Abstract
Alcohol use disorders (AUDs) are a major public health issue and have an enormous social and economic burden in developed, developing, and third-world countries. Current pharmacotherapies for treating AUDs suffer from deleterious side effects and are only effective in preventing relapse in a subset of individuals. This signifies an essential need for improved medications to reduce heavy episodic drinking and alcohol-related problems. Growing literature has provided support for the use of anticonvulsants in suppressing symptoms induced by alcohol withdrawal. Emerging clinical and preclinical evidence suggests that a number of well-tolerated anticonvulsants may also decrease alcohol drinking. This review will focus on recent evidence supporting the efficacy of novel anticonvulsants in reducing voluntary alcohol consumption in rodent models. The data demonstrate that anticonvulsants reduce drinking in standard home cage two-bottle choice paradigms, self-administration of alcohol in operant chambers, and cue- and stress-induced reinstatement of alcohol seeking behaviors in rats and mice. This review also highlights evidence that some anticonvulsants were only moderately effective in reducing drinking in select strains of rodents or models. This suggests that genetics, possible neuroadaptations, or the pharmacological target affect the ability of anticonvulsants to attenuate alcohol consumption. Nonetheless, anticonvulsants are relatively safe, have little abuse potential, and can work in combination with other drugs. The results from these preclinical and clinical studies provide compelling evidence that anticonvulsants are a promising class of medication for the treatment of AUDs.
Keywords: alcohol, consumption, anticonvulsants, novel pharmacotherapy, rodent models
Introduction
Medications for the treatment of alcohol use disorders (AUDs) are limited by poor patient compliance, relatively weak efficacy, potential abuse liability, and serious side-effects. Importantly, these medications are also largely inadequate in preventing relapse to alcohol-seeking behaviors, and some may actually increase relapse risk. Thus, it is necessary to explore additional pharmacotherapies that may be more effective and safer in reducing high rates of relapse, a significant problem in alcoholics. While there is ample evidence that various anticonvulsants are effective in treating many signs and symptoms of the alcohol withdrawal syndrome(1, 2), a number of recent clinical and preclinical studies have demonstrated that anticonvulsant drugs may also be a promising class of compounds that reduce alcohol consumption. As discussed below, many of these anticonvulsant agents that are discussed below have diverse pharmacological actions on a variety of proteins that regulate neuronal excitability(3). These protein targets include voltage sensitive Na+ and Ca2+ channels, the synaptic vesicle glycoprotein SV2A, GABA-A and AMPA-type glutamate receptors, and small-conductance Ca2+-activated K+ (KCa2) channels. While there is some clinical evidence indicating that some of these novel anticonvulsants are efficacious in reducing drinking in individuals with AUDs, the purpose of this mini-review is to highlight emerging evidence on anticonvulsants in rodent models of alcohol drinking behavior.
Animal Models of Alcohol Consumption
A number of rodent strains and models have been used to study the effects of anticonvulsants on voluntary alcohol consumption and alcohol seeking behaviors. In many cases, these studies utilized inbred and outbred lines of rats or mice that have been selectively bred to voluntarily drink high amounts of alcohol (e.g., alcohol-preferring P rats and high alcohol-preferring HAP mice) or naturally exhibit high levels of alcohol consumption (e.g., C57BL/6J mice). These studies also used a variety of rodent drinking models that reflect different aspects of AUDs. These models include an intermittent or continuous access paradigms involving two-bottle choice (alcohol vs. water) home cage drinking, oral self-administration in operant chambers, as well as relapse models such as stress- and cue-induced reinstatement of alcohol-seeking behavior, and drinking after a period of alcohol deprivation (the alcohol deprivation effect (ADE)). While rodents consumed a wide range of alcohol doses (3-20 g/kg) across a 24 hr drinking session, blood alcohol concentrations (BACs) were not reported in the vast majority of these studies. The National Institute on Alcohol Abuse and Alcoholism's Advisory Council defined binge drinking as reaching a BAC of 80 mg/dl or above within a 2 hr period. Most of these models did not produce binge alcohol consumption or BACs that approached 80 mg/dl. Two studies did report that rats that consumed approximately 1.1 g/kg at 1 hr into the drinking session had average BACs of 32-55 mg/dl(4, 5). Thus, as a whole, the studies reviewed below evaluated the ability of anticonvulsants to reduce moderate amounts of alcohol consumption.
