Abstract
Glaucoma is a multifactorial progressive ocular pathology, clinically presenting with damage to the retina and optic nerve, ultimately leading to blindness. Retinal ganglion cell loss in glaucoma ultimately results in vision loss. Vesl/Homer proteins are scaffolding proteins that are critical for maintaining synaptic integrity by clustering, organizing and functionally regulating synaptic proteins. Current anti-glaucoma therapies target IOP as the sole modifiable clinical parameters. Long-term pharmacotherapy and surgical treatment do not prevent gradual visual field loss as the disease progresses, highlighting the need for new complementary, alternative and comprehensive treatment approaches. Vesl/Homer expression was measured in the retinae of DBA/2J mice, a preclinical genetic glaucoma model with spontaneous mutations resulting in a phenotype reminiscent of chronic human pigmentary glaucoma. Vesl/Homer proteins were differentially expressed in the aged, glaucomatous DBA/2J retina, both at the transcriptional and translational level. Immunoreactivity for the long Vesl-1L/Homer 1c isoform, but not of the immediate early gene product Vesl-1S/Homer 1a was increased in the synaptic layers of the retina. This increased protein level of Vesl-1L/Homer 1c was correlated with phenotypes of increased disease severity and a decrease in visual performance. The increased expression of Vesl-1L/Homer 1c in the glaucomatous retina likely results in increased intracellular Ca2+ release through enhancement of synaptic coupling. The ensuing Ca2+ toxicity may thus activate neurodegenerative pathways and lead to the progressive loss of synaptic function in glaucoma. Our data suggest that higher levels of Vesl-1L/Homer 1c generate a more severe disease phenotype and may represent a viable target for therapy development.
Keywords: glaucoma, neurodegeneration, Vesl/Homer, synaptic clustering, calcium channel, DBA/2J
1. Introduction
Glaucoma is a common multifactorial progressive ocular pathology and the most common cause of irreversible blindness worldwide (Cook & Foster, 2012). Clinically, glaucoma presents with damage to the retina and optic nerve, manifesting histologically in a typical appearance of structural damage at the optic nerve head, with neuroretinal rim thinning, excavation (cupping), and sectoral retinal nerve fiber layer defects (Foster, et al., 2002).
Elevated intraocular pressure (IOP) is a major risk factor for glaucomatous optic neuropathy and currently the only target of pharmacological and surgical therapies for glaucoma (Foster et al., 2002). However, estimates suggest that approximately one-third of patients do not have elevated IOP (Dielemans, et al., 1994, Klein, et al., 1992). In addition, IOP-lowering medication typically only slows disease progression delaying the onset of significant vision loss (Pascale, et al., 2012). Thus, novel drugs complementary to IOP-lowering strategies, but targeting different aspects of the disease are urgently needed. These therapies can ideally protect retinal ganglion cells (RGCs) and the structure of the optic nerve head from elevated IOP and other pathological disease processes associated with glaucoma (Casson, et al., 2012, Chang & Goldberg, 2012, Pascale et al., 2012). This led us to measure molecular determinants of the retina that generate elevated susceptibility of RGCs to degeneration during glaucoma. Such new mechanistic insights and the identification of novel drug targets for the protection of the retina and optic nerve in glaucoma are critical for novel therapy development in glaucomatous retinopathies.
Aberrant synaptic signaling is pathophysiologically linked to aging of the nervous system and neurodegenerative disease and provides a highly clinically significant target for future drug discovery efforts. We have previously identified Vesl-1L/Homer 1c as an early indicator of neurodegeneration in a rat model for mild retinal ischemia that mimics a slow-onset neurodegeneration similar to glaucoma (Kaja, et al., 2003).
Vesl (VASP/Ena-related gene up-regulated during seizure and LTP protein)/Homer proteins belong to the family of scaffolding proteins, which are critical for maintaining synaptic integrity (Duncan, et al., 2005) and facilitating the effective conversion of extracellular into intracellular signals at excitatory synapses.
Two Vesl/Homer 1 isoforms are of particular interest in their regulation of synaptic activity: the short isoform Vesl-1S/Homer 1a, an immediate early gene product that is rapidly and transiently induced by high synaptic activity, and the long Vesl-1L/Homer 1c isoform, which is typically expressed constitutively (Duncan et al., 2005). The generally accepted mechanism is that the short immediate early gene Vesl-1S/Homer 1a gene product can competitively disturb multimerization and synaptic clustering of the long Vesl-1L/Homer 1c protein, thereby providing a cellular mechanism for control of synaptic activity (Duncan et al., 2005).
We herein investigated the hypothesis that Vesl/Homer 1 proteins are differentially expressed in the glaucomatous neural retina and that expression levels correlate with visual performance and disease phenotypes in a well-established preclinical model for human pigmentary glaucoma. Our data identify Vesl/Homer 1 proteins as a potential novel target for therapeutic approaches protecting the structure and function of the retina and optic nerve in glaucoma.
2. Methods
2.1 Animals
Two groups of DBA/2J mice were obtained from The Jackson Laboratory (Bar Harbor, ME), six weeks- and nine months-old male animals. Mice were housed in the institutional animal facility with ad libitum access to food and water and maintained on a 12 hour light/dark cycle. All experimental animal procedures were approved by the institutional animal care and use committee and performed in accordance with institutional and federal guidelines and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
2.2 Behavioral analyses
Intraocular pressure was measured by applanation tonometry using a TonoPen XL (Reichert Ophthalmic Instruments, Depew, NY), as described previously (Burroughs, et al., 2011). Three readings at the instrument’s setting at a statistical reliability of 5% standard deviation were taken per eye, and the average of these readings was used for analysis.
