Abstract
Background and Purpose
In rehabilitation, examining how variables change over time can help to define the minimal number of training sessions required to produce a desired change. The purpose of this study was to identify the time course of changes in gait biomechanics and walking function in persons with chronic stroke.
Methods
Thirteen persons > 6 months post-stroke participated in 12 weeks of fast treadmill training combined with plantar- and dorsi-flexor muscle functional electrical stimulation (FastFES). All participants completed testing before the start of intervention, after 4, 8 and 12 weeks of FastFES locomotor training.
Results
Peak limb paretic propulsion, paretic limb propulsive integral, peak paretic limb knee flexion, (p<0.05 for all) and peak paretic trailing limb angle (p<0.01) improved from pre-training to 4 weeks but not between 4 and 12 weeks. Self-selected walking speed and 6-minute walk test distance improved from pre-training to 4 weeks and from 4 to 12 weeks (p<0.01 and p<0.05, respectively for both). Timed Up & Go test time did not improve between pre-training and 4 weeks, but improved by 12 weeks (p=0.24 and p<0.01, respectively).
Discussion and Conclusions
The results demonstrate that walking function improves with a different time course compared to gait biomechanics in response to a locomotor training intervention in persons with chronic stroke. Thirty-six training sessions were necessary to achieve an increase in walking speed that exceeded the MCID. These finding should be considered when designing locomotor training interventions after stroke. Video Abstract available (see Video, Supplemental Digital Content 1) for more insights from the authors.
INTRODUCTION
According to the World Health Organization (WHO), 15 million people worldwide experience a stroke each year. One of the primary concerns for patients who experience stroke is the ability to regain walking function.1 Consequently, significant effort is focused on gait retraining during rehabilitation following a stroke and efforts to develop and improve locomotor retraining programs are a major focus of rehabilitation research.2–5 The primary focus of much of this research has been on the development of novel interventions, using treadmills, body-weight support and robotics.4–6 However, less attention has been paid to the time course of changes in the variety of deficits that are targeted with these interventions.
Based on the specific patient needs, gait training interventions after stroke may target a variety of deficits including walking biomechanics and energetics, walking endurance and/or speed, walking activity or some combination of these and others7. Several studies have examined the time course of improvements in walking speed with intervention after stroke5, 8, however, there are no studies that have simultaneously examined the time course of changes in walking speed, endurance and gait biomechanics. Improvements in gait biomechanics after stroke are thought to be important because of their connection to walking function and safety9–14. Post-stroke intervention studies have associated improvements in specific gait biomechanics with improvements in walking speed after stroke11, 15 and thus, many post-stroke gait interventions focus on improving biomechanics and walking function.16–19 There are likely different mechanisms underlying the change in each of these deficits, therefore, it is anticipated that changes in various deficits will occur on different time scales. For example, changes in the neural activation of muscle can occur on a short time scale (e.g.- several sessions)20, suggesting that changes in gait biomechanics might change with a more rapid time course than walking speed or endurance. To the extent that there is a relationship between improvements in gait biomechanics and improvements in walking function and that these may change on different timescales, it is relevant to examine the time course of changes in both biomechanics and walking function with post-stroke intervention.
By examining how gait-related variables change over the course of training we may be able to determine the minimal number of training sessions required to produce a given change. As an example, in a 6 month treadmill training study focused on improving cardiovascular fitness in persons with chronic stroke, improvements in peak and average VO2 were observed after 3 months of training, but no further improvements were found between 3 and 6 months of training.21 Without the 3 month measurement point, the authors may have erroneously concluded that 6 months of their training intervention was required to see the gains observed.
For these reasons, in the process of developing a novel locomotor training intervention for persons post-stroke, we designed a study that allowed us to examine the time course of changes in a variety of walking parameters. The purpose of this study was to identify the time course of changes in gait biomechanics and walking function in persons with chronic stroke. As indicated above, because changes in neural activation of muscle and motor learning can occur on a relatively short time scale (e.g. several sessions)20, 22, we hypothesized that gait kinematics and kinetics would change with a more rapid time course than measures of walking function with this intervention, but that all variables would show improvement after 12 weeks of training.
METHODS
Participants
Thirteen subjects (Age = 61 ± 8.3 years; 7 males) with post-stroke hemiparesis participated in this study (Table 1). Participants were recruited from local physical therapy clinics, stroke support groups, and newspaper advertisements. All participants were more than 6 months post a single stroke, able to walk continuously for 5 minutes at their self-selected speed without the assistance of another person, and had enough passive range of motion so that their paretic ankle joint could reach within 5 degrees of the neutral position with the knee flexed (i.e., participants could have no more than a 5 degree plantarflexion contracture). Exclusion criteria included congestive heart failure, peripheral artery disease with claudication, diabetes not under control via medication or diet, shortness of breath without exertion, unstable angina, resting heart rate outside of the range of 40–100 beats per minute, resting blood pressure outside of the range of 90/60 to 170/90 mm Hg, inability to communicate with the investigators, pain in lower limbs or spine, total knee replacement, cerebellar involvement, and neglect (star cancellation test23). All participants provided written informed consent to participate in a study that had been approved by the Human Subjects Review Board of ________.