Anticonvulsant Drug Administration
The anticonvulsant drugs used in these studies were typically administered by oral gavage or intraperitoneal (IP) injection 0-120 min prior to the start of the drinking sessions. The majority of these studies administering the compounds 30 min before access to alcohol. For some of the 24 hr drinking models, the amount of alcohol consumed was also determined at an intermediate time point into the drinking session (e.g., 3 or 6 hr). In a few reports, the anticonvulsants and vehicle were administrated using a longitudinal within-subjects repeated measures experimental design. The majority of the studies tested the effects of acute administration of the anticonvulsant drugs on alcohol consumption, with a few testing the effect of chronic treatment on drinking. Findings from these studies are discussed below and are summarized in Table 1.
Table 1.
Effects of Anticonvulsants on Alcohol Consumption and Alcohol-Induced Behaviors.
| Drug | Alcohol Exposure Model | Treatment | Behavioral Effect |
|---|---|---|---|
| Topiramate | C57BL/6J mice6,7 2-bottle choice CAA |
0-90 mg/kg (increasing weekly IP or repeated SC doses) | ↓ Preference & consumption |
| C57BL/6J mice8 Stressed-induced drinking escalation |
0-30 mg/kg (daily IP) for 5 days | ↓ Escalation of consumption & preference | |
| Wistar, P, & Female WHP rats9-11 2- or 3-bottle choice CAA |
0-80 mg/kg (IG or IP) up to 14 days | ↓ Consumption & preference in P & WHP, but not in Wistar rats | |
| Lamotrigine | Wistar rats13 Oral operant SA |
0-15 mg/kg (IP) 30 min before test | ↓ Cue-induced reinstatement for 5 mg/kg dose |
| Wistar rats13, 14 4-bottle choice ADE |
0-15 mg/kg (repeated IP) during withdrawal | ↓ ADE-induced escalation of drinking | |
| Sprague-Dawley rats15 Forced liquid diet |
0 or 30 mg/kg (IP) during withdrawal | No change in consumption | |
| Pregabalin | msP rats18 2-bottle choice & oral operant SA |
0-60 mg/kg (repeated IG) | ↓ Consumption & operant responding ↓ Stress & cue-induced reinstatement |
| Gabapentin | Wistar rats17 Oral operant AS + dependence |
0-120 mg/kg (IP) or 20 μg microinjection into the CeA | ↓ Dependence-induced escalation of operant responding for both |
| Viagabatrin | Wistar or AA rats4,19 2- or 3-bottle choice |
0-500 mg/kg (IP) | ↓ Consumption |
| C57BL/6J mice20 Oral operant SA & 2-bottle choice |
0-600 mg/kg (SC) | ↓ Consumption & operant responding | |
| Chlorzoxazone | Wistar rats5 2-bottle choice IAA & CAA |
0-50 mg/kg (IP) | ↓ Consumption & preference in IAA only |
| Levetiracetam | WHP rats27 2-bottle choice CAA |
0-80 mg/kg (repeated IG) | ↓ Consumption & preference |
| C57BL/6J mice28 2 g/kg or 3 g/kg alcohol (IG) |
0-100 mg/kg (IP) | Prevented change in self-stimulation of the medial forebrain bundle | |
| Carisbamate | P rat29 2-bottle choice CAA & ADE |
0-90 mg/kg (IG) | ↓ Consumption & withdrawal-induced escalation |
| Zonisamide | Wistar rats & C57BL/B6NHsd mice15 1-2 hr limited access |
0-50 mg/kg (repeated IP) | ↓ Consumption in rats & mice |
CAA: Continuous Alcohol Access, IP: Intraperitoneal, SC: Subcutaneous, SA: self-administration, WHP: Warsaw High Preferring, IG: Intragastrically, P: Alcohol preferring, IAA: Intermitted Alcohol Access, ADE: Alcohol Deprivation Effect, msP: Marchigian Sardinian preferring, CeA: Central Nucleus of the Amygdala, AA: Alko Alcohol
Topiramate
Topiramate is perhaps the most widely studied anticonvulsant drug in rodent models of alcohol drinking. A study by Gabriel and Cunningham was the first to examine the ability of topiramate to reduce alcohol intake in C57BL/6J mice. Increasing doses of topiramate administered daily immediately prior to alcohol access decreased preference for alcohol primarily through increased water intake(6). However, it was found that a dose of 25 mg/kg topiramate significantly elevated alcohol consumption, whereas 50 mg/kg decreased intake. In the same strain but using a different dosing pattern, repeated treatment (7 days) with a non-escalating dose of topiramate attenuated alcohol intake when it was administered 60 min prior to alcohol access(7). Topiramate also reduced stress-induced escalation of alcohol consumption and preference in C57BL/6J mice(8). In Warsaw high preferring (WHP) and P rats, repeated treatment (5 - 14 days) with topiramate significantly diminished voluntary consumption and preference for 10% alcohol in a standard two-bottle choice paradigm(9, 10). Tolerance to repeated administration was not observed in these studies, as topiramate was equally effective at reducing drinking throughout the treatment period. An additional study in P rats demonstrated that the combined effects of topiramate and ondanestron, a 5HT3 receptor antagonist, versus either compound alone decreased alcohol consumption(11). In contrast, treatment of Wistar rats with topiramate did not affect home cage drinking(9) and modestly attenuated consumption of a 4.44% alcohol solution at the beginning of a 7 day treatment regime, but no effect was observed on days 2 through 7(12).