For quantification of functional vision we used the Optomotry™ system (CerebralMechanics, Lethbridge, AB), which relies on the optomotor tracking response (Burroughs et al., 2011, Douglas, et al., 2005, Prusky, et al., 2006).
In order to quantify visual acuity, we established the threshold of the maximum spatial frequency that results in an optomotor tracking response. The test was initiated by projecting a grating of low spatial frequency (0.042 cycles/degree [c/d]), rotating at 12°/s at maximum 100% contrast). The contrast sensitivity threshold was determined by varying the contrast with the spatial frequency set at 0.042 c/d.
2.3. Quantitative gene expression analysis
We used the PARISTM kit (Applied Biosystems, Foster City, CA), according to the manufacturer’s recommendations in order to isolate mRNA and total protein from the same tissue sample. cDNA was synthesized from total RNA using the High Capacity cDNA Archive kit (Applied Biosystems) and quantitative PCR was performed using a StepOne Plus instrument (Applied Biosystems) and dual-labeled probes specific for Homer 1a and Homer 1c, exactly as described in (Mackiewicz, et al., 2008). Dual-labeled probes and oligonucleotides were purchased from Sigma-Aldrich (St. Louis, MO). Relative quantification was performed according to the method by Livak and Schmittgen (Livak & Schmittgen, 2001). Data is presented as relative gene expression level (RQ).
2.4. Quantitative immunoblotting
The protein concentration was determined according to the method of Lowry (Lowry, et al., 1951). Thirty-five micrograms of total protein were separated electrophoretically, transferred to nitrocellulose membranes (0.22 μm; Pall Corp., Port Washington, NY, USA), and probed with the following Homer 1 isoform-specific antibodies: goat anti-Homer 1a (1:100; sc-8922) (Giuffrida, et al., 2005, Kaja, et al., 2012b), rabbit anti-Homer 1c (1:100; sc-20807) (Ghasemzadeh, et al., 2009, Kaja et al., 2012b), and rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:5,000; sc-25778) (Kaja et al., 2012b), all from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The two anti-Homer antibodies are isoform-specific as evident by yielding bands of immunoreactivity of approx. 21 kDa and 42 kDa for Homer 1a and Homer 1c, respectively (cf. Suppl. Fig. 1). Secondary antibodies were horseradish peroxidase (HRP)-labeled donkey-anti rabbit IgG (1:10,000; NA9340, GE Healthcare, Piscataway, NJ, USA) and HRP-labeled donkey anti-goat IgG-HRP (1:5,000; sc-2020, Santa Cruz Biotechnology). Quantification was performed by densitometry using ImageJ software (NIH, Bethesda, MD, USA). Density values were corrected for background and Homer 1 expression normalized to endogenous GAPDH expression. At least three separate immunoblots were quantified for each antibody target and the mean for each retina sample utilized for future calculations and correlation analyses.
2.5. Immunohistochemistry and microfluorimetry
Immunhistochemistry was performed essentially as described previously (Kaja, et al., 2012a). Eyecups were fixed in 4% paraformaldehyde in PBS for 15 min, cryprotected in a graded series of sucrose (10–30% in PBS), and sectioned at 16 μm on a CryoCut One cryostat (Vibratome, St. Louis, MO). We used previously validated Homer 1 isoform specific antibodies (rabbit anti-Vesl-1S, 1:200; rabbit anti-Vesl-1L, 1:200) (Kaja et al., 2003) to identify the cell type-specific localization and distribution of Homer 1 isoforms in the retina. These antibodies were different from those used for immunoblotting experiments, and were kindly provided by Dr. K. Inokuchi, University of Toyama, Japan. Images were acquired with a Leica SP5 WLL laser-scanning confocal microscope (Leica Microsystems Inc., Buffalo Grove, IL). Expression levels were quantified by intensity line scan analysis over a 180 μm range using the Leica Application Suite Advance Fluorescence v2.6.2.1 (Leica Microsystems Inc.). Data was exported and plotted in Prism 5.0 software (GraphPad Inc., La Jolla, CA).
2.6. Statistics
Data are expressed as the mean ± standard error of the mean. Statistical significance was determined by Student’s t-test analysis. P values of less than 0.05 were considered statistically significant. For correlation analyses, a Pearson product-moment correlation coefficient (r) calculation to evaluate the strength of the association was used with the strength of the association identified as not, weakly, moderately or strongly correlated for 0.0 ≤ r ≤ 0.2, 0.2 ≤ r ≤ 0.4, 0.4 ≤ r ≤ 0.6, and 0.6 ≤ r ≤ 1.0, respectively (Bartlett, 1993, Burroughs et al., 2011). Prism v5.0 software (Graphpad Software Inc., La Jolla, CA) was used for statistical analysis.
3. Results
3.1. Aged DBA/2J mice have elevated intraocular pressure (IOP) and deficits in functional vision
In order to correlate Homer 1 expression with functional correlates of glaucoma, we measured IOP and functional vision in our experimental cohorts of 6 week-old (young) and 9 months-old (aged) DBA/2J mice.
Aged animals had significantly higher IOPs compared with the young group (29 ± 2 vs. 12 ± 0.4 mm Hg, respectively; n=5, P<0.001; Fig. 1A). Using the OptoMotry™ system, we next measured visual acuity and contrast sensitivity. Functional vision measured as visual acuity was markedly decreased in aged animals compared with young controls (0.503 ± 0.009 c/d vs. 0.257 ± 0.053, respectively; n=5, P<0.01; Fig. 1B). At the same time, contrast sensitivity, the measured threshold for distinction of contrast at a given spatial frequency of 0.042 c/d, was increased in the aged cohort compared with the young (38.6 ± 12.9 vs. 13.3 ± 1.4%, respectively; n=5, P<0.05; Fig. 1C).
