Abstract
The aim of this study was to determine the effect of problem-solving education on self-efficacy and distress in informal caregivers of allogeneic hematopoietic stem cell transplantation patients. Patient/caregiver teams attended three 1-hour problem-solving education sessions to help cope with problems during hematopoietic stem cell transplantation. Primary measures included the Cancer Self-Efficacy Scale–transplant and Brief Symptom Inventory–18. Active caregivers reported improvements in self-efficacy (p < 0.05) and distress (p < 0.01) post-problem-solving education; caregiver responders also reported better health outcomes such as fatigue. The effect of problem-solving education on self-efficacy and distress in hematopoietic stem cell transplantation caregivers supports its inclusion in future interventions to meet the multifaceted needs of this population.
Keywords: anxiety, behavioral medicine, cancer, coping, distress, family, health behavior, intervention
Introduction
Allogeneic hematopoietic stem cell transplantation (A-HSCT) generates multiple symptoms and problems that can vary in complexity (Goldman and Horowitz, 2002; Thomas and Blume, 1999). The challenges of this intense procedure and its recovery mandate the presence of an informal caregiver (CG) who can provide, at minimum, instrumental support to the A-HSCT patient. Without a CG’s support, the transplant experience and patient outcomes may be negatively affected (Foster et al., 2004, 2009; Frey et al., 2002). Although serving as the CG for a loved one may yield benefits, there are multiple physical, social, and emotional problems reported with undetermined effects on patient and CG outcomes (Beattie and Lebel, 2011; Bevans and Sternberg, 2012; Gemmill et al., 2011).
Intervention research to improve quality of life and psychosocial outcomes for CGs of cancer patients has been well reviewed (Northouse et al., 2010; Waldron et al., 2012). This evidence suggests that interventions targeting problem-solving skills can improve CG outcomes, yet few studies have been published testing interventions with this component in CGs of A-HSCT patients (Bevans et al., 2010).
Social problem-solving therapy (SPST) is a specific established cognitive–behavioral therapy defined as “a meta-cognitive process by which individuals understand the nature of problems in living and direct their efforts at altering the difficult nature of the situations themselves, their reaction to them, or both” (Nezu et al., 1999). Problem-solving therapy can improve psychological outcomes in individuals with mental health disorders (e.g. depression) (Dirmaier et al., 2012), spinal cord injury (Dorstyn et al., 2011), parents of children with chronic illness (Eccleston et al., 2012), and cancer patients (Allen et al., 2002; Nezu et al., 2003).
Problem-solving skills have long been recognized as an important resource for successful coping (Lazarus and Folkman, 1984), and successful coping can enhance an individual’s self-efficacy (SE) (Bandura, 1986). CG SE has been shown to improve the following interventions directed at helping cancer (Northouse et al., 2007) and noncancer (Gitlin et al., 2001, 2008) CGs and may be a factor in determining one’s response to an intervention (Rabinowitz et al., 2006) and improving CG health (Harmell et al., 2011; Rabinowitz et al., 2007).
The purpose of this study was to determine the effect of an individualized problem-solving education (PSE) intervention, guided by the principles of SPST, on SE and distress in CGs of patients receiving an A-HSCT. The primary hypothesis was that CGs of A-HSCT patients would report improved SE and distress following a PSE intervention. Although the intervention was provided to the patients and their CGs, the “unit of analysis” was the CG due to the known complexities of the A-HSCT and its impact on patient participation.
Methods
Study design and participants
The study employed a longitudinal repeated measure design in which patients and their CGs at a single center were scheduled for three PSE intervention sessions (Figure 1). This study was approved by the National Heart, Lung and Blood Institute intramural Institutional Review Board, and all patients and CGs provided written informed consent before participation. Potential patient participants were approached during their inpatient or outpatient eligibility screening for an A-HSCT. Consecutive patients who were preparing for their first A-HSCT and were literate in English were approached. To be eligible, the patient needed at least one active CG— someone expecting to spend ≥6 hours/day, for most days during the study period with the patient. If a “team” of active CGs was identified, up to three CGs per patient were invited to participate. CGs were eligible if they were also literate in English. If a CG declined participation, the patient was not enrolled in this study. A baseline-only survey was offered to those who declined because of perceived burden regarding session attendance.
Figure 1.
Study design.
HSCT: hematopoietic stem cell transplantation; PSE: problem-solving education; TP: time point.
Intervention
The intervention originated from the prepared family CG model that was based on SPST (Houts et al., 1996; Loscalzo and Bucher, 1999). The objective was to empower cancer patients and their CGs to cope with day-to-day problems by applying a systematic problem-solving framework (Bucher et al., 2001; McMillan et al., 2006; Meyers et al., 2011). The intervention was administered as three 1-hour sessions (dose) during the first 3 weeks of transition from inpatient to outpatient care (Figure 1). Feedback from A-HSCT patients and CGs who participated in a pilot study (Bevans et al., 2010) indicated that a session prior to transplant was less helpful due to the complexities immediately prior to admission. Therefore, the first session was at initial hospital discharge following A-HSCT. The second and third sessions were scheduled 1 and 3 weeks following initial hospital discharge, respectively. To support attendance, interventions were incorporated into routine care for the patient and their CGs in a private setting on the inpatient or outpatient unit.
The intervention (Appendix 1) was administered face to face with at least one member of the team present. If CG participants were unable to attend in person, they were contacted by phone to join via conference call. If the first session was through conference call, the study materials (Appendix 2) were mailed to the participant. The trained interventionists (nurses and social workers) were guided by a session script. At least one complete case per interventionist was audiotaped for quality control and training purposes. Participants received no financial compensation for their participation.
Measures
Demographic factors were obtained on the CG and patient participants. These included age, gender, race, ethnicity, marital status, education, employment status (CG only), number of CGs, CG and care recipient relationship, and total time of caregiving (number of days as an active CG between the date of hospital discharge and end of study). Clinical and transplant-specific factors were collected on the A-HSCT patient participants including primary disease, conditioning intensity, stem cell source, human leukocyte antigen (HLA) disparity/donor, and performance status (Eastern Cooperative Oncology Group (ECOG)).