Lamotrigine
Lamotrigine is currently available in the United States and Europe for the treatment of epilepsy and bipolar disorder. Recently, Vengeliene and colleagues demonstrated that lamotrigine has potential for treatment of AUDs. Their studies show that treatment of Wistar rats with lamotrigine attenuated the ADE (increased consumption after a period of abstinence/deprivation)(13, 14). Using a drinkometer system, they also demonstrated that the normal pattern of alcohol intake was disrupted by the ADE(14). Rats increased their approaches to the drinking bottles during the first day of post-abstinence drinking over baseline conditions. Interestingly, lamotrigine significantly reduced the amount of alcohol consumed without affecting drinking frequency or the number of approaches to the alcohol bottle. In addition, 5 but not 15 mg/kg lamotrigine attenuated cue-induced reinstatement responding, and the 15 mg/kg dose decreased home cage locomotor activity. An additional study showed that when 30 mg/kg lamotrigine was administered 4 hr into a 48 hr alcohol withdrawal period, it failed to reduce consumption of an alcohol liquid diet in Sprague-Dawley rats (15).
GABA analogs
Gabapentin and pregabalin have a similar structure to the amino acid γ-Aminobutyric acid (GABA), but have potent anticonvulsants actions mediated through voltage sensitive Ca2+ channels(3). To date, there are two preclinical studies that have examined these compounds in rat drinking models. The first of which used two models (i.e., chronic alcohol vapor inhalation and an alcohol liquid diet) to produce dependence in Wistar rats. These models have consistently been shown to produce an escalation of drinking in dependent rats and mice(16). Roberto and colleagues demonstrated that systemic administration as well as microinfusion of gabapentin into the central nucleus of the amygdala prevented alcohol dependence-induced escalation of drinking and attenuated operant responding for alcohol(17). Gabapentin did not alter responding for alcohol in non-dependent rats nor did it affect responding for water. The second preclinical study was an extensive examination of the effectiveness of pregabalin to reduce voluntary alcohol consumption, operant oral alcohol self-administration, and stress- and cue-induced reinstatement in alcohol preferring Marchigian Sardinian rats. After reaching stable baseline consumption in a standard two-bottle choice model, pregabalin was administered at 10, 30 or 60 mg/kg for five consecutive days(18). A significant reduction in alcohol consumption was observed on the first day of administration. Upon cessation of treatment, rats returned to pretreatment consumption levels. Following the administration of yohimbine, a pharmacological stressor that reinstates alcohol seeking behaviors after extinction, pregabalin decreased operant responding for alcohol. Treatment with pregabalin also significantly reduced cue-induced reinstatement of alcohol-seeking behavior. Similar to gabapentin and pregabalin, vigabatrin (gamma-vinyl-GABA) is an analog of GABA. However, vigabatrin influences excitability by inhibiting GABA transaminase. Two studies that used a standard choice model demonstrated that vigabatrin reduced alcohol consumption in alcohol-preferring AA and Wistar rats(4, 19). More recently, Griffin and colleagues reported that vigabatrin decreased operant responding for alcohol as well as home cage drinking in C57BL/6J mice(20). Interestingly, vigabatrin increased food and water intake during treatment, indicating a selective effect in reducing in ethanol reinforcement. It was noted that vigabatrin also enhanced the discriminative-stimulus effect of alcohol and slightly increased BACs in these mice.