Table 1.
Subject characteristics at the pre-training evaluation.
| Subject | Gender | Age (years) |
Side of Hemiparesis (Left or Right) |
Time since Stroke (months) |
Assistive Device Used (Community) |
Orthotic Used (Community) |
Self- selected gait speed (m/s) |
Fugl- Meyer (LE) Score |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 67 | L | 22 | AFO | 0.70 | 21 | |
| 2 | M | 51 | L | 111 | 0.90 | 24 | ||
| 9 | M | 58 | R | 110 | 0.50 | 15 | ||
| 53 | M | 71 | R | 70 | SC | AFO | 0.50 | 14 |
| 98 | M | 66 | R | 19 | SC | 0.30 | 21 | |
| 108 | M | 70 | L | 21 | SC | 0.50 | 13 | |
| 110 | F | 65 | R | 15 | NBQC | 0.70 | 18 | |
| 120 | F | 52 | L | 33 | 0.80 | 19 | ||
| 128 | F | 65 | R | 18 | 0.50 | 18 | ||
| 129 | F | 54 | R | 55 | 0.50 | 17 | ||
| 136 | F | 58 | R | 12 | SC | AFO | 0.30 | 13 |
| 137 | M | 46 | R | 8 | AFO | 0.40 | 15 | |
| 142 | F | 70 | L | 9 | SC | 0.30 | 22 |
L=left, R=right, m/s=meters/second, LE=lower extremity, SC=straight cane, NBQC= narrow based quad cane, AFO= ankle foot orthosis
Testing
All participants completed testing before the start of intervention, and after 4, 8 and 12 weeks of FastFES locomotor training. All assessors were blinded to previous assessment data at each testing session. The clinical evaluation and training was completed by the same tester (MR) while gait analysis testing was completed by another tester (TK).
Clinical evaluations
Participants completed clinical tests to evaluate their walking. These included: 1) the 10-meter walk test to measure short-distance self-selected (comfortable) and fastest walking speeds24; 2) the 6-minute walk test as a measure of walking endurance25; and 3) the Timed Up & GO (TUG) test as a measure of functional mobility that requires participants to stand up from a chair with armrests, walk 3m at their comfortable speed, turn around, return to the chair, and sit down.26 The clinical evaluation and training were completed by the same tester (MR).
Gait analysis
Participants walked at their self-selected overground speed on a split-belt treadmill to measure specific gait impairments. The treadmill was instrumented with two independent 6 degree of freedom force platforms (AMTI, Watertown, MA) from which ground reaction force (GRF) data were collected at 2000Hz. Retro-reflective markers (14-mm diameter) were placed bilaterally over the pelvis, thigh, shank, and foot segments and on the medial and lateral malleoli, at the medial and lateral knee joint line, greater trochanters, and iliac crests. Kinematic data were collected using an 8-camera Vicon Motion Capture System (Vicon MX, Los Angeles, CA) at 100 Hz. Two 20-second trials were collected. For safety, participants held on to a handrail during walking and wore a harness that was attached to an overhead support. No body weight was supported by the harness.
Training
Subjects participated in FastFES training administered by a physical therapist 3×/week for a total of 12 weeks. Training speed was initially determined as the fastest speed the subject could maintain for 4 minutes of continuous walking. This speed was re-evaluated every 4 weeks and increased as possible at each 4 week interval using the same criterion. Each training session consisted of both treadmill and overground walking. First, participants completed 4 treadmill walking bouts of 6 minutes each for a total of 24 minutes of treadmill walking. During each of the 4 bouts, FES to the paretic ankle dorsi- and plantarflexor muscles was delivered during the first, third and fifth minute. During the second, fourth and sixth minute, FES was turned off and participants were encouraged to walk with the same pattern as during FES. This alternating pattern of FES delivery was designed to maximize potential motor learning27 and to minimize muscle fatigue. Seated rest breaks (~5 minutes) were provided between consecutive walking bouts. The 4 bouts were followed by a final bout comprising 3 minutes of walking with FES on the treadmill, followed immediately by 3 minutes of overground walking in the hallway at their fastest possible speed without FES. During overground walking participants were encouraged to reproduce the same walking pattern as practiced with the FES on the treadmill.
Electrical Stimulation
Self-adhesive surface electrical stimulation electrodes were attached over the ankle dorsiflexor (2”×2”, TENS Products, Grand Lake, CO) and plantarflexor (2”×5”, ConMed Corp, New York) muscles. For the dorsiflexor muscles, one electrode was placed over the motor point of the tibialis anterior and the other over the distal portion of the tibialis anterior muscle belly. For the plantarflexors, the electrodes were oriented horizontally and placed on the dorsal aspect of the leg over the proximal and distal portions of the gastrocnemius muscle.28 A Grass S8800 stimulator in combination with a Grass Model SIU8TB stimulus isolation unit was used to deliver electrical stimulation (Grass Instrument Division, Quincy, MA).