Figure 1. Increased IOP and impaired visual function in the glaucomatous DBA/2J retina.
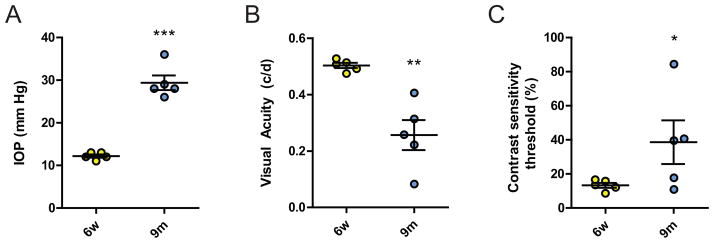
A) IOP is elevated in the aged cohort of 9 months-old DBA/2J mice, compared with young 6 week-old controls (n=5, P<0.001). B) Visual acuity as tested in the OptoMotry™ system was significantly reduced in aged DBA/2J mice (n=5, P<0.001). C) Similarly, the contrast sensitivity threshold showed evidence of impaired vision in the aged cohort and was significantly increased (n=5, P<0.05). * P<0.05; *** P<0.001.
3.2. Expression of Vesl/Homer is increased at the transcriptional and translational level in the retinae of aged DBA/2J mice
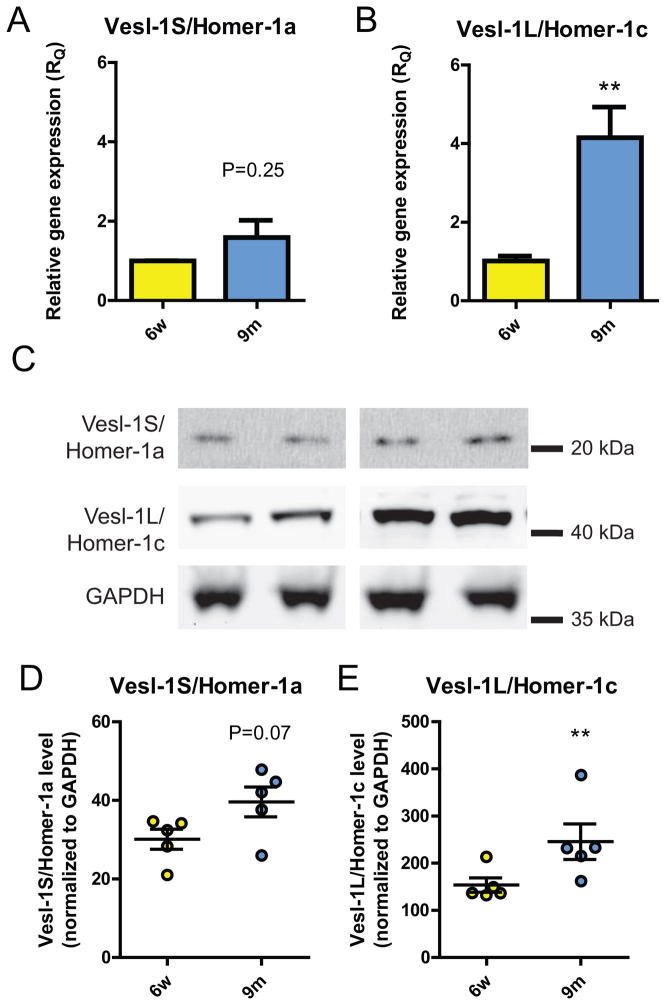
We subsequently investigated both mRNA and protein levels of both the long isoform, the constitutively expressed Vesl-1L/Homer 1c and of the short splice variant, the immediate early gene product Vesl-1S/Homer 1a. Relative gene expression (RQ) of Homer 1a was not statistically significantly different in the retina of 9 months-old when compared with 6-week old DBA/2J mice, even though a trend towards higher transcriptional levels was observed (1.590 ± 0.437 vs. 1.000 ± 0.008; n=3, P=0.25; Fig. 2A). In contrast, Homer 1c mRNA levels assessed as relative gene expression (RQ) were highly statistically significantly elevated, 4.2 ± 0.8-fold higher in the aged cohort compared with young controls (n=3, P<0.01; Fig. 2B).
Figure 2. Vesl-1L/Homer 1c is increased at the transcriptional and translational level in the neural retina.
A) Vesl-1S/Homer 1a mRNA levels were increased by 60% in the neural retina, however, this increase did not reach statistical significance (RQ=1.000 ± 0.008 and RQ=1.590 ± 0.437 for 6w and 9m, respectively; n=3, P=0.25). B) In contrast, normalized gene expression (RQ) for Vesl-1L/Homer 1c was 4-fold higher in the aged, glaucomatous retina compared with young, healthy controls (n=3, P<0.01). C) Representative immunoblots from two animals per cohort showing expression of Vesl-1S/Homer 1a, Vesl-1L/Homer 1c and GAPDH. Expression of Vesl-1L/Homer 1c is increased in the neural retina in aged, glaucomatous DBA/2J mice. D) Protein levels of Vesl-1S/Homer 1a showed a trend towards an increase in the aged DBA/2J retina (n=5, P=0.07). E) Vesl-1L/Homer 1c expression was increased by 60% in the aged cohort, compared with young controls (n=5, P<0.01). ** P<0.01
Subsequently, we assessed whether the observed changes at the transcriptional level were reflected at the protein level based on quantification using GAPDH as an endogenous control (representative immunoblots are shown in Fig. 2C). Similar to our results at the transcriptional level, Homer 1a expression in the aged DBA/2J retina was not different from 6 week-old animals (n=5, P<0.07; Fig. 2D), while a trend towards increased Homer 1a expression (32 ± 13%) was observed. In contrast, normalized Homer 1c expression was statistically highly significantly increased by 60 ± 24% in 9 months-old retina, compared with 6 week-old controls (n=5, P<0.01; Fig. 2E).