KCa2 Channel Positive Modulators
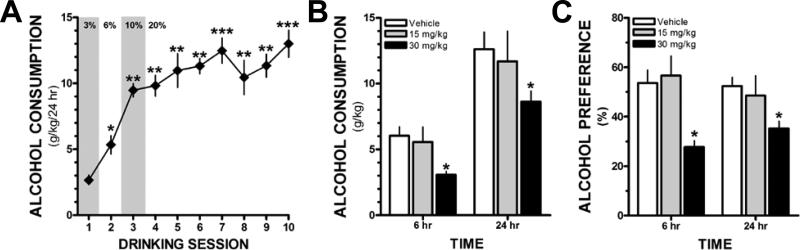
KCa2 channel positive modulators are effective in increasing seizure threshold and reducing hyperexcitability in in-vivo and in-vitro models(21-24). Accumulating evidence suggests that chronic alcohol-associated neuroadaptations in KCa2 channels may contribute to high rates of alcohol consumption and increased alcohol withdrawal severity(22, 24). Accordingly, these data suggest that KCa2 channel positive modulators (i.e., 1-EBIO, chlorzoxazone, and CyPPA) may be novel pharmacotherapies for reducing alcohol drinking. Hopf and colleagues first reported that microinfusion of 1-EBIO into the nucleus accumbens of Wistar rats reduced operant responding for alcohol(25). They next examined the ability of chlorzoxazone to reduce drinking in a standard 2-bottle choice drinking model. Chlorzoxazone is an FDA-approved centrally-acting medication for treating muscle spasms that also activates recombinant KCa2 channels. Systemic administration of chlorzoxazone significantly reduced alcohol consumption and preference in rats with intermittent, but not continuous access to alcohol(5). However, 1-EBIO and chlorzoxazone have off-target actions that confound interpretation of these findings. In a pilot study, we examined the ability of systemic administration of CyPPA (15 or 30 mg/kg in 5% Cremophor (v/v), 10 ml/kg, IP) to reduce voluntary drinking in an intermittent long-access (24 hr) two-bottle choice model that is associated with heavy alcohol consumption in C57BL/6J mice(26). Consistent with published findings, we observed an escalation in voluntary consumption that reached a stable baseline after 5-6 drinking sessions (Figure 1A). Administration of CyPPA (30 mg/kg) 30 min prior to alcohol access significantly reduced the amount of alcohol consumed (Figure 1B) and preference (Figure 1C) for alcohol at the 6 hr and 24 hr time points. CyPPA (30 mg/kg) did not affect the total volume of fluid consumed (two-way ANOVA, F(2,27) = 1.362, p = 0.27, n = 4-6 mice/group). CyPPA (30 mg/kg) increased water consumption at both time points (two-way ANOVA, F(2,27) = 5.352, p < 0.05, n = 4-6 mice/group). These preliminary findings further support the suggestion that positive modulators of KCa2 channel function may be a novel target and pharmacological approach to reducing voluntary alcohol consumption.
Figure 1.
The KCa2.2/3 channel positive modulator CyPPA reduces drinking and alcohol preference in a 24 hr intermittent access mouse model. (A) Escalation of voluntary alcohol consumption across 10 drinking sessions [one-way RM ANOVA, F(9,63) = 24.426, p < 0.001, Student Newman-Keuls (SNK) post-hoc test, *p < 0.01 vs. session 1, **p < 0.001 vs. sessions 1 & 2, ***p < 0.001 vs. sessions 1, 2 & 3 n=8 mice], (B) Administration of CyPPA (30 mg/kg, IP) 30 min prior to the start of the drinking session significantly reduces alcohol consumption at the 6 and 24 hr time points compared with vehicle treated mice [two-way ANOVA, F(2,24) = 3.601, p < 0.05, SNK post hoc, *p < .05 vs. vehicle, n = 4-6/group]. (C) CyPPA (30 mg/kg) treatment also significantly reduces preference for alcohol at both time points [F(2,27) = 9.44, p < 0.001, SNK post-hoc, *p < .01 vs. vehicle & 15 mg/kg].
Levetiracetam
Despite considerable clinical interest in the use of this anticonvulsant for treating AUDs, only two preclinical studies have examined the effects of levetiracetam on alcohol-related behaviors. In a standard two-bottle choice model, repeated doses of levetiracetam (14 days, 40-80 mg/kg, b.i.d.) significantly reduced alcohol intake and preference for alcohol in Warsaw high-preferring rats(27). In a second study, levetiracetam was shown to block the ability of alcohol to reduce intracranial self-stimulation in C57BL/6J mice(28).
Carisbamate
Carisbamate is an antiepileptic agent with demonstrated efficacy in preclinical models of epilepsy, but failed in phase III clinical trials for partial onset seizures. A recent preclinical study by Rezvani and colleagues(29) was conducted to test if acute and chronic treatment with carisbamate reduced voluntary alcohol consumption and the ADE in P rats using a standard two-bottle choice paradigm. When administered acutely, carisbamate dose-dependently decreased alcohol drinking and preference for alcohol without affecting food or water intake. Chronic administration of carisbamate significantly decreased alcohol intake and preference for alcohol across the entire 14 day treatment period. However, partial tolerance developed, and rats treated with carisbamate consumed significantly more alcohol on days 10-14 when compared with the first two days of treatment. Carisbamate completely prevented the ADE, whereas naltrexone administration only partially reduced the increase of alcohol consumption induced by forced abstinence. Interestingly, neither drug affected saccharin preference.