For both the dorsi- and plantar-flexor muscles, the stimulation amplitude was set using a stimulation train that was 300-ms long at a frequency of 30-Hz and with a pulse duration of 300-µs. For the ankle dorsiflexor muscles, stimulation amplitude was set with the subject seated and the foot hanging freely in a plantarflexed position. The stimulation train amplitude was gradually increased until the foot reached a neutral ankle joint position (0°) or the subject’s maximum dorsiflexion range of motion was achieved. The medio-lateral placement of the electrodes was adjusted to minimize ankle eversion / inversion. For the ankle plantarflexor muscles, the stimulation amplitude was set while the subject stood so that the non-paretic foot was a step length ahead of the paretic foot with weight on both the paretic and non-paretic extremities. The amplitude was increased until either the stimulation train produced a plantarflexor force sufficient to lift the paretic heel off the ground or until the subject’s maximal tolerance was reached, whichever occurred first.
Two compression closing foot switches (25-mm diameter MA-153, Motion Lab Systems Inc., Baton Rouge, LA) were attached to the outside sole of the shoe of the paretic limb. One foot switch was placed under the fifth metatarsal head (forefoot switch) and the other under the lateral portion of the heel (hindfoot switch). The foot switches were used to control the timing of the FES during the gait cycle.
Timing of FES During Training
A customized FES system consisting of a real-time controller (cRIO-9004, National Instruments, TX), analog input module (NI 9210), and digital input/output module (NI 9401) were used to control the Grass stimulator and deliver stimulation during the gait cycle.28, 29 The FES system delivered stimulation to the ankle dorsiflexor muscles from the time the paretic foot was off the ground (neither footswitch in contact with ground) to paretic initial contact (either foot switch in contact with ground). The ankle plantarflexor muscles of the paretic limb were stimulated from heel off of the paretic limb (hindfoot foot switch not in contact with ground), until the paretic foot was off the ground (neither footswitch in contact with ground). Recent publications from our lab have shown that this timing algorithm produced the desired effects of increased ankle dorsiflexion, increased anterior ground reaction force and increased knee flexion on the paretic limb during walking.28, 30
The FES used novel, variable-frequency trains (VFTs) consisting of an initial high-frequency (200-Hz) 3-pulse burst followed by a lower frequency (30-Hz) constant frequency portion of the train.28, 29, 31 That is, each time stimulation was delivered, the stimulation train began with three pulses that were each separated by 5 milliseconds; all subsequent pulses within that same train were then separated by 33.3 milliseconds. We used these VFTs because they are physiological-based patterns that take advantage of the catch-like property of muscle and have been shown to enhance gait performance after stroke compared to the more traditionally used constant-frequency train.29
Data Processing
Marker trajectories and GRF data were low-pass filtered (Butterworth fourth order, phase lag) at 6- and 30-Hz, respectively, using commercial software (Visual 3D; C-Motion, Rockville, MD). Lower limb kinematics were calculated using rigid body analysis and Euler angles using Visual 3D. Vertical and antero-posterior GRFs were normalized to body weight. Vertical GRFs were used to identify gait events (initial contact and toe-off). Strides were time normalized to 100% of the gait cycle and averaged across trials for each participant.
We evaluated the following dependent variables prior to training and after 4, 8 and 12 weeks of training:
Peak Paretic Propulsion - peak value of the anterior GRF normalized to body weight.
-
Paretic Propulsive Integral - integral of the anterior GRF from the onset of propulsion through the end of stance phase for the paretic leg.
Variables 1 and 2 were chosen because the purpose of the plantarflexor FES was to increase plantar flexor force during push-off.
Peak Knee Flexion during Swing phase was determined for the paretic leg.
-
Peak Trailing Limb Angle was computed as the peak of the planar angle between the laboratory’s vertical axis (along the sagittal plane) and a vector joining markers located on the lateral malleolus and the greater trochanter of the paretic lower extremity.
Variables 3 & 4 were chosen because the purpose of the plantarflexor FES was to increase plantarflexor force during push-off with the paretic limb in greater extension during pre-swing. This increased plantarflexor force should result in greater knee flexion during swing.
Self-selected walking speed
Distance ambulated during the 6-minute walk test
Time on the TUG test
These measures of walking function (#5–7) were used to examine changes in the functional walking of the participants.
Statistical Analysis
The Kolmogorov-Smirnov test was used to test for normal distribution of data for each of the outcome variables. Because some variables were not normally distributed, non-parametric statistics were utilized. The Wilcoxon signed-rank test was used to compare data between the pre-training and 4 week testing session and between the 4 week and 12 week testing session. We reasoned that if significant differences for a variable were not found between either of these two comparisons, then there was no effect of the intervention on that variable. If, however, there was a difference from pre-training to 4 weeks, but not from 4 to 12 weeks, we would conclude that maximum gains for that variable were achieved by 4 weeks. If there were differences between both time point comparisons, we would conclude that the variable continued to improve through the end of training (12 weeks). Alpha level was set at 0.05. All statistical analyses were performed using SPSS 19.0 (SPSS Inc., Chicago, IL).