3.3. Elevated Vesl-1L/Homer 1c expression correlates with a decrease in visual function in the aged glaucomatous retina
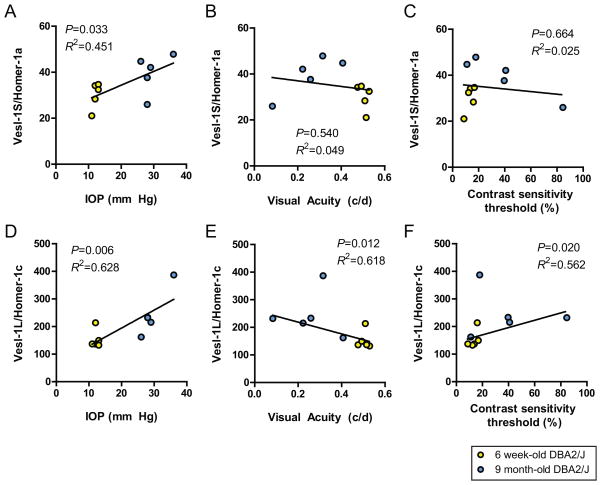
In order to test whether Vesl/Homer isoform expression was correlated with functional disease parameters of glaucoma, we performed a Pearson product-moment correlation coefficient calculation to evaluate the strength of the association.
The short isoform, the immediate early gene product, Vesl-1S/Homer 1a, showed a statistically significant, moderate, positive correlation with IOP (P<0.05, R2=0.451; Fig. 3A), however, no significant correlation was identified with visual acuity (P=0.54, R2=0.049; Fig. 3B) or contrast sensitivity (P=0.66, R2=0.025; Fig. 3D).
Figure 3. Vesl-1L/Homer 1c expression in the neural retina correlates with markers of disease severity in DBA/2J mice.
A–C) Vesl-1S/Homer 1a protein expression showed a positive association with IOP (n=5, P<0.05). In contrast, expression of the short Vesl/Homer isoform did neither correlate with visual acuity nor the contrast sensitivity threshold. D–F) In contrast, Vesl-1L/Homer 1c expression correlated strongly with markers of glaucoma disease severity. Specifically, Vesl-1L/Homer 1c levels were positively associated with IOP (n=5, P<0.01). Furthermore, the long constitutively expressed long Vesl/Homer isoform showed a statistically significant negative association with visual acuity (n=5, P<0.05) and a positive association with the contrast sensitivity threshold (n=5, P<0.05), indicative of progressively higher Vesl-1L/Homer 1c expression as disease severity increased.
In contrast, the constitutively expressed long Vesl-1L/Homer 1c isoform yielded strong associations with measures of disease severity. Specifically, Homer 1c levels were strongly, positively associated with IOP (P<0.01, R2=0.628; Fig. 3D) and contrast sensitivity (P<0.05, R2=0.562; Fig. 3F) and yielded a strong, negative correlation with visual acuity (P<0.05, R2=0.618; Fig. 3E).
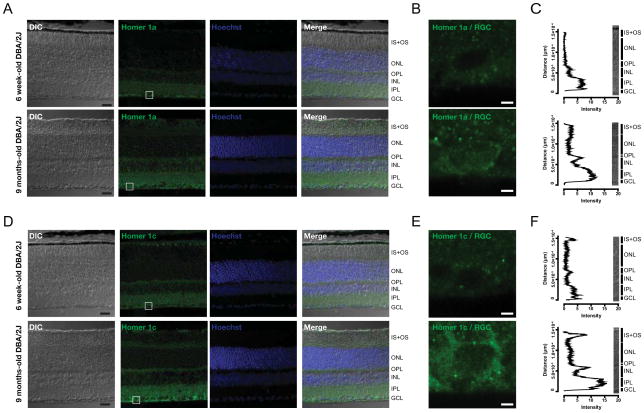
3.4. DBA/2J mice show increased Vesl/Homer 1 immunoreactivity in the neural retina
Given the strong association of increased Vesl-1L/Homer 1c protein levels with a decline in visual function in aging DBA/2J mice, we next performed immunohistochemistry and microfluorimetry in order to identify the cell types responsible for this increase. In 6 week-old DBA/2J mice, Vesl-1S/Homer 1a immunoreactivity was diffuse and strongest in the ganglion cell layer (GCL), and both the inner (IPL) and outer plexiform layer (OPL) (Fig. 4A, B). In the aged, glaucomatous DBA/2J retina, Vesl-1S/Homer 1a immunoreactivity was moderately increased by approx. 50% (Fig. 4A, B), while the cellular localization of Vesl-1S/Homer 1a protein was similar when compared to young controls (Fig. 4A).
Figure 4. Vesl-1/Homer 1 expression is markedly increased in the synaptic layers of the retina.
A) Vesl-1S/Homer 1a immunoreactivity showed a typical expression pattern that did not change in the aged, glaucomatous retina. A representative single confocal section is shown. Scale bar: 20 μm. B) Magnified view of the region in the RGC layer indicated by a square in A). Scale bar: 2.5 μm. C) Quantification using microfluorimetry is presented as the mean intensity of 25 line scans across a length of 180 μm. Intensity of Vesl-1S/Homer 1a immunoreactivity was increased in the synaptic layers, by approx. 60% in the ganglion cell layer (GCL) and the inner plexiform layer (IPL), and approx. 2-fold in the outer plexiform layer (OPL). D) Immunolabeling with an antibody specific for the long Vesl-1L/Homer 1c isoform revealed a significant increase in immunoreactivity in the synaptic layers of the retina of aged DBA/2J mice compared with young controls as evident from a representative single confocal section. Scale bar: 20 μm. E) Magnified view of the region in the RGC layer indicated by a square in D). Scale bar: 2.5 μm. D) The intensity graph represents the mean intensity of 25 line scans across a 180 μm stretch of retina, showing a 3-fold increase in Homer 1c immunoreactivity in the GCL, IPL and OPL.