Study questionnaires were administered at three time-points: (1) pre-HSCT, (2) at initial hospital discharge (both preintervention), and (3) at 6 weeks after the initial discharge (Figure 1) to patients and their CGs, with the exception of the Health-Promoting Lifestyle Profile–II (HPLP-II) that was inappropriate for A-HSCT inpatients. Two preintervention surveys were collected due to the variability in time between initial enrollment and time of discharge. If the length of time between the first and second preintervention surveys was less than 2 weeks, then the second survey was omitted. The survey was mailed to CGs who were not available in person at the time of data collection.
The Cancer Self-Efficacy Scale–transplant (CASE-t) measures confidence in managing the impact of a stem cell transplant. This questionnaire was adapted (with author permission) from a version developed originally for general cancer patients and their CGs (Lewis, 1996). Response options range from 0 to 10 where higher scores (range = 0–170) indicate higher levels of SE. The internal consistency reliability for the total CASE-t scale in this study was 0.96 for CGs and 0.96–0.97 for patients.
The Brief Symptom Inventory–18 (BSI-18) measures psychological distress using a response from 0 to 4; higher scores (range = 0–90) indicate higher levels of psychological distress (Derogatis, 1994). The internal consistency reliability estimates for the Global Severity Index (GSI) in this study ranged from 0.84 to 0.87 for CGs and from 0.82 to 0.88 for A-HSCT patients.
The HPLP-II measures the frequency of healthy behaviors where higher scores (range = 52–208) indicate more frequent engagement in health behaviors (Noble Walker et al., 1987). Cronbach’s alpha coefficients of internal consistency reliability in this study ranged from 0.94 to 0.96 for CGs.
The Pittsburgh Sleep Quality Index (PSQI) measures subjective sleep quality (Buysse et al., 1989) producing a global score (range = 0–21) where higher scores indicate worse sleep quality. Internal consistency reliability estimates from this study ranged from 0.63 to 0.71 in CGs.
The Multidimensional Fatigue Symptom Inventory–Short Form (MFSI-SF) measures fatigue where higher scores (range = −24 to 90) indicate more fatigue (Stein et al., 2004). Estimates of internal consistency reliability for this study range from 0.75 to 0.79 for CGs.
The Family Caregiving Inventory mutuality scale measures relationship quality where higher mean scores (range = 0–4) indicate higher levels of mutuality (Archbold, 1990). Estimates of internal consistency reliability for this study range from 0.93 to 0.97 for CGs.
Analyses
Sample size determination was based on the expected change in the SE score from published data (Northouse et al., 2007). To detect a medium effect size of 0.30 with 80 percent power and a one-sided paired t-test using a 0.05 level of significance, a sample of 71 active CGs was needed. Based on a previous pilot study (Bevans et al., 2010), about one-third of the A-HSCT patients had >1 CG. Therefore, it was estimated that approximately 50 transplant patients were needed to attain a sample of 71 CGs. Significance was set at α = 0.05. Statistical analyses were done using SPSS version 19.
As a single group pretest and posttest design, alternative explanations were considered a priori for any observed effect in this complex clinical environment. Therefore, multiple CG and patient characteristics were collected for consideration in the final analyses. Prior to the primary analyses, the CG’s role as an active CG was validated since study participation was determined prior to A-HSCT. Additional factors that were considered for possible influence on the outcomes were the length of time serving as a CG and the number of sessions attended. The length of time serving as a CG might be a proxy for experience and contribute to the confidence of a CG. The number of sessions that the participants attend might create a dose effect where more sessions yield a larger dose. Therefore, the relationship between SE and distress with length of caregiving and the number of sessions attended was examined and controlled in the analysis if warranted.
The initial data analysis consisted of examining the frequency distributions for all variables at each time point and computing descriptive statistics appropriate for the level of measurement. Missing data were minimal (<5%) and managed in accordance with the guidelines of each questionnaire individually. Only participants with preintervention and postintervention scores were analyzed. A paired t-test was used to compare the two preintervention measures prior to completing the primary analysis. If no significant difference was found between the scores, the first score was used to test the hypotheses; the second score, immediately prior to the study intervention, was used if the difference was significant. Paired t-tests were conducted to assess differences between the preintervention and postintervention scores for CGs and patients. Cohen’s d was calculated to determine intervention effect size.
To examine potential predictors for the primary outcomes of SE and distress, selected baseline variables (gender, relationship to patient (spouse/nonspouse), CG team status (sole/team), education, age, mutuality, and SE or distress (aligned with the outcome)) were regressed in a stepwise approach on postintervention CASE-t and BSI scores separately.
An exploratory analysis was conducted to characterize health outcomes of matched CGs and A-HSCT patients based on the determination of their response to the intervention. Separate responder groups were determined based on the degree of their improvement in SE or distress scores. Chi-square analysis or an independent t-test was used, depending on the level of measurement, to identify the outcomes that might differ between responders and nonresponders.