RESULTS
The participants demonstrated a range of gait and functional impairments prior to training. Average pre-training self-selected walking speed was 0.5 ±0.17 m/s and the lower extremity Fugl-Meyer scores ranged from 13–24 (Table 1). Twelve of the 13 participants who were enrolled completed the 12 weeks of training and follow-up testing. The subject who did not complete training dropped out due to knee pain from an incident unrelated to the training.
Kinematics and kinetics
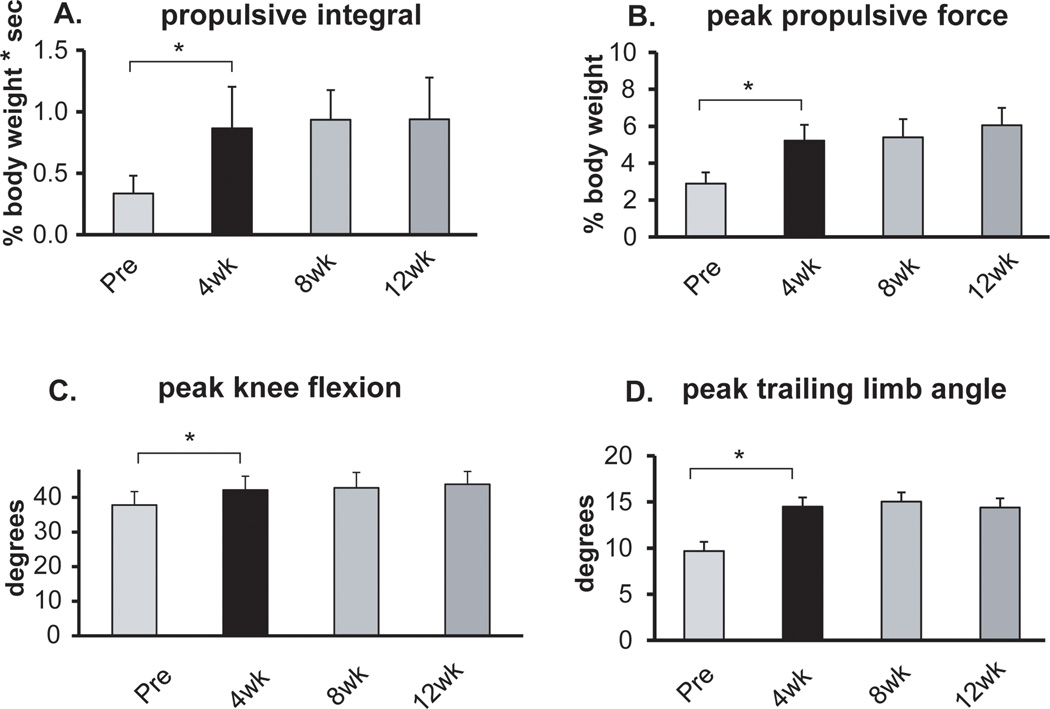
Due to technical problems, ground reaction force data from 3 participants were not available at all time points and therefore these participants’ data were not included in the analysis of peak paretic propulsion and paretic propulsive integral. Both peak paretic propulsion and the paretic propulsive integral improved from pre-training to 4 weeks (Figure 1A and B), but no differences were found between 4 and 12 weeks (p>0.05 for both). Similarly, peak knee flexion and peak trailing limb angle improved from pre-training to 4 weeks, (p<0.05 and p<0.01, respectively), but no differences were found between 4 and 12 weeks (Figure 1C and D).
Figure 1.
Results for the targeted kinematic and kinetic variables at self-selected walking speed. From left to right, the bars in each figure represent the average results across participants prior to training (Pre) and after 4, 8 and 12 weeks of training. Error bars are 1 SE. * indicates p<0.05 between time points.
Clinical measures of walking function
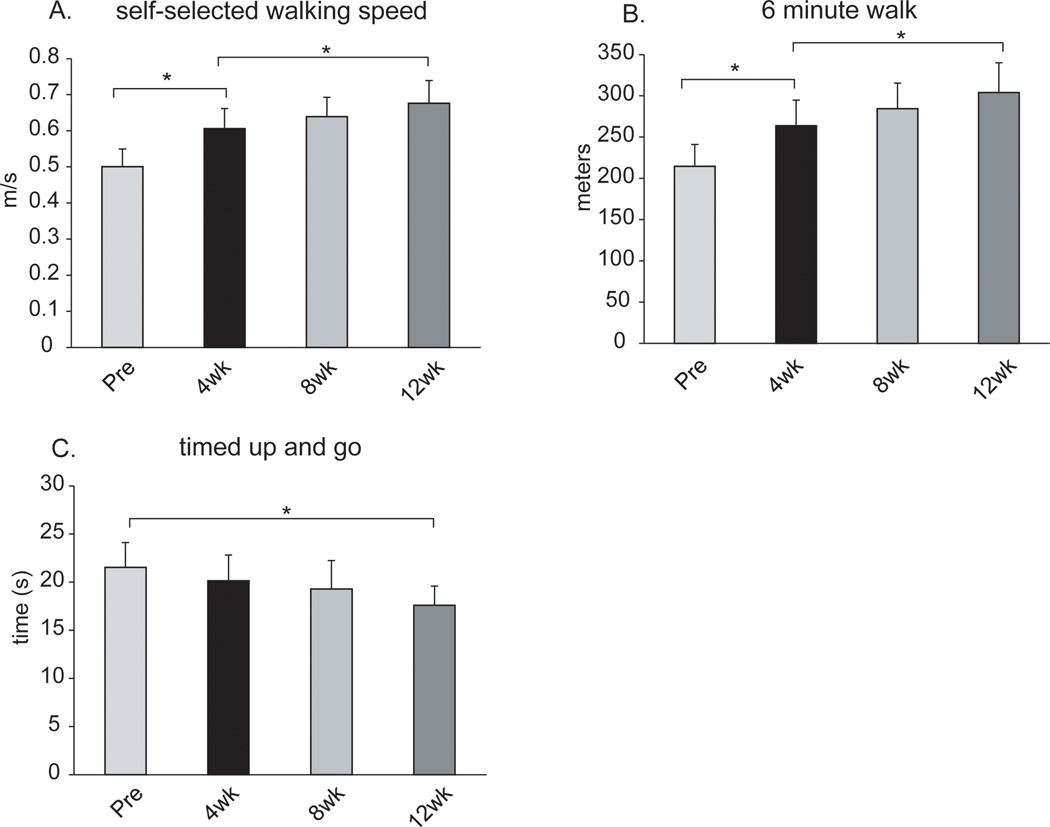
Self-selected walking speed improved from pre-training (0.50±0.17) to 4 weeks (0.61±0.19) and again from 4 weeks to 12 weeks (0.68±0.22) (p<0.01 and p<0.05, respectively, Figure 2A). Similarly, distance covered during the 6-minute walk test improved from pre-training (214±92) to 4 weeks (264±107) and again from 4 weeks to 12 weeks (304±125; p<0.01 and p<0.05, respectively, Figure 2B). Time to complete the TUG test did not improve significantly between pre-training (21.5±8.9) and 4 weeks (20.1±9.3), but was improved by 12 weeks (17.6±6.8; p=0.24 and p<0.01, respectively, Figure 2C).
Figure 2.
Results for the measures of walking function. From left to right, the bars in each figure represent the average results across participants prior to training (Pre) and after 4, 8 and 12 weeks of training. Error bars are 1 SE. * indicates p<0.05 between time points.
DISCUSSION
The results of this study demonstrate that after 12 weeks of fast speed locomotor training with functional electrical stimulation to the plantar- and dorsi-flexor muscles, improvements were observed in kinematic and kinetic gait patterns and in functional walking. However, the time course of the improvements in the gait biomechanics was shorter than the time course of the improvements in functional measures. This information can assist clinicians in setting expectations for the time course of improvements with post-stroke locomotor rehabilitation.