Immunoreactivity for the long Vesl-1L/Homer 1c isoform strongly increased in the glaucomatous retina as assessed by microflourimetry (Fig. 4C, D). Immunoreactivity was punctate, indicative of synaptic staining, and strongest in the GCL, IPL and OPL (Fig. 4C) and 2–3 fold higher in the aged compared with the young DBA/2J retina (Fig. 4D).
4. Discussion
Herein we tested the hypothesis that expression levels of Vesl-1/Homer 1 isoforms are altered in the glaucomatous neural retina and that such changes are correlated with functional markers of disease severity. Our hypothesis was based on the rationale that Vesl-1/Homer 1 plays a critical role in synaptic and intracellular calcium signaling (Duncan et al., 2005, Hwang, et al., 2003, Kaja et al., 2012b, Tanaka, et al., 2006, Westhoff, et al., 2003), signaling mechanisms critical for the pathogenesis of glaucoma and based on previous reports describing changes in Vesl/Homer concentrations during pathological processes and aging of other parts of the CNS (Kaja et al., 2012b, Luo, et al., 2012, Menard & Quirion, 2012). We identified a significant increase in the expression levels of Vesl-1L/Homer 1c, a constitutively expressed central component of glutamatergic synapses that was highly correlated with clinically relevant behavioral markers for disease progression and deterioration of visual function.
4.1. Evidence for glaucomatous retinopathy in the aged, 9 months-old cohort of DBA/2J mice
Given the variable onset and progression of the glaucomatous phenotype in patients, and, similarly, in preclinical models such as the DBA/2J mouse used in the present study (Heijl, et al., 2012, Schlamp, et al., 2006), it is critical to quantitatively assess disease state in any experimental cohort (Burroughs et al., 2011). Herein, we used the Optomotry™ system, which exploits the optomotor tracking response to measure functional vision (Douglas, et al., 2006, McGill, et al., 2004, Prusky, et al., 2004). The left-right asymmetry of the optomotor reflex in rodents permits quantification of functional vision independently for each eye (Douglas et al., 2005, Douglas et al., 2006), thereby providing for accurate, eye-specific correlational analysis. Thus, this type of analysis takes into account data points that represent a range of different levels of disease severity with respect to the physiological parameters assayed in both the young healthy and middle-aged glaucomatous animals. Our focus on young and middle aged animals representing healthy and early to mid-stage glaucomatous retinopathy conditions thereby avoids end-stage disease with its hallmarks of severe neuronal loss in the retina and absence of retina function (Schuettauf, et al., 2004), mirroring more closely what is observed clinically in glaucoma patients with respect to functional impairment (Sena, et al., 2010, Wilensky & Hawkins, 2001, Zulauf & Flammer, 1993).
Functional vision and IOP in our cohorts of young and aged DBA/2J mice were similar to those reported for this strain previously (Burroughs et al., 2011, Nagaraju, et al., 2007, Zhang, et al., 2006).
4.2. Physiological consequences of increased Vesl/Homer expression
Our results show a strong negative association between Vesl-1L/Homer 1c expression and visual function in aged, glaucomatous DBA/2J mice. Vesl-1L/Homer 1c is the long, constitutively expressed isoform (Duncan et al., 2005), which enhances synaptic coupling through multimerization (Hayashi, et al., 2009) and anchoring synaptic proteins (Duncan et al., 2005) (Fig. 5). Binding partners of Vesl/Homer proteins include inositol 1,4,5-trisphosphate receptors (IP3Rs) and ryanodine receptors (RyRs) in the membranes of the endoplasmic reticulum and metabotropic glutamate receptors at the plasma membrane (Hwang et al., 2003, Mao, et al., 2005, Menard & Quirion, 2012, Tu, et al., 1998, Westhoff et al., 2003). In addition to providing a critical clustering role, Homer isoforms also differentially alter the biophysical properties of RyRs (Feng, et al., 2002, Hwang et al., 2003, Westhoff et al., 2003). Expression of the neuronal type 2 RyR is unaltered in the retina of aged, glaucomatous DBA/2J mice (Huang, et al., 2011). It can therefore be speculated that increased Vesl-1S/Homer 1c expression will result in increased Ca2+ release, if Vesl-1L/Homer 1c exerts similar effects on intracellular Ca2+ channels in the retina. Therefore, an increased ratio of Homer 1c over Homer 1a in the aged DBA/2J retina would favor synaptic coupling (as depicted on the right on Fig. 5), whereas a lower Homer 1c over Homer 1a ratio as observed in young, non-glaucomatous DBA/2J mice would promote a homeostatic condition as shown on the left in Fig. 5. Increased release of intracellular Ca2+ in the aged glaucomatous retina of DBA/2J mice is thus likely to contribute to overall hyperexcitability and Ca2+ toxicity (Chrysostomou, et al., 2012) (Fig. 5).
Figure 5. Proposed mechanism of Vesl-1/Homer 1-mediated changes in the neural retina.