Results
Sample description
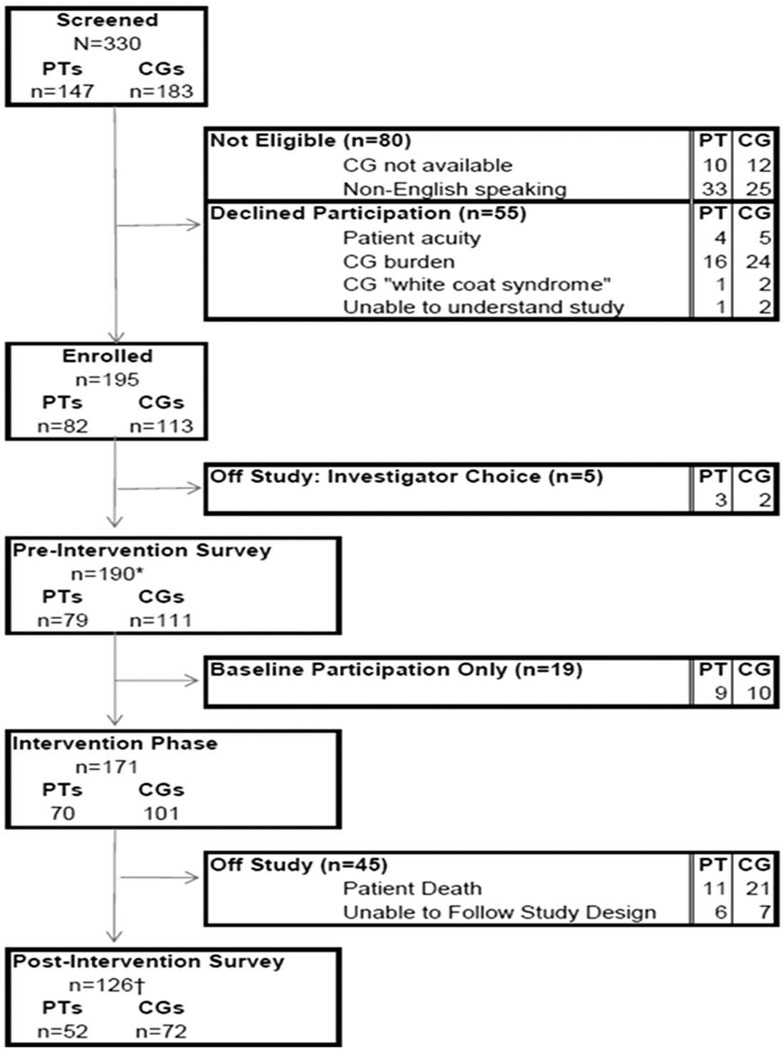
Participants were accrued from October 2008 through September 2010 (Figure 2). Three hundred and thirty participants were screened for study participation with 195 participants enrolled on study (CGs, n = 113; patients, n = 82). Five participants were removed from this study following enrollment when the CG retracted their interest in study participation. Nineteen participants initially declined participation in the study related to scheduling difficulties but accepted a baseline survey only participation option. Of the 171 participants (CGs, n = 101; patients, n = 70) who participated in the study intervention phase, 126 participants (CGs, n = 71; patients, n = 55) completed all study procedures (Table 1). There were no significant differences between the CG participants who completed baseline-only participation and those who completed study participation on demographic variables (data not shown). Relative to the primary study outcomes, CGs who declined to participate in the study intervention reported significantly higher scores on the BSI-18 (14.6 ± 12.7 vs 6.9 ± 6.6) and lower scores on the CASE-t (128.0 ± 40 vs 150.6 ± 17) compared to participants who completed the study intervention.
Figure 2.
Study enrollment and attrition (<2 weeks between the first and second preintervention surveys (n = 5)). The symbols “*” and “†” denote four PTs missing the preintervention survey and one PT/CG set missing due to CG incomplete data, respectively. PT: patient; CG: caregiver.
Table 1.
Caregivers demographic and clinical characteristics at study enrollment
| Caregivers (n = 72) |
Active caregivers (n = 66) |
|
|---|---|---|
| n (%) | n (%) | |
| Age, years, mean (±SD) | 53.3 (±12.6) | 52.8 (±12.9) |
| Gender, female | 52 (72.2) | 47 (71.2) |
| Race/ethnicity | ||
| White | 56 (77.8) | 50 (75.8) |
| African-American | 7 (9.7) | 7 (10.6) |
| Asian | 2 (2.8) | 2 (3.0) |
| Hispanic or Latino | 5 (6.9) | 5 (7.6) |
| Education | ||
| Bachelor’s or graduate degree | 32 (44.4) | 30 (45.5) |
| Employment status | ||
| Full-time/part-time | 29 (40.3) | 27 (40.9) |
| Relationship to patient | ||
| Spouse | 34 (47.2) | 34 (51.5) |
| Family member/nonspouse | 32 (44.4) | 27 (40.9) |
| Friend | 6 (8.3) | 5 (7.6) |
| Caregiver team | ||
| Sole caregiver | 36 (50.0) | 35 (53.0) |
| Caregiver team | 36 (50.0) | 31 (47.0) |
| Total caregiving days, mean (±SD) | 32.4 (±15.8) | 35.4 (±12.9) |
| Chronic health problems ≥ 1 | 46 (63.9) | 44 (66.7) |
SD: standard deviation.
The majority of CGs who participated in the study intervention were female (72.2%) and white (77.8%) with an average age of 53 years (standard deviation (SD) = 12.6 years). One-half served as the sole CG (50%). Sixty-six of the 72 CG participants (92%) served as active CGs during the study and are also characterized in Table 1. Patients (n = 53) who completed the study were on average 45.4 years (SD = 14.4) and predominately male (n = 35, 66.0%) (Table 2). Session characteristics are presented in Table 3 for all participants.
Table 2.
Transplant patient’s demographic and clinical characteristics at study enrollment
| Patientsa (n = 53) |
|
|---|---|
| n (%) | |
| Age, years, mean (±SD) | 45.4 (±14.4)b |
| Gender, female | 18 (34.0) |
| Race | |
| White | 37 (69.8) |
| African American | 7 (13.2) |
| Other | 9 (17.0) |
| Ethnicity | |
| Hispanic or Latino | 5 (9.4) |
| Education | |
| Bachelor’s or graduate degree | 28 (53.9)b |
| Baseline ECOG | |
| 0 | 22 (41.5) |
| 1 | 30 (56.6) |
| 2 | 1 (1.9) |
| Primary disease | |
| Lymphoma/MM | 27 (50.9) |
| Nonmalignant disease | 6 (11.3) |
| CLL/CML | 8 (15.1) |
| ALL/AML | 5 (9.4) |
| MDS | 4 (7.5) |
| Solid tumor | 2 (3.8) |
| CML and Hodgkin’s (dual diagnosis) | 1 (1.9) |
| Conditioning intensity | |
| Reduced intensity conditioning | 45 (86.5)b |
| Myeloablative | 7 (13.5)b |
| Stem cell source | |
| Peripheral blood | 51 (98.1)b |
| Cord | 1 (1.9)b |
| HLA disparity/donor | |
| HLA—well or partially matched related | 29 (55.8)b |
| HLA—well or partially matched unrelated | 20 (38.5)b |
| HLA—mismatched related | 3 (5.8)b |
| Disease status | |
| CR | 10 (18.9) |
| PR | 8 (15.1) |
| Stable | 14 (26.4) |
| PD | 12 (22.6) |
| Severe diseasec | 9 (17.0) |
MM: multiple myeloma; CLL: chronic lymphocytic leukemia; AML: acute myeloid leukemia; MDS: myelodysplastic syndrome; CML: chronic myelogenous leukemia; ALL: acute lymphoblastic leukemia; ECOG: Eastern Cooperative Oncology Group; HLA: human leukocyte antigen; SD: standard deviation; CR: complete remission; PR: partial remission PD: progressive disease.