Zonisamide
To our knowledge, there is only one preclinical study that has examined the ability of zonisamide to reduce alcohol drinking. Knapp and colleagues used a limited-access design in Wistar rats and C57BL/B6NHsd mice, and compared the ability of zonisamide and topiramate 10-day treatment regimens to decrease consumption(15). For the first 5 days of treatment, rodents were administered 25 mg/kg, and the dose was increased to 50 mg/kg for the next 5 days. While the high dose of topiramate modestly reduced drinking in rats, treatment with 50 mg/kg zonisamide produced a more robust decrease in alcohol consumption. Similarly, these authors also reported a significant reduction in drinking when mice were administered the high dose of zonisamide. However, drinking levels in rats and mice quickly returned to baseline levels once zonisamide treatment was discontinued. Chronic treatment of zonisamide did not induce weight loss in rats, and only slightly increased weight loss in mice.
Conclusions & Future Directions
As discussed above, the vast majority of the preclinical studies have shown that treatment using several different anticonvulsant agents reduces alcohol consumption and operant self-administration of alcohol. Additionally, these anticonvulsants were shown to decrease relapse-like behavior, including stress- and cue-induced reinstatement of alcohol responding and the ADE effect. However, there are a number of caveats to consider as the field progresses with studies using anticonvulsants. Future studies should address the issues that most of the drugs only reduced drinking when the drug was onboard, not many studies have examined anticonvulsants in relapse models, and some of the studies demonstrated tolerance to the effects of the drugs. Some of the anticonvulsants only reduced consumption in alcohol-preferring(9, 10), high-drinking(5), or alcohol-dependent rodents(17). This suggests that genetics and/or chronic alcohol induced neuroadaptations may affect the efficacy of these drugs. Further studies are necessary to characterize what genetic underpinnings or neuroadaptations contribute to the effectiveness of anticonvulsants. Most of these preclinical studies examined the efficacy of anticonvulsants to reduce moderate alcohol consumption. Since binge drinking and relapse pose serious health threats to society, additional preclinical studies are needed to address the efficacy of anticonvulsants to prevent binge drinking and relapse to heavy drinking, particularly excessive drinking associated with dependence.
In support of the results from preclinical models, there is a growing literature supporting the use of anticonvulsants for AUDs in clinical studies(1, 30, 31). Despite the efficacy in the majority of preclinical and clinical studies, several recent trials have reported that some anticonvulsants (i.e., lamotrigine, levetiracetam) do not reduce heavy drinking or prevent relapse(32, 33). Furthermore, levetiracetam actually increased consumption in self-reported moderate drinkers(34). The inconsistency in the literature suggests that individuals respond differently to some medications, though the reason for this is unclear and deserves further consideration. Compared to single drug medications, combinations of therapeutic agents may be more efficacious for treating AUDs(11). Anticonvulsants might be considered adjunctive therapy and be prescribed with therapeutics such as naltrexone or acamprosate, which are FDA-approved for treatment of AUDs. Interestingly, anticonvulsants appear to reduce alcohol consumption and relapse by affecting novel and sometimes multiple molecular targets. These molecular targets may regulate neuroadaptations that are critical for driving uncontrolled drinking and relapse. Another contributing factor relates to the fact that anticonvulsants have the capacity to alleviate some withdrawal symptoms and this, in turn, may reduce the negative reinforcing effects of ethanol. Alternatively, anticonvulsants may re-establish the homeostasis of the reward neurocircuitry. Of course, none of these possibilities are mutual exclusive, and it is likely that depending on the drug and treatment regimen, a number of factors may play a role in mediating therapeutic effects (i.e., reduction of excessive and risky drinking). Further work is necessary to determine the molecular targets that are important for decreasing drinking and reducing relapse vulnerability.
Finally, while there is ample evidence indicating that use of anticonvulsants is relatively safe in individuals suffering with AUDs, many of these drugs also have considerable side effects, such as sedation, cognitive impairments, and weight loss. Thus, additional research aimed at achieving an appropriate balance between maximizing therapeutic efficacy and minimizing untoward effects of anticonvulsants is critical for advancing these compounds in clinical trials and ultimately, use in the clinical management of AUDs. In sum, there is a growing body of preclinical literature indicating that numerous anticonvulsant agents are not only effective in treating symptoms of alcohol withdrawal during periods of abstinence, but these drugs also may be effective in reducing alcohol consumption in various rodent models of alcohol self-administration. There is a clear need for more preclinical and clinical studies to address these important issues because anticonvulsants appear to be a promising class of compounds that warrant further exploration for treating individuals with AUDs
Acknowledgement
Supported by NIH grants U01 AA020930 (PJM), R00 AA017922 (PJM), and F31 AA021618 (NSM).