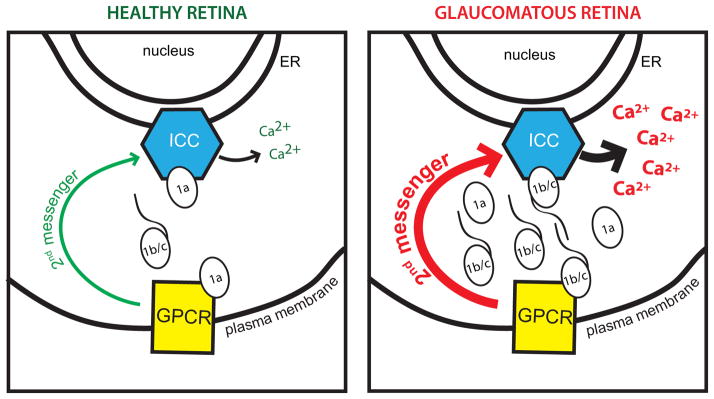
In the young, non-glaucomatous retina, Vesl-1S/Homer 1a prevents synaptic coupling of intracellular calcium channels (ICC) in the membranes of the endoplasmic reticulum with G-protein coupled receptors (GPCR) at the plasma membrane, resulting in tightly controlled intracellular Ca2+ release mediated by 2nd messenger pathways (green arrow). Up-regulation of Vesl-1L/Homer 1c in the aged, glaucomatous retina increases synaptic coupling, leading to increased intracellular Ca2+ release due to increased strength of second messenger pathways (red arrow) and the direct modulation of ICCs by Vesl-1L/Homer 1c.
Furthermore, this effect generated by elevated Vesl-1L/Homer 1c levels is potentially exacerbated by increased release of Ca2+ from intracellular stores following cellular oxidative stress (Andersson, et al., 2011, Kaja, et al., 2011, Lencesova & Krizanova, 2012, Smith, et al., 2005), as occurs during glaucoma and related neurodegenerative pathologies (Chrysostomou et al., 2012, Droge & Schipper, 2007, Smaili, et al., 2009), and likely amplified through polycystin-2 intracellular Ca2+ release channels present on the ER in the mouse retina (Kaja et al., 2012a).
4.3. Vesl/Homer proteins as potential targets for the development of intervention therapy in glaucoma
Mechanistically, the long Vesl-1L/Homer 1c isoform serves as an important scaffolding molecule that brings synaptic proteins into proximity (Duncan et al., 2005), while the short Vesl-1S/Homer 1a isoform can disturb this interaction through its lack of a multimerization domain and dominant negative competition for Vesl/Homer binding sites (Duncan et al., 2005). This makes Vesl/Homer proteins a potentially highly relevant clinical target for pharmaceutical intervention in glaucoma.
Previous studies have shown feasibility for such intervention in vitro and in vivo (Chen, et al., 2012, Klugmann, et al., 2005).
5. Conclusion
We identified the synaptic clustering Vesl-1L/Homer 1c as strongly correlated with functional markers of disease severity of glaucoma in DBA/2J mice. Vesl-1L/Homer 1c represents a novel, druggable target for the future development of anti-glaucoma therapies aimed at reducing hyperexcitability and aberrant neuronal Ca2+ signaling in the glaucomatous retina.
Supplementary Material
Highlights.
Vesl-1L/Homer-1c expression in increased in the retina of glaucomatous DBA/2J mice
Vesl-1L/Homer-1c expression correlates with functional markers of glaucoma severity
Vesl-1L/Homer 1c is a novel target for the development of anti-glaucoma therapies
Acknowledgments
Research reported in this publication was supported by grants from the National Eye Institute (EY014227 and EY022774), the Institute on Aging (AG010485, AG022550 and AG027956), the National Center for Research Resources and National Institute of General Medical Sciences (RR022570 and RR027093) of the National Institutes of Health (PK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support by the Felix and Carmen Sabates Missouri Endowed Chair in Vision Research and the Vision Research Foundation of Kansas City is gratefully acknowledged. The authors thank Dr. K. Inokuchi, University of Toyama, Japan, for the generous gift of antisera to Vesl-1 proteins and Margaret, Richard and Sara Koulen for generous support and encouragement.
Abbreviations
- HRP
horseradish peroxidase
- IOP
intraocular pressure
- RGCs
retinal ganglion cells
- Vesl
VASP/Ena-related gene up-regulated during seizure and LTP protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson DC, Betzenhauser MJ, Reiken S, Meli AC, Umanskaya A, Xie W, Shiomi T, Zalk R, Lacampagne A, Marks AR. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab. 2011;14(2):196–207. doi: 10.1016/j.cmet.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett RF. Linear Modelling of Pearson’s Product Moment Correlation Coefficient: An Application of Fisher’s $z$-Transformation. Journal of the Royal Statistical Society. Series D (The Statistician) 1993;42(1):45–53. [Google Scholar]
- Burroughs SL, Kaja S, Koulen P. Quantification of deficits in spatial visual function of mouse models for glaucoma. Invest Ophthalmol Vis Sci. 2011;52(6):3654–3659. doi: 10.1167/iovs.10-7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casson RJ, Chidlow G, Ebneter A, Wood JP, Crowston J, Goldberg I. Translational neuroprotection research in glaucoma: a review of definitions and principles. Clin Experiment Ophthalmol. 2012;40(4):350–357. doi: 10.1111/j.1442-9071.2011.02563.x. [DOI] [PubMed] [Google Scholar]
- Chang EE, Goldberg JL. Glaucoma 2.0: neuroprotection, neuroregeneration, neuroenhancement. Ophthalmology. 2012;119(5):979–986. doi: 10.1016/j.ophtha.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Fei F, Jiang XF, Zhang L, Qu Y, Huo K, Fei Z. Down-regulation of Homer1b/c attenuates glutamate-mediated excitotoxicity through endoplasmic reticulum and mitochondria pathways in rat cortical neurons. Free Radic Biol Med. 2012;52(1):208–217. doi: 10.1016/j.freeradbiomed.2011.10.451. [DOI] [PubMed] [Google Scholar]