Patients (n = 53) correspond to caregivers (n = 73). 52 patients correspond to active caregivers (n = 66).
n = 52.
Nonmalignant diseases.
Table 3.
Session characteristics
| Session 1 | Session 2 | Session 3 | |
|---|---|---|---|
| Visit type, n (%) | |||
| Inpatient | 20 (39.2) | 40 (78.4) | 13 (25.0) |
| Outpatient | 31 (60.8) | 11 (21.6) | 39 (75.0) |
| Length, mean (SD), minimum | 52.4 (11.5) | 43.5 (12.6) | 39.0 (15.2) |
| Method, n (%) | |||
| In-person | 109 (88.6) | 97 (79.5) | 91 (16.4) |
| Phone | 7 (5.7) | 13 (10.7) | 11 (9.0) |
| Attendance, n (%) | |||
| Patient | 48 (96.0) | 46 (92.0) | 45 (90.0) |
| Caregiver | 67 (94.4) | 62 (88.6) | 56 (80.0) |
| Satisfactiona, mean (SD) | |||
| Patient | 4.4 (0.6) | ||
| Caregiver | 4.5 (0.7) | ||
SD: standard deviation.
Caregiver, n = 72; patient, n = 52.
Range: from 1 (not satisfied) to 5 (very satisfied); from exit interview.
Self-Efficacy
The two baseline CASE-t scores for CGs did not differ (M = 147.8, SD = 2.34 vs M = 150.2, SD = 2.35, t = 1.31, p = 0.19); therefore, the first baseline was used as the preintervention score. Also, there was no correlation between CASE-t scores and the length of time caregiving (preintervention: r = 0.03, p = 0.82; postintervention: r = −0.07, p = 0.56) or the number of sessions attended (r = −0.01, p = 0.96; r = 0.04, p = 0.78, respectively). CGs, who were active during the study period and completed study participation, reported improvement in post-PSE intervention SE scores (Table 4). Patients, who were matched with active CGs who completed the study, reported no significant change in post-PSE intervention CASE-t scores (Table 4).
Table 4.
Comparison of self-efficacy scores preintervention and postintervention
| Active caregivers (n = 65) |
Patients (n = 50) |
|||
|---|---|---|---|---|
| Preinterventiona | Postintervention | Preinterventionb | Postintervention | |
| Mean (±SD) | 149.7 (±16.8) | 154.0 (±15.0) | 152.0 (±16.2) | 151.2 (±17.1) |
| Mean difference (SE) | 4.3 (1.9) | 0.76 (2.13) | ||
| t-score | 2.25 | 0.36 | ||
| p-value | 0.028 | 0.72 | ||
| Cohen’s d | 0.27 | 0.05 | ||
SD: standard deviation; SE: standard error; CASE-t: Cancer Self-Efficacy Scale–transplant.
The number of sessions attended was not related to the CASE-t scores preintervention and postintervention (r = 0.24, p = 0.10; r = −0.13, p = 0.39, respectively).
Pretransplant baseline.
Posttransplant baseline.
The CGs’ postintervention CASE-t scores were regressed on selected baseline variables to examine possible predictors. With all potential predictors included, the model was significant (F = 25.01, df = 1, 63, p < 0.001) with 28 percent of the variability in postintervention CASE-t explained by the model (adjusted R2 = 0.28). Baseline SE (B = 0.48, p < 0.001) was the only significant predictor in the model: active CGs with more SE at baseline tended to have greater SE postintervention.
CG SE scores ranged from −40 to 39 (M = 4.0, SD = 15.45). Responders were categorized by a positive change score or 5 points above the mean (CASE-t score > 8; n = 23), while nonresponders were identified by a negative change score or 5 points below the mean (CASE-t score < 0; n = 20) (Table 5). The mean CASE-t score pre-HSCT for SE responders was significantly lower than nonresponders (p < 0.001). CG responders also reported better health outcomes compared to nonresponders, although the differences were not statistically significant. Of interest, the A-HSCT patients of the CG nonresponder were more likely to be inpatient, a proxy for acuity, at the end of the study period.
Table 5.
Caregiver and patient outcomes by caregiver response category
| Self-efficacya |
Distressb |
|||
|---|---|---|---|---|
| Responders (n = 23) |
Nonresponders (n = 20) |
Responders (n = 35) |
Nonresponders (n = 17) |
|
| M (±SD) | M (±SD) | M (±SD) | M (±SD) | |
| Caregiver | ||||
| Fatigue | 2.65 (±18.6) | 6.35 (±12.9) | 1.26 (±14.1) | 10.65 (±16.5) |
| Sleep | 5.71 (± 3.8)c | 6.71 (±2.9)d | 5.37 (±3.3) | 6.19 (± 2.8) |
| Health behaviors | 2.70 (±0.5) | 2.42 (±0.5) | 2.68 (±0.5) | 2.48 (±0.4) |
| Baseline CASE-t | 138.5 (±18.1) | 156.5 (±10.2)e | 10.1 (±6.9) | 5.5 (±4.3)f |
| Patient (end of study) | ||||
| Readmissions daysg | 5.0 (±5.4) | 4.1 (±4.5) | 3.3 (±4.7) | 5.2 (±5.2) |
| n (%) | n (%) | n (%) | n (%) | |
| Progressive disease | 2 (9) | 3 (15) | 4 (11.4) | 4 (23.5) |
| Inpatient (yes) | 5 (22) | 10 (50) | 6 (17.7) | 7 (41.2) |
SD: standard deviation; CASE-t: Cancer Self-Efficacy Scale–transplant.
Responders = scores > 5 above the mean difference; nonresponders = scores > 5 below the mean difference.
Responders = mean difference scores < 0; nonresponders = mean difference scores > 0.
n = 21.
n = 17.
Compared to responders, t = 4.08 (35), p < 0.001.