Footnotes
All authors contributed to conception and design, manuscript preparation, read and approved the final manuscript.
All authors abide by the Association for Medical Ethics (AME) ethical rules of disclosure.
Competing interests: none declared
Conflict of interests: none declared
References
- 1.Book SW, Myrick H. Novel anticonvulsants in the treatment of alcoholism. Expert Opin Investig Drugs. 2005;14(4):371–6. doi: 10.1517/13543784.14.4.371. Epub 2005/05/11. doi: 10.1517/13543784.14.4.371. PubMed PMID: 15882114. [DOI] [PubMed] [Google Scholar]
- 2.Malcolm R, Myrick H, Brady KT, Ballenger JC. Update on anticonvulsants for the treatment of alcohol withdrawal. Am J Addict. 2001;10(Suppl):16–23. doi: 10.1080/10550490150504100. Epub 2001/03/28. PubMed PMID: 11268817. [DOI] [PubMed] [Google Scholar]
- 3.Brodie MJ, Covanis A, Gil-Nagel A, Lerche H, Perucca E, Sills GJ, et al. Antiepileptic drug therapy: does mechanism of action matter? Epilepsy & behavior : E&B. 2011;21(4):331–41. doi: 10.1016/j.yebeh.2011.05.025. doi: 10.1016/j.yebeh.2011.05.025. PubMed PMID: 21763207. [DOI] [PubMed] [Google Scholar]
- 4.Stromberg MF, Mackler SA, Volpicelli JR, O'Brien CP, Dewey SL. The effect of gamma-vinyl-GABA on the consumption of concurrently available oral cocaine and ethanol in the rat. Pharmacology, biochemistry, and behavior. 2001;68(2):291–9. doi: 10.1016/s0091-3057(00)00456-1. PubMed PMID: 11267634. [DOI] [PubMed] [Google Scholar]
- 5.Hopf FW, Simms JA, Chang SJ, Seif T, Bartlett SE, Bonci A. Chlorzoxazone, an SK-type potassium channel activator used in humans, reduces excessive alcohol intake in rats. Biol Psychiatry. 2011;69(7):618–24. doi: 10.1016/j.biopsych.2010.11.011. doi: 10.1016/j.biopsych.2010.11.011. PubMed PMID: 21195386; PubMed Central PMCID: PMC3062269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabriel KI, Cunningham CL. Effects of topiramate on ethanol and saccharin consumption and preferences in C57BL/6J mice. Alcohol Clin Exp Res. 2005;29(1):75–80. doi: 10.1097/01.alc.0000150014.79657.64. PubMed PMID: 15654294. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen SA, Malcolm R, Middaugh LD. Topiramate reduces ethanol consumption by C57BL/6 mice. Synapse. 2007;61(3):150–6. doi: 10.1002/syn.20350. doi: 10.1002/syn.20350. PubMed PMID: 17146766. [DOI] [PubMed] [Google Scholar]
- 8.Farook JM, Lewis B, Littleton JM, Barron S. Topiramate attenuates the stress-induced increase in alcohol consumption and preference in male C57BL/6J mice. Physiology & behavior. 2009;96(1):189–93. doi: 10.1016/j.physbeh.2008.08.011. doi: 10.1016/j.physbeh.2008.08.011. PubMed PMID: 18786555. [DOI] [PubMed] [Google Scholar]
- 9.Breslin FJ, Johnson BA, Lynch WJ. Effect of topiramate treatment on ethanol consumption in rats. Psychopharmacology. 2010;207(4):529–34. doi: 10.1007/s00213-009-1683-4. doi: 10.1007/s00213-009-1683-4. PubMed PMID: 19823810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zalewska-Kaszubska J, Bajer B, Gorska D, Andrzejczak D, Dyr W, Bienkowski P. Effect of repeated treatment with topiramate on voluntary alcohol intake and beta-endorphin plasma level in Warsaw alcohol high-preferring rats. Psychopharmacology. 2013;225(2):275–81. doi: 10.1007/s00213-012-2812-z. doi: 10.1007/s00213-012-2812-z. PubMed PMID: 22847457; PubMed Central PMCID: PMC3536943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lynch WJ, Bond C, Breslin FJ, Johnson BA. Severity of drinking as a predictor of efficacy of the combination of ondansetron and topiramate in rat models of ethanol consumption and relapse. Psychopharmacology. 2011;217(1):3–12. doi: 10.1007/s00213-011-2253-0. doi: 10.1007/s00213-011-2253-0. PubMed PMID: 21424693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hargreaves GA, McGregor IS. Topiramate moderately reduces the motivation to consume alcohol and has a marked antidepressant effect in rats. Alcohol Clin Exp Res. 2007;31(11):1900–7. doi: 10.1111/j.1530-0277.2007.00485.x. doi: 10.1111/j.1530-0277.2007.00485.x. PubMed PMID: 17877781. [DOI] [PubMed] [Google Scholar]