- Chrysostomou V, Rezania F, Trounce IA, Crowston JG. Oxidative stress and mitochondrial dysfunction in glaucoma. Curr Opin Pharmacol. 2012 doi: 10.1016/j.coph.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Cook C, Foster P. Epidemiology of glaucoma: what’s new? Can J Ophthalmol. 2012;47(3):223–226. doi: 10.1016/j.jcjo.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Dielemans I, Vingerling JR, Wolfs RC, Hofman A, Grobbee DE, de Jong PT. The prevalence of primary open-angle glaucoma in a population-based study in The Netherlands. The Rotterdam Study. Ophthalmology. 1994;101(11):1851–1855. doi: 10.1016/s0161-6420(94)31090-6. [DOI] [PubMed] [Google Scholar]
- Douglas RM, Alam NM, Silver BD, McGill TJ, Tschetter WW, Prusky GT. Independent visual threshold measurements in the two eyes of freely moving rats and mice using a virtual-reality optokinetic system. Vis Neurosci. 2005;22(5):677–684. doi: 10.1017/S0952523805225166. [DOI] [PubMed] [Google Scholar]
- Douglas RM, Neve A, Quittenbaum JP, Alam NM, Prusky GT. Perception of visual motion coherence by rats and mice. Vision Res. 2006;46(18):2842–2847. doi: 10.1016/j.visres.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Droge W, Schipper HM. Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell. 2007;6(3):361–370. doi: 10.1111/j.1474-9726.2007.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan RS, Hwang SY, Koulen P. Effects of Vesl/Homer proteins on intracellular signaling. Exp Biol Med(Maywood) 2005;230(8):527–535. doi: 10.1177/153537020523000803. [DOI] [PubMed] [Google Scholar]
- Feng W, Tu J, Yang T, Vernon PS, Allen PD, Worley PF, Pessah IN. Homer regulates gain of ryanodine receptor type 1 channel complex. J Biol Chem. 2002;277(47):44722–44730. doi: 10.1074/jbc.M207675200. [DOI] [PubMed] [Google Scholar]
- Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86(2):238–242. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Mueller C, Vasudevan P. Behavioral sensitization to cocaine is associated with increased glutamate receptor trafficking to the postsynaptic density after extended withdrawal period. Neuroscience. 2009;159(1):414–426. doi: 10.1016/j.neuroscience.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Giuffrida R, Musumeci S, D’Antoni S, Bonaccorso CM, Giuffrida-Stella AM, Oostra BA, Catania MV. A reduced number of metabotropic glutamate subtype 5 receptors are associated with constitutive homer proteins in a mouse model of fragile X syndrome. J Neurosci. 2005;25(39):8908–8916. doi: 10.1523/JNEUROSCI.0932-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi MK, Tang C, Verpelli C, Narayanan R, Stearns MH, Xu RM, Li H, Sala C, Hayashi Y. The postsynaptic density proteins Homer and Shank form a polymeric network structure. Cell. 2009;137(1):159–171. doi: 10.1016/j.cell.2009.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijl A, Buchholz P, Norrgren G, Bengtsson B. Rates of visual field progression in clinical glaucoma care. Acta Ophthalmol. 2012 doi: 10.1111/j.1755-3768.2012.02492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Xing W, Ryskamp DA, Punzo C, Krizaj D. Localization and phenotype-specific expression of ryanodine calcium release channels in C57BL6 and DBA/2J mouse strains. Exp Eye Res. 2011;93(5):700–709. doi: 10.1016/j.exer.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SY, Wei J, Westhoff JH, Duncan RS, Ozawa F, Volpe P, Inokuchi K, Koulen P. Differential functional interaction of two Vesl/Homer protein isoforms with ryanodine receptor type 1: a novel mechanism for control of intracellular calcium signaling. Cell Calcium. 2003;34(2):177–184. doi: 10.1016/s0143-4160(03)00082-4. [DOI] [PubMed] [Google Scholar]
- Kaja S, Duncan RS, Longoria S, Hilgenberg JD, Payne AJ, Desai NM, Parikh RA, Burroughs SL, Gregg EV, Goad DL, Koulen P. Novel mechanism of increased Ca2+ release following oxidative stress in neuronal cells involves type 2 inositol-1,4,5-trisphosphate receptors. Neuroscience. 2011;175:281–291. doi: 10.1016/j.neuroscience.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaja S, Mafe OA, Parikh RA, Kandula P, Reddy CA, Gregg EV, Xin H, Mitchell P, Grillo MA, Koulen P. Distribution and function of polycystin-2 in mouse retinal ganglion cells. Neuroscience. 2012a;202:99–107. doi: 10.1016/j.neuroscience.2011.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaja S, Sumien N, Borden PK, Khullar N, Iqbal M, Collins JL, Forster MJ, Koulen P. Homer-1a immediate early gene expression correlates with better cognitive performance in aging. Age (Dordr) 2012b doi: 10.1007/s11357-11012-19479-11356. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaja S, Yang SH, Wei J, Fujitani K, Liu R, Brun-Zinkernagel AM, Simpkins JW, Inokuchi K, Koulen P. Estrogen protects the inner retina from apoptosis and ischemia-induced loss of Vesl-1L/Homer 1c immunoreactive synaptic connections. Invest Ophthalmol Vis Sci. 2003;44(7):3155–3162. doi: 10.1167/iovs.02-1204. [DOI] [PubMed] [Google Scholar]
- Klein BE, Klein R, Sponsel WE, Franke T, Cantor LB, Martone J, Menage MJ. Prevalence of glaucoma. The Beaver Dam Eye Study. Ophthalmology. 1992;99(10):1499–1504. doi: 10.1016/s0161-6420(92)31774-9. [DOI] [PubMed] [Google Scholar]