Compared to responders, t = −2.52 (50), p = 0.015.
Readmission days = number of inpatient days from initial discharge to end of study.
Distress
The two baseline BSI-18 scores for CGs did not differ (M = 8.2, SD = 0.97 vs M = 7.2, SD = 0.95, t = 1.15, p = 0.25); therefore, the first baseline was used as the preintervention distress score. Also, there was no significant correlation between preintervention and postintervention BSI-18 scores and the length of time caregiving (r = −0.002, p = 0.98; r = −0.12, p = 0.34, respectively) or the number of sessions attended (r = 0.01, p = 0.94; r = 0.01, p = 0.94, respectively). CGs, who were active during the study period and completed study participation, reported improvement in post-PSE intervention BSI-18 scores (Table 6). Patients, who were matched with active CGs who completed the study, reported a significant decrease in post-PSE intervention BSI-18 scores (Table 6).
Table 6.
Comparison of distress scores preintervention and postintervention
| Active caregivers (n = 65) |
Patients (n = 50) |
|||
|---|---|---|---|---|
| Preinterventiona | Postintervention | Preinterventiona | Postintervention | |
| Mean (±SD) | 7.4 (±6.7) | 5.0 (±5.2) | 8.5 (±8.2) | 5.8 (±5.8) |
| Mean difference (SE) | 2.4 (0.76) | 2.7 (1.1) | ||
| t-score | 3.14 | 2.49 | ||
| p-value | 0.003 | 0.016 | ||
| Cohen’s d | 0.40 | 0.38 | ||
SD: standard deviation; SE: standard error; BSI-18: Brief Symptom Inventory–18.
The number of sessions attended was not related to the preintervention and postintervention BSI-18 scores (r = −0.01, p = 0.95; r = −0.10, p = 0.49, respectively).
Pretransplant baseline.
The postintervention BSI-18 scores were regressed on selected baseline variables to examine possible predictors of lower BSI scores. With all potential predictors included, the model was significant (F = 21.71, df = 1, 63, p < 0.001) with 26 percent of the variability in postintervention BSI explained by the set of predictors (adjusted R2 = 0.26). Baseline distress (B = 0.39, p < 0.001) was the only predictor that was significant in the model where active CGs with less distress at baseline tended to have less distress postintervention.
CG distress scores ranged from −28 to 10 (M = −2.37, SD = 6.09). Responders were categorized by a negative change scores indicating less distress (BSI-18 score < 0; n = 35) while nonresponders were identified by a positive change score (BSI-18 score > 0; n = 17) (Table 5). The mean CASE-t score pre-HSCT for distress responders was significantly lower than nonresponders (p = 0.015). CG responders also reported better health outcomes compared to nonresponders, although the differences were not statistically significant except for fatigue. CG responders had lower mean postintervention MFSI scores compared to nonresponders (1.3 vs 10.6; t = 2.12, p = 0.04). Analysis of the sub-scales of MFSI indicated a significantly lower mean score on the emotional subscale postintervention for responders compared to nonresponders (3.6 vs 6.3, t = 2.54, p = 0.01). Of interest, the A-HSCT patients of the CGs who were defined as nonresponder relative to distress level had more readmission days between discharge and end of study. Readmission following initial hospital discharge is common after A-HSCT (Bejanyan et al., 2012). However, the evidence for CGs’ influence on readmission is limited, with only one study suggesting that CG difficulty or absence predicts readmission in a general cancer sample (Weaver et al., 2006).
Discussion
CGs of individuals with advanced cancer or those receiving intense cancer treatments juggle the complex needs of their loved one along with the resulting life adjustments for self and their family. Although most CGs would have it no other way, this burden is associated with increased emotional distress (Ferrario et al., 2003; Kim et al., 2005; Northouse et al., 2002) often associated with a lack of confidence in their new role (Nijboer et al., 1999). The findings from this study suggest that informal CGs who are providing direct support to A-HSCT patients report an increase in SE and reduction in distress after participating in a PSE intervention. This supports previous research demonstrating that interventions including a problem-solving component can preserve or improve cancer CG outcomes (Demiris et al., 2010; Meyers et al., 2011; Northouse et al., 2010). This positive effect, however, seems less evident when the burden of cancer care (e.g. hours/day) is less and in noncancer caregiving populations (Hartmann et al., 2010; Meyers et al., 2011). SE and distress prior to A-HSCT might also be helpful to identify individuals at risk and who would benefit from a problem-solving intervention. The participants in this study who reported benefit from the intervention (responders) reported less confidence and high distress prior to A-HSCT. Providing an intervention earlier in the treatment trajectory may provide for improvement in CG outcomes (Choi et al., 2012).
The objective of this intervention was to teach a systematic approach to problem-solve the complexities of an intense treatment. Relative to other published CG interventions with a problem-solving component (Northouse et al., 2010), the dose of this intervention was rather low (3 hours), which was designed to “fit” an already busy phase where the caregiving burden shifts to the informal CG during the transition from inpatient to outpatient care. Previous research suggests that skill training focused closer to the time of application along with concise materials, such as the brochure developed for this study, might improve clinical effectiveness (Bevans et al., 2010). Despite the low dose, however, the burden to the health-care provider was not insignificant, not an uncommon finding for multisession interventions (Hack et al., 2011). Although the time commitment by the providers was notable, the length of each session did decrease across the series, suggesting that the problem-solving method Creativity, Optimism, Planning, and Expert Information (COPE) was readily understood. COPE was also well received across intervention formats. Attention to the burden of attendance for the CG was considered a priori allowing for teleconferencing as well as face-to-face attendance.
Interventions for CGs of cancer patients have primarily focused on improving CG outcomes. However, there have been a few studies of CG interventions with a problem-solving component that evaluated patient outcomes. Overall, the studies to date have shown no conclusive evidence that improving CG skills will improve the care patient’s outcomes (Meeker et al., 2011). In a randomized controlled trial (RCT), CGs who received three telephone encounters to improve problem-solving skills in the assistance of managing patient’s symptoms, there was no effect on symptom assistance at 10 weeks (Sherwood et al., 2011). In the same study, CGs with less depression in the problem-solving arm were more likely to provide assistance at 10 weeks, suggesting that improving CG distress may improve the patient’s management. Although the link between CG response and patient outcomes was not the primary objective of this study, both CG and patient participants reported improved distress. Despite these findings, the patients for the CG nonresponders had an acuity that was consistently worse than that of the responders. The relationship between patient acuity and CG outcomes has not been well explored. These findings suggest that exploring this relationship might be informative.