- 13.Vengeliene V, Heidbreder CA, Spanagel R. The effects of lamotrigine on alcohol seeking and relapse. Neuropharmacology. 2007;53(8):951–7. doi: 10.1016/j.neuropharm.2007.09.006. Epub 2007/11/03. doi: 10.1016/j.neuropharm.2007.09.006. PubMed PMID: 17976664. [DOI] [PubMed] [Google Scholar]
- 14.Vengeliene V, Noori HR, Spanagel R. The use of a novel drinkometer system for assessing pharmacological treatment effects on ethanol consumption in rats. Alcohol Clin Exp Res. 2013;37(Suppl 1):E322–8. doi: 10.1111/j.1530-0277.2012.01874.x. Epub 2012/07/05. doi: 10.1111/j.1530-0277.2012.01874.x. PubMed PMID: 22757984. [DOI] [PubMed] [Google Scholar]
- 15.Knapp CM, Mercado M, Markley TL, Crosby S, Ciraulo DA, Kornetsky C. Zonisamide decreases ethanol intake in rats and mice. Pharmacology, biochemistry, and behavior. 2007;87(1):65–72. doi: 10.1016/j.pbb.2007.04.001. doi: 10.1016/j.pbb.2007.04.001. PubMed PMID: 17482246; PubMed Central PMCID: PMC2867456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker HC. Animal models of excessive alcohol consumption in rodents. Current topics in behavioral neurosciences. 2013;13:355–77. doi: 10.1007/7854_2012_203. doi: 10.1007/7854_2012_203. PubMed PMID: 22371267. [DOI] [PubMed] [Google Scholar]
- 17.Roberto M, Gilpin NW, O'Dell LE, Cruz MT, Morse AC, Siggins GR, et al. Cellular and behavioral interactions of gabapentin with alcohol dependence. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(22):5762–71. doi: 10.1523/JNEUROSCI.0575-08.2008. doi: 10.1523/JNEUROSCI.0575-08.2008. PubMed PMID: 18509038; PubMed Central PMCID: PMC2493536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stopponi S, Somaini L, Cippitelli A, de Guglielmo G, Kallupi M, Cannella N, et al. Pregabalin reduces alcohol drinking and relapse to alcohol seeking in the rat. Psychopharmacology. 2012;220(1):87–96. doi: 10.1007/s00213-011-2457-3. doi: 10.1007/s00213-011-2457-3. PubMed PMID: 21887495. [DOI] [PubMed] [Google Scholar]
- 19.Wegelius K, Halonen T, Korpi ER. Gamma-vinyl GABA decreases voluntary alcohol consumption in alcohol-preferring AA rats. Pharmacology & toxicology. 1993;73(3):150–2. doi: 10.1111/j.1600-0773.1993.tb01554.x. PubMed PMID: 8265518. [DOI] [PubMed] [Google Scholar]
- 20.Griffin WC, 3rd, Nguyen SA, Deleon CP, Middaugh LD. Effects of vigabatrin, an irreversible GABA transaminase inhibitor, on ethanol reinforcement and ethanol discriminative stimuli in mice. Behavioural pharmacology. 2012;23(2):178–90. doi: 10.1097/FBP.0b013e3283512c56. doi: 10.1097/FBP.0b013e3283512c56. PubMed PMID: 22336593; PubMed Central PMCID: PMC3296837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson NJ, Slough S, Watson WP. In vivo characterisation of the small-conductance KCa (SK) channel activator 1-ethyl-2-benzimidazolinone (1-EBIO) as a potential anticonvulsant. European journal of pharmacology. 2006;546(1-3):48–53. doi: 10.1016/j.ejphar.2006.07.007. doi: 10.1016/j.ejphar.2006.07.007. PubMed PMID: 16925994. [DOI] [PubMed] [Google Scholar]
- 22.Mulholland PJ. K(Ca)2 channels: novel therapeutic targets for treating alcohol withdrawal and escalation of alcohol consumption. Alcohol. 2012;46(4):309–15. doi: 10.1016/j.alcohol.2011.11.002. doi: 10.1016/j.alcohol.2011.11.002. PubMed PMID: 22464787; PubMed Central PMCID: PMC3358436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulholland PJ, Becker HC, Woodward JJ, Chandler LJ. Small conductance calcium-activated potassium type 2 channels regulate alcohol-associated plasticity of glutamatergic synapses. Biol Psychiatry. 