To our knowledge, this is the first study that has simultaneously examined the time course of changes in walking speed, endurance and gait biomechanics following a locomotor training intervention in individuals after stroke. The data show that changes in the biomechanics of walking took place early in the training period and leveled off after subjects had trained for 4 weeks (12 sessions). However, the measures of function showed continued improvement which has also been demonstrated by a number of studies that have examined the time course of improvements in walking function. Sullivan et al. (2007) studied the effect of body-weight supported treadmill training in combination with upper extremity exercises that were administered 4 days/week for 24 sessions in persons after stroke. The results demonstrated a linear increase in walking speed between baseline and 12 sessions and between 12 sessions and 24 sessions of training.5 A 12-week intervention study that included body-weight supported treadmill training, strength training, and aerobic training 5 days/week in persons with stroke found that after 8 weeks of training (40 sessions), walking speed improvements during the 6-minute walk test appeared to plateau.8 A 12 week intervention including body-weight supported treadmill training and overground walking training 3×/week in individuals with chronic stroke found that short distance walking speed improved across all 12 weeks (36 sessions).24 These findings are similar to those of the present study and support the assertion that walking speed and endurance continues to improve well after 12 sessions (4 weeks) of locomotor training in those with chronic stroke.
With respect to the functional measures, it is necessary to ask whether the improvements beyond 4 weeks (12 sessions) were not only statistically significant, but also clinically meaningful. In the case of walking speed, the minimally clinically important difference (MCID) is 0.16 m/s in persons with stroke.32 This value was not exceeded until the 12 week time point (after 36 sessions), indicating that to achieve a meaningful change in walking speed in these participants, 12 weeks of training were needed. MCID values do not exist for those with stroke for the 6-minute walk test or the TUG test. We do, however, know that the minimal detectable change (MDC) value for the TUG test is 3.7 seconds.33 A reduction in the time taken to complete the test did not exceed this value until the 12 week time point (36 sessions). In the case of the 6-minute walk test, the change in distance exceeded the MDC value of 52 meters34 at the 8 week time point (24 sessions). Taken together these results suggest that to achieve meaningful and detectable changes in functional walking, 12 weeks (36 sessions) of intervention were required.
MCID values do not exist for those with stroke for the gait biomechanical variables presented. Evaluation of our results relative to MDC values for the gait biomechanics indicate that the changes observed exceed the MDC for trailing limb position and peak anterior ground reaction force, but not for propulsive integral or peak knee flexion during swing35.