This study offers some preliminary evidence that improving CG distress and SE may result in improved CG health outcomes such as lower levels of fatigue, improved sleep, and health prevention behavior choices, for example, staying physically active and eating well. This has been documented previously where CGs with higher levels of SE have improved health behaviors and physical health (Rabinowitz et al., 2007; Zeiss et al., 1999). In addition to health behaviors directed at illness prevention, CGs often have their own health concerns that may or may not be easy to manage while their focus is directed at the care recipient (Northouse et al., 2010). If CGs of patients experiencing complex treatments become more confident in their role, there may be a shift in their perception of caregiving as a barrier to self-care creating an avenue for behaviors that might preserve their own health.
Conclusions
A-HSCT CGs are involved in complex patient care. A systematic process of problem-solving to manage the complexities of the experience can help them cope with this significant life stressor and may preserve or improve their general health. Improving the effect of the intervention may be possible with an expanded focus on other dimensions such as social support and stress reduction. However, attention must be given to the translation of any intervention into practice that is complicated during an intense treatment with multiple transition points. To design the most effective intervention, determining a minimally effective dose and method for intervention administration would be valuable. This would be the foundation for striking a balance between the services provided by the health-care team and self-care strategies that can be reinforced independently by a CG. The variability in how family rally to provide support, alone or within a network, needs to be considered in the provision of services to HSCT patients and their CGs. The needs of CGs are multifaceted, and the services provided to them should be designed with this in mind (Northouse et al., 2010; Waldron et al., 2012).
Future research should include the development of a multisite RCT to validate the results of this single-center, single-arm study that has limited generalizability beyond this sample. In addition, the inclusion of CGs from diverse cultures through purposive sampling of ethnic and racial minorities would also strengthen the future research. Despite the limited generalizability, the implementation of this study in a clinical setting, with members of the interdisciplinary clinical team, reveals multiple critical elements that should be considered. A study such as this provides insight regarding the feasibility and acceptability of this or similar patient and CG interventions for translation into practice.
Acknowledgements
We thank the NHLBI and NCI transplant teams, Olena Prachenko, Julie Kohn, Stephen Klagholz, and the staff of 3NE, 3SES, OP7, and OP12.
Funding
This research received was funded by the National Institutes of Health Intramural Research Program.
Appendix 1
Problem-solving education intervention content
- Introduction
- The interventionist acknowledges and thanks the participants for participating in the intervention.
- The interventionist introduces “problems” as a common part of life. Highlighting that when people are under stress, it is more difficult to solve problems effectively, especially when confronted with complex situations.
- Introduce Creativity, Optimism, Planning, and Expert Information (COPE)
- COPE is a systematic problem-solving method with the objective to help participants solve problems better and reduce stress levels during difficult times in life.
- Key points for COPE method are as follows:
- Teamwork (patient and caregiver (CG)/family).
- Systematic method to aid in memory of the process.
- Provide the COPE pamphlet that summarized the COPE method of problem-solving. Each section concisely describes elements to consider when approaching problems. For example, “C” Creativity prompts readers to find creative ways to approach unique situations and lists tips for thinking creatively when faced with obstacles.
- Review the process of problem-solving using COPE
- To apply the model, the acronym is applied backward: Expert, Planning, Optimism, and Creativity Information (EPOC)
- One starts with the “E” Expert Information in order to obtain the correct information that is essential to successful problem-solving.
- With the expert information, a feasible “P” Plan is developed
- “O” Optimism and “C” Creativity are attitudes that are important in moving a plan forward and being aware that adjustments may be required.
- Role play the application of COPE
- To apply the model, the patient/CG (team) identifies a problem; a problem that is currently or likely to cause distress if not solved. The interventionist provides assistance to define the problem clearly and to define a desired outcome. If multiple problems are shared, one is selected and the participants are encouraged to document the others for a later time. If a problem is not easily identified, one is selected that is common for the population/participants (e.g. communication with healthcare team). The model continues with a review of each element specific to the problem identified:
- Expert Information. What is known about the problem? The participants determine whether professional help is required and where to obtain “expert information,” for example, from physicians, nurses, and legitimate websites. The interventionist reinforces that the participants have expertise as well.
- Planning. The participants brainstorm creative solutions without judgment. The interventionist encourages the participants to write the plan on paper, to provide a record of the plan, and assists with identifying any possible obstacles.
- Optimism. A goal for the participants is to foster an attitude of hopefulness to solve the problem grounded in the reality that a developed plan is critical for successful problem-solving.
- Creativity. The participants are encouraged to be creative in planning and to allow for adjustments if obstacles arise.
- Close and summarize the session
- In summary, the participants teach the interventionalist the COPE model reviewing the major principles and elements of the COPE method.
Appendix 2
Intervention materials, including a padfolio and study pamphlet, were provided to study participants during the first session. The padfolio provided a place for participants to organize their thoughts and ideas related to their transplant experience and study participation. The study pamphlet described the “COPE” method of problem-solving and was used throughout the intervention phase to guide study participants. The “COPE” method of problem-solving was originally described in “The Home Care Guide for Cancer, How to care for family and friends at home” (Houts, 1994). Participant feedback (Bevans et al., 2010) indicated that the “The Home Care Guide for Cancer” was cumbersome, and an abbreviated version would be more meaningful. The study pamphlet was developed by the investigators and adapted with permission from Dr. Peter Houts who offered a review of the document along with a purposeful group of hematopoietic stem cell transplantation (HSCT) patients and their CGs.
Footnotes
Conflict of interest
No conflicting interests.