2011;69(7):625–32. doi: 10.1016/j.biopsych.2010.09.025. doi: 10.1016/j.biopsych.2010.09.025. PubMed PMID: 21056409; PubMed Central PMCID: PMC3103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mulholland PJ, Hopf FW, Bukiya AN, Martin GE, Liu J, Dopico AM, et al. Sizing up ethanol-induced plasticity: the role of small and large conductance calcium-activated potassium channels. Alcohol Clin Exp Res. 2009;33(7):1125–35. doi: 10.1111/j.1530-0277.2009.00936.x. doi: 10.1111/j.1530-0277.2009.00936.x. PubMed PMID: 19389201; PubMed Central PMCID: PMC2760381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hopf FW, Bowers MS, Chang SJ, Chen BT, Martin M, Seif T, et al. Reduced nucleus accumbens SK channel activity enhances alcohol seeking during abstinence. Neuron. 2010;65(5):682–94. doi: 10.1016/j.neuron.2010.02.015. doi: 10.1016/j.neuron.2010.02.015. PubMed PMID: 20223203; PubMed Central PMCID: PMC2847608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA. Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res. 2011;35(11):1938–47. doi: 10.1111/j.1530-0277.2011.01545.x. doi: 10.1111/j.1530-0277.2011.01545.x. PubMed PMID: 21631540; PubMed Central PMCID: PMC3166538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zalewska-Kaszubska J, Bajer B, Czarnecka E, Dyr W, Gorska D. Voluntary alcohol consumption and plasma beta-endorphin levels in alcohol preferring rats chronically treated with levetiracetam: a preliminary study. Physiology & behavior. 2011;102(5):538–41. doi: 10.1016/j.physbeh.2010.12.021. doi: 10.1016/j.physbeh.2010.12.021. PubMed PMID: 21187108. [DOI] [PubMed] [Google Scholar]
- 28.Robinson JE, Chen M, Stamatakis AM, Krouse MC, Howard EC, Faccidomo S, et al. Levetiracetam Has Opposite Effects on Alcohol- and Cocaine-Related Behaviors in C57BL/6J Mice. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.30. doi: 10.1038/npp.2013.30. PubMed PMID: 23353709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rezvani AH, Overstreet DH, Vaidya AH, Zhao B, Levin ED. Carisbamate, a novel antiepileptic candidate compound, attenuates alcohol intake in alcohol-preferring rats. Alcohol Clin Exp Res. 2009;33(8):1366–73. doi: 10.1111/j.1530-0277.2009.00966.x. doi: 10.1111/j.1530-0277.2009.00966.x. PubMed PMID: 19413647. [DOI] [PubMed] [Google Scholar]
- 30.Clapp P. Current progress in pharmacologic treatment strategies for alcohol dependence. Expert review of clinical pharmacology. 2012;5(4):427–35. doi: 10.1586/ecp.12.31. doi: 10.1586/ecp.12.31. PubMed PMID: 22943122. [DOI] [PubMed] [Google Scholar]
- 31.De Sousa A. The role of topiramate and other anticonvulsants in the treatment of alcohol dependence: a clinical review. CNS & neurological disorders drug targets. 2010;9(1):45–9. doi: 10.2174/187152710790966696. PubMed PMID: 20201814. [DOI] [PubMed] [Google Scholar]
- 32.Fertig JB, Ryan ML, Falk DE, Litten RZ, Mattson ME, Ransom J, et al. A double-blind, placebo-controlled trial assessing the efficacy of levetiracetam extended-release in very heavy drinking alcohol-dependent patients. Alcohol Clin Exp Res. 2012;36(8):1421–30. doi: 10.1111/j.1530-0277.2011.01716.x. doi: 10.1111/j.1530-0277.2011.01716.x. PubMed PMID: 22324516; PubMed Central PMCID: PMC3355217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richter C, Effenberger S, Bschor T, Bonnet U, Haasen C, Preuss UW, et al. Efficacy and safety of levetiracetam for the prevention of alcohol relapse in recently detoxified alcohol-dependent patients: a randomized trial. Journal of clinical psychopharmacology. 2012;32(4):558–62. doi: 10.1097/JCP.0b013e31825e213e. doi: 10.1097/JCP.0b013e31825e213e. PubMed PMID: 22722516. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell JM, Grossman LE, Coker AR, Messing RO. The anticonvulsant levetiracetam potentiates alcohol consumption in non-treatment seeking alcohol abusers. Journal of clinical psychopharmacology. 2012;32(2):269–72. doi: 10.1097/JCP.0b013e318248ba69. doi: 10.1097/JCP.0b013e318248ba69. PubMed PMID: 22367657. [DOI] [PubMed] [Google Scholar]