an aggregate drug resistance profile for index patients could overestimate resistance. When we performed the analysis using a single DST result for index patients, however, results were nearly identical.
Presumptive treatment according to the index patient's DST results is an important intervention to accelerate appropriate treatment for MDR tuberculosis in a household contact, pending DST results from that contact's isolate. Presumptive treatment, however, is not a permanent substitute for a regimen tailored to the contact's DST because a contact may be susceptible (or resistant) to drugs to which the index MDR tuberculosis patient is resistant (or susceptible). We recently highlighted the importance of an MDR tuberculosis regimen containing at least 5 likely effective drugs to reduce the risk of death and recurrence [8, 9]. The results presented here further serve to inform decisions about the choice of drugs that should be used in a presumptive MDR tuberculosis regimen for contacts of patients with MDR tuberculosis.
In conclusion, presumptive treatment of tuberculosis disease in close contacts of patients with MDR tuberculosis should be guided by the DST results of the index MDR tuberculosis patient. In many index–contact pairs, some discordance in the resistance profile can be expected. For this reason, every effort should be made to obtain DST results for each contact who develops tuberculosis disease in order to design an MDR tuberculosis regimen with the best chance of achieving cure in each patient.
Notes
Acknowledgments. The authors thank Ted Cohen for helpful advice in framing this analysis.
Financial support. This work was supported by Thomas J. White, the Doris and Howard Hiatt Residency in Global Health Equity, the Charles H. Hood Foundation, the David Rockefeller Center for Latin American Studies at Harvard University, the Bill & Melinda Gates Foundation, and a career development award from the National Heart, Lung, and Blood Institute (K01 HL080939 to M. C. B.).
Potential conflicts of interest. C. D. M. has received institutional grant funding through the National Institute of Allergy and Infectious Diseases (1 K01 AI065836). K. C., M. C. B., and J. B. have received institutional grant funding through the Charles H. Hood Foundation and the Bill & Melinda Gates Foundation. M. C. B. has also received institutional grant funding from the National Institutes of Health. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Orenstein EW, Basu S, Shah NS, et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis. 2009;9:153–61. doi: 10.1016/S1473-3099(09)70041-6. [DOI] [PubMed] [Google Scholar]
- 2.Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Recomm Rep. 2000;49(RR-6):1–51. [PubMed] [Google Scholar]
- 3.Tuberculosis Coalition for Technical Assistance. International standards for tuberculosis care. The Hague: Tuberculosis Coalition for Technical Assistance; 2006. [Google Scholar]
- 4.World Health Organization. Geneva, Switzerland: WHO; 2008. Guidelines for the programmatic management of drug-resistant tuberculosis: emergency update. [PubMed] [Google Scholar]
- 5.Bayona J, Chavez-Pachas AM, Palacios E, Llaro K, Sapag R, Becerra MC. Contact investigations as a means of detection and timely treatment of persons with infectious multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2003;7(12 suppl 3):S501–9. [PubMed] [Google Scholar]
- 6.Becerra MC, Appleton SC, Franke MF, et al. Tuberculosis burden in households of patients with multidrug-resistant and extensively drug-resistant tuberculosis: a retrospective cohort study. Lancet. 2011;377:147–52. doi: 10.1016/S0140-6736(10)61972-1. [DOI] [PubMed] [Google Scholar]
- 7.Mitnick CD, Shin SS, Seung KJ, et al. Comprehensive treatment of extensively drug-resistant tuberculosis. N Engl J Med. 2008;359:563–74. doi: 10.1056/NEJMoa0800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitnick CD, Franke MF, Rich ML, et al. Aggressive regimens for multidrug-resistant tuberculosis decrease all-cause mortality. PLoS One. 2013;8:e58664. doi: 10.1371/journal.pone.0058664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franke MF, Appleton SC, Mitnick CD, et al. Aggressive regimens for multidrug-resistant tuberculosis reduce recurrence. Clin Infect Dis. 2013;56:770–6. doi: 10.1093/cid/cis1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seddon JA, Godfrey-Faussett P, Hesseling AC, Gie RP, Beyers N, Schaaf HS. Management of children exposed to multidrug-resistant Mycobacterium tuberculosis. Lancet Infect Dis. 2012;12:469–79. doi: 10.1016/S1473-3099(11)70366-8. [DOI] [PubMed] [Google Scholar]
- 11.Seddon JA, Jordaan AM, Victor TC, Schaaf HS. Discordant drug susceptibility for Mycobacterium tuberculosis within families. Pediatr Infect Dis J. 2012;31:783–5. doi: 10.1097/INF.0b013e3182567c20. [DOI] [PubMed] [Google Scholar]
- 12.Furin JJ, Becerra MC, Shin SS, Kim JY, Bayona J, Farmer PE. Effect of administering short-course, standardized regimens in individuals infected with drug-resistant Mycobacterium tuberculosis strains. Eur J Clin Microbiol Infect Dis. 2000;19:132–6. doi: 10.1007/s100960050445. [DOI] [PubMed] [Google Scholar]
- 13.Cohen T, Murray M, Abubakar I, et al. Multiple introductions of multidrug-resistant tuberculosis into households, Lima, Peru. Emerg Infect Dis. 2011;17:969–75. doi: 10.3201/eid1706.101471. [DOI] [PMC free article] [PubMed] [Google Scholar]