References
- Allen SM, Shah AC, Nezu AM, et al. A problem-solving approach to stress reduction among younger women with breast carcinoma: A randomized controlled trial. Cancer. 2002;94:3089–3100. doi: 10.1002/cncr.10586. [DOI] [PubMed] [Google Scholar]
- Archbold PG. Mutuality and preparedness as predictors of caregiver role strain. Research in Nursing & Health. 1990;13:375–384. doi: 10.1002/nur.4770130605. [DOI] [PubMed] [Google Scholar]
- Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall, Inc; 1986. [Google Scholar]
- Beattie S, Lebel S. The experience of caregivers of hematological cancer patients undergoing a hematopoietic stem cell transplant: A comprehensive literature review. Psycho-Oncology. 2011;20:1137–1150. doi: 10.1002/pon.1962. [DOI] [PubMed] [Google Scholar]
- Bejanyan N, Bolwell BJ, Lazaryan A, et al. Risk factors for 30-day hospital readmission following myeloablative allogeneic hematopoietic cell transplantation (allo-HCT) Biology of Blood and Marrow Transplantation: Journal of the American Society for Blood and Marrow Transplantation. 2012;18:874–880. doi: 10.1016/j.bbmt.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Bevans M, Sternberg EM. Caregiving burden, stress, and health effects among family caregivers of adult cancer patients. JAMA: The Journal of the American Medical Association. 2012;307:398–403. doi: 10.1001/jama.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevans M, Castro K, Prince P, et al. An individualized dyadic problem-solving education intervention for patients and family caregivers during allogeneic hematopoietic stem cell transplantation: A feasibility study. Cancer Nursing. 2010;33:E24–E32. doi: 10.1097/NCC.0b013e3181be5e6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher JA, Loscalzo M, Zabora J, et al. Problem-solving cancer care education for patients and caregivers. Cancer Practice. 2001;9:66–70. doi: 10.1046/j.1523-5394.2001.009002066.x. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Choi CW, Stone RA, Kim KH, et al. Group-based trajectory modeling of caregiver psychological distress over time. Annals of Behavioral Medicine: A Publication of the Society of Behavioral Medicine. 2012;44:73–84. doi: 10.1007/s12160-012-9371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demiris G, Oliver DP, Washington K, et al. A Problem Solving Intervention for hospice caregivers: A pilot study. Journal of Palliative Medicine. 2010;13:1005–1011. doi: 10.1089/jpm.2010.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR. Brief Symptom Inventory (BSI) Administration, Scoring, and Procedures Manual. 3rd edn. Minneapolis, MN: National Computer Systems; 1994. [Google Scholar]
- Dirmaier J, Steinmann M, Krattenmacher T, et al. Non-pharmacological treatment of depressive disorders: A review of evidence-based treatment options. Reviews on Recent Clinical Trials. 2012;7:141–149. doi: 10.2174/157488712800100233. [DOI] [PubMed] [Google Scholar]
- Dorstyn D, Mathias J, Denson L. Efficacy of cognitive behavior therapy for the management of psychological outcomes following spinal cord injury: A meta-analysis. Journal of Health Psychology. 2011;16:374–391. doi: 10.1177/1359105310379063. [DOI] [PubMed] [Google Scholar]
- Eccleston C, Palermo TM, Fisher E, et al. Psychological interventions for parents of children and adolescents with chronic illness. Cochrane Database of Systematic Reviews. 2012;8 doi: 10.1002/14651858.CD009660.pub2. CD009660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario SR, Zotti AM, Massara G, et al. A comparative assessment of psychological and psychosocial characteristics of cancer patients and their caregivers. Psycho-Oncology. 2003;12:1–7. doi: 10.1002/pon.626. [DOI] [PubMed] [Google Scholar]
- Foster FM, Owens TW, Tanianis-Hughes J, et al. Targeting inhibitor of apoptosis proteins in combination with ErbB antagonists in breast cancer. Breast Cancer Research: BCR. 2009;11:R41. doi: 10.1186/bcr2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster LW, McLellan LJ, Rybicki LA, et al. Survival of patients who have undergone allogeneic bone marrow transplantation: The relative importance of in-hospital lay care-partner support. Journal of Psychosocial Oncology. 2004;22:1–20. [Google Scholar]
- Frey P, Stinson T, Siston A, et al. Lack of caregivers limits use of outpatient hematopoietic stem cell transplant program. Bone Marrow Transplantation. 2002;30:741–748. doi: 10.1038/sj.bmt.1703676. [DOI] [PubMed] [Google Scholar]
- Gemmill R, Cooke L, Williams AC, et al. Informal caregivers of hematopoietic cell transplant patients: A review and recommendations for interventions and research. Cancer Nursing. 2011:E13–E21. doi: 10.1097/NCC.0b013e31820a592d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin LN, Corcoran M, Winter L, et al. A randomized, controlled trial of a home environmental intervention: Effect on efficacy and upset in caregivers and on daily function of persons with dementia. The Gerontologist. 2001;41:4–14. doi: 10.1093/geront/41.1.4. [DOI] [PubMed] [Google Scholar]
- Gitlin LN, Winter L, Burke J, et al. Tailored activities to manage neuropsychiatric behaviors in persons with dementia and reduce caregiver burden: A randomized pilot study. The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry. 2008;16:229–239. doi: 10.1097/JGP.0b013e318160da72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JM, Horowitz MM. The international bone marrow transplant registry. International Journal of Hematology. 2002;76:393–397. doi: 10.1007/BF03165291. [DOI] [PubMed] [Google Scholar]
- Hack TF, Carlson L, Butler L, et al. Facilitating the implementation of empirically valid interventions in psychosocial oncology and supportive care. Supportive Care in Cancer: Official Journal of the Multinational Association of Supportive Care in Cancer. 2011;19:1097–1105. doi: 10.1007/s00520-011-1159-z. [DOI] [PubMed] [Google Scholar]
- Harmell AL, Mausbach BT, Roepke SK, et al. The relationship between self-efficacy and resting blood pressure in spousal Alzheimer’s caregivers. British Journal of Health Psychology. 2011;16:317–328. doi: 10.1348/135910710X504932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann M, Bazner E, Wild B, et al. Effects of interventions involving the family in the treatment of adult patients with chronic physical diseases: A meta-analysis. Psychotherapy and Psychosomatics. 2010;79:136–148. doi: 10.1159/000286958. [DOI] [PubMed] [Google Scholar]