Walking function after stroke is thought to be influenced by a variety of factors including gait biomechanics, cardiovascular fitness, and biopsychosocial factors.10, 36–38 Improvements in gait biomechanics are thought to be important because of the connection between biomechanics and walking function and safety after stroke10–12, 14, 39, 40. Many cross-sectional studies have demonstrated a relationship between specific deficits in gait biomechanics and walking speed after stroke12, 39, 41, 42. Moreover, intervention studies have associated improvements in specific gait biomechanics with improvements in walking speed after stroke11, 15. For example, cross-sectional studies have shown that increased plantarflexor power generation is associated with faster walking speed after stroke39 and greater changes in this power generation are associated with greater improvements in walking speed15. While the present study was not designed to test a direct relationship between changes in gait biomechanics and walking function, our results do suggest that if biomechanical changes are important for improving walking speed or function, individuals after a stroke must devote additional time to learn to utilize the biomechanical changes to improve their walking speed and function. This is supported by the results of a recent study showing that improvements in a global measure of gait biomechanics (step length symmetry) at the end of a post-stroke locomotor training intervention were associated with improved walking speed 6 months later18.
The results from this study provide clinicians with information about expected time frames for improvements in walking with rehabilitation after stroke that can be used in treatment planning and goal setting. Specifically, our results indicate that improvements in gait biomechanics will plateau prior to improvements in walking speed and endurance and thus, expectations for each outcome should be set accordingly.
Limitations
The sample in this study is small. Future studies should examine the time course of changes in gait biomechanics and walking function during locomotor interventions in larger groups of persons with chronic stroke and should include follow-up testing.
It is possible that the specific time course of change for the various outcomes depends on factors such as type, intensity and progression of training. Other studies have found a similar pattern of change in walking speed with differing locomotor training intensities and progressions8, 24 suggesting that the results found here may be representative.
Biomechanical data were collected at the subject’s self-selected speed at each time point. While this congruence is important for relating the biomechanical data to the functional data, changes in speed could have played a role in the biomechanical changes43. However, the finding that improvements in biomechanics and function diverged after 4 weeks of training, suggests that there may be a limit to the relationship between changes in walking speed and changes in biomechanics after stroke.
Conclusions
The results of this study demonstrate that after stroke, gait biomechanics and walking function both improved following a novel locomotor training intervention, but that each improved on a different time scale. Thirty-six training sessions were necessary to achieve an increase in walking speed that exceeded the MCID. This finding should be considered when designing locomotor training interventions after stroke.
Supplementary Material
Supplemental Digital Content 1. Video abstract
Acknowledgments
Funding: This study was supported by the National Institute of Nursing Research grant R01NR010786; the National Institute of Health Shared Instrumentation grant S10RR022396; and the National Institute of Child Health & Human Development grant K01HD050582.
Key
- AFO
Ankle Foot Orthosis
- SC
Straight Cane
Footnotes
Results were presented in part at the Combined Sections meeting of the American Physical Therapy Association in February, 2011.
References
- 1.Mayo NE, Wood-Dauphinee S, Ahmed S, et al. Disablement following stroke. Disabil Rehabil. 1999 May-Jun;21(5–6):258–268. doi: 10.1080/096382899297684. [DOI] [PubMed] [Google Scholar]
- 2.Hidler J, Nichols D, Pelliccio M, et al. Multicenter randomized clinical trial evaluating the effectiveness of the Lokomat in subacute stroke. Neurorehabil Neural Repair. 2009 Jan;23(1):5–13. doi: 10.1177/1545968308326632. [DOI] [PubMed] [Google Scholar]
- 3.Jette DU, Latham NK, Smout RJ, Gassaway J, Slavin MD, Horn SD. Physical therapy interventions for patients with stroke in inpatient rehabilitation facilities. Phys Ther. 2005 Mar;85(3):238–248. [PubMed] [Google Scholar]
- 4.Macko RF, Ivey FM, Forrester LW, et al. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke: a randomized, controlled trial. Stroke. 2005 Oct;36(10):2206–2211. doi: 10.1161/01.STR.0000181076.91805.89. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan KJ, Brown DA, Klassen T, et al. Effects of task-specific locomotor and strength training in adults who were ambulatory after stroke: results of the STEPS randomized clinical trial. Phys Ther. 2007 Dec;87(12):1580–1602. doi: 10.2522/ptj.20060310. discussion 1603-1587. [DOI] [PubMed] [Google Scholar]