- Klugmann M, Symes CW, Leichtlein CB, Klaussner BK, Dunning J, Fong D, Young D, During MJ. AAV-mediated hippocampal expression of short and long Homer 1 proteins differentially affect cognition and seizure activity in adult rats. Mol Cell Neurosci. 2005;28(2):347–360. doi: 10.1016/j.mcn.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Lencesova L, Krizanova O. IP(3) receptors, stress and apoptosis. Gen Physiol Biophys. 2012;31(2):119–130. doi: 10.4149/gpb_2012_014. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- Luo P, Li X, Fei Z, Poon W. Scaffold protein Homer 1: implications for neurological diseases. Neurochem Int. 2012;61(5):731–738. doi: 10.1016/j.neuint.2012.06.014. [DOI] [PubMed] [Google Scholar]
- Mackiewicz M, Paigen B, Naidoo N, Pack AI. Analysis of the QTL for sleep homeostasis in mice: Homer1a is a likely candidate. Physiol Genomics. 2008;33(1):91–99. doi: 10.1152/physiolgenomics.00189.2007. [DOI] [PubMed] [Google Scholar]
- Mao L, Yang L, Tang Q, Samdani S, Zhang G, Wang JQ. The scaffold protein Homer1b/c links metabotropic glutamate receptor 5 to extracellular signal-regulated protein kinase cascades in neurons. J Neurosci. 2005;25(10):2741–2752. doi: 10.1523/JNEUROSCI.4360-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill TJ, Douglas RM, Lund RD, Prusky GT. Quantification of spatial vision in the Royal College of Surgeons rat. Invest Ophthalmol Vis Sci. 2004;45(3):932–936. doi: 10.1167/iovs.03-0964. [DOI] [PubMed] [Google Scholar]
- Menard C, Quirion R. Successful cognitive aging in rats: a role for mGluR5 glutamate receptors, homer 1 proteins and downstream signaling pathways. PLoS One. 2012;7(1):e28666. doi: 10.1371/journal.pone.0028666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraju M, Saleh M, Porciatti V. IOP-dependent retinal ganglion cell dysfunction in glaucomatous DBA/2J mice. Invest Ophthalmol Vis Sci. 2007;48(10):4573–4579. doi: 10.1167/iovs.07-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascale A, Drago F, Govoni S. Protecting the retinal neurons from glaucoma: lowering ocular pressure is not enough. Pharmacol Res. 2012;66(1):19–32. doi: 10.1016/j.phrs.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci. 2004;45(12):4611–4616. doi: 10.1167/iovs.04-0541. [DOI] [PubMed] [Google Scholar]
- Prusky GT, Alam NM, Douglas RM. Enhancement of vision by monocular deprivation in adult mice. J Neurosci. 2006;26(45):11554–11561. doi: 10.1523/JNEUROSCI.3396-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlamp CL, Li Y, Dietz JA, Janssen KT, Nickells RW. Progressive ganglion cell loss and optic nerve degeneration in DBA/2J mice is variable and asymmetric. BMC Neurosci. 2006;7:66. doi: 10.1186/1471-2202-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuettauf F, Rejdak R, Walski M, Frontczak-Baniewicz M, Voelker M, Blatsios G, Shinoda K, Zagorski Z, Zrenner E, Grieb P. Retinal neurodegeneration in the DBA/2J mouse-a model for ocular hypertension. Acta Neuropathol. 2004;107(4):352–358. doi: 10.1007/s00401-003-0816-9. [DOI] [PubMed] [Google Scholar]
- Sena DF, Ramchand K, Lindsley K. Neuroprotection for treatment of glaucoma in adults. Cochrane Database Syst Rev. 2010;2:CD006539. doi: 10.1002/14651858.CD006539.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaili S, Hirata H, Ureshino R, Monteforte PT, Morales AP, Muler ML, Terashima J, Oseki K, Rosenstock TR, Lopes GS, Bincoletto C. Calcium and cell death signaling in neurodegeneration and aging. An Acad Bras Cienc. 2009;81(3):467–475. doi: 10.1590/s0001-37652009000300011. [DOI] [PubMed] [Google Scholar]
- Smith IF, Hitt B, Green KN, Oddo S, LaFerla FM. Enhanced caffeine-induced Ca2+ release in the 3xTg-AD mouse model of Alzheimer’s disease. J Neurochem. 2005;94(6):1711–1718. doi: 10.1111/j.1471-4159.2005.03332.x. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Duncan RS, McClung N, Yannazzo JA, Hwang SY, Marunouchi T, Inokuchi K, Koulen P. Homer proteins control neuronal differentiation through IP(3) receptor signaling. FEBS Lett. 2006;580(26):6145–6150. doi: 10.1016/j.febslet.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21(4):717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Westhoff JH, Hwang SY, Duncan RS, Ozawa F, Volpe P, Inokuchi K, Koulen P. Vesl/Homer proteins regulate ryanodine receptor type 2 function and intracellular calcium signaling. Cell Calcium. 2003;34(3):261–269. doi: 10.1016/s0143-4160(03)00112-x. [DOI] [PubMed] [Google Scholar]
- Wilensky JT, Hawkins A. Comparison of contrast sensitivity, visual acuity, and Humphrey visual field testing in patients with glaucoma. Trans Am Ophthalmol Soc. 2001;99:213–217. discussion 217–218. [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang M, Avila MY, Ge J, Laties AM. Time course of age-dependent changes in intraocular pressure and retinal ganglion cell death in DBA/2J mouse. Yan Ke Xue Bao. 2006;22(3):184–189. 194. [PubMed] [Google Scholar]
- Zulauf M, Flammer J. Correlation of spatial contrast sensitivity and visual fields in glaucoma. Graefes Arch Clin Exp Ophthalmol. 1993;231(3):146–150. doi: 10.1007/BF00920937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.