- Houts PS. Home Care Guide for Cancer: How to Care for Family and Friends at Home: The College. Philadelphia PA: American College of Physician; 1994. [Google Scholar]
- Houts PS, Nezu AM, Nezu CM, et al. The prepared family caregiver: A problem-solving approach to family caregiver education. Patient Education and Counseling. 1996;27:63–73. doi: 10.1016/0738-3991(95)00790-3. [DOI] [PubMed] [Google Scholar]
- Kim Y, Duberstein PR, Sörensen S, et al. Levels of depressive symptoms in spouses of people with lung cancer: Effects of personality, social support, and caregiving burden. Psychosomatics. 2005;46:123–130. doi: 10.1176/appi.psy.46.2.123. [DOI] [PubMed] [Google Scholar]
- Lazarus R, Folkman S. Stress, Appraisal, and Coping. New York: Macmillan Publishers Limited; 1984. [Google Scholar]
- Lewis FM. Family Home Visitation Study Final Report. Bethesda MD: National Cancer Institute, National Institutes of Health; 1996. [Google Scholar]
- Loscalzo MJ, Bucher JA. The COPE model: Its clinical usefulness in solving pain-related problems. Journal of Psychosocial Oncology. 1999;16:93–117. [Google Scholar]
- McMillan SC, Small BJ, Weitzner M, et al. Impact of coping skills intervention with family caregivers of hospice patients with cancer: A randomized clinical trial. Cancer. 2006;106:214–222. doi: 10.1002/cncr.21567. [DOI] [PubMed] [Google Scholar]
- Meeker MA, Finnell D, Othman AK. Family caregivers and cancer pain management: A review. Journal of Family Nursing. 2011;17:29–60. doi: 10.1177/1074840710396091. [DOI] [PubMed] [Google Scholar]
- Meyers FJ, Carducci M, Loscalzo MJ, et al. Effects of a problem-solving intervention (COPE) on quality of life for patients with advanced cancer on clinical trials and their caregivers: Simultaneous care educational intervention (SCEI): Linking palliation and clinical trials. Journal of Palliative Medicine. 2011;14:465–473. doi: 10.1089/jpm.2010.0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezu AM, Nezu CM, Felgoise SH, et al. Project Genesis: Assessing the efficacy of problem-solving therapy for distressed adult cancer patients. Journal of Consulting and Clinical Psychology. 2003;71:1036–1048. doi: 10.1037/0022-006X.71.6.1036. [DOI] [PubMed] [Google Scholar]
- Nezu CM, Nezu AM, Friedman SH, et al. Cancer and psychological distress: Two investigations regarding the role of social problem-solving. Journal of Psychosocial Oncology. 1999;16:27–40. [Google Scholar]
- Nijboer C, Triemstra M, Tempelaar R, et al. Measuring both negative and positive reactions to giving care to cancer patients: Psychometric qualities of the Caregiver Reaction Assessment (CRA) Social Science & Medicine. 1999;48:1259–1269. doi: 10.1016/s0277-9536(98)00426-2. [DOI] [PubMed] [Google Scholar]
- Noble Walker SN, Sechrist KR, Pender NJ. The Health-Promoting Lifestyle Profile: Development and psychometric characteristics. Nursing Research. 1987;36:76–80. [PubMed] [Google Scholar]
- Northouse LL, Katapodi MC, Song L, et al. Interventions with family caregivers of cancer patients: Meta-analysis of randomized trials. CA: Cancer Journal for Clinicians. 2010;60:317–339. doi: 10.3322/caac.20081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northouse LL, Mood D, Kershaw T, et al. Quality of life of women with recurrent breast cancer and their family members. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2002;20:4050–4064. doi: 10.1200/JCO.2002.02.054. [DOI] [PubMed] [Google Scholar]
- Northouse LL, Mood DW, Schafenacker A, et al. Randomized clinical trial of a family intervention for prostate cancer patients and their spouses. Cancer. 2007;110:2809–2818. doi: 10.1002/cncr.23114. [DOI] [PubMed] [Google Scholar]
- Rabinowitz YG, Mausbach BT, Coon DW, et al. The moderating effect of self-efficacy on intervention response in women family caregivers of older adults with dementia. The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry. 2006;14:642–649. doi: 10.1097/01.JGP.0000192496.73673.e5. [DOI] [PubMed] [Google Scholar]
- Rabinowitz YG, Mausbach BT, Thompson LW, et al. The relationship between self-efficacy and cumulative health risk associated with health behavior patterns in female caregivers of elderly relatives with Alzheimer’s dementia. Journal of Aging and Health. 2007;19:946–964. doi: 10.1177/0898264307308559. [DOI] [PubMed] [Google Scholar]
- Sherwood P, Hricik A, Donovan H, et al. Changes in caregiver perceptions over time in response to providing care for a loved one with a primary malignant brain tumor. Oncology Nursing Forum. 2011;38:149–155. doi: 10.1188/11.ONF.149-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein KD, Jacobsen PB, Blanchard CM, et al. Further validation of the multidimensional fatigue symptom inventory-short form. Journal of Pain and Symptom Management. 2004;27:14–23. doi: 10.1016/j.jpainsymman.2003.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas ED, Blume KG. Historical markers in the development of allogeneic hematopoietic cell transplantation. Biology of Blood and Marrow Transplantation: Journal of the American Society for Blood and Marrow Transplantation. 1999;5:341–346. doi: 10.1016/s1083-8791(99)70010-8. [DOI] [PubMed] [Google Scholar]
- Waldron EA, Janke EA, Bechtel CF, et al. A systematic review of psychosocial interventions to improve cancer caregiver quality of life. Psycho-Oncology. 2012 doi: 10.1002/pon.3118. Epub ahead of press. [DOI] [PubMed] [Google Scholar]
- Weaver C, Schiech L, Held-Warmkessel J, et al. Risk for unplanned hospital readmission of patients with cancer: Results of a retrospective medical record review. Oncology Nursing Forum. 2006;33:E44–E52. doi: 10.1188/06.ONF.E44-E52. [DOI] [PubMed] [Google Scholar]
- Zeiss AM, Gallagher-Thompson D, Lovett S, et al. Self-efficacy as a mediator of caregiver coping: Development and testing of an assessment model. Journal of Clinical Geropsychology. 1999;5:221–230. [Google Scholar]