- 6.Hornby TG, Campbell DD, Kahn JH, Demott T, Moore JL, Roth HR. Enhanced gait-related improvements after therapist- versus robotic-assisted locomotor training in subjects with chronic stroke: a randomized controlled study. Stroke. 2008 Jun;39(6):1786–1792. doi: 10.1161/STROKEAHA.107.504779. [DOI] [PubMed] [Google Scholar]
- 7.Richards CL, Malouin F, Dean C. Gait in stroke: assessment and rehabilitation. Clin Geriatr Med. 1999 Nov;15(4):833–855. [PubMed] [Google Scholar]
- 8.Jorgensen JR, Bech-Pedersen DT, Zeeman P, Sorensen J, Andersen LL, Schonberger M. Effect of intensive outpatient physical training on gait performance and cardiovascular health in people with hemiparesis after stroke. Phys Ther. 2010 Apr;90(4):527–537. doi: 10.2522/ptj.20080404. [DOI] [PubMed] [Google Scholar]
- 9.Hall AL, Peterson CL, Kautz SA, Neptune RR. Relationships between muscle contributions to walking subtasks and functional walking status in persons with post-stroke hemiparesis. Clin Biomech (Bristol, Avon) 2011 Jan 18; doi: 10.1016/j.clinbiomech.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mulroy S, Gronley J, Weiss W, Newsam C, Perry J. Use of cluster analysis for gait pattern classification of patients in the early and late recovery phases following stroke. Gait Posture. 2003 Aug;18(1):114–125. doi: 10.1016/s0966-6362(02)00165-0. [DOI] [PubMed] [Google Scholar]
- 11.Mulroy SJ, Klassen T, Gronley JK, Eberly VJ, Brown DA, Sullivan KJ. Gait parameters associated with responsiveness to treadmill training with body-weight support after stroke: an exploratory study. Phys Ther. 2010 Feb;90(2):209–223. doi: 10.2522/ptj.20090141. [DOI] [PubMed] [Google Scholar]
- 12.Peterson CL, Hall AL, Kautz SA, Neptune RR. Pre-swing deficits in forward propulsion, swing initiation and power generation by individual muscles during hemiparetic walking. J Biomech. 2010 Aug 26;43(12):2348–2355. doi: 10.1016/j.jbiomech.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Swigchem R, van Duijnhoven HJ, den Boer J, Geurts AC, Weerdesteyn V. Deficits in motor response to avoid sudden obstacles during gait in functional walkers poststroke. Neurorehabil Neural Repair. Mar;27(3):230–239. doi: 10.1177/1545968312462070. [DOI] [PubMed] [Google Scholar]
- 14.Kluding PM, Dunning K, O'Dell MW, et al. Foot drop stimulation versus ankle foot orthosis after stroke: 30-week outcomes. Stroke. 2013 Jun;44(6):1660–1669. doi: 10.1161/STROKEAHA.111.000334. [DOI] [PubMed] [Google Scholar]
- 15.Brincks J, Nielsen JF. Increased power generation in impaired lower extremities correlated with changes in walking speeds in sub-acute stroke patients. Clin Biomech (Bristol, Avon) 2012 Feb;27(2):138–144. doi: 10.1016/j.clinbiomech.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Embrey DG, Holtz SL, Alon G, Brandsma BA, McCoy SW. Functional electrical stimulation to dorsiflexors and plantar flexors during gait to improve walking in adults with chronic hemiplegia. Arch Phys Med Rehabil. 2010 May;91(5):687–696. doi: 10.1016/j.apmr.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 17.Lewek MD, Feasel J, Wentz E, Brooks FP, Jr., Whitton MC. Use of visual and proprioceptive feedback to improve gait speed and spatiotemporal symmetry following chronic stroke: a case series. Phys Ther. 2012 May;92(5):748–756. doi: 10.2522/ptj.20110206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall AL, Bowden MG, Kautz SA, Neptune RR. Biomechanical variables related to walking performance 6-months following post-stroke rehabilitation. Clin Biomech (Bristol, Avon) 2012 Dec;27(10):1017–1022. doi: 10.1016/j.clinbiomech.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Routson RL, Clark DJ, Bowden MG, Kautz SA, Neptune RR. The influence of locomotor rehabilitation on module quality and post-stroke hemiparetic walking performance. Gait Posture. 2013 Mar 12; doi: 10.1016/j.gaitpost.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabriel DA, Kamen G, Frost G. Neural adaptations to resistive exercise: mechanisms and recommendations for training practices. Sports Med. 2006;36(2):133–149. doi: 10.2165/00007256-200636020-00004. [DOI] [PubMed] [Google Scholar]
- 21.Macko RF, Smith GV, Dobrovolny CL, Sorkin JD, Goldberg AP, Silver KH. Treadmill training improves fitness reserve in chronic stroke patients. Arch Phys Med Rehabil. 2001 Jul;82(7):879–884. doi: 10.1053/apmr.2001.23853. [DOI] [PubMed] [Google Scholar]
- 22.Malone LA, Vasudevan EV, Bastian AJ. Motor adaptation training for faster relearning. J Neurosci. 2011 Oct 19;31(42):15136–15143. doi: 10.1523/JNEUROSCI.1367-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azouvi P, Samuel C, Louis-Dreyfus A, et al. Sensitivity of clinical and behavioural tests of spatial neglect after right hemisphere stroke. J Neurol Neurosurg Psychiatry. 2002 Aug;73(2):160–166. doi: 10.1136/jnnp.73.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plummer P, Behrman AL, Duncan PW, et al. Effects of stroke severity and training duration on locomotor recovery after stroke: a pilot study. Neurorehabil Neural Repair. 2007 Mar-Apr;21(2):137–151. doi: 10.1177/1545968306295559. [DOI] [PubMed] [Google Scholar]
- 25.Pohl PS, Duncan PW, Perera S, et al. Influence of stroke-related impairments on performance in 6-minute walk test. J Rehabil Res Dev. 2002 Jul-Aug;39(4):439–444. [PubMed] [Google Scholar]
- 26.Ng SS, Hui-Chan CW. The timed up & go test: its reliability and association with lower-limb impairments and locomotor capacities in people with chronic stroke. Arch Phys Med Rehabil. 2005 Aug;86(8):1641–1647. doi: 10.1016/j.apmr.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Cirstea MC, Levin MF. Improvement of arm movement patterns and endpoint control depends on type of feedback during practice in stroke survivors. Neurorehabil Neural Repair. 2007 Sep-Oct;21(5):398–411. doi: 10.1177/1545968306298414. [DOI] [PubMed] [Google Scholar]
- 28.Kesar TM, Perumal R, Reisman DS, et al. Functional electrical stimulation of ankle plantarflexor and dorsiflexor muscles: effects on poststroke gait. Stroke. 2009 Dec;40(12):3821–3827. doi: 10.1161/STROKEAHA.109.560375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kesar TM, Perumal R, Jancosko A, et al. Novel patterns of functional electrical stimulation have an immediate effect on dorsiflexor muscle function during gait for people poststroke. Phys Ther. 2010 Jan;90(1):55–66. doi: 10.2522/ptj.20090140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kesar TM, Reisman DS, Perumal R, et al. Combined effects of fast treadmill walking and functional electrical stimulation on post-stroke gait. Gait Posture. 2011 Feb;33(2):309–313. doi: 10.1016/j.gaitpost.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Binder-Macleod S, Kesar T. Catchlike property of skeletal muscle: recent findings and clinical implications. Muscle Nerve. 2005 Jun;31(6):681–693. doi: 10.1002/mus.20290. [DOI] [PubMed] [Google Scholar]
- 32.Tilson JK, Sullivan KJ, Cen SY, et al. Meaningful gait speed improvement during the first 60 days poststroke: minimal clinically important difference. Phys Ther. 2010 Feb;90(2):196–208. doi: 10.2522/ptj.20090079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faria CD, Teixeira-Salmela LF, Neto MG, Rodrigues-de-Paula F. Performance-based tests in subjects with stroke: outcome scores, reliability and measurement errors. Clin Rehabil. 2011 Oct 18; doi: 10.1177/0269215511423849. [DOI] [PubMed] [Google Scholar]
- 34.Flansbjer UB, Holmback AM, Downham D, Patten C, Lexell J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J Rehabil Med. 2005 Mar;37(2):75–82. doi: 10.1080/16501970410017215. [DOI] [PubMed] [Google Scholar]
- 35.Kesar TM, Binder-Macleod SA, Hicks GE, Reisman DS. Minimal detectable change for gait variables collected during treadmill walking in individuals post-stroke. Gait Posture. 2011 Feb;33(2):314–317. doi: 10.1016/j.gaitpost.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lord SE, Rochester L. Measurement of community ambulation after stroke: current status and future developments. Stroke. 2005 Jul;36(7):1457–1461. doi: 10.1161/01.STR.0000170698.20376.2e. [DOI] [PubMed] [Google Scholar]
- 37.Ivey FM, Hafer-Macko CE, Macko RF. Task-oriented treadmill exercise training in chronic hemiparetic stroke. J Rehabil Res Dev. 2008;45(2):249–259. doi: 10.1682/JRRD.2007.02.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richards CL, Olney SJ. Hemiparetic gait following stroke: Part II. Recovery and physical therapy. Gait Posture. 1996;1996(4):149–162. [Google Scholar]
- 39.Hall AL, Peterson CL, Kautz SA, Neptune RR. Relationships between muscle contributions to walking subtasks and functional walking status in persons with post-stroke hemiparesis. Clin Biomech (Bristol, Avon) 2011 Jun;26(5):509–515. doi: 10.1016/j.clinbiomech.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Swigchem R, van Duijnhoven HJ, den Boer J, Geurts AC, Weerdesteyn V. Deficits in motor response to avoid sudden obstacles during gait in functional walkers poststroke. Neurorehabil Neural Repair. 2013 Mar-Apr;27(3):230–239. doi: 10.1177/1545968312462070. [DOI] [PubMed] [Google Scholar]
- 41.Jonkers I, Delp S, Patten C. Capacity to increase walking speed is limited by impaired hip and ankle power generation in lower functioning persons post-stroke. Gait Posture. 2008 Sep 10; doi: 10.1016/j.gaitpost.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cruz TH, Lewek MD, Dhaher YY. Biomechanical impairments and gait adaptations post-stroke: multi-factorial associations. J Biomech. 2009 Aug 7;42(11):1673–1677. doi: 10.1016/j.jbiomech.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tyrell CM, Roos MA, Rudolph KS, Reisman DS. Influence of systematic increases in treadmill walking speed on gait kinematics after stroke. Phys Ther. 2011 Mar;91(3):392–403. doi: 10.2522/ptj.20090425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Video abstract